Abstract
Several anaerobic metal-reducing bacteria have been shown to be able to donate electrons directly to an electrode. This property is of great interest for microbial fuel cell development. To date, microbial fuel cell design requires avoiding O2 diffusion from the cathodic compartment to the sensitive anodic compartment. Here, we show that Acidiphilium sp. strain 3.2 Sup 5 cells that were isolated from an extreme acidic environment are able to colonize graphite felt electrodes. These bacterial electrodes were able to produce high-density electrocatalytic currents, up to 3 A/m2 at a poised potential of +0.15 V (compared to the value for the reference standard calomel electrode) in the absence of redox mediators, by oxidizing glucose even at saturating air concentrations and very low pHs.
Río Tinto, a river located in the Iberian Pyritic Belt (Spain), is an unusual ecosystem with a rather constant acidic pH (mean pH of 2.3), a high concentration of heavy metals (Fe, Cu, Zn, As, Mn, Cr, etc.), and a high level of microbial diversity, mainly eukaryotic (22, 23, 37). One important characteristic of Río Tinto is the high concentration of ferric iron and sulfates found in its waters, which are the products of the biooxidation of pyrite, the main mineral component of the system. Ferric iron is maintained in solution due to the acidic pH of the river and is responsible for the constant pH due to the buffer characteristics of this cation according to the following equilibrium: Fe3+ + 3H2O ⇄ Fe(OH)3 + 3H+.
The combined use of conventional microbial ecology methods and molecular ecology techniques has confirmed that 80% of water column prokaryotic diversity corresponds to three bacterial groups, Leptospirillum spp., Acidothiobacillus ferrooxidans, and Acidiphilium spp., with all of them being conspicuous members of the iron cycle (11). All Leptospirillum isolates from the Río Tinto are aerobic iron oxidizers. A. ferrooxidans can oxidize iron aerobically and reduce it anaerobically. All Acidiphilium isolates can use ferric iron as an electron acceptor and can use reduced organic compounds as electron donors. Although some other microorganisms able to oxidize (i.e., Ferroplasma spp.) or reduce iron (i.e., “Ferrimicrobium” spp.) have been detected in the River Tinto ecosystem, their low numbers suggest that they play a minor role in the function of this cycle, at least in the water column (26).
Members of the Acidiphilium genus are acidophilic, gram-negative, rod-shaped bacteria that originally were reported as obligate aerobes (13). However, more recent research has identified a significant number of Acidiphilium and Acidiphilium-related bacteria that carry out a dissimilatory reduction of ferric iron under anoxic as well as microaerophilic conditions (15, 29).
Several metal-reducing bacteria have been shown to be able to donate electrons directly to the electrode without the need to add redox mediators to the solution (5, 8, 16). This functional property is of great interest for developing microbial fuel cells (25, 21). In this work, we studied the interaction of Acidiphilium cells with carbon electrodes and the influence of O2 in the electrocatalytic properties of the bacterial electrodes.
MATERIALS AND METHODS
Isolation and growth of Acidiphilium spp.
Samples were collected from the Tinto River in 1-liter bottles and kept on ice until they were inoculated. The solid medium that was used to isolate Acidiphilium contained the following components (per liter): 0.25 g K2HPO4, 0.25 g MgSO4·7H2O, 0.1 g KCl, 1 g glucose, 1 g yeast extract, and 1% agar, with the pH adjusted to 4.6 with 1 N H2SO4 before sterilization. Colonies were inoculated in liquid medium in aerobic conditions at 30°C in a chemically defined medium as reported by Hiraishi and Kitamura (13). The pH was adjusted to 2.5 with 1 N H2SO4 prior to sterilization at 120°C and 0.5 atm for 30 min.
Acidiphilium sp. strain 3.2 Sup 5 has been deposited in the Spanish Type Culture Collection (CECT 7285).
Acidiphilium SJH cells were kindly supplied by Barry Johnson.
Ferric iron reduction.
Ferric iron reduction was measured in liquid medium containing 50 mM ferric sulfate, 10 mM glucose, and 0.025% tryptone soya broth-basal salts, pH 2.0 (Fe-TSB). Inoculated cultures were incubated (120 ml of medium in 250-ml Erlenmeyer flasks that were shaken at 120 rpm) under aerobic or anaerobic conditions (in universal tubes completely filled with pure N2 and CO2 and capped with gas-tight stoppers). Ferrous iron concentrations were measured as reported previously (27).
