Abstract
The intraspecific relationships among a collection of Enterococcus faecium isolates comprising probiotic cultures and human clinical isolates were investigated through the combined use of two high-resolution DNA-fingerprinting techniques. In addition, the incidences of antimicrobial resistance and virulence traits were investigated. A total of 128 E. faecium isolates from human clinical or nonclinical sources or used as probiotic cultures were subjected to fluorescent amplified fragment length polymorphism (FAFLP) fingerprinting and pulsed-field gel electrophoresis (PFGE) analysis of SmaI macrorestriction patterns. Susceptibilities to 16 antimicrobial agents were tested using broth microdilution, and the presence of the corresponding resistance genes was investigated using PCR. Multiplex PCR was used to detect the presence of the enterococcal virulence genes asa1, gelE, cylA, esp, and hyl. The results of the study showed that two intraspecific genomic groups (I and II) were obtained in FAFLP analysis. PFGE analysis demonstrated high variability within these two groups but also indicated that some probiotic cultures were indistinguishable and that a number of clinical isolates may be reisolations of commercial probiotic cultures. Compared to group II, which contained the majority of the probiotic isolates and fewer human clinical isolates, higher phenotypic and genotypic resistance frequencies were observed in group I. Two probiotic isolates were phenotypically resistant to erythromycin, one of which contained an erm(B) gene that was not transferable to enterococcal recipients. None of the probiotic E. faecium isolates demonstrated the presence of the tested virulence genes. The previously reported observation that E. faecium consists of two intraspecific genomic groups was further substantiated by FAFLP fingerprinting of 128 isolates. In combination with antimicrobial resistance and virulence testing, this grouping might represent an additional criterion in assessing the safety of new potential probiotic E. faecium isolates.
Enterococci are normal inhabitants of the gastrointestinal tracts of both humans and animals. In the human intestine, Enterococcus faecium and Enterococcus faecalis are the two predominant species (11, 26). On the other hand, enterococci also occur in or are deliberately added to fermented foods, in which they contribute to the organoleptic properties, and have also been used as probiotics (16). According to the FAO/WHO definition, a “probiotic” is a live microorganism that, when administered in adequate amounts, confers a health benefit on the host (57). Enterococci used as probiotics may improve the microbial balance of the intestine or can be used in the treatment of gastroenteritis in humans and animals (13). Enterococcal strains used in food and as probiotics mainly belong to the species E. faecium (13).
In contrast to most other genera of the lactic acid bacteria, not all enterococcal species have “generally recognized as safe” status (11). Indeed, enterococci have been recognized as important nosocomial pathogens causing endocarditis, bacteremia, and central nervous system infections, as well as neonatal, respiratory tract, urinary tract, and other infections (25, 26), which may in part be linked to the presence of antibiotic resistance and virulence properties. Resistance of E. faecium and E. faecalis to therapeutically important antibiotics is emerging, in particular, resistance to the glycopeptides vancomycin and teicoplanin, which is often associated with high-level resistance to aminoglycosides (11). The emergence of vancomycin-resistant enterococci, belonging predominantly to E. faecium, has resulted in cases of untreatable infections (28). Antibiotic resistance may confer a selective advantage on enterococci in the hospital environment, thereby supporting their virulence potential (26). In addition, dissemination of antimicrobial resistance genes through clonal expansion and horizontal transmission causes great concern for infectious disease specialists.
The origin of enterococcal pathogenicity has been linked to a range of virulence traits involved in adhesion, translocation, and immune evasion (20, 26). Several putative virulence factors have been identified in enterococci, such as aggregation substance (encoded by asa1) (14), cytolysin (encoded by cylA) (18), gelatinase (encoded by gelE) (40), hyaluronidase (encoded by the hyl gene) (36), and enterococcal surface protein (encoded by esp) (38).
Among other criteria, the FAO-WHO have recommended that antimicrobial resistance patterns and opportunistic virulence properties should be tested to document the safety of probiotic strains (58). However, because both characteristics are strain specific, molecular strain typing should also be considered for safety assessment of potential probiotics. Previous typing studies (34, 56) have indicated that antibiotic-resistant E. faecium isolates from different sources tend to cluster according to their sources and hosts. Based on amplified fragment length polymorphism (AFLP) and randomly amplified polymorphic DNA-PCR analysis, Vancanneyt et al. (47) delineated two intraspecific genomic groups (I and II) among E. faecium isolates from various sources. The authors suggested that subclusters of group I could to some extent be correlated with the origins and pathogenicities of the strains. In all of the above-mentioned studies, however, characterization of antibiotic resistance and virulence genes was not performed, and only a few human clinical isolates were investigated.
The aim of the present study was to investigate the intraspecific relationships among a total of 128 E. faecium isolates comprising human clinical isolates and commercial probiotic cultures through the combined use of pulsed-field gel electrophoresis (PFGE) and AFLP. In addition, the incidences of antimicrobial resistance and virulence traits were investigated.
MATERIALS AND METHODS
Bacterial isolates.
A total of 128 E. faecium isolates were collected in the framework of a European Union-funded research project, PROSAFE (48), including 37 isolates from human feces, 79 isolates from different human clinical samples, and 12 isolates commercially used as probiotics, 6 of which were isolated from products with probiotic claims (41) while the remaining 6 were received directly from the manufacturer or the depositor (Table 1). A representative subset of these isolates has been deposited in the BCCM/LMG Bacteria Collection (Ghent University, Ghent, Belgium [http://www.belspo.be/bccm/lmg.htm]). The isolates were routinely grown on Columbia agar (Becton-Dickinson, Sparks, MD) supplemented with 5% defibrinated horse blood at 37°C for 24 h.
TABLE 1.
