Abstract
The use of genetically modified (Bt) crops expressing lepidopteran-specific Cry proteins derived from the soil bacterium Bacillus thuringiensis is an effective method to control the polyphagous pest Helicoverpa armigera. As H. armigera potentially develops resistance to Cry proteins, Bt crops should be regarded as one tool in integrated pest management. Therefore, they should be compatible with biological control. Bioassays were conducted to understand the interactions between a Cry2Aa-expressing chickpea line, either a susceptible or a Cry2A-resistant H. armigera strain, and the entomopathogenic fungus Metarhizium anisopliae. In a first concentration-response assay, Cry2A-resistant larvae were more tolerant of M. anisopliae than susceptible larvae, while in a second bioassay, the fungus caused similar mortalities in the two strains fed control chickpea leaves. Thus, resistance to Cry2A did not cause any fitness costs that became visible as increased susceptibility to the fungus. On Bt chickpea leaves, susceptible H. armigera larvae were more sensitive to M. anisopliae than on control leaves. It appeared that sublethal damage induced by the B. thuringiensis toxin enhanced the effectiveness of M. anisopliae. For Cry2A-resistant larvae, the mortalities caused by the fungus were similar when they were fed either food source. To examine which strain would be more likely to be exposed to the fungus, their movements on control and Bt chickpea plants were compared. Movement did not appear to differ among larvae on Bt or conventional chickpeas, as indicated by the number of leaflets damaged per leaf. The findings suggest that Bt chickpeas and M. anisopliae are compatible to control H. armigera.
Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is one of the most important insect pests in the Old World due to its mobility, high polyphagy, short generation duration, and high reproductive rate (11, 45). Currently, the application of chemical spray insecticides is the most common method of controlling this pest on crops, including cotton (7, 22) and chickpea (44, 46). H. armigera is known to develop resistance to almost all the insecticides used for its control (14, 23). These chemical sprays are also of environmental concern and are responsible for human health problems (34, 35). Thus, alternative control methods are increasingly being employed. The use of genetically modified (GM) crops that express insecticidal genes, such as those derived from the soil bacterium Bacillus thuringiensis (Bt crops), provide a powerful option to control pest Lepidoptera (48). This technology, for example, is applied to protect cotton plants by the expression of B. thuringiensis cry genes, i.e., cry1Ac and cry2Ab, either alone or in combination, from damage by the budworm/bollworm complex [Helicoverpa/Heliothis spp. and Pectinophora gossypiella (Saunders)]. These B. thuringiensis-transgenic cotton plants are highly resistant to damage by lepidopteran pests, and consequently, the application of chemical insecticides has been greatly reduced (12, 32). This makes Bt cotton a valuable component of integrated pest management programs, with many environmental, economic, and health benefits (34, 35).
As with cotton, the expression of B. thuringiensis cry genes is an option to protect chickpeas from damage by H. armigera (41). Chickpea plants that express either Cry1Ac or Cry2Aa, or both proteins, are currently under development and could become commercially available in the future (28, 43).
The deployment of insect-resistant GM plants poses two potential problems. First, the target pest may develop resistance to the expressed insecticidal protein(s) due to the strong and continued selection pressure imposed on the insect populations (16, 48, 49). This is particularly the case for H. armigera, for which populations resistant to single Cry proteins have been selected in the laboratory (9). To manage insect resistance development, the use of high-dose-expressing Bt plants, along with an adjacent refuge of non-Bt plants, is considered to be the most effective strategy (9, 49). Most resistance alleles are recessive, and the frequency of such alleles in pest populations is generally very low before resistance becomes evident (9). However, recently, a relatively high baseline frequency of resistance alleles for Cry2Ab (0.0033) has been reported in an Australian H. armigera population prior to the widespread adoption of Bollgard II (Monsanto Company, St. Louis, MO) cotton, which expresses this protein in combination (pyramided) with Cry1Ac (26, 27).
The second area of concern is the possible effect of insect-resistant GM crops on nontarget organisms, especially those that provide important ecological services, such as biological control (39, 42). These organisms are important, since they help to keep other herbivores that are not affected by the insecticidal GM protein under their economic thresholds, but also because they potentially help to kill target insects that have developed resistance against the GM trait. Biological control of arthropods is thus considered during the environmental-risk assessment of insecticidal GM crops (42), and a great deal of research has been conducted to assess the impact of B. thuringiensis-transgenic crops on arthropod predators and parasitoids (40). Overall, studies have not revealed any direct effects of the B. thuringiensis Cry proteins on natural enemies. In contrast to arthropod natural enemies, insect pathogens have received little attention. This needs to be addressed, since it is known that the activity of entomopathogens is affected by host plant resistance factors. Hare (19) has compiled a comprehensive literature review of the interactions between host plants, herbivores, and pathogens. Additive effects of these interactions were reported in over half of the published studies, while approximately one-third reported synergistic effects. However, little attention has been given to the interactions of Bt plants with entomopathogenic fungi, despite evidence that H. armigera is attacked by a variety of entomopathogens, such as Nomuraea rileyi, Beauveria bassiana, and Metarhizium anisopliae (18). Since the application of M. anisopliae is a promising method to control H. armigera in India (29, 30, 31), it was selected for our investigations.
To evaluate the complementarity of a pathogen and a Bt plant for insect pest control, we studied the interaction of the entomopathogenic fungus M. anisopliae, a susceptible and a Cry2A-resistant strain of H. armigera, and Cry2Aa-expressing chickpea plants (transformation line BS 5A). Previous bioassays have shown that this line caused approximately 36% mortality among neonate H. armigera larvae (S. Acharjee, B. K. Sarmah, P. A. Kumar, K. M. Olsen, R. J. Mahon, W. J. Moar, A. Moore, and T. J. V. Higgins, unpublished data). This low-cry2Aa-expressing line was chosen in order to examine the combined effects of M. anisopliae and Bt chickpea plants on the susceptible H. armigera strain. To determine the responses of susceptible and Cry2A-resistant H. armigera larvae to M. anisopliae on control (non-Bt) plants, a concentration-response curve was established. Using this knowledge, we examined whether the fungus could complement the mortality induced by the toxin in the Bt plant in laboratory and greenhouse studies. Subsequently, we evaluated the feeding behavior of susceptible and Cry2A-resistant H. armigera larvae when exposed to control and Bt chickpeas.
MATERIALS AND METHODS
Plants.
B. thuringiensis-transgenic chickpea (Cicer arietinum var. Desi) plants (transformation line BS 5A; families, 5A.6.14.1, 5A.6.17.3, and 5A.6.17.4), (Acharjee et al., unpublished) expressing a full-length Cry2Aa toxin (Bt plants) and the corresponding nontransformed near-isoline cv. Semsen (control plants) were used in the bioassays. Seeds were germinated in a climate chamber at 24°C ± 1°C before being planted. The plants were grown individually in sandy soil (70% compost, 15% sand, 15% perlite) in plastic pots (15 cm in diameter; 14 cm high) at a temperature of 25 to 32°C during the day and 15 to 20°C at night with an ∼12-h photoperiod in the greenhouse. Plants 5 to 8 weeks old were used in all bioassays.
