Abstract
Almonds are known to have a number of nutritional benefits, including cholesterol-lowering effects and protection against diabetes. They are also a good source of minerals and vitamin E, associated with promoting health and reducing the risk for chronic disease. For this study we investigated the potential prebiotic effect of almond seeds in vitro by using mixed fecal bacterial cultures. Two almond products, finely ground almonds (FG) and defatted finely ground almonds (DG), were subjected to a combined model of the gastrointestinal tract which included in vitro gastric and duodenal digestion, and the resulting fractions were subsequently used as substrates for the colonic model to assess their influence on the composition and metabolic activity of gut bacteria populations. FG significantly increased the populations of bifidobacteria and Eubacterium rectale, resulting in a higher prebiotic index (4.43) than was found for the commercial prebiotic fructooligosaccharides (4.08) at 24 h of incubation. No significant differences in the proportions of gut bacteria groups were detected in response to DG. The increase in the numbers of Eubacterium rectale during fermentation of FG correlated with increased butyrate production. In conclusion, we have shown that the addition of FG altered the composition of gut bacteria by stimulating the growth of bifidobacteria and Eubacterium rectale.
Functional foods are known as dietary components that may cause physiological effects on the consumer, leading to justifiable claims of health benefits (36). According to the European consensus document “Scientific concepts of functional foods,” a food ingredient may be regarded as functional if it beneficially affects one or more target functions in the body, beyond its nutritional effects, in order to improve the state of health and/or reduce the risk of disease (9). A prebiotic is defined as “a nondigestible food ingredient which beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improving host health” (15). Prebiotics of proven efficacy are able to modulate the gut microbiota by stimulating indigenous beneficial flora while inhibiting the growth of pathogenic bacteria, such as proteolytic bacteroides and clostridia (43). Bifidobacteria and lactobacilli are able to inhibit the growth of clostridia and pathogenic Enterobacteriaceae by the production of short-chain fatty acids and antimicrobial compounds, as well as by competition for growth substrate and adhesion sites (16, 19, 23). As such, these beneficial gut bacteria, together with mucins and antimicrobial peptides, represent part of the host's front line of defense against harmful microorganisms (24, 40). In order to be effective as a prebiotic, an ingredient must neither be hydrolyzed nor absorbed in the upper part of the gastrointestinal tract (GIT). Although any dietary material that enters the large intestine can be considered as potentially prebiotic, currently the best-known prebiotics are nondigestible oligosaccharides (17). Different oligosaccharides with prebiotic properties, such as inulin, fructooligosaccharides (FOS), galactooligosaccharides, and lactulose, are commercially available, but currently there is increasing interest in the identification and development of new prebiotic compounds, perhaps with added functionality (26, 29, 33, 42).
The almond nut (Amygdalus communis L.) is a species of Prunus belonging to the family Rosaceae. The global production of almonds is around 1.7 million metric tons, with California producing 80% of the world's almonds. Lipid, the main storage component in almond seeds, constituting over 50% of the total weight of the seeds, is located as intracellular oil bodies (35). Proteins comprise about 22 to 25% of the seeds, while 11 to 12% is represented by dietary fiber. Recent results have shown that the encapsulation of intracellular lipids by the cell walls restricts their digestion in the stomach and small intestine (13, 27). Furthermore, if undigested lipid from almond tissue reaches the large intestine, it could be used by resident microbiota, and evidence of bacterial fermentation was previously shown (13). Almond cell wall material contains pectic substances that are rich in arabinose, and the observation of their partial degradation by the gut microbiota in the fecal samples can be explained by the erosion of the middle lamella (10, 13). The results of our recent study have demonstrated that the bioaccessibility of nutrients and phytochemicals from almond seeds is improved by increased residence time in the gut and is regulated by almond cell walls. The results of these in vitro and ileostomy digestibility studies have shown high amounts of lipid and protein remaining in the almond tissue after duodenal digestion and therefore available for fermentation in the colon by the gut microbiota (27).
Here we describe the investigation of the potential prebiotic effect of almond seeds by using a full model of GIT digestion, including gastric and small intestinal environments and a colonic model consisting of in vitro fermentation systems with representative human gut bacteria.
MATERIALS AND METHODS
Almond products.
Blanched finely diced and powdered almonds (Amygdalus communis L; variety Nonpareil) were kindly provided by the Almond Board of California. Commercial FG did not contain skin coat and had a mean particle size of 200 μm. DG was prepared by extracting 25 g of FG three times with 400 ml of n-hexane for 2 h on each occasion, as previously described (27).
Chemicals and enzymes.
Egg l-α-phosphatidylcholine (PC; lecithin grade 1, 99% purity) was purchased from Lipid Products (South Nutfield, Surrey, United Kingdom). Porcine gastric mucosa pepsin (activity of 3,300 U/mg of protein calculated by using hemoglobin as substrate), bovine α-chymotrypsin (activity of 40 U/mg of protein using benzoyl-L-tyrosine ethyl ester as substrate), porcine trypsin (activity of 13,800 U/mg of protein using benzoyl-l-arginine ethyl ester as substrate), porcine pancreatic lipase (activity of 25,600 U/mg protein), porcine colipase, sodium taurocholate, and sodium glycodeoxycholate were obtained from Sigma (Poole, Dorset, United Kingdom). The lipase for the simulated gastric phase of digestion was a gastric lipase analogue from Rhizopus oryzae (F-AP15; activity of ≥150 U/mg) obtained from Amano Enzyme (Nagoya, Japan). All other chemicals were of Analar quality.
In vitro digestion studies.
The protocol previously developed to study almond digestion under gastric and duodenal conditions (27) was used to simulate gastrointestinal processing for both FG and DG. Each simulated digestion was performed at least four times, and the solid material recovered for analysis. Control digestions of the two almond products (FG and DG) were performed in saline solution (150 mM NaCl, pH 2.5 or 6.5 for gastric and duodenal digestion, respectively) without enzyme additions.
In vitro gastric digestion.
Phospholipid vesicles were prepared as previously described (27). Briefly, solvent was removed from 0.94 ml of PC stock solution (63.5 mM), and the thin film of phospholipids was then suspended in 12.2 ml of warmed saline (150 mM NaCl, pH 2.5, at 37°C). The suspension was then sonicated at 5°C in a coolant-jacketed vessel, using a sonication probe (Status US 200; Avestin) with a pulsed cycle of 30% full power on for 0.9 s and off for 0.1 s. The single-shelled liposome suspension was filtered through a 0.2-μm nylon syringe filter (Nalgene, United Kingdom) and equilibrated in an orbital shaking incubator (170 rpm) at 37°C. Each almond product (1.5 g) was suspended in 12.4 ml acidic saline (150 mM NaCl, pH 2.5) in the presence of the PC vesicle suspension, pepsin, and the gastric lipase analogue at concentrations of 2.4 mM, 146 U/ml, and 0.56 mg/ml, respectively. In vitro gastric digestion was performed for 2 h at pH 2.5.
In vitro duodenal digestion.
Following gastric digestion, the pH was immediately raised to 6.5 in order to simulate the duodenal conditions. Bile salt solution (4 mM sodium taurocholate and 4 mM sodium glycodeoxycholate), CaCl2 (11.7 mM), and bis-Tris buffer, pH 6.5 (0.73 mM) were also added. Duodenal digestions were initiated by the addition of α-chymotrypsin (5.9 U/ml), trypsin (104 U/ml), colipase (3.2 μg/ml), and pancreatic lipase (54 U/ml) and performed in a shaking incubator (170 rpm) at 37°C for 1 h.
Lipid content determination.
Total lipid and vitamin E extraction of FG and of FG after in vitro gastric and gastric plus duodenal digestion was performed as previously described (27).
Total protein assays.
Original almond materials (FG and DG) and solid residues recovered after in vitro gastric and duodenal digestion were analyzed for total nitrogen by using the micro-Kjeldahl method, as previously reported (27).
Cell wall analysis.
Cell wall material was prepared from FG and FG after gastric plus duodenal digestion by using the method previously described (27). The alditol acetates were quantified by gas-liquid chromatography, and total uronic acids determined colorimetrically at 580 nm (2, 3).
Fecal batch culture fermentations.
Water-jacketed fermenter vessels (300 ml) were filled with 135 ml of presterilized basal growth medium (2 g/liter peptone water, 2 g/liter yeast extract, 0.1 g/liter NaCl, 0.04 g/liter K2HPO4, 0.04 g/liter KH2PO4, 0.01 g/liter MgSO4·7H2O, 0.01 g/liter CaCl2·6H2O, 2 g/liter NaHCO3, 2 ml Tween 80, 0.02 g/liter hemin, 10 μl vitamin K1, 0.5 g/liter cysteine HCl, 0.5 g/liter bile salts, pH 7.0) and inoculated with 15 ml of fecal slurry. Before the addition of the fecal slurry, prepared by homogenizing 10% (wt/vol) freshly voided fecal material from one healthy donor in 0.1 M phosphate-buffered saline (PBS), pH 7.0, the almond extract (FG or DG after gastric and duodenal digestion) or FOS was added to give a final concentration of 1% (wt/vol). Each vessel was magnetically stirred, the pH automatically controlled and maintained at pH 6.8, and the temperature set at 37°C. Anaerobic conditions were maintained by sparging the vessels with oxygen-free nitrogen gas at 15 ml/min. Samples (5 ml) were removed over 24 h for the enumeration of bacteria and short-chain fatty acid analysis. Fermentations were run on three separate occasions.
Enumeration of bacteria.
Bacteria were counted by using fluorescent in situ hybridization (FISH) (37). Duplicate fermentation samples were diluted four times in 4% (wt/vol) filtered paraformaldehyde and fixed overnight at 4°C. Samples were then washed twice with filtered PBS (0.1 M, pH 7.0) and stored at −20°C in PBS-ethanol (1:1, vol/vol) until further analysis. Hybridization was performed at an appropriate temperature by using genus-specific 16S rRNA-targeted oligonucleotide probes labeled with the fluorescent dye Cy3 for the different bacterial groups or with 4′,6-diamidino-2-phenylindole (DAPI) for total cell counts. The probes used were Bif164, specific for Bifidobacterium (22); Bac303, specific for bacteroides (28); Lab158, specific for Lactobacillus/Enterococcus spp. (18); His150, specific for most species of the Clostridium histolyticum group (Clostridium clusters I and II) (14); and EREC482, specific for most of the Clostridium coccoides-Eubacterium rectale group (Clostridium clusters XIVa and XIVb) (5). The hybridized mixture was then vacuum filtered using a 0.2-μm membrane filter (Millipore, Watford, United Kingdom), and the filter was mounted on a microscope slide. At least 15 random fields were counted on each slide by using a Nikon Microphot fluorescent microscope.
Short-chain fatty acid analysis.
One-milliliter samples removed from the batch culture fermenter were centrifuged at 15,000 × g for 5 min, and 20 μl of the supernatant was injected into a high-pressure liquid chromatography system equipped with a refractive index detector. We used an ion exclusion Aminex HPX-87H column (7.8 by 300 mm; Bio-Rad, Watford, United Kingdom), maintained at 50°C, and 5 mM H2SO4 as eluent at a flow rate of 0.6 ml/min. Quantification of the organic acids was carried out by using calibration curves of acetic, propionic, butyric, and lactic acids in concentrations between 0.5 and 100 mM, and the results expressed in mmol/liter (37).
Statistical analysis.
Differences between bacterial numbers at 0, 8, and 24 h of fermentation for each batch culture were checked for significance by paired t test, assuming normal distribution and equal variances and considering both sides of the distribution. The differences were considered significant when the P value was <0.05.
RESULTS
Almond product characterization after in vitro digestion.
The compositions of the two almond extracts (FG and DG) obtained after in vitro gastric plus duodenal digestion are shown in Table 1. These fractions were subsequently used as substrates for the colonic model. As previously reported (13, 27), almond cell walls are not degraded in the upper GIT, and therefore, the mass loss during digestion is mostly related to loss of intracellular components, such as lipid and protein. The simulated gastric digestion step was responsible for the majority of the gravimetric losses and the highest extent of lipolysis and proteolysis (27). FG after duodenal digestion still contained 57% of the start lipid and 34 mg of vitamin E per 100 g total almond mass, of which 96% was α-tocopherol, 1.3% β-tocopherol, and the remainder γ-tocopherol. The sugar composition indicated that almond cell walls are mainly composed of arabinose-rich polysaccharides, including the pectic substances, encasing cellulose microfibrils. The monomeric sugar concentrations did not change significantly after digestion: the sugar contents (as percentage of the total) were 39.9 and 39.5 arabinose, 12.0 and 12.6 xylose, 4.7 and 4.7 galactose, 16.7 and 16.8 glucose, and 21.1 and 20.7 galacturonic acid for FG and for FG after gastric plus duodenal digestion, respectively. This suggests that almond cell walls were not degraded during digestion.
TABLE 1.
Chemical composition of FG and DG before and after in vitro gastric plus duodenal digestiona
| Time of analysis | Almond product used | Amt of nutrient per 100 gm
|
|||
|---|---|---|---|---|---|
| Lipid (g) | Vitamin E (mg) | Protein (g) | Dietary fiber (g) | ||
| Before digestion | FG | 54.9 ± 2.5 | 28.2 ± 1.5 | 25.9 ± 2.6 | 9.8 ± 1.3 |
| DG | 0 | 0 | 57.2 ± 2.4 | 32.5 ± 1.1 | |
| After digestion | FG | 57.4 ± 3.8 | 34.0 ± 1.8 | 24.7 ± 1.9 | 13.3 ± 0.9 |
| DG | 0 | 0 | 58.0 ± 2.8 | 31.2 ± 0.8 | |
Values are the means ± standard deviations of the results. Other components include carbohydrates, minerals, and vitamins.
Batch culture fermentations.
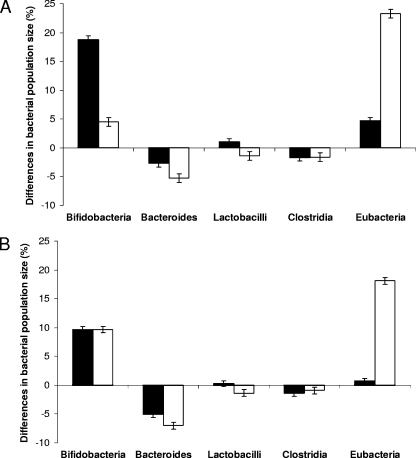
Batch fermentations were used to monitor the effects of predigested FG, DG, and FOS on the growth of a mixed bacterial population of the human colon. Samples were removed at intervals, and FISH was used to quantify the levels of different bacterial groups. The results shown in Table 2 indicate that a significant increase in the level of total bacteria was seen with both FG and FOS after 8 and 24 h of incubation, whereas the total bacterial number was largely unaffected by the addition of DG. Generally, an increase in the numbers of bifidobacteria, lactobacilli, and Eubacterium rectale was observed in response to the addition of FG and FOS at both the 8- and 24-h incubation time points. Compared to the control vessel, the bacteroides population with FG decreased significantly after 24 h, whereas their numbers were similar to those of the control at 8 and 24 h of incubation in the presence of DG. An increase in the number of Eubacterium rectale was observed in the presence of both FOS and FG, the latter showing a greater increase after 24 h of incubation. Both fractions also stimulated the growth of bifidobacteria, with a 0.61 and 0.68 log increase in their numbers at 24 h with FG and FOS, respectively. The effect of FG on bifidobacteria, lactobacilli, and Eubacterium rectale numbers was optimal after 8 h of incubation and did not evolve toward the end of incubation, whereas a smaller prebiotic effect was observed with FOS after 24 h than after 8 h. This suggests a slower fermentation with FG, which will further the prebiotic effect in the colon. In comparison to the results with the control, the addition of DG did not alter the bacterial numbers of any of the groups examined. The relative changes in the numbers of different groups of bacteria after 8 h and 24 h of incubation as a result of FG and FOS addition are shown in Fig. 1.
TABLE 2.
Changes in bacterial populations in batch cultures after 8 and 24 h of incubationa
| Organism(s) | Incubation time (h) | No. (log 10 cells/ml) with:
|
|||
|---|---|---|---|---|---|
| Control | FOS | FG | DG | ||
| Total bacteria | 0 | 9.20 ± 0.03 | |||
| 8 | 9.17 ± 0.04 | 9.36 ± 0.11 | 9.39 ± 0.05b | 9.23 ± 0.02 | |
| 24 | 9.19 ± 0.02 | 9.47 ± 0.01c | 9.40 ± 0.01c | 9.21 ± 0.01 | |
| Bifidobacteria | 0 | 8.03 ± 0.03 | |||
| 8 | 8.14 ± 0.06 | 8.81 ± 0.05b | 8.54 ± 0.02b | 8.22 ± 0.05 | |
| 24 | 7.99 ± 0.04 | 8.67 ± 0.01c | 8.60 ± 0.11c | 8.01 ± 0.11 | |
| Bacteroides | 0 | 8.23 ± 0.01 | |||
| 8 | 8.30 ± 0.04 | 8.39 ± 0.01 | 8.31 ± 0.02 | 8.31 ± 0.06 | |
| 24 | 8.34 ± 0.12 | 8.42 ± 0.04c | 8.24 ± 0.01c | 8.38 ± 0.06 | |
| Clostridia | 0 | 7.47 ± 0.09 | |||
| 8 | 7.54 ± 0.07 | 7.18 ± 0.04b | 7.29 ± 0.07b | 7.51 ± 0.03 | |
| 24 | 7.54 ± 0.02 | 7.37 ± 0.04 | 7.52 ± 0.02 | 7.57 ± 0.07 | |
| Eubacterium rectale | 0 | 8.28 ± 0.01 | |||
| 8 | 8.33 ± 0.01 | 8.64 ± 0.13 | 8.97 ± 0.08b | 8.45 ± 0.04b | |
| 24 | 8.34 ± 0.08 | 8.64 ± 0.08c | 8.91 ± 0.02c | 8.46 ± 0.08c | |
| Lactobacilli | 0 | 7.58 ± 0.02 | |||
| 8 | 7.72 ± 0.09 | 8.02 ± 0.01b | 7.73 ± 0.04 | 7.66 ± 0.05 | |
| 24 | 7.68 ± 0.07 | 7.90 ± 0.04c | 7.63 ± 0.01 | 7.66 ± 0.08 | |
Bacterial counts were obtained by using FISH. Values are the means ± standard deviations of the results.
Significantly different from control at 8 h (P < 0.05).
Significantly different from control at 24 h (P < 0.05).
FIG. 1.
Differences in the bacterial population sizes (black bars, FOS; white bars, predigested FG) compared to the total numbers of bacteria counted at 8 h [(selected bacterial numbers at 8 h/total bacteria counted at 8 h) − (selected bacterial numbers at 0 h/total bacteria counted at 0 h)] (A) and 24 h [(selected bacterial numbers at 24 h/total bacteria counted at 24 h) − (selected bacterial numbers at 0 h/total bacteria counted at 0 h)] (B). Error bars show standard deviations.