E. faecium isolates included in this study
| PRSF no. | Other strain no. | Depositora | Originb | Source; geographical origin; yr of isolation | FAFLP group | PFGE group |
|---|---|---|---|---|---|---|
| PRSF-E001 | 6254 | H. Dupont | H | Peritonitis; France | I | 32a′ |
| PRSF-E002 | 21771 | H. Dupont | H | Peritonitis; France | II | 45a |
| PRSF-E003 | 22183 | H. Dupont | H | Peritonitis; France | I | 30a |
| PRSF-E004 | 31505 | H. Dupont | H | Peritonitis; France | I | 9c |
| PRSF-E005 | 37215 | H. Dupont | H | Peritonitis; France | I | 37b |
| PRSF-E006 | 43169 | H. Dupont | H | Peritonitis; France | I | 28a |
| PRSF-E007 | 44849; LMG 24169 | H. Dupont | H | Peritonitis; France | I | 37a |
| PRSF-E008 | 45414 | H. Dupont | H | Peritonitis; France | II | 82a |
| PRSF-E009 | 45780 | H. Dupont | H | Peritonitis; France | I | 38a |
| PRSF-E010 | 46741 | H. Dupont | H | Peritonitis; France | I | 48a |
| PRSF-E011 | 47271 | H. Dupont | H | Peritonitis; France | I | 25a |
| PRSF-E012 | 59910 | H. Dupont | H | Peritonitis; France | II | 27a |
| PRSF-E013 | 65233 | H. Dupont | H | Peritonitis; France | I | 37a |
| PRSF-E015 | 74254 | H. Dupont | H | Peritonitis; France | I | 98a |
| PRSF-E016 | 75133; LMG 24170 | H. Dupont | H | Peritonitis; France | I | 32a |
| PRSF-E017 | 78601 | H. Dupont | H | Peritonitis; France | I | 18a |
| PRSF-E018 | 83123 | H. Dupont | H | Peritonitis; France | I | 19c |
| PRSF-E019 | 90056 | H. Dupont | H | Peritonitis; France | I | 15a |
| PRSF-E020 | 960018; LMG 24171 | UZA | H | Blood; Belgium; 2000 | I | 31a |
| PRSF-E021 | 960050 | UZA | H | Blood; Belgium; 2000 | I | 19b |
| PRSF-E022 | 1-20 | UZA | H | Blood; Belgium; 1995 | I | 16a |
| PRSF-E023 | 1-9 | UZA | H | Blood; Belgium; 1995 | I | 17a′ |
| PRSF-E024 | 11/4 | UZA | H | Blood; Belgium; 1995 | I | 23a |
| PRSF-E025 | 13/1; LMG 24172 | UZA | H | Blood; Belgium; 1995 | II | 26b |
| PRSF-E026 | 13/11 | UZA | H | Blood; Belgium; 1995 | II | 74a |
| PRSF-E027 | 13/5 | UZA | H | Blood; Belgium; 1995 | II | 26a |
| PRSF-E028 | 14/1 | UZA | H | Blood; Belgium; 1995 | I | 94a |
| PRSF-E029 | 17/7 | UZA | H | Blood; Belgium; 1995 | II | 101a |
| PRSF-E030 | 18/2 | UZA | H | Blood; Belgium; 1995 | I | 35a |
| PRSF-E031 | 18/7 | UZA | H | Blood; Belgium; 1995 | I | 93a |
| PRSF-E032 | 2/5 | UZA | H | Blood; Belgium; 1995 | I | 49a |
| PRSF-E033 | 20/8; LMG 23226 | UZA | H | Blood; Belgium; 1995 | I | 22a |
| PRSF-E034 | 22/1; LMG 23227 | UZA | H | Blood; Belgium; 1995 | I | 92a |
| PRSF-E035 | 3-26; LMG 23228 | UZA | H | Blood; Belgium; 1995 | I | 95a |
| PRSF-E036 | 4/10; LMG 23229 | UZA | H | Blood; Belgium; 1995 | I | 17a |
| PRSF-E037 | 4/19; LMG 23230 | UZA | H | Blood; Belgium; 1995 | I | 17a |
| PRSF-E038 | 5/5; LMG 23231 | UZA | H | Blood; Belgium; 1995 | I | 36b |
| PRSF-E039 | 6-8; LMG 23232 | UZA | H | Blood; Belgium; 1995 | II | 83a |
| PRSF-E040 | 7/6; LMG 23233 | UZA | H | Blood; Belgium; 1995 | I | 41a |
| PRSF-E041 | 8/1; LMG 23234 | UZA | H | Blood; Belgium; 1995 | I | 4a |
| PRSF-E042 | 9/4; LMG 23235 | UZA | H | Blood; Belgium; 1995 | II | 44a |
| PRSF-E043 | 01SE05 LMG 23236 | UA | H | Fecal flora; Belgium; 1997 | I | 20b |
| PRSF-E044 | 01VHM19; LMG 23237 | UA | H | Fecal flora; Belgium; 1996 | II | 66a |
| PRSF-E045 | 02bVHM05; LMG 23238 | UA | H | Fecal flora; Belgium; 1997 | II | 12a |
| PRSF-E046 | 02VHM03; LMG 23239 | UA | H | Fecal flora; Belgium; 1996 | I | 100a |
| PRSF-E047 | 04VHM06; LMG 23240 | UA | H | Fecal flora; Belgium; 1996 | II | 59a |
| PRSF-E048 | 04VHM09; LMG 23241 | UA | H | Fecal flora; Belgium; 1996 | II | 88a |
| PRSF-E049 | 04VWK14; LMG 23242 | UA | H | Fecal flora; Belgium; 1997 | II | 2a |
| PRSF-E050 | 06VHM11; LMG 23243 | UA | H | Fecal flora; Belgium; 1996 | II | 87a |
| PRSF-E051 | 06VWK04; LMG 23244 | UA | H | Fecal flora; Belgium; 1997 | II | 76c |
| PRSF-E052 | 07SS01; LMG 23245 | UA | H | Fecal flora; Belgium; 1997 | I | 22b |
| PRSF-E053 | 07TB04; LMG 24173 | UA | H | Fecal flora; Belgium; 1997 | I | 23b |
| PRSF-E054 | 09SS01; LMG 24174 | UA | H | Fecal flora; Belgium; 1997 | II | 9b |
| PRSF-E055 | 09VHM05 | UA | H | Fecal flora; Belgium; 1997 | II | 85a |
| PRSF-E057 | 10SS05 | UA | H | Fecal flora; Belgium; 1997 | I | 3a |
| PRSF-E058 | 11T | UA | H | Fecal flora; Belgium; 1996 | I | 97a |
| PRSF-E059 | 126T | UA | H | Fecal flora; Belgium; 1996 | I | 89a |
| PRSF-E060 | 13/13 | UA | H | Blood; Belgium; 1995 | II | 17a′ |
| PRSF-E061 | 162V | UA | H | Fecal flora; Belgium; 1996 | I | 7a |
| PRSF-E062 | 175V | UA | H | Fecal flora; Belgium; 1996 | I | 53a |
| PRSF-E063 | 24/16 | UA | H | Blood; Belgium; 1995 | I | 47a |
| PRSF-E064 | 24/19 | UA | H | Blood; Belgium; 1995 | II | 103a′ |
| PRSF-E065 | 302V | UA | H | Fecal flora; Belgium; 1996 | I | 68a |
| PRSF-E066 | 325T | UA | H | Fecal flora; Belgium; 1996 | I | 69a |
| PRSF-E067 | 360V | UA | H | Fecal flora; Belgium; 1996 | I | 8a′ |
| PRSF-E068 | 39771a | H. Dupont | H | Peritonitis; France | I | 14a |
| PRSF-E069 | 398T | UA | H | Fecal flora; Belgium; 1996 | I | 96a |
| PRSF-E070 | 406T | UA | H | Fecal flora; Belgium; 1996 | I | 8a |
| PRSF-E072 | 615V; LMG 24175 | UA | H | Fecal flora; Belgium; 1996 | I | 8b |
| PRSF-E073 | 7532b; LMG 24176 | H. Dupont | H | Peritonitis; France | I | 98d |