Total DNA extraction and phylogenetic analysis.
DNA was extracted from cells using the FastDNA spin kit for soil according to the instructions supplied by the manufacturer. Bacterial 16S rRNA gene fragments were amplified by PCR using bacterial primers 341F and 907RM.
Sequences of 16S rRNA genes initially were compared to reference sequences contained in the EMBL nucleotide sequence database by using the BLAST program and were subsequently aligned with 16S rRNA reference sequences in the ARB package (http://www.mikro.biologie.tu-muenchen.de). A 16S rRNA gene sequence phylogenetic tree was obtained by using the DNAPARS parsimony tool included in the ARB software.
Electrochemical measurements.
A sterilized electrochemical glass cell from Radiometer that had been thermostatized at 30°C was used. The working electrodes were graphite felt (9-μm fiber diameter; 3,500 cm2 g−1 surface area; RVG 4000; Le Carbon Lorraine) disks of approximately 13 mm in diameter and 10 mm in thickness (6.5 cm2 total geometric area). Before use, the disks were cleaned in 2 N H2SO4 and then sterilized at 120°C and 1 atm for 30 min. A standard calomel electrode (SCE) was used as the reference electrode, and the counter electrode was a platinum electrode; both were supplied by Radiometer. The electrochemical cell contained a pH 2.5 solution of 2.0 g/liter (NH4)2SO4, 0.1 g/liter KCl, 0.25 g/liter K2HPO4, 0.25 g/liter MgSO4·7H2O, and 0.01 g/liter Ca(NO3)2. Glucose was added to a final concentration of 10 mM, Acidiphilium sp. was inoculated into the electrochemical cell, and the solution was stirred. Air was diffused into the cell through a permeable membrane. The oxygen concentration in the electrochemical cell was determined with a Sylant Simplair F-15 oxymeter (Syland Scientific GmbH). Cyclic voltammetry experiments were performed with an Autolab PGSTAT 30 potentiostat (Eco-Chemie) at 50 mV/s in an air atmosphere, except when purging with N2 was indicated.
Electron microscopy.
The scanning electron microscopy (SEM) of modified electrodes was done according to a protocol described previously (1) with a JEOL-5600LV scanning electron microscope.
RESULTS
Isolation, identification, and phylogenetic analysis of Acidiphilium sp. strain 3.2 Sup 5.
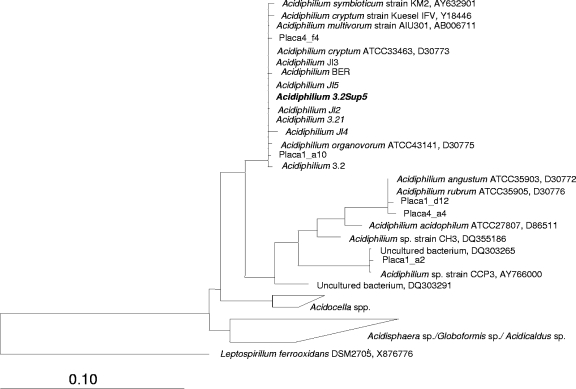
Different Acidiphilium isolates from Río Tinto were grown in the conditions described in Materials and Methods. Their purities were checked by pulsed-field gel electrophoresis and characterized by comparisons of 16S rRNA gene sequences (Fig. 1). The different species that belong to the Acidiphilium genus cluster in two groups. The sequence obtained from strain 3.2 Sup 5, the strain used in this work, clustered with those of “Acidiphilium symbioticum,” Acidiphilium cryptum, Acidiphilium multivorum, and Acidiphilium organovorum.
FIG. 1.
Neighbor-joining phylogenetic tree based on almost-complete 16S rRNA gene sequences of Acidiphilium isolates from Río Tinto and Acidiphilium reference species. The strain used in this work, Acidiphilium sp. strain 3.2 Sup 5, is in boldface.
Ferric iron reduction.