Expression of Cry2Aa protein in plants.
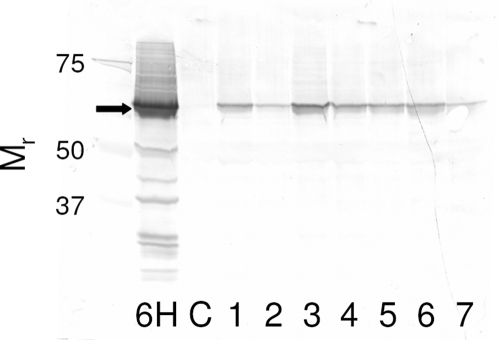
To ensure that presumptive transgenic chickpea plants were expressing the Cry2Aa protein, Western blot analyses were conducted on a total of 152 plants.
In the laboratory bioassay examining the complementarity of Bt chickpeas and M. anisopliae, 47 presumptive transgenic plants of the families used in the bioassay were analyzed. In 6% of the plants, no Cry2Aa protein could be detected. Because of the presence of nonexpressors, all transgenic plants were tested before use in subsequent bioassays. A two- to threefold variation in expression of the Cry2Aa toxin among expressors was also observed (Fig. 1).
FIG. 1.
Western blot of chickpea leaf proteins (40 μg per lane). Lane 6H contained protein from a high-expressing line that caused 98% mortality in H. armigera larvae, lane C contained protein from a nontransgenic chickpea leaf, and lanes 1 to 7 contained proteins from individual Bt chickpea plants used in the bioassays. The numbers on the y axis (Mr) refer to the relative molecular masses of markers (103). The arrow indicates the position of full-length Cry2Aa protein.
Leaves of untransformed chickpea plants were used as a negative control, while a high-expressing line, BS 6H, which caused 98% mortality in H. armigera larvae (Acharjee et al., unpublished) was used as a positive control. Protein was extracted from 80 to 100 mg of a young, fully expanded leaf into 400 μl of extraction buffer {0.1 M TES (N-tris[hydroxymethyl]methyl-2-aminoethane-sulfonic acid) (Sigma catalog no. T-5691) pH 7.6, 0.2 M NaCl, 1 mM PMSF, 1 mM EDTA}. The suspension was centrifuged at 13,000 rpm for 5 min, and the resultant supernatant was used for protein determination (6). Forty micrograms of protein (with disulfide bonds reduced in the presence of β-mercaptoethanol) from each sample was separated by size fractionation in a NuPage precast 10% Bis-Tris polyacrylamide gel system (Invitrogen catalog no. NP0315), using a MOPS (morpholinepropanesulfonic acid)-sodium dodecyl sulfate running buffer (50 mM MOPS, 50 mM Tris base, 3.5 mM sodium dodecyl sulfate, 1 mM EDTA). The protein was transferred electrophoretically to a nitrocellulose membrane (200 mA for 90 min) using transfer buffer (25 mM Bicine, 25 mM Bis-Tris, 1 mM EDTA, 10% methanol). The nitrocellulose membrane was blocked in Tris-buffered saline solution (20 mM Tris, pH 7.5, 0.5 M NaCl) and 5% skim milk powder for 1 h. The membrane was washed in Tris-buffered saline solution and 0.1% Tween 20 (TTBS). The primary anti-Cry2Aa antibody, raised in rabbit, was diluted in TTBS and incubated with the membrane for 1 h before being washed briefly with TTBS. The secondary antibody, anti-rabbit immunoglobulin G (Fc)-alkaline phosphatase conjugate (Promega catalog no. S3731), was diluted in TTBS and incubated with the membrane for 1 h before being washed briefly in TTBS. Cry2Aa protein bands were detected by the addition of BCIP (5-bromo-4-chloro-3-indolylphosphate)/Nitro Blue Tetrazolium substrate (Sigma catalog no. B5655).
Insect material.
Strains of susceptible and Cry2A-resistant H. armigera were provided by CSIRO Entomology, Canberra, Australia. The Cry2A-resistant strain (SP15) was established from a single H. armigera pair collected as eggs on corn near Griffith, NSW (27). To maintain fitness vigor, the Cry2A-resistant strain was outcrossed with a susceptible strain (GR). After three outcrosses to the susceptible strain, the Cry2A-resistant colony was genetically very similar (87% isogenic) to the susceptible strain (with the exception of the linkage group containing the gene conferring resistance) (27).
Larvae were reared as described by Teakle and Jensen (50), except that three or four neonates were kept in each well (3 cm by 3 cm by 2 cm) of a 32-well plastic tray (Oliver Products Company, Grand Rapids, MI) until they reached the third instar. Subsequently, the larvae were separated and kept in individual wells on a fresh diet. Larvae used to evaluate the sensitivity of H. armigera to M. anisopliae were treated as described above. For bioassays involving Bt chickpea plants, ∼50 neonates were reared in plastic boxes (12 cm in diameter; 6 cm high) on control chickpea leaves (10 to 12 weeks old) until they reached the third instar. Two pieces (ca. 5 ml) of 2% agar were included in the box to raise the humidity in order to limit leaf desiccation. The larvae were refed after 2 or 3 days if necessary. Early-third-instar larvae were used in all bioassays. Adults were housed as described by Mahon et al. (26).
Fungus.
M. anisopliae var. anisopliae (FI-1248) from the CSIRO Insect Pathogen Culture collection was used in the experiments. The strain was originally isolated from a termite, Mastotermes darwiniensis, collected near Darwin, Northern Territory, Australia, in 1997. M. anisopliae was grown on Oxoid Sabouraud's dextrose agar plus 1% yeast extract for 3 weeks at ∼24°C under natural daylight conditions. Spores were harvested by scraping them from the agar surface using a loop and stored at 4°C until they were used. Clumps of spores were then dispersed in 0.5% Tween 80 using a magnetic stirrer for 1 h. The concentration of conidia was estimated using a Petroff-Hausser counting chamber (Hausser Scientific Partnership, Horsham, PA; 1/400 mm2; 0.02 mm deep). The initial suspension was serially diluted with 0.5% Tween 80 to the concentrations used in the experiments.
Prior to each bioassay, a sample of spores was taken to determine viability by germinating conidia on thin plates of Sabouraud's dextrose agar with 0.1% chloramphenicol. A droplet of spore suspension (∼107 spores/ml) was pipetted onto the plate, covered with a thin coverslip, and incubated at ∼28°C in the dark for 24 h. The plate was examined using a phase-contrast microscope (Leitz, Wetzlar, Germany) at ×400 magnification. One hundred spores were examined at three locations on each plate and scored as either germinated (viable) or not germinated (dead). A spore was considered to have germinated if the germ tube was clearly visible. Germination was >90% in all bioassays.
Sensitivities of susceptible and Cry2A-resistant H. armigera larvae to M. anisopliae: first laboratory bioassay.