| PRSF-E074 | 80A | UA | H | Fecal flora; Belgium; 1996 | I | 105a |
| PRSF-E075 | 8489b | H. Dupont | H | Peritonitis; France | I | 51a |
| PRSF-E076 | 88156a | H. Dupont | H | Peritonitis; France | I | 13b |
| PRSF-E077 | 95-04-3212 | UA | H | Fecal flora; Belgium; 1995 | I | 95b |
| PRSF-E078 | 95-12-6002 | UA | H | Fecal flora; Belgium; 1995 | I | 90a |
| PRSF-E079 | 95-12-6037 | UA | H | Fecal flora; Belgium; 1995 | I | 6b |
| PRSF-E080 | 95-12-6076; LMG 24177 | UA | H | Fecal flora; Belgium; 1995 | I | 7b |
| PRSF-E081 | 9727/1700 | UA | H | Urine; Belgium; 1997 | I | 98c |
| PRSF-E083 | A.N.07/27/99 | UA | H | Wound; Belgium; 1999 | I | 36a |
| PRSF-E084 | D.A.072999 | UA | H | Wound; Belgium; 1999 | I | 19e |
| PRSF-E085 | M4/05/0203 | UZA | H | Blood; Belgium; 1994 | II | 86a |
| PRSF-E086 | M4/06/0908; LMG 24178 | UZA | H | Blood; Belgium; 1994 | II | 62a |
| PRSF-E087 | M5/01/0874; LMG 24179 | UZA | H | Blood; Belgium; 1995 | I | 24a |
| PRSF-E088 | M5/04/0316 | UZA | H | Blood; Belgium; 1995 | I | 19b′ |
| PRSF-E089 | M5/04/0363 | UZA | H | Blood; Belgium; 1995 | I | 31b |
| PRSF-E090 | M5/04/0408; LMG 24180 | UZA | H | Blood; Belgium; 1995 | I | 6a |
| PRSF-E092 | M5/10/0447 | UZA | H | Blood; Belgium; 1995 | I | 21a |
| PRSF-E093 | M6/02/0521; LMG 24181 | UZA | H | Blood; Belgium; 1996 | II | 57a |
| PRSF-E094 | M6/02/0956 | UZA | H | Blood; Belgium; 1996 | I | 24b |
| PRSF-E095 | M6/03/1007 | UZA | H | Blood; Belgium; 1996 | I | 9a |
| PRSF-E097 | S.E.072899 | UA | H | Wound; Belgium; 1999 | I | 19d |
| PRSF-E098 | V.K.07/27/99 | UA | H | Wound; Belgium; 1999 | II | 19b′ |
| PRSF-E099 | V.K.072999; LMG 24182 | UA | H | Wound; Belgium; 1999 | I | 21b |
| PRSF-E100 | VL.F.080499; LMG 24183 | UA | H | Wound; Belgium; 1999 | I | 19a |
| PRSF-E101 | W.R.032400; LMG 24184 | UA | H | Wound; Belgium; 2000 | I | 25b |
| PRSF-E102 | D38-P18 | P | Probiotic product | II | 75a | |
| PRSF-E103 | D29-P07 | P | Probiotic product | II | 57b | |
| PRSF-E104 | D29-P29 | P | Probiotic product | II | 62b | |
| PRSF-E105 | D42-P17 | P | Probiotic product | II | 63a | |
| PRSF-E106 | D28-P06 | P | Probiotic product | II | 76b | |
| PRSF-E107 | D27-P05 | P | Probiotic product | II | 63a | |
| PRSF-E110 | UW 1952 | RKI | H | Peritonitis | II | 43a |
| PRSF-E113 | UW 2434 | RKI | H | Blood | I | Bad profile |
| PRSF-E114 | UW 2493 | RKI | H | Blood | I | Bad profile |
| PRSF-E115 | UW 2742 | RKI | H | Liver | I | 61a |
| PRSF-E116 | UW 3181 | RKI | H | Blood | I | 46a |
| PRSF-E117 | D05 | PHA | Fecal flora; Sweden; 1968 | II | 63a | |
| PRSF-E121 | LMG 20734 | LMG/BCCM | H | Fecal flora; The Netherlands; 1995 | I | 42a |
| PRSF-E122 | LMG 20735 | LMG/BCCM | H | Fecal flora; The Netherlands; 1995 | I | 11a |
| PRSF-E123 | LMG 20939 | LMG/BCCM | H | Fecal flora; Ireland; 1997 | II | 56a |
| PRSF-E124 | LMG 20946 | LMG/BCCM | H | Fecal flora; Ireland; 1997 | II | 56a |
| PRSF-E125 | CCUG 31387 | CCUG | H | Blood; Sweden; 1993 | I | 39a |
| PRSF-E126 | CCUG 32171 | CCUG | H | Blood; Sweden; 1993 | I | 58a |
| PRSF-E127 | CCUG 32655 | CCUG | H | Blood; Sweden; 1994 | II | 50a |
| PRSF-E128 | CCUG 34324 | CCUG | H | Blood; Sweden; 1995 | I | 51c |
| PRSF-E129 | CCUG 45539 | CCUG | H | Blood; Sweden; 2001 | I | 13a |
| PRSF-E130 | CCUG 29743 | CCUG | H | Blood; Sweden; 1992 | I | 50b |
| PRSF-E131 | CCUG 36838 | CCUG | H | Blood; Sweden; 1996 | II | 1a |
| PRSF-E132 | D04 | PH | Fecal flora; Italy; 1977 | II | 63a | |
| PRSF-E133 | D04 | PH | Fecal flora; Italy; 1977 | II | 63a | |
| PRSF-E134 | D10 | PH | Non-human; Canada; 1988 | II | 76a | |
| PRSF-E140 | D15 | PH | Animal; United Kingdom | I | 29a | |
| PRSF-E142 | H 437 | P. Moreillon | H | Blood; Switzerland; 1996 | I | 10a |
| PRSF-E144 | 03WS09 | C. Vael | H | Fecal flora | I | 20a |
| PRSF-E146 | 01SS07 | C. Vael | H | Fecal flora | II | 60a |
| PRSF-E149 | C68 | L. Rice | H | Fecal flora | I | 40a |
| PRSF-E162 | FH 02001 | H | Fecal flora; France; 2002 | I | 5a | |
| PRSF-E164 | LMG 23514 | D50 | PHA | Dairy product, cheese; Italy | II | 63a |
BCCM/LMG, BCCM/LMG Bacteria Collection, Laboratorium voor Microbiologie, Ghent University, Ghent, Belgium; CCUG, Culture Collection University of Göteborg, Department of Clinical Bacteriology, Göteborg, Sweden; P. Moreillon, CHUV, Centre de Collection de Type Microbien, Centre Hospitalier Universitaire Vaudois, Institut de Microbiologie, Université de Lausanne, Lausanne, Switzerland; Dx, probiotic strain collected from company x; Dx-Py, probiotic strain collected from product y from company x; UA, University of Antwerp, Antwerp, Belgium; UZA, University Hospital Antwerp, Antwerp, Belgium; C. Vael, ZNA, Antwerp, Belgium; L. Rice, Research and Medical Services, Louis Stokes Cleveland Veterans Affairs Medical Center, and Department of Medicine, Case Western Reserve University, Cleveland, OH; RKI, Robert Koch Institute, Wernigerode Branch, Wernigerode, Germany; H. Dupont, CHU D'Amiens, France.
P, strain used commercially as a probiotic; PH, strain used commercially as a probiotic for human consumption; PHA, strain used commercially as a probiotic for human and animal consumption; H, human isolate.
Identification and typing.
For identification purposes, the isolates were first subjected to fluorescent AFLP (FAFLP) analysis as described below.