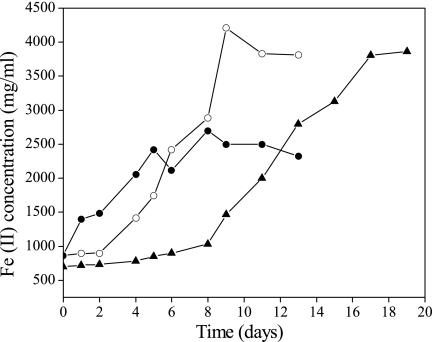
The ability of the Acidiphilium sp. strain 3.2 Sup 5 culture to reduce ferric iron using glucose as the energy source was tested with different concentrations of O2 in the medium. The isolated culture was able to reduce a significant amount of ferric ion under all conditions, although under anaerobic conditions the rate was significantly slower (Fig. 2). These results are in agreement with those of previous reports (15, 29).
FIG. 2.
Reduction of ferric ions by Acidiphilium sp. strain 3.2 Sup 5 cells incubated under aerobic (closed circles), microaerobic (open circles), and anaerobic conditions (closed triangles).
Growth of Acidiphilium spp. on graphite felt electrodes.
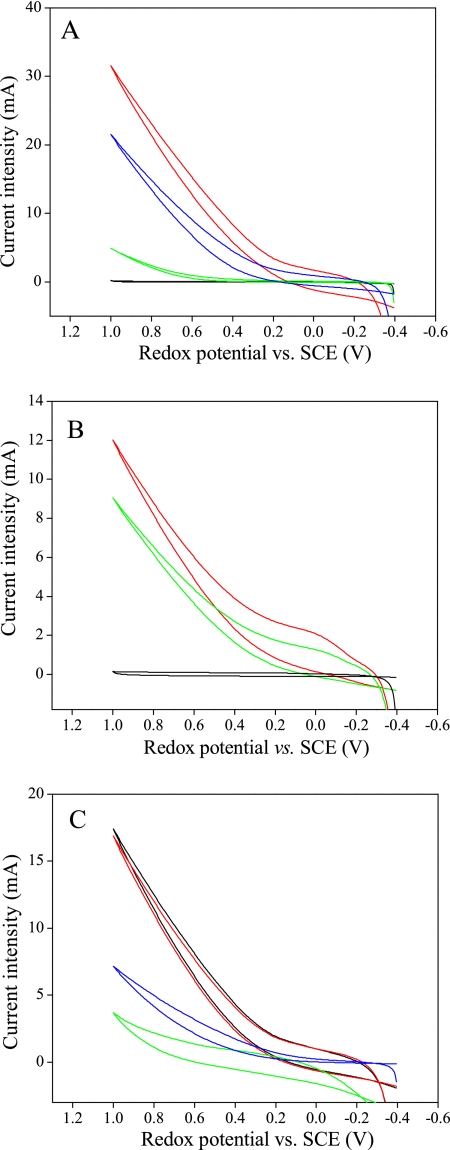
The electrochemical cell containing 10 mM glucose as the energy source was inoculated with Acidiphilium sp. strain 3.2 Sup 5 culture. Additional glucose was added at intervals to keep its initial concentration constant. After 6 days of inoculation at 30°C and aerobic conditions, the number of cells in the solution had increased considerably: 4 × 109 cells ml−1 were measured by microscopy observation and the counting of cells stained by 4′,6′-diaminophenylindol. In parallel, cyclic voltammetry experiments were done in order to detect the electrocatalytic oxidation of glucose at the graphite felt electrode without purging the solution with N2. Before the addition of the bacterial culture, only background current was observed in the cyclic voltammetry, because glucose does not oxidize directly on electrodes. The electrocatalytic oxidation of glucose was gradually detected after the inoculation of Acidiphilium sp. strain 3.2 Sup 5 in the electrochemical cells under aerobic conditions (Fig. 3A). This electrocatalytic effect is shown in the cyclic voltammograms (Fig. 3) as a positive current that begins at approximately 0 V and increases as the potential was scanned toward higher redox potentials, whereas in the reverse scan no negative currents due to a reduction process are observed. The formal redox potential of glucose at pH 2.5 is −0.136 V; thus, there is not a great overpotential for the electrocatalytic process. The maximum electrocatalytic effect was measured after 28 days of bacterial inoculation. Ten electrodes were prepared in this form (although in most cases the incubation period was less than 10 days), and the final electrocatalytic current measured at +0.15 V above the value for the reference SCE ranged between 2.0 and 3.0 A/m2, considering the geometric area of the electrodes. When a carbon felt electrode modified with Acidiphilium sp. strain 3.2 Sup 5 cells was changed to a new glucose solution free of cells, a significant amount of the catalytic effect was observed by cyclic voltammetry (Fig. 3B). Different parts of the working electrode were cut and analyzed by SEM. The images show that the bacterial cells had colonized the graphite felt electrode, as individual cells clearly can be observed attached to the fibers of the electrode (Fig. 4).