Control chickpea leaves (8 leaflets each) were dipped into six M. anisopliae Tween 80-based spore suspensions prepared as fivefold serial dilutions ranging from 9.6 × 105 to 3 × 109 spores/ml. A 0.5% Tween 80 solution was used as a control. The dipped chickpea leaves were placed on the surface of 4 to 5 ml cooled 2% agar in wells of 32-well plastic trays. After 2 to 3 h of exposure to air (to allow the leaves to dry), a single early-third-instar H. armigera larva was placed on each leaf. The trays were then heat sealed with a vented acetate cover and maintained at 28°C ± 1°C. To give the fungus optimal conditions for germination, the trays were wrapped in damp tissues and enclosed in a plastic bag to provide a humid environment for the first 24 h. On days 2, 4, and 6, dead larvae were removed and fresh untreated leaves (8 to 10 leaflets each) were provided to each living larva. On day 8, survivors from each treatment were pooled, and their fresh weight and larval stage were recorded. Dead larvae were incubated at 28°C and >90% rH for up to 10 days and examined regularly for evidence of conidial growth. The bioassay was repeated twice, resulting in the exposure of a total of 55 to 61 larvae to each spore concentration. Slopes, intercepts, and 50% lethal concentration (LC50) estimates for the two H. armigera strains were calculated using the software package POLO-PC (LeOra Software, Berkeley, CA).
Bt chickpeas and M. anisopliae in combination to control H. armigera. (i) Second laboratory bioassay.
Control and Bt chickpea leaves (eight leaflets each) were dipped either in a Tween 80-based suspension of a “low” (L) spore suspension (1.2 × 108 spores/ml), in a “medium” (M) spore suspension (5.7 × 108 spores/ml), or in 0.5% Tween 80 (0) as a control. The L and M spore suspensions were chosen to lie approximately midway between the LC30s (L) or LC50s (M) of the two H. armigera strains, respectively, as determined in the concentration-response assay. The bioassays were set up as described above, except that the larvae used in the experiments were reared on control chickpea leaves until they reached the third instar. The larvae were fed with eight leaflets each (four leaflets each from two plants to provide a mixture of plants that might have different expression levels). The leaves were changed on days 2, 4, and 6. The parameters assessed were larval survival, weight, and instar after 8 days and the proportion of larvae producing M. anisopliae spores. The bioassay was repeated four times, with 30 to 32 larvae tested at each spore concentration.
Larval survival was analyzed using the Cox proportional-hazard model. Bonferroni-Holm correction was performed when required. Each run was analyzed separately, comparing the fungus treatments (L and M) and the controls (susceptible/Cry2A-resistant strain on control/Bt leaves with no fungus application). Where no larvae died in a control group, one additional dead larva was added to each treatment to enable statistical analysis. This procedure was necessary for susceptible larvae, run B on control plants, and for Cry2A-resistant larvae, runs C and D on Bt plants. Data on larval weights after 8 days of feeding were checked for normality and homogeneity of variances prior to analysis. Since all assumptions were met, the data were analyzed for all repeated experiments (runs), together with a three-way analysis of variance (ANOVA) (factors: plant, fungus, and H. armigera strain; n = 125 to 128). For all tests, the α-level was set at 5%. Statistical analyses were conducted using the software package Statistica (version 6; StatSoft Inc., Tulsa, OK).
(ii) Greenhouse bioassay.
Half of the available control and Bt chickpea plants were sprayed until runoff with an M. anisopliae spore suspension containing 5.4 × 108 spores/ml. The remainder were sprayed with 0.5% Tween 80 as a control. After the plants were allowed to dry for 1 h, 10 susceptible early-third-instar larvae were placed on each plant, each on a separate leaf. The plants were then enclosed in a cloth bag, which was sealed to the pot to ensure that the larvae could not escape. To provide humid conditions, the plants were enclosed in a plastic bag for the first 24 h. The plants were watered every 2 days by placing the pots into water-filled dishes (17 cm in diameter; 2 cm deep) for 2 to 3 h. The mortality of the larvae was evaluated after 10 days. In total, three to five plants were used for each of the four treatments. The plants were placed at randomized positions in the greenhouse. During the experiment, the greenhouse temperature varied between 15°C and 35°C with an ∼12-h photoperiod and ∼40% rH.
Feeding behavior of susceptible and Cry2A-resistant H. armigera larvae on control and Bt chickpeas.
Control or Bt chickpea leaves of similar sizes and structures (10 leaflets each) were placed in petri dishes (9 cm in diameter; 2 cm high). Subsequently, one early-third-instar H. armigera larva, either susceptible or Cry2A resistant, was placed on the lowest leaflet of either control or Bt leaves. The petri dishes were stored at 25 ± 1°C, 40 ± 5% rH, and a 14-h photoperiod. After 24 h, the larvae were removed and leaf feeding activity was evaluated using a nine-category scale according to the damage inflicted by the feeding larvae (categories: 0, 0% damage; 1, <1%; 2, 2 to 5%; 3, 5 to 10%; 4, 10 to 20%; 5, 20 to 30%; 6, 30 to 50%; 7, 50 to 70%; 8, 70 to 80%; 9, >80%). Feces produced during the exposure period by each larva were collected and stored in a desiccator containing silica gel for at least 24 h before storage at −80°C. Samples were dried further at 50°C in an oven for at least 4 days before being weighed on a microbalance (Mettler Toledo MX5; division, 1 μg; tolerance, ±2 μg). The experiment was repeated twice, resulting in a total of 32 to 43 larvae per treatment. The data on feeding damage and feces weight were evaluated by Kruskal-Wallis ANOVA, followed by pairwise comparisons using the Mann-Whitney U test adjusted for ties and Bonferroni-Holm correction. The importance of two factors, plant (control or Bt) and strain (susceptible or Cry2A-resistant), was evaluated.
RESULTS
Sensitivities of susceptible and Cry2A-resistant H. armigera larvae to M. anisopliae: first laboratory bioassay.
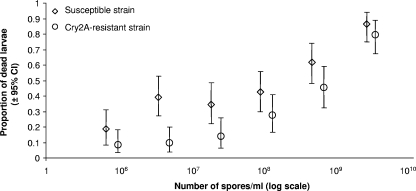
The concentration response of the two H. armigera strains to M. anisopliae is given in Fig. 2, and details of the mortality, sporulation, weight, and larval instar of survivors are shown in Table 1. The LC50 for the susceptible strain was determined to be 1.9 × 108 spores/ml (95% confidence interval [CI], 1.5 × 107, 8.1 × 108), while for the Cry2A-resistant strain, it was 7.8 × 108 spores/ml (95% CI, 4.5 × 108, 1.3 × 109). The slope of the line for susceptible larvae (0.95 ± 0.187) differed significantly from that of the Cry2A-resistant strain (1.94 ± 0.370; P = 0.002).
FIG. 2.
Proportions of dead susceptible and Cry2A-resistant H. armigera larvae (±95% CI) fed on control chickpea leaves treated with six different spore concentrations of M. anisopliae differing fivefold (n = 55 to 61). Third-instar larvae were treated with the fungus.