For FAFLP analysis, total chromosomal DNA was prepared using a modification of the method described by Pitcher et al. (31). Template preparation was carried out as described previously (19). Essentially, purified genomic DNA was digested by two restriction enzymes, a 4-bp cutter EcoRI and a 6-bp cutter TacI. Small double-stranded DNA molecules (adaptors; 15 to 20 bp) containing one compatible end were ligated to the corresponding “sticky ends” of the restriction fragments. These adaptors served as binding sites for selective amplification with the primer combination E01/T01 (primers extended with an additional A) (19). PCR products were separated according to their lengths on a high-resolution polyacrylamide gel using a DNA sequencer (ABI 377). Fragments that contained an adaptor specific for the restriction half-site created by the 6-bp cutter were visualized due to the 5′ end labeling of the corresponding primer with the fluorescent dye 6-carboxyfluorescein. The resulting electrophoretic patterns were numerically analyzed with Bionumerics software version 4.01 (Applied Maths, Belgium), using the Dice coefficient and unweighted-pair group method using average linkages cluster analysis.
The intraspecific diversity among the collection of E. faecium isolates was also studied by PFGE, as previously described (7). Briefly, bacterial cells from an overnight culture were embedded in low-melting-point preparative agarose (Bio-Rad Laboratories, Nazareth, Belgium). After cell wall and protein digestion, the plugs were digested overnight with 30 U of SmaI (MBI Fermentas, St. Leon-Rot, Germany) at 25°C. PFGE was performed with a 1% agarose gel by using a CHEF Mapper apparatus (Bio-Rad Laboratories) in 0.5× Tris-borate-EDTA buffer at 14°C at 6 V/cm. A linearly ramped switching time from 5 to 35 s was applied for 24 h. The DNA band profiles were stained with ethidium bromide, and the image was digitized with the Gel Doc 1000 System (Bio-Rad Laboratories). Conversion, normalization, and further analysis of the DNA band patterns were performed using GelCompar software version 4.0b (Applied Maths, Kortrijk, Belgium) as described previously (33). Similarity between PFGE patterns was expressed using the Dice band-based correlation coefficient.
Phenotypic and genotypic characterization of antimicrobial susceptibility.
Antimicrobial susceptibility testing of the isolates was performed using broth microdilution following CLSI guidelines (5) to determine the MICs of the following agents (with the concentration ranges [mg/liter] tested given in parentheses): penicillin (0.032 to 64), ampicillin (0.032 to 64), ampicillin/sulbactam (sulbactam was tested as a fixed concentration of 8 mg/liter; 0.032 to 64), vancomycin (0.125 to 256), teicoplanin (0.125 to 256), gentamicin (1 to 2,048), streptomycin (2 to 4,096), erythromycin (0.016 to 32), clindamycin (0.032 to 32), quinupristin-dalfopristin (Q/D; tested as a 30:70 ratio; 0.032 to 64), oxytetracycline (0.063 to 128), chloramphenicol (0.125 to 256), and linezolid (0.016 to 32). For cotrimoxazole (tested as a 1:19 ratio; 0.25 to 512), MIC breakpoints according to CLSI guidelines for Staphylococcus (5) were used. For fusidic acid (0.063 to 128), breakpoints as defined by Toma and Barriault (43) were used, while for trimethoprim (0.25 to 512), European Food Safety Authority guidelines (9) were followed. According to the CLSI guidelines, enterococci are considered naturally resistant to clindamycin. Occasionally, however, strains with MICs in the susceptibility range have been observed (personal observation). Except for sulbactam and linezolid (Pfizer), teicoplanin and Q/D (Sanofi-Aventis), and erythromycin (Abbott), all tested antibiotics originated from Sigma.
For isolates displaying phenotypic resistance to one or more of the tested agents, the presence of the following acquired (and potentially transferable) resistance genes was investigated: the tet(M), tet(L), tet(K), tet(O), tet(P), tet(Q), tet(T), tet(S), tet(W), and tet(M) group; van(A), van(B), erm(A), erm(B), erm(C), cat(pC194), cat(piP501), aad(E), aph(2″)-aac(6′), aad(E)-aph(A), vat(D), and vat(E). For this purpose, DNA was isolated using the DNeasy tissue kit (Qiagen), and amplification of the corresponding gene fragments was performed in a DNA Engine Thermal Cycler “PTC-200” (MJ Research), as previously described (22). The following positive control strains were used: E. faecium UW 1342 for vat(D) (52); E. faecium UW 1965 for vat(E), erm(B), aad(E), aad(E)-aph(A), cat(pC194), and cat(piP501) (50, 51, 53, 55); Staphylococcus aureus 694/01 for erm(A), erm(C), tet(K), tet(M), and aac(A)-aph(D) (39); E. faecium UW 1873 for tet(L) (54); E. faecium BM4147 for van(A) (3, 29); E. faecium V583 for van(B) (29, 35); and Streptococcus pyogenes A498 for tet(T) (4). For the remaining genes, the following control plasmids were used: pGEM-tet(O) for tet(O) (2); pJIR667 for tet(P) (23a); pBT-1 for tet(Q) (27); pVP2 for tet(S) (30); and pGEM-tetW for tet(W) (2). The amplification products were detected by electrophoresis in a 1.5% agarose gel and subsequent ethidium bromide staining.
In vitro transfer experiments were performed by conjugation (filter mating) as previously described (22). Possible transconjugants were identified in several steps, selecting for the selective and nonselective markers. Probiotic E. faecium isolates representing non-wild-type isolates with acquired antibiotic resistance(s) were used as donors, whereas the well-documented strains E. faecium 64/3 and E. faecalis JH2-2 were chosen as recipients. Possible transconjugants were further characterized by MIC determination, PCR-based detection of resistance genes, PFGE, and (GTG)5-PCR (15, 21).
Multiplex PCR virulence genes.
Multiplex PCR for the detection of the virulence genes asa1, gelE, cylA, esp, and hyl was performed as described previously (49). Briefly, each 50-μl PCR mixture consisted of 5 μl of bacterial suspension; 0.1 μM primers for the detection of asa1, gelE, and hyl; 0.2 μM primers for the detection of cylA and esp; 25 μl HotStarTaq Master Mix (Qiagen, Germany); and an additional 1.0 mM MgCl2. PCR was performed in a GeneAmp PCR System 9600 (Perkin Elmer, Wellesley, MA). An initial activation step at 95°C for 15 min, during which the HotStarTaq DNA polymerase was activated, was followed by 30 cycles of denaturation (94°C for 1 min), annealing (56°C for 1 min), and extension (72°C for 1 min), followed by 1 cycle consisting of 10 min at 72°C. The PCR products were electrophoresed in a 1.5% pronarose D1 gel (SphaeroQ, Burgos, Spain) for 1 h at 150 V in 0.5× Tris-borate-EDTA containing 0.05 mg/liter ethidium bromide (positive and negative controls were included in each set of amplifications) (49). A 100-bp DNA ladder (Invitrogen, Merelbeke, Belgium) was used as molecular size marker.
Statistical analysis.
Student's t test was used for statistical analysis. A P value of <0.05 was considered statistically significant.
RESULTS
Identification and typing.