FIG. 3.
Cyclic voltammograms of electrocatalytic oxidation of 10 mM glucose with different carbon felt electrodes (A) before (black) and 3 (green line), 13 (blue line), and 28 days (red line) after the inoculation of Acidiphilium sp. strain 3.2 Sup 5 into the electrochemical cell or (B) 23 days after the inoculation of Acidiphilium sp. strain 3.2 Sup 5 cells into the electrochemical cell (red line) and then put into a new solution without Acidiphilium sp. cells (green line). (C) Carbon felt electrode modified with Acidiphilium sp. strain 3.2 Sup 5 cells in the presence of 6.2 ppm O2 in solution (black line), after 20 min of being bubbled with pure N2 (red line), and 4 (blue line) or 6 h (green line) after the addition of 5% phenol to the electrochemical cell.
FIG. 4.
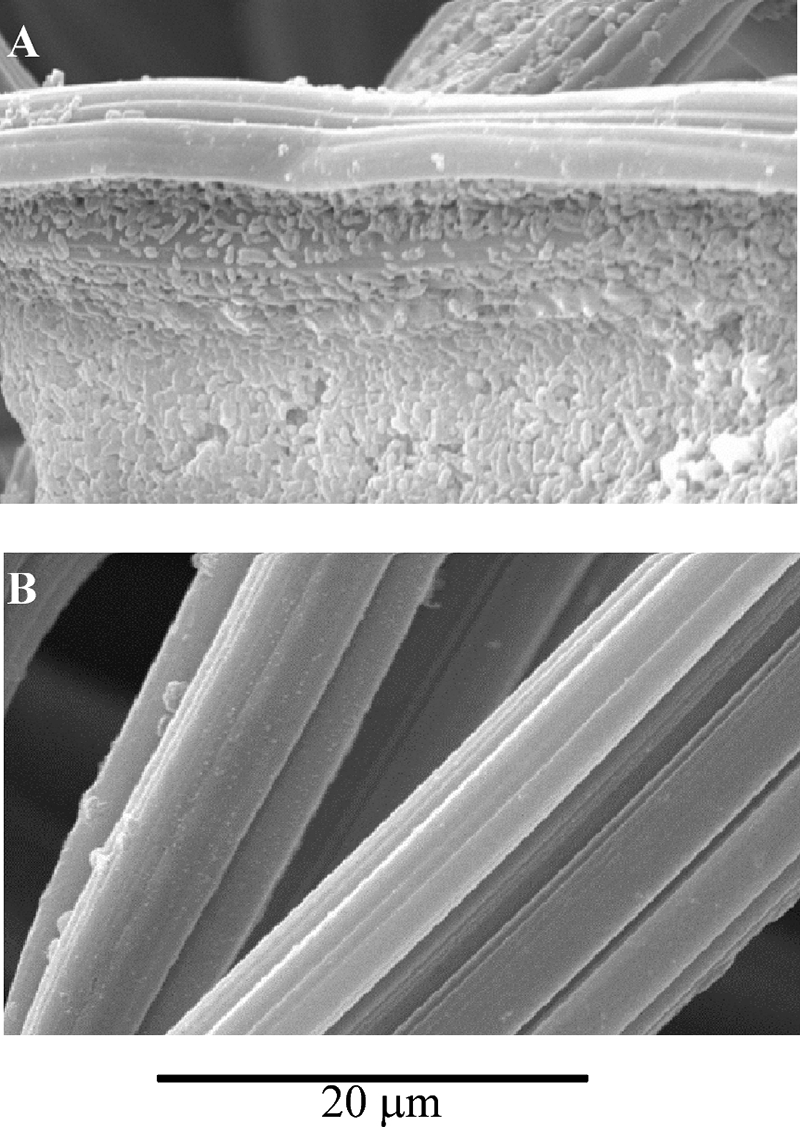
Bacterial electrode characterization by SEM. (A) Image of a fiber of a carbon felt electrode 28 days after the inoculation of the electrochemical cell with Acidiphilium sp. strain 3.2 Sup 5. (B) Control image of a carbon felt electrode fiber before inoculation with Acidiphilium sp. strain 3.2 Sup 5 cells.