TABLE 1.
Evaluation of the sensitivities of susceptible and Cry2A-resistant H. armigera larvae to M. anisopliaea
| Spore concn (no. of spores/ml) | H. armigera strain | No. of larvae exposed | No. of dead larvae (no. of larvae producing spores) | Mortality (%) | Mean wt (mg) | Distribution of larval instars (%)
|
||
|---|---|---|---|---|---|---|---|---|
| L3 | L4 | L5 | ||||||
| 0 | Susceptible | 60 | 6 (0) | 10.0 | 68.0 | 0 | 48.2 | 51.9 |
| Cry2A resistant | 60 | 6 (0) | 10.0 | 77.7 | 0 | 37.0 | 63.0 | |
| 9.6 × 105 | Susceptible | 59 | 11 (0) | 18.6 | 68.1 | 0 | 51.1 | 48.9 |
| Cry2A resistant | 59 | 5 (0) | 8.5 | 67.2 | 0 | 59.3 | 40.7 | |
| 4.8 × 106 | Susceptible | 61 | 24 (6) | 39.3 | 66.7 | 0 | 75.7 | 24.3 |
| Cry2A resistant | 61 | 6 (1) | 9.8 | 75.9 | 0 | 65.5 | 34.6 | |
| 2.4 × 107 | Susceptible | 55 | 19 (6) | 34.5 | 63.3 | 0 | 81.1 | 18.9 |
| Cry2A resistant | 57 | 8 (2) | 17.0 | 83.1 | 0 | 69.4 | 30.6 | |
| 1.2 × 108 | Susceptible | 59 | 25 (15) | 42.4 | 59.3 | 0 | 88.2 | 11.8 |
| Cry2A resistant | 58 | 16 (8) | 27.6 | 76.9 | 0 | 63.4 | 36.6 | |
| 6 × 108 | Susceptible | 60 | 37 (27) | 61.7 | 63.2 | 12.0 | 48.0 | 40.0 |
| Cry2A resistant | 57 | 26 (24) | 45.6 | 72.4 | 0 | 56.7 | 43.3 | |
| 3 × 109 | Susceptible | 59 | 51 (48) | 86.4 | 51.2 | 0 | 100 | 0 |
| Cry2A resistant | 59 | 47 (46) | 79.7 | 57.9 | 9.1 | 72.7 | 18.2 | |
Larvae were exposed to chickpea leaves treated with six different spore concentrations for 2 days, differing fivefold in their spore concentrations. Subsequently, the larvae were provided with untreated chickpea leaves every other day until day 8. Weight is shown as the mean weight of all survivors per treatment. The larval instar reached by surviving larvae at the completion of the experiment is given as the percentage of survivors. n = 55 to 61.
Bt chickpeas and M. anisopliae in combination to control H. armigera. (i) Second laboratory bioassay.
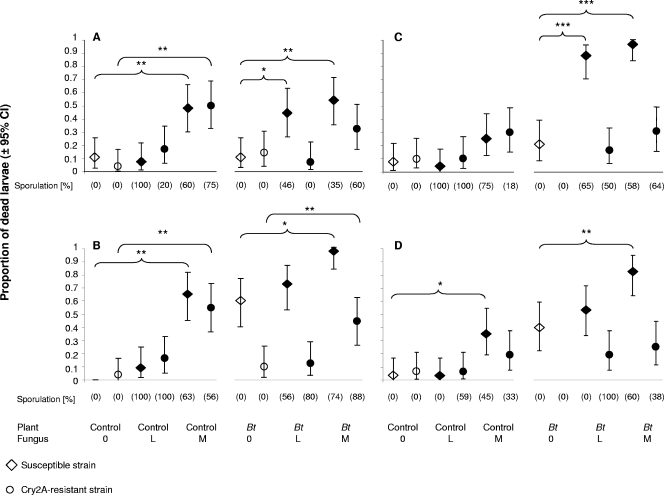
The performances of larvae on control and Bt chickpea leaves at different concentrations of fungal spores are shown in Fig. 3. For the control chickpea leaves, mortality rates in the H. armigera strains were similar (P > 0.05); however, a marked fungus effect (P < 0.0001) was observed. For the Bt chickpea leaves, both strain (P < 0.0001) and fungus (P < 0.0001) effects were recorded. The data were thus analyzed separately for each H. armigera strain. Since larval survival differed significantly between bioassay runs for control and Bt leaves (P = 0.003; P = 0.018), each run was evaluated separately (runs A to D in Fig. 3).
FIG. 3.
Proportions of dead susceptible and Cry2A-resistant H. armigera larvae (±95% CI) fed on control or Bt chickpea leaves treated with different concentrations of M. anisopliae spores [0 (0.5% Tween 80), L (1.2 × 108 spores/ml), and M (5.7 × 108 spores/ml)]. The experiment was repeated four times (runs A to D) with an n of 30 to 32 per run. Statistical comparisons were made separately for each H. armigera strain and for control or Bt chickpea leaves. Statistical significances are shown between the fungus treatments (L and M) and the controls (susceptible/Cry2a-resistant strains on control/Bt leaves with no fungus application). No control mortality occurred for susceptible larvae in run B and on Bt leaves for Cry2A-resistant larvae in runs C and D. *, P < 0.05; **, P < 0.01; ***, P < 0.001; Cox proportional-hazard model. Sporulation (percent) in dead larvae is given for each treatment. Open symbols refer to the control treatments; filled symbols refer to the fungus treatments.
While the L spore concentration did not increase mortality among susceptible H. armigera larvae feeding on control leaves (P > 0.05), a significant increase was observed in two of the four runs when the larvae were fed Bt leaves (run A, P = 0.006; run C, P < 0.0001). In contrast, the M spore concentration caused a significant level of mortality in susceptible H. armigera larvae on control leaves in three of the four runs (run A, P = 0.009; run B, P < 0.001; run D, P = 0.033) and in all four runs on Bt leaves (run A, P = 0.035; run B, P = 0.022; run C, P < 0.0001; run D, P = 0.002). Mortality was not increased at the L spore concentration on control plants in the Cry2A-resistant strain in any of the runs (P > 0.05). The M spore concentration caused significant mortality among Cry2A-resistant larvae in two runs (run A, P = 0.009; run B, P = 0.005) when control leaves were fed and in one run (run B, P = 0.014) when the larvae were feeding on Bt leaves.
A three-way ANOVA evaluating the factors fungus (0, L, or M), H. armigera strain (susceptible or Cry2A resistant), and plant (control or Bt) indicated that a significant decrease in larval weight occurred after 8 days due to the factors strain and plant (P < 0.0001). However, the factor fungus did not significantly contribute to this decrease (P > 0.05) (Table 2).
TABLE 2.