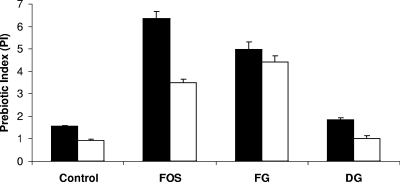
In order to obtain a general quantitative measure of the prebiotic effect, a prebiotic index (PI) was calculated for the oligosaccharide fractions (31). The PI equation is described as follows: PI = (Bif/total) + (Lac/total) + Erec/total) − (Bac/total) − (Clos/total), where Bif is bifidobacterial numbers at sample time divided by numbers at inoculation, Lac is lactobacilli numbers at sample time divided by numbers at inoculation, Erec is Eubacterium rectale numbers at sample time divided by numbers at inoculation, Bac is bacteroides numbers at sample time divided by numbers at inoculation, Clos is clostridia numbers at sample time divided by numbers at inoculation, and total is total bacteria number at sample time divided by numbers at inoculation. The PI represents a comparative relationship between the growth of “beneficial” bacteria, such as bifidobacteria, lactobacilli, and Eubacterium rectale, and that of the “less desirable” ones, such as clostridia and bacteroides, in relation to the change in the total number of bacteria (Fig. 2). For both substrates, the PI values obtained at 8 h of incubation were higher than those at 24 h. The FOS fraction produced the highest PI value after 8 h of incubation, 6.36, with that of FG being 4.98, whereas FG produced the highest PI value at the 24-h time point, 4.43, with that of FOS being 3.49. Low PI values were obtained with DG and the control at both the 8- and 24-h incubation time points.
FIG. 2.
Prebiotic index (PI) scores from batch cultures at 8 h (black bars) and 24 h (white bars) using FOS, predigested FG, DG, and untreated (control) cultures. Error bars show standard deviations.
Short-chain fatty acid production during fermentation.
The concentrations of lactic, acetic, propionic, and butyric acids produced during fermentation are shown in Table 3. FOS gave the highest total short-chain fatty acid production at all time points tested. However, butyrate production significantly increased after 8 h of incubation with FG and peaked at 24 h, coinciding with the highest number of Eubacterium rectale bacteria. Fermentation with FOS resulted in the highest production of lactic and acetic acids: their concentrations increased at 4 h and remained elevated up to 24 h. These increases correlated with changes in the numbers of bifidobacteria and lactobacilli. The concentrations of propionic and butyric acids were higher after 8 and 24 h of fermentation with FG and DG, again correlating with Eubacterium rectale population changes. In the absence of the added carbon source, an increase in acetic acid was observed after 24 h, although the amounts of the other organic acids did not change significantly.
TABLE 3.
Concentrations at 0, 4, 8, and 24 h of short-chain fatty acids and lactate produced during fermentation
| Fatty acid(s) | Incubation time (h) | Concn (mM)a with:
|
|||
|---|---|---|---|---|---|
| Control | FOS | FG | DG | ||
| Total fatty acids | 0 | 4.19 | 5.58 | 4.10 | 4.22 |
| 4 | 7.44 | 40.35 | 14.44 | 11.29 | |
| 8 | 14.80 | 74.02 | 39.13 | 33.21 | |
| 24 | 24.20 | 91.23 | 62.36 | 61.36 | |
| Lactic acid | 0 | 0.53 ± 0.03 | 0.54 ± 0.05 | 0.25 ± 0.02 | 0.22 ± 0.05 |
| 4 | 0.51 ± 0.29 | 12.23 ± 0.36 | 4.96 ± 0.03 | 1.18 ± 0.20 | |
| 8 | 0.68 ± 0.21 | 16.58 ± 1.36 | 4.79 ± 0.27 | 0.45 ± 0.38 | |
| 24 | 0.69 ± 0.25 | 16.91 ± 2.02 | 7.18 ± 0.44 | 1.58 ± 0.17 | |
| Acetic acid | 0 | 1.42 ± 0.22 | 2.15 ± 0.28 | 1.24 ± 0.32 | 1.16 ± 0.28 |
| 4 | 3.38 ± 1.03 | 21.97 ± 0.36 | 5.25 ± 1.06 | 7.00 ± 0.88 | |
| 8 | 8.81 ± 1.89 | 46.15 ± 1.21 | 14.09 ± 0.16 | 20.77 ± 1.72 | |
| 24 | 15.05 ± 2.43 | 50.77 ± 3.91 | 26.88 ± 0.01 | 34.79 ± 2.90 | |
| Propionic acid | 0 | 0.90 ± 0.21 | 1.05 ± 0.16 | 1.27 ± 0.04 | 1.21 ± 0.06 |
| 4 | 1.80 ± 0.56 | 2.67 ± 0.36 | 2.44 ± 1.06 | 1.04 ± 0.06 | |
| 8 | 2.42 ± 0.19 | 4.00 ± 0.62 | 8.09 ± 1.31 | 7.06 ± 1.60 | |
| 24 | 3.90 ± 0.70 | 11.11 ± 2.27 | 12.09 ± 2.23 | 14.77 ± 0.70 | |
| Butyric acid | 0 | 1.34 ± 0.04 | 1.85 ± 0.21 | 1.35 ± 0.04 | 1.63 ± 0.08 |
| 4 | 1.75 ± 0.66 | 3.47 ± 0.36 | 1.79 ± 0.54 | 2.07 ± 0.09 | |
| 8 | 2.89 ± 0.25 | 7.29 ± 0.71 | 12.16 ± 0.33 | 4.93 ± 0.24 | |
| 24 | 4.56 ± 0.17 | 12.44 ± 4.73 | 16.21 ± 0.87 | 10.22 ± 1.07 | |
Values for individual acids are means ± standard deviations of the results.
DISCUSSION
In the present study, we have demonstrated the prebiotic potential of almond seeds. As far as we are aware, this is the first study that has used combined models of human digestion which include gastric and duodenal digestion followed by colonic fermentation to study the effects of almond extracts on the modulation of gut microbiota. Commercially available prebiotics (such as FOS and galactooligosaccharides) are not sensitive to gastric acid and do not serve as substrate for hydrolytic enzymes in the upper digestive tract. The evaluation of novel prebiotic compounds should take into account the available ingredients which can be digested by human enzymes and adsorbed, thus entering into intermediary metabolism. On the contrary, food components able to reach the colon can potentially provide the body with additional energy via microbial fermentation, and the production of short-chain fatty acids may have potential prebiotic functionality.
In our previous study, we showed that relatively small amounts of almond lipids and proteins are bioavailable during gastric and small intestinal digestion and that nutrient encapsulation by cell walls is likely to prevent their digestion in the upper GIT (27). However, bioaccessibility is improved when the number of fractured cells is increased by processing or by increased residence time in the gut. Evidence of bacterial fermentation of the almond tissue was provided by micrographs of fecal samples collected from healthy subjects consuming an almond-rich diet (13). By using transmission electron microscopy, it was possible to document the presence of bacteria both on the cell wall surface and within the cells. Therefore, almond intracellular lipids, together with nonstarch polysaccharides from cell walls, which are known to be metabolized to a variable degree in the large intestine, could represent a suitable carbon source for bacterial fermentation (12, 13). The erosion of the middle lamella observed in fecal samples was considered to be further evidence of pectic degradation by gut microbiota. Our data presented in this study show that when almond lipids are available for fermentation in the large bowel, modulation of the composition of the bacterial population is observed, with a significant increase in the numbers of bifidobacteria and Eubacterium rectale (Fig. 1). However, when the almond lipid source was removed (defatted almond product) no significant changes in the bacterial population were detected (Table 1). These results suggest that the lipid component of almond seeds is relevant in the alteration of bacterial growth and metabolism.
The unique role of dietary fiber, essentially the plant cell wall, has been evaluated in many physiological processes and in disease prevention (7). Pectins with different degrees of esterification have previously been shown to increase Eubacterium rectale numbers, and this group of gut bacteria is known to produce relatively large amounts of butyrate (1, 11). The anti-inflammatory (4) and antineoplastic (25, 39) properties of butyrate have been demonstrated on cell tissue cultures in vitro, increasing the interest in the potential effect of butyrate in inflammatory bowel disease and colorectal cancer. However, butyrate production from oligofructose fermentation was shown to be mainly derived from interconversion of extracellular lactate and acetate (30). In the present study, FG produced more butyrate than FOS and can therefore be considered a butyrogenic prebiotic.
The kinetics of almond lipid digestion and absorption is an important factor for postprandial lipemia and has implications for the regulation of body weight (41). The results of a dose-response study have shown that almond consumption improved the serum profile of healthy and middle-hypercholesterolemic adults, thus reducing risk factors for coronary heart disease (38). The predominant fatty acid of almond triacylglycerols is oleic acid, comprising more than 65% of the total oil fraction and contributing to the high monounsaturated fat content of almonds. Prebiotics have also been reported to indirectly lead to a reduction in serum triglyceride levels (44), and short-chain fatty acid production can modulate the expression of multiple genes involved in the atherosclerosis process (32). An in vivo murine study investigating the effects of prebiotics on atherosclerotic plaques showed that inulin and FOS were able to reduce plasma and hepatic cholesterol (34). In the present study, we have shown that almond extracts can act as prebiotics, and this may add functionality to almonds in improving serum cholesterol levels.
In this study, we have not investigated whether oleic acid, which is present in the FG fraction, was utilized by the gut bacteria. However, there is evidence to indicate that oleic acid may be metabolized by the ruminal bacterium Selenomonas ruminantium and strains of Streptococcus, Enterococcus, and Lactobacillus (20, 21). Linoleic acid, another unsaturated fatty acid, was also metabolized in the human colon by a number of Roseburia species (8). In addition, by being incorporated into the bacterial membrane of specific gut bacteria, oleic acid may contribute to their increased survival rate in gastric juice (6).
In conclusion, we have shown that almond seeds exhibited the potential to be used as a novel source of prebiotics, increasing the populations of bifidobacteria and Eubacterium rectale with the subsequent increase in butyrate concentrations. More-detailed studies on the digestibility of almonds and the role played by lipids in the potential prebiotic effect need to be performed using human volunteers.
Acknowledgments
We gratefully acknowledge the help provided by Yvan Lemarc (IFR) with statistic analyses. We thank Karen Lapsley (ABC) for providing the almond products and for useful discussions.
This research was funded by the Almond Board of California (ABC).
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakeney, A. B., P. J. Harris, and B. A. A. Stone. 1983. A simple and rapid preparation of alditol acetates for monosaccharides analysis. Carbohydr. Res. 113:291-299. [Google Scholar]
- 3.Blumenkrantz, N., and G. Asboe-Hansen. 1973. New method for quantitative determination of uronic acids. Anal. Biochem. 54:484-489. [DOI] [PubMed] [Google Scholar]
- 4.Cavaglieri, C. R., A. Nishiyama, L. C. Fernandez, R. Curi, E. A. Miles, and P. C. Calder. 2003. Differential effects of short chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 73:1683-1690. [DOI] [PubMed] [Google Scholar]
- 5.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran, B. M., C. Stanton, G. F. Fitzgerald, and R. P. Ross. 2007. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 153:291-299. [DOI] [PubMed] [Google Scholar]
- 7.Cummings, J. H., L. M. Edmond, and E. A. Magee. 2004. Dietary carbohydrates and health: do we still need the fibre concept? Clin. Nutr. Suppl. 1:5-17. [Google Scholar]
- 8.Devillard, E., F. M. McIntosh, S. H. Duncan, and R. J. Wallace. 2007. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J. Bacteriol. 189:2566-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diplock, A. T., P. J. Aggett, M. Ashwell, F. Bornet, E. B. Fern, and M. B. Roberfroid. 1999. Scientific concepts of functional foods in Europe: consensus document. Br. J. Nutr. 81:(Suppl. 1):S1-S27. [PubMed] [Google Scholar]
- 10.Dourado, F., A. Barros, M. Mota, M. A. Coimbra, and F. M. Gama. 2004. Anatomy and cell wall polysaccharides of almond (Prunus dulcis D. A. Webb) seeds. J. Agric. Food Chem. 52:1364-1370. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, S. H., K. P. Scott, A. G. Ramsay, H. J. M. Harmsen, G. W. Welling, C. S. Stewart, and J. H. Flint. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, C. A., and I. R. Rowland. 1992. Bacterial fermentation in the colon and its measurement, p. 119-136. In T. F. Schweizer and C. A. Edwards (ed.), Dietary fiber: a component of food. Springer, London, United Kingdom.
- 13.Ellis, P. R., C. W. C. Kendall, Y. Ren, C. Parker, J. F. Pacy, K. W. Waldron, and D. J. A. Jenkins. 2004. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 80:604-613. [DOI] [PubMed] [Google Scholar]
- 14.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concepts of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G. R., and X. Wang. 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77:412-420. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, G. R., P. B. Ottaway, and R. A. Rastall. 2000. Prebiotics: new developments in functional foods. Chandos Publishing Limited, Oxford, United Kingdom.
- 18.Harmsen, H. J. M., P. Elferrich, F. Schut, and G. W. Welling. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 11:3-12. [Google Scholar]
- 19.Hudault, S., S. V. Liévin, M.-F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson, J. A., C. A. MacKenzie, and K. N. Joblin. 1995. Conversion of oleic acid to 10-hydroxystearic acid by two species of ruminal bacteria. Appl. Microbiol. Biotechnol. 44:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Hudson, J. A., Y. Cai, R. J. Corner, B. Morvan, and K. N. Joblin. 2000. Identification and enumeration of oleic acid and linoleic acid hydrating bacteria in the rumen of sheep and cows. J. Appl. Microbiol. 88:286-292. [DOI] [PubMed] [Google Scholar]
- 22.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in faecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lievin, V., L. Pfeiffer, S. Hudault, F. Rochat, D. Brassart, J.-R. Neeser, and A. L. Servin. 2000. Bifidobacterium strains from resident infant human gastrointestinal microbiota exert antimicrobial activity. Gut 47:646-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lievin, V., and A. L. Servin. 2006. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 19:315-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupton, J. R. 2004. Microbial degradation products influence colon cancer risk: the butyrate controversy. J. Nutr. 134:479-482. [DOI] [PubMed] [Google Scholar]
- 26.Mandalari, G., C. Nueno Palop, K. Tuohy, G. R. Gibson, R. N. Bennett, K. W. Waldron, G. Bisignano, A. Narbad, and C. B. Faulds. 2007. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microb. Biotechnol. 73:1173-1179. [DOI] [PubMed] [Google Scholar]
- 27.Mandalari, G., R. M. Faulks, G. T. Rich, V. Lo Turco, D. R. Picout, R. B. Lo Curto, G. Bisignano, P. Dugo, G. Dugo, K. W. Waldron, P. R. Ellis, and M. S. J. Wickham. 2008. Release of protein, lipid and vitamin E from almond seeds during digestion. J. Agric. Food Chem. 56:3409-3416. [DOI] [PubMed] [Google Scholar]
- 28.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 29.Menne, E., N. Guggenbuhl, and M. Roberfroid. 2000. Fn-type chicory inulin hydrolysate has a prebiotic effect in humans. J. Nutr. 130:1197-1199. [DOI] [PubMed] [Google Scholar]
- 30.Morrison, D. J., W. G. Mackay, C. A. Edwards, T. Preston, B. Dodson, and L. T. Weaver. 2006. Butyrate production from oligofructose fermentation by the human faecal flora: what is the contribution of extracellular acetate and lactate? Br. J. Nutr. 96:570-577. [PubMed] [Google Scholar]
- 31.Palframan, R., G. R. Gibson, and R. A. Rastall. 2003. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett. Appl. Microbiol. 37:281-284. [DOI] [PubMed] [Google Scholar]
- 32.Ranganna, K., F. M. Yatsu, B. E. Hayes, S. G. Milton, and A. Jayakumar. 2000. Butyrate inhibits proliferation-induced proliferating cell nuclear antigen expression (PCNA) in rat vascular smooth muscle cells. J. Clin. Investig. 102:910-918. [DOI] [PubMed] [Google Scholar]
- 33.Rao, V. A. 2001. The prebiotic properties of oligofructose at low intake levels. Nutr. Res. 6:843-848. [Google Scholar]
- 34.Rault-Nania, M. H., E. Gueux, C. Demougeot, C. Demigné, E. Rock, and A. Mazur. 2006. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br. J. Nutr. 96:840-844. [DOI] [PubMed] [Google Scholar]
- 35.Ren, Y., K. W. Waldron, J. F. Pacy, A. Brain, and P. R. Ellis. 2001. Chemical and histochemical characterisation of cell wall polysaccharides in almond seeds in relation to lipid bioavailability, p. 448-452. In W. Pfannhauser, G. R. Fenwick, and S. Khokhar (ed.), Biologically-active phytochemicals in food. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 36.Roberfroid, M. B. 1996. Functional effects of food components and the gastrointestinal system: chicory fructooligosaccharides. Nutr. Rev. 54:S38-S42. [DOI] [PubMed] [Google Scholar]
- 37.Rycroft, C. E., M. R. Jones, G. R. Gibson, and R. A. Rastall. 2001. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 91:878-887. [DOI] [PubMed] [Google Scholar]
- 38.Sabate, J., E. Haddad, J. S Tanzman, P. Jambazian, and S. Rajaram. 2003. Serum lipid response to the graduated enrichment of a step I diet with almonds: a randomized feeding trial. Am. J. Clin. Nutr. 77:1379-1384. [DOI] [PubMed] [Google Scholar]
- 39.Scheppach, W., and F. Weiler. 2004. The butyrate story: old wine in new bottles? Curr. Opin. Clin. Nutr. Metab. Care 7:563-567. [DOI] [PubMed] [Google Scholar]
- 40.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 41.Sethi, S., M. J. Gibney, and C. M. Williams. 1993. Postprandial lipoprotein metabolism. Nutr. Res. Rev. 6:161-183. [DOI] [PubMed] [Google Scholar]
- 42.Tuohy, K. M., C. J. Ziemer, A. Klinder, Y. Knöbel, B. L. Pool-Zobel, and G. R. Gibson. 2002. A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microb. Ecol. Health Dis. 14:165-173. [Google Scholar]
- 43.Tuohy, K. M., S. Kolida, A. M. Lustenberger, and G. R. Gibson. 2001. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides: a human volunteer study. Br. J. Nutr. 86:341-348. [DOI] [PubMed] [Google Scholar]
- 44.Williams, C. M., and K. G. Jackson. 2002. Inulin and oligofructose: effects on lipid metabolism from human studies. Br. J. Nutr. 87:S261-S264. [DOI] [PubMed] [Google Scholar]