Acidiphilium sp. strain SJH cells also were grown on a graphite felt electrode. In this case, the cyclic voltammogram measured after 20 days of inoculation was almost equal to the background one (not shown).
Figure 3C shows how the medium conditions within the electrochemical cell affected the electrocatalytic properties of the Acidiphilium sp. strain 3.2 Sup 5 electrode. A highly catalytic current of glucose oxidation is measured even if the O2 concentration in solution was almost saturating. Sparging the cell solution with pure N2 for 40 min in order to establish anaerobic conditions seldom affected the catalytic effect observed in the voltammogram. On the contrary, when 5% phenol, which is an inhibitor of Acidiphilium spp. cells, was added to the solution, the electrocatalytic current gradually decreased with time and after 6 h was almost suppressed.
DISCUSSION
In this work, we have shown that Acidiphilium sp. strain 3.2 Sup 5 cells colonized graphite felt disks when glucose is the energy source and O2 is the only redox acceptor. Moreover, the attached cells were able to establish electronic communication with the carbon support when they were used as a working electrode in an electrochemical cell configuration, as high catalytic current densities due to the oxidation of glucose were measured by cyclic voltammetry. This electrocatalytic phenomenon most probably is due to the cells of Acidiphilium spp. that are attached to the electrode surface and not to planktonic cells, because when the carbon felt electrode modified with Acidiphilium spp. cells was changed to a new glucose solution free of cells, a significant amount of the catalytic effect was observed by cyclic voltammetry. The suppression of glucose electrocatalysis by the addition of phenol, which is a strong inhibitor of Acidiphilium spp., was a further indication of bacterial cells acting as the catalysts of the system. Because no redox mediators were added to the electrolyte solution or immobilized onto the electrode surface, we can conclude that bacterial cells were able to directly donate the electrons obtained from glucose metabolism to the electrode.
Direct electron exchange between microbial cells and a carbon electrode was first reported by Kim et al. in 1999 (16). Since then, several more microorganisms have been shown to be able to donate electrons directly to carbon electrodes (4, 5, 8, 17, 30). This functional property has spurred research in microbial fuel cell development, in which bacteria act as the electrocatalysts of the anodic compartment (7, 21, 25). A novel aspect of the bacterial anode of the present work is that it operates at low pH, whereas all other reported exoelectrogenic bacteria require nearly neutral pH conditions. This is of interest, because low proton concentrations can limit the overall reaction rate in microbial fuel cells (9).
The electrocatalytic currents measured with the bacterial electrode of this work are very high; a maximum density current of 3 A/m2 was measured at a poised potential of +0.15 V compared to that for the reference SCE and taking into account the geometric area of the electrode. This value is approximately three times higher than the maximum value reported by Bond and Lovley for Geobacter sulfurreducens cells that were attached to graphite electrodes that were poised at the same redox potential under anaerobic conditions and with acetate as the electron donor (5). The high electrocatalytic current density of our system could be attributed to the highly electroactive surface area of the carbon felt electrode, but in spite of this, the high-density currents indicate an efficient electron transfer between the bacterial cells and the electrode. Three different routes for direct electron transfer between bacteria and electrodes have been proposed: (i) via redox proteins located on the bacterial membrane (5, 18); (ii) via redox compounds (i.e., phenazines or quinones) excreted from the bacteria (30, 31, 36); and (iii) via conductive nanowires that interconnect bacterial cells to the electrode surface (12, 32, 33). At this stage, we do not know which of these mechanisms efficiently transfers the electrons from the Acidiphilium sp. cells to the carbon electrode. However, the shape of the electrocatalytic cyclic voltammograms measured, in which the current increases greatly with the overpotential at the electrode without reaching a plateau (Fig. 3), suggests that interfacial electron transfer between the biocatalyst (Acidiphilium sp. cells in our case) and the electrode is the rate-limiting step (19, 28). Thus, routes (i) and (ii) for electron transfer are more probable in our system.