Weights and developmental stages of susceptible and Cry2A-resistant H. armigera larvae after 8 days of feeding on either control or Bt chickpea leaves treated with different spore concentrations of M. anisopliaea
| Plant | Fungusb | H. armigera strain | Mean wt (mg) ± SEc | Distribution of larval instars (%)d
|
||
|---|---|---|---|---|---|---|
| L3 | L4 | L5 | ||||
| Control | 0 | Susceptible | 42.0 ± 0.7 a | 2.5 | 41.0 | 56.6 |
| Cry2A resistant | 45.1 ± 0.5 a | 47.1 | 52.9 | |||
| L | Susceptible | 38.6 ± 0.7 a | 5.0 | 57.9 | 37.2 | |
| Cry2A resistant | 35.8 ± 0.6 a | 63.1 | 36.9 | |||
| M | Susceptible | 34.3 ± 0.7 a | 12.3 | 53.4 | 34.2 | |
| Cry2A resistant | 35.6 ± 0.4 a | 5.1 | 73.1 | 21.8 | ||
| Bt | 0 | Susceptible | 16.8 ± 0.8 b | 41.2 | 48.2 | 10.6 |
| Cry2A resistant | 42.3 ± 0.9 a | 1.7 | 46.6 | 51.7 | ||
| L | Susceptible | 28.0 ± 0.2 b | 37.8 | 53.3 | 8.9 | |
| Cry2A resistant | 41.7 ± 1.1 a | 4.5 | 53.6 | 41.8 | ||
| M | Susceptible | 16.0 ± 0.7 b | 17.4 | 60.9 | 21.7 | |
| Cry2A resistant | 37.8 ± 1.1 a | 3.4 | 50.6 | 46.0 | ||
Data from the four runs (Fig. 3) were pooled (n = 125 to 128).
0, control; L, 1.2 × 108 spores/ml; M, 5.7 × 108 spores/ml.
Weight is shown as the mean weight of all surviving larvae per treatment. Different letters indicate significant differences (P < 0.05) between means of a group (i.e., a certain “plant” and “fungus” treatment).
The larval instar reached after 8 days is given as a percentage of the survivors.
(ii) Greenhouse bioassay.
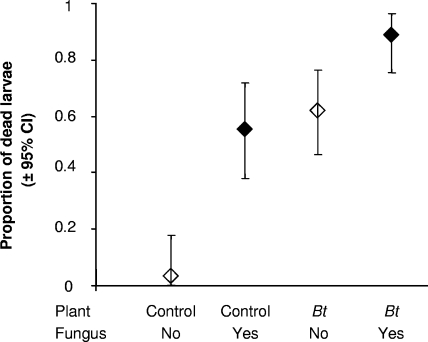
The greenhouse bioassay was conducted only with susceptible H. armigera larvae, since the second laboratory bioassays revealed no interaction of Bt plants and fungal efficacy for the Cry2A-resistant H. armigera strain. Hardly any larval mortality was observed on the untreated control plants. Approximately 50% of the H. armigera larvae died on untreated Bt plants and fungus-treated control plants. Combining the B. thuringiensis protein and the fungus caused a mortality rate of 89%. Consequently, the greenhouse bioassay suggested an additive effect of the B. thuringiensis toxin and the fungus on the mortality of susceptible H. armigera larvae (Fig. 4). Of the larvae that had fed on fungus-treated leaves, 25 to 100% of the larvae from the control plants and 20 to 86% of the larvae from the Bt plants produced fungal spores. None of the dead larvae on the control treatments produced M. anisopliae spores.
FIG. 4.
Proportions of dead susceptible H. armigera larvae (±95% CI) fed on control or Bt chickpea leaves treated with M. anisopliae (5.4 × 108 spores/ml) or 0.5% Tween 80 in the greenhouse. The mortality of susceptible H. armigera larvae was recorded after 10 days. n = 3 to 5 plants per treatment.
Toward the end of the experimental period, an incursion of an unknown insect pathogen, or perhaps insecticide use in neighboring glasshouses, caused mortality to insects in all treatments, including controls. This prevented planned replication of this bioassay. Thus, no statistical analyses were performed.
Feeding behavior of susceptible and Cry2A-resistant H. armigera larvae on control and Bt chickpeas.
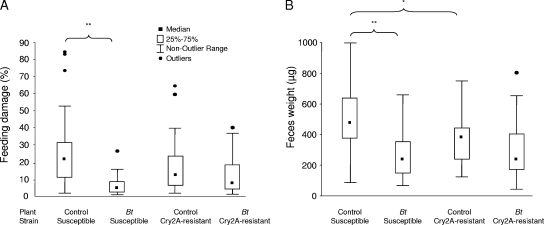
There was a high correlation between feeding damage caused by H. armigera larvae on the chickpea leaves and the weight of feces they excreted (R2 = 0.716). For the susceptible H. armigera strain, leaf damage was significantly higher for control leaves than for Bt chickpea leaves after 24 h of feeding (P < 0.001) (Fig. 5A). The difference in feeding activities on the two plant types was also evident in the feces weight measurements (P < 0.001) (Fig. 5B). In contrast, the Cry2A-resistant strain inflicted similar levels of feeding damage (P > 0.05) and produced similar amounts of feces (P > 0.05). On control plants, the two strains inflicted similar levels of leaf damage; however, significantly more feces were produced by susceptible larvae (P = 0.007). Data for the two experimental runs were combined for the analysis, since they revealed similar patterns.
FIG. 5.
Boxplots showing the distribution of feeding damage (percent; n = 32 to 43) (A) and feces weights (μg; n = 27 to 40) (B) for susceptible and Cry2A-resistant H. armigera larvae fed for 24 h on control or Bt chickpea leaves. *, P < 0.01; **, P < 0.001. The nonoutlier range is the range of values that fall above the upper outlier limit (+1.5 × the height of the box) and above the below-outlier limit (−1.5 × the height of the box).
No significant difference was observed in the number of leaflets damaged per leaf provided to the two strains on either plant type during 24 h (means for the susceptible strain, 8.1 to 9.3 leaflets damaged/leaf; Cry2A-resistant strain, 8.7 to 9.1 leaflets damaged/leaf).
DISCUSSION
Our studies revealed that M. anisopliae is effective at killing both susceptible and Cry2A-resistant H. armigera larvae on control and Bt chickpeas and that the number of larvae producing M. anisopliae spores did not differ between the two H. armigera strains. In some treatments, 20 to 100% of larvae that were apparently killed by the fungus did not produce fungal spores. It is known that entomopathogenic fungi can cause high mortality with little sporulation (47). It was suggested in earlier studies that the insects were killed by toxins, such as destruxins, produced by M. anisopliae (21, 51).
The Cry2A-resistant strain of H. armigera appeared to be more tolerant of M. anisopliae than the susceptible strain in the concentration-response bioassay in which larvae were fed control chickpea leaves treated with various spore concentrations of the entomopathogenic fungus. However, this finding was not confirmed in the second laboratory bioassay, in which larvae received control or Bt chickpea leaves treated with no fungus or an L or an M spore concentration. In this bioassay, no difference in larval susceptibility to M. anisopliae was observed between the two H. armigera strains while feeding on fungus-treated control chickpea leaves.