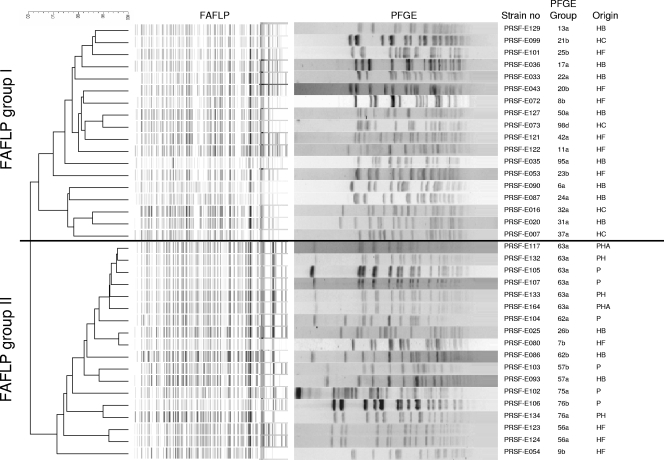
All strains were identified at the species level as E. faecium by FAFLP (data not shown). The dendrogram obtained from numerical analysis of digitized FAFLP generated with primer combination E01/T01 clearly showed the presence of two intraspecific genomic groups (denoted I and II) among the set of 128 E. faecium isolates. The delineation of these two groups is shown for a selection of isolates in Fig. 1. A total of 87 E. faecium isolates belonged to FAFLP group I, whereas 41 E. faecium isolates belonged to FAFLP group II. FAFLP group I consisted of 86 isolates of human origin (25 fecal and 61 clinical) and 1 probiotic culture. In FAFLP group II, 30 isolates were of human origin (12 fecal and 18 clinical), and 11 isolates were received as probiotic cultures.
FIG. 1.
Dendrogram based on numerical analysis of FAFLP patterns obtained with the primer combination E01/T01 with the corresponding SmaI PFGE patterns of selected E. faecium isolates. The dendrogram was constructed using the unweighted-pair group method using average linkages and the band-based Dice similarity coefficient. P, probiotic; PH, probiotic human consumption; PHA, probiotic human and animal consumption; HB, human blood isolate; HC, human clinical isolate; HF, human fecal isolate.
Cluster analysis and visual inspection of the PFGE profiles revealed high variability within the two genomic FAFLP groups. Based on the criterion that isolates exhibiting a maximum of six band position differences (42) in their respective PFGE patterns belonged to the same PFGE group, a total of 25 PFGE groups containing more than 1 isolate and 59 single isolates were recognized. In case no differences in band number and position were observed upon visual inspection, isolates were considered indistinguishable. The PFGE patterns of a selection of isolates are shown in Fig. 1. A total of 13 groups (i.e., groups 13, 17, 19, 21, 24, 25, 26, 31, 32, 37, 50, 51, and 98) contained only clinical isolates, 3 groups (i.e., groups 8, 20, and 56) contained only fecal isolates, and 5 groups (i.e., groups 6, 9, 22, 23, and 95) contained both clinical and fecal isolates. High similarity was observed between a number of isolates used commercially as probiotics. These isolates belonged to PFGE groups 57, 62, 63, and 76, of which group 63 contained six isolates received from five different depositors (i.e., D04, D05, D27, D42, and D50) with indistinguishable SmaI macrorestriction profiles (i.e., PRSF-E133, PRSF-E132, PRSF-E164, PRSF-E105, PRSF-E117, and PRSF-E107). In PFGE groups 62 and 57, a probiotic isolate clustered with a clinical isolate (i.e., PRSF-E103 with PRSF-E093 and PRSF-E104 with PRSF-E086) but could not be considered indistinguishable based on two band differences (Fig. 1). In PFGE group 76, fecal isolate PRSF-E051 clustered with the two probiotic isolates PRSF-E106 and PRSF-E134, but these isolates were also not considered indistinguishable (Fig. 1).
Phenotypic and genotypic characterization of antimicrobial susceptibilities.
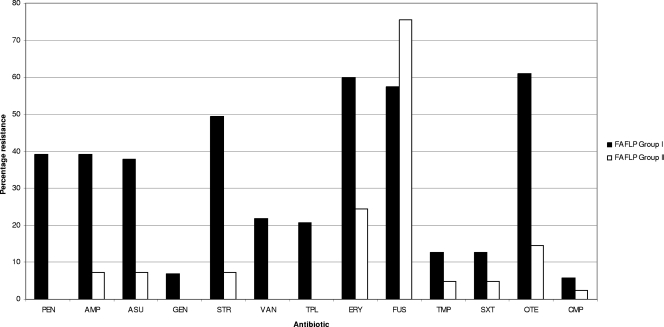
Using broth microdilution, phenotypic resistances to 13 antimicrobial agents were determined, after which the genetic basis of the observed resistance was investigated (Table 2). All isolates were susceptible to linezolid, but only 15 (12%) out of 128 isolates were susceptible to all agents tested. Overall, the highest phenotypic resistance frequencies were observed for erythromycin (62 of 128 isolates; 48%) and oxytetracycline (59 of 128 isolates; 46%). In Fig. 2, the distribution of antimicrobial resistances in FAFLP groups I and II is depicted. Resistance to gentamicin, penicillin, vancomycin, and teicoplanin was observed only in group I, and at least twice as many isolates (P < 0.001) in group I were resistant to the other tested antibiotics (Q/D, ampicillin, ampicillin/sulbactam, streptomycin, erythromycin, trimethoprim, cotrimoxazole, oxytetracycline, and chloramphenicol) compared to the isolates in group II. On the other hand, a larger number of group II isolates (31 out of 41 isolates; 76%) were resistant to fusidic acid compared to group I (50 out of 87 isolates; 57%), although the difference was not statistically significant (P = 0.09).
TABLE 2.
Frequencies of antimicrobial resistance and virulence genes in 128 E. faecium isolates
| Phenotypic resistance or virulence gene | No. (%) with indicated phenotypic resistance or virulence gene
|
Antibiotic resistance gene | No. (%) with indicated phenotypic resistance that also possess the indicated antibiotic resistance gene
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolates (n = 128) | Probiotics (n = 12) | Human isolates
|
Isolates | Probiotics | Human isolates
|
||||
| Nonfecala (n = 79) | Fecal (n = 37) | Nonfecala | Fecal | ||||||
| Penicillin | 37 (28) | 35 (44) | 2 (3) | —b | |||||
| Ampicillin | 37 (28) | 35 (44) | 2 (3) | — | |||||
| Ampicillin/sulbactam | 36 (28) | 35 (44) | 1 (3) | — | |||||
| Tetracycline | 59 (46) | 41 (52) | 18 (49) | tet(M) | 57 (97) | 39 (68) | 18 (32) | ||
| tet(L) | 41 (69) | 29 (71) | 12 (29) | ||||||
| tet(K) | 1 (2) | 1 (100) | 0 (0) | ||||||
| Vancomycin | 19 (15) | 3 (4) | 16 (43) | van(A) | 18 (95) | 3 (17) | 15 (93) | ||
| van(B) | 1 (5) | 0 (0) | 1 (100) | ||||||
| Teicoplanin | 18 (14) | 3 (4) | 15 (41) | van(A) | 18 (100) | 3 (17) | 15 (83) | ||
| Erythromycin | 62 (48) | 2 (17) | 42 (53) | 18 (49) | erm(A) | 1 (2) | 1 (100) | 0 (0) | |
| erm(B) | 51 (82) | 1 (2) | 36 (71) | 14 (27) | |||||
| Chloramphenicol | 5 (4) | 3 (4) | 2 (3) | cat(pC194) | 10 (?) | 7 (70) | 3 (30) | ||
| cat(piP501) | 11 (?) | 7 (64) | 4 (36) | ||||||
| Streptomycin | 46 (36) | 37 (47) | 9 (24) | aad(E) | 14 (30) | 7 (50) | 7 (50) | ||
| aad(E)-aph(A) | 28 (61) | 26 (93) | 2 (7) | ||||||
| Gentamicin | 6 (5) | 5 (6) | 1 (3) | aph(2″)-aac(6′) | 6 (100) | 5 (83) | 1 (17) | ||
| Quinupristin-dalfopristin | 16 (13) | 9 (11) | 7 (19) | vat(D) | 3 (19) | 2 (67) | 1 (33) | ||
| Linezolid | |||||||||
| Fusidic acid | 81 (63) | 11 (92) | 47 (59) | 23 (62) | |||||
| Virulence genes | |||||||||
| asa1 | |||||||||
| gelE | |||||||||
| cylA | |||||||||
| esp | 11 (9) | 11 (14) | |||||||
| hyl | |||||||||
| esp+hyl | 1 (1) | 1 (3) | |||||||
| None | 116 (90) | 12 (100) | 68 (86) | 36 (97) | |||||
Sterile sites (blood, liver, others), wound fluid, urine.