In microbial fuel cell designs, the anode compartment has to be separated from the cathode compartment, generally by a proton exchange membrane, in order to avoid the diffusion of O2 to the anodic compartment, which either inhibits the growth of the microbial electrocatalyst (5, 8, 30, 35) or acts as a competitor to the anode by taking electrons from the bacteria, which decreases the current intensity measured in the biofuel cell (14, 20, 34). A remarkable property of the Acidiphilium sp. strain 3.2 Sup 5 electrode is that the electrocatalytic oxidation of glucose was not affected by the presence of O2 in the electrolyte solution (Fig. 3C). This result has not been reported before for any microorganism. This behavior of the bacterial electrode concurs with the ability of Acidiphilium sp. cells to reduce the level of ferric ions in the presence of O2 that was shown in this work and previously by other authors (15, 29). This physiological property is quite uncommon for metal-reducing bacteria, which generally are anaerobic organisms (3, 5, 10, 24). Nevertheless, this ability is a necessary condition, but is not sufficient, for the direct electrocatalytic oxidation of glucose in the presence of O2, because Acidiphilium sp. strain SJH grown on the electrode gave negative results. Besides, a very recent article has shown that Acidiphilium cryptum cells were unable to transfer electrons directly to a graphite felt electrode (6). Both of these species are highly effective iron reducers. Therefore, we conclude that adequate electrical connectivity between the adhered bacterial cells and the electrode surface is an essential parameter for the preferential donation of electrons to the electrode instead of to O2. Genetic differences between Acidiphilium spp. will be studied in order to explain why those isolated from Río Tinto have this special property.
In summary, this work shows that Acidiphilium sp. strain 3.2 Sup 5 cells, which were isolated from an extremely acidic environment, are able to donate electrons obtained from the oxidation of an organic substrate directly to an electrode, producing high-density electrocatalytic currents in the presence of O2, a property of interest for the further development of microbial fuel cells (2). Future work will involve studying the performance of this Acidiphilium sp. strain 3.2 Sup 5 electrode in a fuel cell configuration and the further characterization of the bacteria in order to understand better its noteworthy functional properties.
Acknowledgments
This work was supported by the Comunidad de Madrid (PICOMICRO project S0505/AMB-0259).
We thank González-Toril for her help on the phylogenetic analysis.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Alphenaar, P. A., N. Groeneveld, and A. C. van Aelst. 1994. Scanning electron microscopic method for internal structure analysis of anaerobic granular sludge. Micron 25:129-133. [Google Scholar]
- 2.Amils, R., A. L. De Lacey, V. M. Fernandez, and M. Malki. June 2007. Spanish patent P2007011534.
- 3.Arnold, R. G., M. R. Hoffmann, T. J. DiChristina, and F. W. Picardal. 1990. Regulation of dissimilatory Fe(III) reduction activity in Shewanella putrefaciens. Appl. Environ. Microbiol. 56:2811-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffinger, J. C., J. Pietron, R. Ray, B. Little, and B. R. Ringeisen. 2007. A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens. Bioelectron. 22:1672-1679. [DOI] [PubMed] [Google Scholar]
- 5.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borole, A. P., H. O'Neill, C. Tsouris, and S. Cesar. 2008. A microbial fuel cell operating at low pH using the acidophile Acidiphilium cryptum. Biotechnol. Lett. doi: 10.1007/s10529-008-9700-y. [DOI] [PubMed]
- 7.Bullen, R. A., T. C. Arnot, J. B. Lakeman, and F. C. Walsh. 2006. Biofuel cells and their development. Biosens. Bioelectron. 21:2015-2045. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, S., and B. E. Logan. 2007. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 104:18871-188873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finneran, K. T., C. V. Johnsen, and D. R. Lovley. 2003. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 53:669-673. [DOI] [PubMed] [Google Scholar]
- 11.González-Toril, E., F. Gómez, M. Malki, and R. Amils. 2006. Methods for the isolation and study of acidophilic microorganisms. Methods Microbiol. 35:436-502. [Google Scholar]
- 12.Gorby, Y. A., S. Yanina, J. S. Mclean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraishi, A., and H. Kitamura. 1984. Distribution of phototrophic purple non-sulfur bacteria in activated-sludge systems and other aquatic environments. Bull. Jpn. Soc. Sci. Fisheries 50:1929-1937. [Google Scholar]
- 14.Jang, J. K., T. H. Pham, I. S. Chang, K. H. Kang, H. Moon, K. S. Cho, and B. H. Kim. 2004. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process Biochem. 39:1007-1012. [Google Scholar]
- 15.Johnson, D. B., and S. McGinness. 1991. Ferric iron reduction by acidophilic heterotrophic bacteria. Appl. Environ. Microbiol. 57:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, B. H., H. J. Kim, M. S. Hyun, and D. H. Park. 1999. Direct electrode reaction of Fe(III) reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 17.Kim, B. H., H. S. Park, H. J. Kim, G. T. Kim, I. S. Chang, J. Lee, and N. T. Phung. 2004. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 63:672-681. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 19.Leger, C., A. K. Jones, S. P. J. Albracht, and F. A. Armstrong. 2002. Effect of a dispersion of interfacial electron transfer rates on steady state catalytic electron transport in [NiFe]-hydrogenase and other enzymes. J. Phys. Chem. B 106:13058-13063. [Google Scholar]
- 20.Liu, H., and B. E. Logan. 2004. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 38:4040-4046. [DOI] [PubMed] [Google Scholar]
- 21.Logan, B. E., and J. M. Regan. 2006. Microbial challenges and fuel cells applications. Environ. Sci. Technol. 40:5172-5180. [PubMed] [Google Scholar]
- 22.López-Archilla, A. I., I. Marín, and R. Amils. 2001. Microbial community composition and ecology of an acidic aquatic environment: the Tinto River, Spain. Microb. Ecol. 41:20-35. [DOI] [PubMed] [Google Scholar]
- 23.López-Archilla, A. I., A. E. González, M. C. Terron, and R. Amils. 2004. Ecological study of the fungal populations of the acidic Tinto River in southwest Spain. Can. J. Microbiol. 50:923-934. [DOI] [PubMed] [Google Scholar]
- 24.Lovley, D. R., S. J. Giovannoni, D. C. White, J. E. Champine, E. J. P. Phillips, Y. A. Gorby, and S. Goodwin. 1993. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron, and other metals. Arch. Microbiol. 159:336-344. [DOI] [PubMed] [Google Scholar]
- 25.Lovley, D. R. 2006. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4:497-508. [DOI] [PubMed] [Google Scholar]
- 26.Malki, M., E. González-Toril, J. L. Sanz, F. Gómez, N. Rodríguez, and R. Amils. 2006. Importance of the iron cycle in biohydrometallurgy. Hydrometallurgy 83:223-228. [Google Scholar]
- 27.Nakanishi, T., and M. Otomo. 1986. Solvent extraction and spectrophotometric determination of iron(II) with di-2-pyridyl ketone benzoylhydrazone. Microchem. J. 33:172-178. [Google Scholar]
- 28.Patolsky, F., Y. Weizmann, and I. Willner. 2004. Long-range electrical contacting of redox enzymes by SWCNT connectors. Angew. Chem. Int. Ed. 43:2113-2117. [DOI] [PubMed] [Google Scholar]
- 29.Pronk, J. T., and D. B. Johnson. 1992. Oxidation and reduction of iron by acidophilic bacteria. Geomicrobiol. J. 10:153-171. [Google Scholar]
- 30.Rabaey, K., N. Boon, S. D. Siciliano, M. Verhaege, and W. Verstraete. 2004. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 70:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabaey, K., N. Boon, M. Höfte, and W. Verstraete. 2005. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 39:3401-3408. [DOI] [PubMed] [Google Scholar]
- 32.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 33.Reguera, G., K. P. Nevin, J. S. Nicoll, S. F. Covalla, T. L. Woodard, and D. R. Lovley. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72:7345-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ringeisen, B. R., R. Ray, and B. Little. 2007. A miniature microbial fuel cell operating with an aerobic anode chamber. J. Power Sources 165:591-597. [Google Scholar]
- 35.Scott, K., and C. Murano. 2007. Microbial fuel cells utilising carbohydrates. J. Chem. Technol. Biotechnol. 82:92-100. [Google Scholar]
- 36.Wang, Y. F., S. Tsujimura, S. S. Cheng, and K. Kano. 2007. Self-excreted mediator from Escherichia coli K-12 for electron transfer to carbon electrodes. Appl. Microbiol. Biotechnol. 76:1439-1446. [DOI] [PubMed] [Google Scholar]
- 37.Zettler, L. A. A., F. Gómez, E. Zettler, B. G. Keenan, R. Amils, and M. L. Sogin. 2002. Eukaryotic diversity in Spain's river of fire: this ancient and hostile ecosystem hosts a surprising variety of microbial organisms. Nature 417:137. [DOI] [PubMed] [Google Scholar]