A number of studies have shown significant fitness costs with some laboratory-selected B. thuringiensis-resistant strains of different species of Lepidoptera (2). Fitness costs may be expressed in a variety of forms, e.g., reduced survival rates; diminished fertility, fecundity, and mating ability; and increased overwintering mortality and developmental rates. For larvae of a Cry1Ac-resistant strain of H. armigera, a reduced survival rate and an increased development time on different host plants were observed (1, 5). These fitness costs can also be expressed as an increased susceptibility to natural enemies, such as entomopathogenic nematodes (15) or insect viruses (38). However, we did not detect a higher susceptibility of Cry2A-resistant H. armigera larvae to infection by M. anisopliae, which is supported by the fact that previous studies had revealed that the two H. armigera strains used in our study are indistinguishable in a number of life table parameters (R. J. Mahon, unpublished data). Our findings are consistent with the study by Johnson et al. (20), who did not find a higher fungal infection rate with N. rileyi in B. thuringiensis-resistant Heliothis virescens larvae. Likewise, susceptibility to nucleopolyhedrovirus infection was not increased in a B. thuringiensis-resistant Plutella xylostella strain (37) and B. thuringiensis resistance in larvae of the flour moth Ephestia kuehniella had no effect on parasitism by an endoparasitoid (36).
Interestingly, a greater susceptibility to M. anisopliae occurred when susceptible H. armigera larvae fed on Bt chickpea leaves than when they fed on control leaves. On control leaves, the L spore concentration of the fungus did not cause mortality, while significantly increased mortality (31 to 65%) due to the M spore concentration was observed in three out of four bioassays (Fig. 3A, B, and D) (the L spore concentration refers to the approximate LC30 calculated in the concentration-response curve and the M spore concentration refers to the LC50). When susceptible H. armigera larvae fed on Bt chickpea leaves treated with M. anisopliae, an additive effect occurred at an M spore concentration, with larval mortalities between 53 and 97%, whereas at an L spore concentration, the effect was more than additive in two out of four bioassays, resulting in 72 and 87% mortality (Fig. 3A and C). Interestingly, this was observed when the untreated Bt leaves caused little mortality to susceptible H. armigera larvae (9 and 20%). In the two runs (runs B and D) in which larval mortality was already substantial on the Bt leaves (39 and 59%,) no significant increase in larval mortality due to an L M. anisopliae spore concentration was observed (52 and 72%). Varying levels of mortality on Bt chickpea leaves in susceptible H. armigera larvae probably reflected varying expression levels in the Bt chickpea plants to which they were exposed. The variation may be due to segregation of at least two copies of the gene present at different loci (Acharjee et al., unpublished). As the leaves provided to the H. armigera larvae were always taken from two different plants during each feeding regime and a weight reduction in survivors was measured in each experimental run, it can be concluded that larvae always ingested at least low doses of B. thuringiensis toxin, which caused sublethal damage that subsequently resulted in the enhanced efficacy of the entomopathogenic fungus. The Cry2A-resistant larvae showed no indication of deleterious effects of very high levels of Cry2Ab toxin and were cross-resistant to Cry2Aa (27). Therefore, it was not surprising that mortalities of resistant insects induced by M. anisopliae did not differ when larvae fed on either control or Bt chickpea leaves. Once higher-expressing Bt chickpeas are available that are appropriate for H. armigera control in the field, additional studies should examine the effect of the entomopathogenic fungus on Cry2A-resistant larvae in more detail. Those data would also be valuable in the context of resistance management.
The application of pathogens as a biopesticide in combination with B. thuringiensis-transgenic plants to control pest Lepidoptera has previously been examined in the laboratory. For susceptible H. virescens larvae, a synergistic effect was observed between Cry1Ab-expressing tobacco plants and the entomopathogenic fungus N. rileyi (20). Unlike bacteria and viruses, fungi can infect insects not only through the gut, but also through spiracles and, in particular, through the surface of the integument (10). This leads to the possibility of infecting insects independently of their feeding activity. A previous study reported that susceptible larvae of H. virescens moved more than resistant larvae on B. thuringiensis (Cry1Ab)-expressing tobacco plants (20). Consequently, when the plants were treated with a pathogen, susceptible larvae were more likely to be infected than B. thuringiensis-resistant larvae. Similarly, a higher level of activity was reported for B. thuringiensis-susceptible Spodoptera exigua larvae when feeding on a B. thuringiensis-containing diet than on a control diet (4). Interestingly, in some cases of orally active pathogens, such as nucleopolyhedrovirus, antagonistic effects by B. thuringiensis were reported (8, 25, 37). This could be due to the feeding-deterrent effect of the B. thuringiensis toxin, which reduces the consumption rate of plant material and thus ingestion of the virus.
In our studies, the movements of larvae of both H. armigera strains were similar on both Bt and non-Bt chickpea leaves, as indicated by the number of leaflets damaged per leaf. This finding is supported by behavioral observations made over an 11-h period of the movements of susceptible and Cry2A-resistant larvae on either control or Bt chickpea leaves, which did not reveal any obvious differences (data not presented). One possible explanation for the lack of activity differences observed in this study is that the B. thuringiensis expression level in the chickpea plants employed may have been simply too low to cause behavioral effects in H. armigera, as has been suggested for H. virescens on low-expressing Bt cotton lines (3). However, the chickpea plants were clearly expressing at some level, as susceptible H. armigera larvae caused significantly less damage to Bt chickpea leaves than to control leaves. In the case of the Cry2A-resistant strain, no difference in feeding activity between the plant types was observed. In contrast to the leaf damage data, our study revealed a difference in feces production within 24 h by both H. armigera strains while feeding on control chickpea leaves. A reason for this difference is not obvious, but it could be a discrepancy in food utilization between strains. If food utilization differences were responsible, one would expect to see differential larval and pupal weights. However, such differences were not seen when the two genotypes were fed an artificial diet, cotton or pigeonpea plants (Mahon, unpublished).
Our laboratory/glasshouse studies with low-expressing Bt chickpea plants and M. anisopliae have shown that the two control methods are generally complementary for the control of H. armigera. Bt chickpea plants that are developed for commercial release will need to provide much greater control than the plants used in our study. Furthermore, they are likely to express two Cry proteins that are sufficiently different that insects resistant to one would still be susceptible to the other. Such a pyramid of cry genes should provide good control, as well as reduce the likelihood of the development of resistance by the target pest (2, 9). Nevertheless, it is likely that some H. armigera larvae will survive in a Bt chickpea crop. First, we do not expect that the Bt technology will provide 100% control, and therefore, occasional susceptible larvae will survive. This could occur, for example, through selective feeding on lower-expressing tissues, or the toxin concentration could decline in chickpea plants after flowering, as has been documented in cotton crops (13, 24, 33). Secondly, larvae might survive on Bt chickpeas through possession of a level of tolerance or resistance to the expressed Cry proteins. In both cases, the impact of natural enemies, such as M. anisopliae, will help to kill survivors and potentially decrease the speed of resistance development (17, 38).
Acknowledgments
We thank Andy Moore from CSIRO Plant Industry for conducting the Western blot analyses and the B. thuringiensis resistance group at CSIRO Entomology for support and advice on Lepidoptera rearing, especially Su Young, who always knew an easier way.
The study was funded by the Pulse Network of the Indo-Swiss Collaboration in Biotechnology (ISCB) and the graduate school of the National Centre of Competence in Research (NCCR) “Plant Survival.”
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Akhurst, R. J., W. James, L. J. Bird, and C. Beard. 2003. Resistance to the Cry1Ac δ-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96:1290-1299. [DOI] [PubMed] [Google Scholar]
- 2.Bates, S. L., J.-Z. Zhao, R. T. Roush, and A. M. Shelton. 2005. Insect resistance management in GM crops: past, present and future. Nat. Biotechnol. 23:57-62. [DOI] [PubMed] [Google Scholar]
- 3.Benedict, J. H., D. W. Altman, P. F. Umbeck, and D. R. Ring. 1992. Behavior, growth, survival, and plant injury by Heliothis virescens (F) (Lepidoptera, Noctuidae) on transgenic Bt cotton. J. Econ. Entomol. 85:589-593. [Google Scholar]
- 4.Berdegué, M., J. T. Trumble, and W. J. Moar. 1996. Effect of Cry1C toxin from Bacillus thuringiensis on larval feeding behavior of Spodoptera exigua. Entomol. Exp. Appl. 80:389-401. [Google Scholar]
- 5.Bird, L. J., and R. J. Akhurst. 2007. Effects of host plant species on fitness costs of Bt resistance in Helicoverpa armigera (Lepidoptera: Noctuidae). Biol. Contr. 40:196-203. [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Durairay, C., G. V. Subbaratnam, T. V. K. Singh, and T. G. Shanower. 2005. Helicoverpa in India: spatial and temporal dynamics and managment options, p. 91-117. In H. C. Sharma (ed.), Heliothis/Helicoverpa management: emerging trends and strategies for future research. IBH Publishing Co., New Delhi, India.
- 8.Farrar, R. R., M. Shapiro, and B. M. Shepard. 2004. Activity of the nucleopolyhedrovirus of the fall armyworm (Lepidoptera: Noctuidae) on foliage of transgenic sweet corn expressing a Cry1A(b) toxin. Environ. Entomol. 33:982-989. [Google Scholar]
- 9.Ferré, J., J. van Rie, and S. C. MacIntosh. 2008. Insecticidal genetically modified crops and insect resistance management (IRM), p. 41-85. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, The Netherlands.
- 10.Ferron, P. 1978. Biological control of insect pests by entomogenous fungi. Annu. Rev. Entomol. 23:409-442. [Google Scholar]
- 11.Fitt, G. P. 1989. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 34:17-52. [Google Scholar]
- 12.Fitt, G. P. 2008. Have Bt crops led to changes in insecticide use patterns and impacted IPM?, p. 303-328. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, The Netherlands.
- 13.Fitt, G. P., C. L. Mares, and D. J. Llewellyn. 1994. Field evaluation and potential ecological impact of transgenic cotton (Gossypium hirsutum) in Australia. Biocontr. Sci. Technol. 4:535-548. [Google Scholar]
- 14.Forrester, N. W., M. Cahill, L. Bird, and J. K. Layland. 1993. Management of pyrethoid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bull. Entomol. Res. Suppl. Ser. 1:1-132. [Google Scholar]
- 15.Gassmann, A. J., S. P. Stock, Y. Carriere, and B. E. Tabashnik. 2006. Effect of entomopathogenic nematodes on the fitness cost of resistance to Bt toxin Cry1Ac in pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 99:920-926. [DOI] [PubMed] [Google Scholar]
- 16.Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 43:701-726. [DOI] [PubMed] [Google Scholar]
- 17.Gould, F., G. G. Kennedy, and M. T. Johnson. 1991. Effects of natural enemies on the rate of herbivore adaptation to resistant host plants. Entomol. Exp. Appl. 58:1-14. [Google Scholar]
- 18.Grzywacz, D., A. Richards, R. J. Rabindra, H. Saxena, and O. P. Rupela. 2005. Efficacy of biopesticides and natural plant products for Heliothis/Helicoverpa control, p. 371-389. In H. C. Sharma (ed.), Heliothis/Helicoverpa management. Emerging trends and strategies for future research. IBH Publishing Co., New Delhi, India.
- 19.Hare, J. D. 2002. Plant genetic variation in tritrophic interactions, p. 8-43. In T. Tscharntke, and B. A. Hawkins (ed.), Multitrophic interactions level. Cambridge University, Cambridge, United Kingdom.
- 20.Johnson, M. T., F. Gould, and G. G. Kennedy. 1997. Effects of natural enemies on relative fitness of Heliothis virescens genotypes adapted and not adapted to resistant host plants. Entomol. Exp. Appl. 82:219-230. [Google Scholar]
- 21.Kershaw, M. J., E. R. Moorhouse, R. Bateman, S. E. Reynolds, and A. K. Charnley. 1999. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 74:213-223. [DOI] [PubMed] [Google Scholar]
- 22.King, A. B. S. 1994. Heliothis/Helicoverpa (Lepidoptera: Noctuidae), p. 39-106. In G. A. Matthews and J. P. Tunstall (ed.), Insect pests of cotton. CAB International, Wallingford, Oxon, United Kingdom.
- 23.Kranthi, K. R., D. R. Jadhav, S. Kranthi, R. R. Wanjari, S. S. Ali, and D. A. Russell. 2002. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 21:449-460. [Google Scholar]
- 24.Kranthi, K. R., S. Naidu, C. S. Dhawad, A. Tatwawadi, K. Mate, E. Patil, A. A. Bharose, G. T. Behere, R. M. Wadaskar, and S. Kranthi. 2005. Temporal and intra-plant variability of Cry1Ac expression in Bt cotton and its influence on the survival of the cotton bollworm Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera). Curr. Sci. 89:291-298. [Google Scholar]
- 25.Liu, X. X., Q. W. Zhang, B. L. Xu, and J. C. Li. 2006. Effects of Cry1Ac toxin of Bacillus thuringiensis and nuclear polyhedrosis virus of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on larval mortality and pupation. Pest Manag. Sci. 62:729-737. [DOI] [PubMed] [Google Scholar]
- 26.Mahon, R. J., K. M. Olsen, S. Downes, and S. Addison. 2007. Frequency of alleles conferring resistance to the Bt toxins Cry1Ac and Cry2Ab in Australian populations of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Econ. Entomol. 100:1844-1853. [DOI] [PubMed] [Google Scholar]
- 27.Mahon, R. J., K. M. Olsen, K. A. Garsia, and S. R. Young. 2007. Resistance to Bacillus thuringiensis toxin Cry2Ab in a strain of Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. J. Econ. Entomol. 100:894-902. [DOI] [PubMed] [Google Scholar]
- 28.McPhee, K. E., J. Croser, B. Sarmah, S. S. Ali, D. V. Amla, P. N. Rajesh, H.-B. Zhang, and T. J. Higgins. 2007. Development of transgenics in chickpea, p. 458-473. In S. S. Yadav, R. J. Redden, W. Chen, and B. Sharma (ed.), Chickpea breeding and management. CAB International, New Delhi, India.
- 29.Nahar, P., V. Ghormade, and M. V. Deshpande. 2004. The extracellular constitutive production of chitin deacetylase in Metarhizium anisopliae: possible edge to entomopathogenic fungi in the biological control of insect pests. J. Invertebr. Pathol. 85:80-88. [DOI] [PubMed] [Google Scholar]
- 30.Nahar, P., M. Kulye, P. Yadav, M. Hassani, U. Tuor, S. Keller, and M. V. Deshpande. 2003. Comparative evaluation of indigenous fungal isolates, Metarhizium anisopliae M34412, Beauveria bassiana B3301 and Nomuraea rileyi N812 for the control of Helicoverpa armigera (Hüb.) on chickpea. J. Mycol. Plant Pathol. 33:372-377. [Google Scholar]
- 31.Nahar, P., P. Yadav, M. Kulye, A. Hadapad, M. Hassani, U. Tuor, S. Keller, A. G. Chandele, B. Thomas, and M. V. Deshpande. 2004. Evaluation of indigenous fungal isolates, Metarhizium anisopliae M34412, Beauveria bassiana B3301 and Nomuraea rileyi N812 for the control of Helicoverpa armigera (Hübner) in pigeonpea fields. J. Biol. Contr. 18:1-8. [Google Scholar]
- 32.Naranjo, S. E., J. R. Ruberson, H. C. Sharma, L. Wilson, and K.-M. Wu. 2008. The present and future role of insect-resistant GM crops in cotton IPM, p. 159-194. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, The Netherlands.
- 33.Olsen, K. M., J. C. Daly, H. E. Holt, and E. J. Finnegan. 2005. Season-long variation in expression of Cry1Ac gene and efficiency of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 98:1007-1017. [DOI] [PubMed] [Google Scholar]
- 34.Pray, C. E., J. Huang, R. Hu, and S. Rozelle. 2002. Five years of Bt cotton in China—the benefits continue. Plant J. 31:423-430. [DOI] [PubMed] [Google Scholar]
- 35.Qaim, M., C. E. Pray, and D. Zilberman. 2008. Economic and social considerations in the adoption of Bt crops, 329-356. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, The Netherlands.
- 36.Rahman, M. M., H. L. S. Roberts, and O. Schmidt. 2004. The development of the endoparasitoid Venturia canescens in Bt-tolerant, immune induced larvae of the flour moth Ephestia kuehniella. J. Invertebr. Pathol. 87:129-131. [DOI] [PubMed] [Google Scholar]
- 37.Raymond, B., A. H. Sayyed, and D. J. Wright. 2006. The compatibility of a nucleopolyhedrosis virus control with resistance management for Bacillus thuringiensis: co-infection and cross-resistance studies with the diamondback moth, Plutella xylostella. J. Invertebr. Pathol. 93:114-120. [DOI] [PubMed] [Google Scholar]
- 38.Raymond, B., A. H. Sayyed, R. S. Hails, and D. J. Wright. 2007. Exploiting pathogens and their impact on fitness costs to manage the evolution of resistance to Bacillus thuringiensis. J. Appl. Ecol. 44:768-780. [Google Scholar]
- 39.Romeis, J., D. Bartsch, F. Bigler, M. P. Candolfi, M. M. C. Gielkens, S. E. Hartley, R. L. Hellmich, J. E. Huesing, P. C. Jepson, R. Layton, H. Quemada, A. Raybould, R. I. Rose, J. Schiemann, M. K. Sears, A. M. Shelton, J. Sweet, Z. Vaituzis, and J. D. Wolt. 2008. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 26:203-208. [DOI] [PubMed] [Google Scholar]
- 40.Romeis, J., M. Meissle, and F. Bigler. 2006. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat. Biotechnol. 24:63-71. [DOI] [PubMed] [Google Scholar]
- 41.Romeis, J., H. C. Sharma, K. K. Sharma, S. Das, and B. K. Sarmah. 2004. The potential of transgenic chickpeas for pest control and possible effects on non-target arthropods. Crop Prot. 23:923-938. [Google Scholar]
- 42.Romeis, J., R. G. van Driesche, B. I. P. Barratt, and F. Bigler. 2008. Insect-resistant transgenic crops and biological control, p. 87-117. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer, Dordrecht, The Netherlands.
- 43.Sanyal, I., A. K. Singh, M. A. Kaushik, and D. V. Amla. 2005. Agrobacterium-mediated transformation of chickpea (Cicer arietinum L.) with Bacillus thuringiensis Cry1Ac gene for resistance against pod borer insect Helicoverpa armigera. Plant Sci. 168:1135-1146. [Google Scholar]
- 44.Shanower, T. G., T. G. Kelley, and S. E. Cowgill. 1998. Development of effective and environmentally sound strategies to control Helicoverpa armigera in pigeonpea and chickpea production systems, p. 239-260. In R. K. Saini (ed.), Tropical entomology. Proceedings of the 3rd International Conference on Tropical Entomology. ICIPE Science Press, Nairobi, Kenya.
- 45.Sharma, H. C. (ed.). 2005. Heliothis/Helicoverpa management: emerging trends and strategies for future research. IBH Publishing Co., New Delhi, India.
- 46.Sharma, H. C., C. L. L. Gowda, P. C. Stevenson, T. J. Ridsdill-Smith, S. L. Clement, G. V. Ranga Rao, J. Romeis, M. Miles, and M. Bouhssini. 2007. Host plant resistance and insect pest management in chickpea, p. 520-537. In S. S. Yadav, R. R. Redden, W. Chen, and B. Sharma (ed.). Chickpea breeding and management. CAB International, Wallingford, United Kingdom.
- 47.Shaw, K. E., G. Davidson, S. J. Clark, B. V. Ball, J. K. Pell, D. Chandler, and K. D. Sunderland. 2002. Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol. Contr. 24:266-276. [Google Scholar]
- 48.Shelton, A. M., J. Z. Zhao, and R. T. Roush. 2002. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 47:845-881. [DOI] [PubMed] [Google Scholar]
- 49.Tabashnik, B. E., Y. Carrière, T. J. Dennehy, S. Morin, M. S. Sisterson, R. T. Roush, A. M. Shelton, and J. Z. Zhao. 2003. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. J. Econ. Entomol. 96:1031-1038. [DOI] [PubMed] [Google Scholar]
- 50.Teakle, J., and J. M. Jensen. 1985. Heliothis punctigera, p. 312-322. In R. Singh and R. F. Moore (ed.), Handbook of insect rearing, vol. 2. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- 51.Zimmermann, G. 2007. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontr. Sci. Technol. 17:879-920. [Google Scholar]