—, no PCR performed.
FIG. 2.
Antibiotic resistances in FAPLP groups I and II. PEN, penicillin; AMP, ampicillin; ASU, ampicillin-sulbactam; GEN, gentamicin (only high-level resistance was reported); STR, streptomycin (only high-level resistance was reported); VAN, vancomycin; TPL, teicoplanin; ERY, erythromycin; FUS, fusidic acid; TMP, trimethoprim; SXT, cotrimoxazole; OTE, oxytetracycline; CMP, chloramphenicol.
The majority of the isolates (87 out of 113 isolates; 77%) displaying phenotypic resistance also possessed the corresponding antibiotic resistance gene (Table 2). Overall, out of the 59 tetracycline-resistant E. faecium isolates, the tet(M) and tet(L) genes were detected in 57 (97%) and 41 (69%) human isolates, respectively. Out of the 53 tetracycline-resistant isolates in group I, 51 (96%) carried the tet(M) gene and 37 (69%) carried the tet(L) gene. In group II, all six tetracycline-resistant isolates carried the tet(M) gene and four (67%) carried the tet(L) gene. The tet(K) gene was detected in only one human isolate belonging to group I, which also harbored the tet(L) gene. In all isolates displaying phenotypic resistance to chloramphenicol, streptomycin, and gentamicin, corresponding genes could be detected (Table 2). On the other hand, 11 nonfecal isolates and three fecal isolates possessed a cat gene, while none of these were classified as phenotypically resistant to chloramphenicol. All 18 vancomycin- and teicoplanin-resistant isolates of group I carried the van(A) gene. In addition, one isolate with phenotypic resistance to vancomycin but not to teicoplanin also contained the van(B) gene. The following genes were not detected: the tet(O), tet(P), tet(Q), tet(T), tet(S), tet(W), and tet(M) group; erm(C); and vat(E).
Among the probiotic isolates, 11 out of 12 (92%) displayed phenotypic resistance to fusidic acid. Two out of these 11 isolates, PRSF-E105 and PRSF-E140, were also resistant to erythromycin, but only isolate PRSF-E140 carried an erm(B) gene. Using filter mating, transfer of erm(B) from PRSF-E140 to recipients E. faecium 64/3 and E. faecalis JH2-2 could not be detected (data not shown).
Multiplex PCR virulence genes.
A total of 12 out of 128 (9%) E. faecium isolates were positive for the enterococcal surface protein gene esp, including the fecal isolate PRSF-E149 from a hospitalized patient, which also contained the hyaluronidase gene, hyl. Of the remaining 11 (9%) esp-positive E. faecium isolates, 7 were blood isolates and 4 were wound isolates. More specifically, a total of 7 out of 87 (8%) isolates in FAFLP group I demonstrated the presence of the esp gene, of which 1 isolate also harbored the hyl gene, whereas in group II, the number of isolates positive for esp was slightly higher (5 out of 41; 12%). None of the probiotic isolates possessed any of the virulence factors tested. Table 2 gives an overview of the presence of virulence genes in the E. faecium isolates tested.
DISCUSSION
A broad collection of E. faecium isolates from different human origins (sterile sites, wound fluid, urine, and fecal origin), as well as commercial probiotic cultures, was included in the study. PFGE analysis of SmaI macrorestriction profiles, which is considered to be the gold standard for genotyping of enterococci (1, 23, 44), was used to determine the strain diversity among human clinical isolates and commercial probiotic cultures of E. faecium. A number of probiotic E. faecium isolates (Fig. 1) from different producers of probiotic products did not differ by a single band in PFGE using SmaI, indicating that they belonged to the same PFGE clone. Although, this is the reference method for typing enterococci, use of a second restriction enzyme or multilocus sequence typing (17) could provide even stronger evidence. In a few other cases, human E. faecium isolates from sterile body sites and feces were highly related to but not indistinguishable from a specific probiotic isolate. Provided that additional PFGE analyses with other restriction enzymes could further substantiate these results, this may suggest that some human isolates in the studied collection may be reisolations of commercial isolates. Similar conclusions were formulated by Vancanneyt et al. (46) for (potentially) probiotic L. rhamnosus isolates.
In line with a previous study (47), FAFLP analysis revealed the presence of two intraspecific genomic groups in E. faecium. Vancanneyt and colleagues (47) delineated two genomic groups among a collection of 78 E. faecium isolates from various human, animal, and food origins on the basis of randomly amplified polymorphic DNA-PCR and AFLP analyses. Four isolates were tested in both studies and were also allocated to the same genomic groups, i.e., PRSF-E122 and PRSF-E123 to group I and PRSF-E124 and PRSF-E125 to group II. In contrast to the former study (47), in which all human clinical isolates belonged to group I, our results showed that both FAFLP groups contained clinical isolates, which might be explained by the larger number of clinical isolates investigated. However, it should be mentioned that there might be a selection bias for the clinical isolates, as the majority of the isolates in our study were isolated in Belgium. In comparison, the 19 clinical isolates included in the study by Vancanneyt et al. (47) mainly originated from The Netherlands (n = 8) but also included isolates from Ireland, Belgium, Italy, and Germany. Notably, all but one (PRSF-E140) of the probiotic cultures belonged to FAFLP group II, which contained considerably fewer clinical isolates than FAFLP group I.
Although the enterococcal isolates in this study were in general susceptible to clinically relevant antibiotics, such as vancomycin, teicoplanin, gentamicin, and linezolid, comparison of the two intraspecific groups showed that the isolates in FAFLP group I displayed higher resistance frequencies to all agents tested except fusidic acid (Fig. 2). Possibly, these differences reflect strain origin and selective pressure, because antibiotic resistance was mainly observed in isolates of human origin, irrespective of the FAFLP grouping. The probiotic E. faecium isolates were highly susceptible to all tested antimicrobials except fusidic acid, resistance to which was demonstrated in a high percentage (92%) of probiotic isolates. However, fusidic acid has relatively poor activity against enterococci (6, 43), and the MICs of the enterococcal strains tested in the present study were located around the breakpoint. Importantly, two probiotic E. faecium isolates were phenotypically resistant to erythromycin, one of which (PRSF-E140) carried an erm(B) gene that was not transferable to enterococcal recipients. The previously reported involvement of msr, mef, or vga genes in erythromycin efflux or other resistance mechanisms (10, 32) might explain the erythromycin resistance phenotype that lacked the erm(B) gene. We speculate that probiotic cultures belonging to FAFLP group II may display a better safety record than probiotic members of FAFLP group I (i.e., PRSF-E140), because overall fewer phenotypic and genotypic resistances were detected in isolates from the former group. Furthermore, it is relevant to mention that, based on the descriptive information received from the original strain depositors, PRSF-E140 was the only probiotic E. faecium culture of animal origin included in this study. After the presence of acquired antibiotic resistance genes is verified, their transferabilities also need to be investigated in terms of safety evaluation of probiotic bacteria.
Overall, the virulence determinants present were highly similar in the human isolates in both groups. None of the E. faecium isolates in either genomic group was found to be positive for asa1, gelE, and cylA genes. Likewise, previous studies of E. faecium did not demonstrate the presence of any of these genes (8, 24, 37, 49). None of the probiotic E. faecium isolates, which mainly clustered in group II, contained any of the virulence genes tested. To date, esp and hyl genes have mainly been detected in clinical E. faecium isolates (8, 12, 24, 36, 49).
In order to obtain further insights into the evolutionary history and biological importance of intraspecific groups I and II in E. faecium, it would be interesting to challenge current FAFLP fingerprinting data with sequence-based approaches, such as multilocus sequence typing (17) and multilocus variable-number tandem-repeat analysis (45).
In conclusion, whole-genome FAFLP fingerprinting confirmed the previously reported intraspecific subdivision of E. faecium into two genomic groups. Although the virulence genes present were similar in both groups, FAFLP group II differed from group I because it contained only a minority of clinical isolates and because fewer antibiotic resistances were detected. Combined with phenotypic and genotypic assays investigating the presence of (transferable) antibiotic resistance and virulence traits, this intraspecific genomic grouping might be useful to document the safety records of new probiotic candidates of E. faecium.
Acknowledgments
The work described in this article was funded by the European Commission's 5th Framework Programme “PROSAFE”: Biosafety Evaluation of Lactic Acid Bacteria used for Human Consumption (QLRT-2001-01273). G. Huys is a postdoctoral fellow of the Fund for Scientific Research-Flanders (Belgium) (F.W.O.-Vlaanderen).
We also acknowledge all strain depositors.
Footnotes
Published ahead of print on 16 May 2008.
REFERENCES
- 1.Barbier, N., P. Saulnier, E. Chachaty, S. Dumontier, and A. Andremont. 1996. Random amplified polymorphic DNA typing versus pulsed-field gel electrophoresis for epidemiological typing of vancomycin-resistant enterococci. J. Clin. Microbiol. 34:1096-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbosa, T. M., K. P. Scott, and H. J. Flint. 1999. Evidence for recent intergeneric transfer of a new tetracycline resistance gene, tet(W), isolated from Butyrivibrio fibrisolvens, and the occurrence of tet(O) in ruminal bacteria. Environ. Microbiol. 1:53-64. [DOI] [PubMed] [Google Scholar]
- 3.Brisson-Noel, A., S. Dutka-Malen, C. Molinas, R. Leclercq, and P. Courvalin. 1990. Cloning and heterospecific expression of the resistance determinant vanA encoding high-level resistance to glycopeptides in Enterococcus faecium BM4147. Antimicrob. Agents Chemother. 34:924-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clermont, D., O. Chesneau, C. G. De, and T. Horaud. 1997. New tetracycline resistance determinants coding for ribosomal protection in streptococci and nucleotide sequence of tet(T) isolated from Streptococcus pyogenes A498. Antimicrob. Agents Chemother. 41:112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement, M100-S15, vol. 25, no. 1. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Collignon, P., and J. Turnidge. 1999. Fusidic acid in vitro activity. Int. J. Antimicrob. Agents 12(Suppl. 2):S45-S58. [DOI] [PubMed] [Google Scholar]
- 7.Descheemaeker, P., C. Lammens, B. Pot, P. Vandamme, and H. Goossens. 1997. Evaluation of arbitrarily primed PCR analysis and pulsed-field gel electrophoresis of large genomic DNA fragments for identification of enterococci important in human medicine. Int. J. Syst. Bacteriol. 47:555-561. [DOI] [PubMed] [Google Scholar]
- 8.Eaton, T. J., and M. J. Gasson. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Food Safety Authority. 2005. Opinion of the Scientific Panel on Additives and Products or Substance Used in Animal Feed on the updating of the criteria used in the assessment of bacteria for resistance to antibiotics of human or veterinary importance. EFSA J. 223:1-12. [Google Scholar]
- 10.Fluit, A. C., M. R. Visser, and F. J. Schmitz. 2001. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 14:836-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz, C. M., W. H. Holzapfel, and M. E. Stiles. 1999. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 47:1-24. [DOI] [PubMed] [Google Scholar]
- 12.Franz, C. M., A. B. Muscholl-Silberhorn, N. M. Yousif, M. Vancanneyt, J. Swings, and W. H. Holzapfel. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franz, C. M., M. E. Stiles, K. H. Schleifer, and W. H. Holzapfel. 2003. Enterococci in foods—a conundrum for food safety. Int. J. Food Microbiol. 88:105-122. [DOI] [PubMed] [Google Scholar]
- 14.Galli, D., F. Lottspeich, and R. Wirth. 1990. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 4:895-904. [DOI] [PubMed] [Google Scholar]
- 15.Gevers, D., G. Huys, and J. Swings. 2001. Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiol. Lett. 205:31-36. [DOI] [PubMed] [Google Scholar]
- 16.Giraffa, G., D. Carminati, and E. Neviani. 1997. Enterococci isolated from dairy products: a review of risks and potential technological use. J. Food Prot. 60:732-738. [DOI] [PubMed] [Google Scholar]
- 17.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ike, Y., H. Hashimoto, and D. B. Clewell. 1987. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J. Clin. Microbiol. 25:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, A. P. 1994. The pathogenicity of enterococci. J. Antimicrob. Chemother. 33:1083-1089. [DOI] [PubMed] [Google Scholar]
- 21.Klare, I., C. Konstabel, S. Müller-Bertling, R. Reissbrodt, G. Huys, M. Vancanneyt, J. Swings, H. Goossens, and W. Witte. 2005. Evaluation of new broth media for microdilution antibiotic susceptibility testing of lactobacilli, pediococci, lactococci, and bifidobacteria. Appl. Environ. Microbiol. 71:8982-8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klare, I., C. Konstabel, G. Werner, G. Huys, V. Vankerckhoven, G. Kahlmeter, B. Hildebrandt, S. Müller-Bertling, W. Witte, and H. Goossens. 2007. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 59:900-912. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn, I., L. G. Burman, S. Haeggman, K. Tullus, and B. E. Murray. 1995. Biochemical fingerprinting compared with ribotyping and pulsed-field gel electrophoresis of DNA for epidemiological typing of enterococci. J. Clin. Microbiol. 33:2812-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Lyras, D., and J. I. Rood. 1996. Genetic organization and distribution of tetracycline resistance determinants in Clostridium perfringens. Antimicrob. Agents Chemother. 40:2500-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannu, L., A. Paba, E. Daga, R. Comunian, S. Zanetti, I. Dupre, and L. A. Sechi. 2003. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. Int. J. Food Microbiol. 88:291-304. [DOI] [PubMed] [Google Scholar]
- 25.Moellering, R. C., Jr. 1992. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 14:1173-1176. [DOI] [PubMed] [Google Scholar]
- 26.Murray, B. E. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolich, M. P., N. B. Shoemaker, and A. A. Salyers. 1992. A Bacteroides tetracycline resistance gene represents a new class of ribosome protection tetracycline resistance. Antimicrob. Agents Chemother. 36:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel, R. 2003. Clinical impact of vancomycin-resistant enterococci. J. Antimicrob. Chemother. 51(Suppl. 3):iii13-iii21. [DOI] [PubMed] [Google Scholar]
- 29.Patel, R., J. R. Uhl, P. Kohner, M. K. Hopkins, J. M. Steckelberg, B. Kline, and F. R. Cockerill III. 1998. DNA sequence variation within vanA, vanB, vanC-1, and vanC-2/3 genes of clinical Enterococcus isolates. Antimicrob. Agents Chemother. 42:202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perreten, V., F. Schwarz, L. Cresta, M. Boeglin, G. Dasen, and M. Teuber. 1997. Antibiotic resistance spread in food. Nature 389:801-802. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Bacteriol. 8:151-156. [Google Scholar]
- 32.Portillo, A., F. Ruiz-Larrea, M. Zarazaga, A. Alonso, J. L. Martinez, and C. Torres. 2000. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemother. 44:967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pot, B., P. Vandamme, and K. Kersters. 1994. Analysis of electrophoretic whole-organism protein fingerprints, p. 493-521. In M. Goodfellow and A. G. O'Donnell (ed.), Modern microbial methods. Chemical methods in prokaryotic systematics. J. Wiley and Sons Ltd., Chichester, United Kingdom.
- 34.Quednau, M., S. Ahrné, and G. Molin. 1999. Genomic relationships between Enterococcus faecium strains from different sources and with different antibiotic resistance profiles evaluated by restriction endonuclease analysis of total chromosomal DNA using EcoRI and PvuII. Appl. Environ. Microbiol. 65:1777-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintiliani, R., Jr., S. Evers, and P. Courvalin. 1993. The vanB gene confers various levels of self-transferable resistance to vancomycin in enterococci. J. Infect. Dis. 167:1220-1223. [DOI] [PubMed] [Google Scholar]
- 36.Rice, L. B., L. Carias, S. Rudin, C. Vael, H. Goossens, C. Konstabel, I. Klare, S. R. Nallapareddy, W. Huang, and B. E. Murray. 2003. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 187:508-512. [DOI] [PubMed] [Google Scholar]
- 37.Semedo, T., M. A. Santos, M. F. Lopes, J. J. Figueiredo Marques, M. T. Barreto Crespo, and R. Tenreiro. 2003. Virulence factors in food, clinical and reference enterococci: a common trait in the genus? Syst. Appl. Microbiol. 26:13-22. [DOI] [PubMed] [Google Scholar]
- 38.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strommenger, B., C. Kettlitz, G. Werner, and W. Witte. 2003. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 41:4089-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su, Y. A., M. C. Sulavik, P. He, K. K. Makinen, P. L. Makinen, S. Fiedler, R. Wirth, and D. B. Clewell. 1991. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis subsp. liquefaciens. Infect. Immun. 59:415-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Temmerman, R., B. Pot, G. Huys, and J. Swings. 2003. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int. J. Food Microbiol. 81:1-10. [DOI] [PubMed] [Google Scholar]
- 42.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toma, E., and D. Barriault. 1995. Antimicrobial activity of fusidic acid and disk diffusion susceptibility testing criteria for gram-positive cocci. J. Clin. Microbiol. 33:1712-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomayko, J. F., and B. E. Murray. 1995. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:2903-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Top, J., L. M. Schouls, M. J. Bonten, and R. J. Willems. 2004. Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. J. Clin. Microbiol. 42:4503-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vancanneyt, M., G. Huys, K. Lefebvre, V. Vankerckhoven, H. Goossens, and J. Swings. 2006. Intraspecific genotypic characterization of Lactobacillus rhamnosus strains intended for probiotic use and isolates of human origin. Appl. Environ. Microbiol. 72:5376-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vancanneyt, M., A. Lombardi, C. Andrighetto, E. Knijff, S. Torriani, K. J. Bjorkroth, C. M. Franz, M. R. Foulquie Moreno, H. Revets, L. De Vuyst, J. Swings, K. Kersters, F. Dellaglio, and W. H. Holzapfel. 2002. Intraspecies genomic groups in Enterococcus faecium and their correlation with origin and pathogenicity. Appl. Environ. Microbiol. 68:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vankerckhoven, V., T. Van Autgaerden, G. Huys, M. Vancanneyt, J. Swings, and H. Goossens. 2004. Establishment of the PROSAFE collection of probiotic and human lactic acid bacteria. Microb. Ecol. Health Dis. 16:131-136. [Google Scholar]
- 49.Vankerckhoven, V., T. Van Autgaerden, C. Vael, C. Lammens, S. Chapelle, R. Rossi, D. Jabes, and H. Goossens. 2004. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. 42:4473-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner, G., B. Hildebrandt, I. Klare, and W. Witte. 2000. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int. J. Med. Microbiol. 290:543-548. [DOI] [PubMed] [Google Scholar]
- 51.Werner, G., B. Hildebrandt, and W. Witte. 2001. Aminoglycoside-streptothricin resistance gene cluster aadE-sat4-aphA-3 disseminated among multiresistant isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 45:3267-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Werner, G., I. Klare, and W. Witte. 1998. Association between quinupristin/dalfopristin resistance in glycopeptide-resistant Enterococcus faecium and the use of additives in animal feed. Eur. J. Clin. Microbiol. Infect. Dis. 17:401-402. [DOI] [PubMed] [Google Scholar]
- 53.Werner, G., I. Klare, and W. Witte. 2002. Molecular analysis of streptogramin resistance in enterococci. Int. J. Med. Microbiol. 292:81-94. [DOI] [PubMed] [Google Scholar]
- 54.Werner, G., R. J. Willems, B. Hildebrandt, I. Klare, and W. Witte. 2003. Influence of transferable genetic determinants on the outcome of typing methods commonly used for Enterococcus faecium. J. Clin. Microbiol. 41:1499-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werner, G., and W. Witte. 1999. Characterization of a new enterococcal gene, satG, encoding a putative acetyltransferase conferring resistance to streptogramin A compounds. Antimicrob. Agents Chemother. 43:1813-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willems, R. J., J. Top, N. van Den Braak, A. van Belkum, H. Endtz, D. Mevius, E. Stobberingh, A. van Den Bogaard, and J. D. van Embden. 2000. Host specificity of vancomycin-resistant Enterococcus faecium. J. Infect. Dis. 182:816-823. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization and Food and Agricultural Organization of the United Nations. 2001. Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. London, Ontario, Canada.
- 58.World Health Organization and Food and Agricultural Organization of the United Nations. 2002. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada.