Abstract
Culture-dependent PCR-amplified rRNA gene restriction analysis and culture-independent (PCR-denaturing gradient gel electrophoresis) methodologies were used to examine vaginal lactobacilli from Brazilian women who were healthy or had been diagnosed with vulvovaginal candidiasis (VVC) or bacterial vaginosis. Only Lactobacillus crispatus was detected accordingly by both methods, and H2O2-producing lactobacilli were not associated with protection against VVC.
Lactobacillus species are the predominant organisms in the healthy vagina, as determined by fermentation profiles (6), PCR and amplified rRNA gene restriction analysis (ARDRA) (10), and denaturing gradient gel electrophoresis (DGGE) (5).
Vulvovaginal candidiasis (VVC) affects up to 70 to 75% of women once in their life and presents with pruritus and vaginal discharge (13). Bacterial vaginosis (BV) is a common aberrant condition associated with depletion of lactobacilli, elevated vaginal pH, and overgrowth of Atopobium, Mobiluncus, Prevotella, Gardnerella, and Megasphaera species (1, 8, 17).
Hydrogen peroxide (H2O2) has been considered a key factor in Lactobacillus antagonism against pathogens. This compound generates cytotoxic reactive oxygen species, superoxide anions, and hydroxyl radicals in the vaginal fluid (6, 9).
In the present study, 64 healthy women (control group), 68 women diagnosed with VVC, and 64 women diagnosed with BV signed an informed consent statement (Ethics Review Board of the Centro de Saúde Escola da Faculdade de Medicina de Ribeirão Preto—Universidade de São Paulo; CSE-FMRP-USP protocol 0146). This study was registered online at “Comissão Nacional de Ética em Pesquisa” (CONEP document 070202), Brazil. Exclusion criteria included immunosuppression, pregnancy, current use of antibiotics or antifungals, menses during sample collection, and diagnosis of trichomoniasis.
The examining physician detected the presence of vaginal discharge, determined vaginal pH (Acilit indicator strip, pH 0 to 6; Merck, Germany), and collected three vaginal samples. Healthy subjects had no vaginal discharge or signs or symptoms of infections and had vaginal Gram-stained smears dominated by lactobacilli. Subjects were diagnosed with VVC by the presence of vaginal discharge and/or vaginal itching and burning plus being positive for Candida by wet mount preparations with 10% potassium hydroxide, Gram staining, or culture. Subjects diagnosed with BV fulfilled the criteria proposed by Amsel et al. (3) and Nugent et al. (11).
Samples were diluted with saline, plated on de Man Rogosa Sharpe (MRS) agar (Oxoid, Basingstoke, United Kingdom), and incubated for 48 h at 37°C aerobically and anaerobically. Colonies with different morphologies that were catalase- and oxidase-negative, gram-positive rods were processed by PCR-ARDRA.
Chromosomal bacterial DNA was obtained from overnight cultures of Lactobacillus sp. grown in 10 ml of MRS broth (Difco Laboratories, Detroit, MI) (10). For DNA extraction, the Wizard SV genomic DNA purification system kit (Promega Corporation, Madison, WI) was used. Amplification of the 16S-to-23S intergenic spacer region was performed as reported previously (10, 16). Restriction digestion of 16S-to-23S short intergenic spacer regions of the lactobacilli was performed with SphI, NcoI, NheI, SspI, SfuI, EcoRV, DraI, VspI, HincII, EcoRI, HindIII, and AvrII (Promega, New England Biolabs, Roche, and Invitrogen). The presence of three intergenic spacer regions (corresponding to long, medium, or short) was confirmatory for identification of Lactobacillus (see Table S1 in the supplemental material). For Lactobacillus reuteri and Lactobacillus vaginalis, two different restriction sites were observed in the 16S-to-23S spacer region.
DGGE was performed as previously described by Burton and Reid (5). Lactobacillus primers and the amplification conditions utilized were described previously (19). Sequences of the reamplified fragments were determined by the dideoxy chain termination method (Robarts Institute, London, Canada). Analysis of the partial 16S rRNA sequences was conducted by using the GenBank database and the BLAST algorithm. Identities of isolates were determined on the basis of the highest score.
H2O2 production by lactobacilli (expressed as negative, 1 to 3, 3 to 10, 10 to 30, or 30 to 100 mg/liter) was measured was with Merckoquant peroxide test strips (Merck, Darmstadt, Germany) (20).
One-way analysis of variance (P < 0.05) and the chi-square test (P < 0.25) were used for comparisons. Pairwise between-group comparisons were performed with differences being considered statistically significant at the 0.017 level to allow for a Bonferroni adjustment for multiple comparisons. Comparisons of Lactobacillus species per patient and production of H2O2 were assessed by Kruskal-Wallis test (P < 0.05). Whenever differences were observed, the Wilcoxon two-sample test was performed with a critical level of P < 0.025. Agreement between PCR-ARDRA and PCR-DGGE was assessed using the κ agreement coefficient. SAS software, version 9.1 (SAS Institute Inc., Cary, NC), was used for all tests.
The three groups of patients had similar mean ages and behavioral characteristics (P > 0.05) (data not shown).
Control and VVC groups did not differ in Lactobacillus counts (P = 0.543) (Table 1), in agreement with Sobel and Chaim (14), but contrary to Zdolsek et al. (21), who found more lactobacilli in VVC patients. Healthy and VVC groups exhibited higher counts than the BV group (P < 0.001).
TABLE 1.
Categorized Lactobacillus counts in vaginal samples obtained from healthy control women and those with vaginal infections
| Group | No. (%) of women with Lactobacillus count (CFU/ml):
|
Total (%) | ||
|---|---|---|---|---|
| ≤103 | 104-106 | ≥107 | ||
| Control | 13 (20.3) | 8 (12.5) | 43 (67.2) | 64 (100.0) |
| VVC | 9 (13.2) | 10 (14.7) | 49 (72.1) | 68 (100.0) |
| BV | 27 (42.2) | 19 (29.7) | 18 (28.1) | 64 (100.0) |
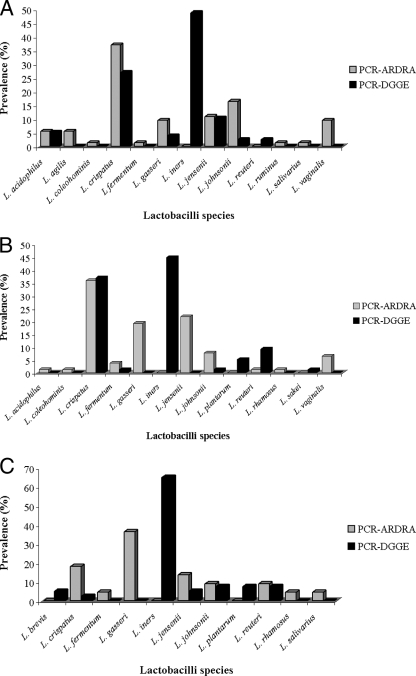
Of the 426 isolates Gram stained and evaluated biochemically, 262 bacilli were gram positive and catalase and oxidase negative: 173 Lactobacillus isolates were identified by PCR-ARDRA, with at least one species in 87.5%, 88.2%, and 32.8% of the control, VVC, and BV groups, respectively (maximum of three Lactobacillus species per subject). This compares with 98.4%, 94.1%, and 57.8% for DGGE (maximum of two species per subject). No statistical difference was observed in the rate of vaginal Lactobacillus colonization in normal and VVC groups (P = 0.897); but these differed from the BV group (P < 0.001). The prevalence of each species is shown in Fig. 1A, B, and C.
FIG. 1.
Prevalence of vaginal lactobacilli obtained from three groups of Brazilian patients comprising 64 healthy women (A), 68 women diagnosed with VVC (B), and 64 women diagnosed with BV (C), according to results assessed by PCR-ARDRA and PCR-DGGE.
In this first-ever study of vaginal lactobacilli from Brazilian women, Lactobacillus species detected were similar to those from subjects with distinctly different environments, diets, and geographic locations, namely Nigeria, Canada, and Sweden (4, 5, 18). This is quite remarkable and warrants studies on the origins of these organisms. PCR-ARDRA did not detect L. brevis, L. iners, L. plantarum, and L. sakei, whereas PCR-DGGE did not identify L. agilis, L. coleohominis, L. ruminus, and L. salivarius in any sample. L. iners does not grow on MRS (7), which explains failure to detect it by PCR-ARDRA.
Few lactobacilli did not produce H2O2 (1.4% in healthy subjects and 2.6% in VVC subjects) (P < 0.05) (Table 2), in agreement with others (2, 6, 12), suggesting that H2O2 does not per se protect against yeast infection. Possibly antifungal cyclic dipeptides, pyroglutamic acid, and lactones produced by Lactobacillus spp. (15) help protect the host. In contrast, 31.8% of isolates from the BV group did not produce H2O2 (P > 0.05).
TABLE 2.
Semiquantification of H2O2 production by different vaginal Lactobacillus species obtained from three groups of Brazilian patientsa
| Group and Lactobacillus species (no. of isolates tested)b | No. of isolates that produced H2O2 amt (mg/liter):
|
||||
|---|---|---|---|---|---|
| None | 1-3 | 3-10 | 10-30 | 30-100 | |
| Healthy | |||||
| L. acidophilus (4) | 0 | 0 | 2 | 1 | 1 |
| L. agilis (4) | 0 | 0 | 2 | 1 | 1 |
| L. coleohominis (1) | 0 | 0 | 1 | 0 | 0 |
| L. crispatus (27) | 1 | 0 | 9 | 15 | 2 |
| L. fermentum (1) | 0 | 1 | 0 | 0 | 0 |
| L. gasseri (7) | 0 | 0 | 3 | 4 | 0 |
| L. jensenii (8) | 0 | 0 | 1 | 7 | 0 |
| L. johnsonii (12) | 0 | 0 | 2 | 3 | 7 |
| L. reuteri (0) | 0 | 0 | 0 | 0 | 0 |
| L. rhamnosus (0) | 0 | 0 | 0 | 0 | 0 |
| L. ruminus (1) | 0 | 0 | 1 | 0 | 0 |
| L. salivarius (1) | 0 | 0 | 0 | 1 | 0 |
| VVC | |||||
| L. acidophilus (1) | 0 | 0 | 0 | 0 | 1 |
| L. agilis (0) | 0 | 0 | 0 | 0 | 0 |
| L. coleohominis (1) | 0 | 0 | 0 | 1 | 0 |
| L. crispatus (28) | 0 | 3 | 9 | 9 | 7 |
| L. fermentum (3) | 0 | 1 | 1 | 1 | 0 |
| L. gasseri (15) | 2 | 0 | 6 | 3 | 4 |
| L. jensenii (17) | 0 | 1 | 0 | 6 | 10 |
| L. johnsonii (6) | 0 | 0 | 0 | 2 | 4 |
| L. reuteri (1) | 0 | 0 | 1 | 0 | 0 |
| L. rhamnosus (1) | 0 | 0 | 0 | 0 | 1 |
| L. ruminus (0) | 0 | 0 | 0 | 0 | 0 |
| L. salivarius (0) | 0 | 0 | 0 | 0 | 0 |
| L. vaginalis (5) | 0 | 0 | 3 | 1 | 1 |
| BV | |||||
| L. acidophilus (0) | 0 | 0 | 0 | 0 | 0 |
| L. agilis (0) | 0 | 0 | 0 | 0 | 0 |
| L. coleohominis (0) | 0 | 0 | 0 | 0 | 0 |
| L. crispatus (4) | 1 | 3 | 0 | 0 | 0 |
| L. fermentum (1) | 0 | 1 | 0 | 0 | 0 |
| L. gasseri (8) | 3 | 1 | 3 | 1 | 0 |
| L. jensenii (3) | 1 | 0 | 0 | 1 | 1 |
| L. johnsonii (2) | 0 | 0 | 1 | 0 | 1 |
| L. reuteri (2) | 1 | 1 | 0 | 0 | 0 |
| L. rhamnosus (1) | 1 | 0 | 0 | 0 | 0 |
| L. ruminus (0) | 0 | 0 | 0 | 0 | 0 |
| L. salivarius (1) | 0 | 1 | 0 | 0 | 0 |
| L. vaginalis (0) | 0 | 0 | 0 | 0 | 0 |
There were 64 women in the healthy group, 68 in the VVC group, and 64 in the BV group.
Values for healthy women, women diagnosed with VVC, and women diagnosed with BV are plotted in Fig. 1A, B, and C, respectively.
In summary, there are relatively few Lactobacillus species which are commonly found in the vagina. The importance of H2O2 as a key anti-infective remains to be fully determined.
Supplementary Material
Acknowledgments
We are grateful to Augusto C. C. Spadaro and Sérgio de Albuquerque from FCFRP-USP (Brazil) for administrative support throughout this study. We are thankful for the collaboration of the excellent team of physicians and nurses and for the study volunteers recruited at public health centers in the city of Ribeirão Preto, São Paulo State. The input of Andrew W. Bruce into the design is appreciated.
R. C. R. Martinez is grateful to the São Paulo State Foundation for Support of Science (FAPESP; process 04/14580-0) and to the Brazilian Post Graduate Federal Agency (process 6159/06-2) for supporting his Ph.D. studies in Brazil and Canada, respectively. We also acknowledge FAPESP for financial support (process 06/06595-2). Assistance from a grant from the Natural Sciences and Engineering Research Council of Canada is also appreciated.
Footnotes
Published ahead of print on 23 May 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allsworth, J. E., and J. F. Peipert. 2007. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet. Gynecol. 109:114-120. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mushrif, S., and B. M. Jones. 1998. A study on the prevalence of hydrogen-peroxide generating lactobacilli in bacterial vaginosis: the determination of H2O2 concentrations generated, in vitro, by isolated strains and the levels found in vaginal secretions of women with and without infection. J. Obstet. Gynaecol. 18:63-67. [DOI] [PubMed] [Google Scholar]
- 3.Amsel, R., P. A. Totten, C. A. Spiegel, K. C. Chen, D. Eschenbach, and K. K. Holmes. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14-22. [DOI] [PubMed] [Google Scholar]
- 4.Anukam, K. C., E. O. Osazuwa, I. Ahonkhai, and G. Reid. 2006. Lactobacillus vaginal microbiota of women attending a reproductive health care service in Benin City, Nigeria. Sex. Transm. Dis. 33:59-62. [DOI] [PubMed] [Google Scholar]
- 5.Burton, J. P., and G. Reid. 2002. Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J. Infect. Dis. 186:1770-1780. [DOI] [PubMed] [Google Scholar]
- 6.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsen, E., C. Pauscaul, B. Sjoden, M. Ohlen, and M. D. Collins. 1999. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int. J. Syst. Bacteriol. 49:217-221. [DOI] [PubMed] [Google Scholar]
- 8.Fredricks, D. N., T. L. Fiedler, K. K. Thomas, B. B. Oakley, and J. M. Marrazzo. 2007. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J. Clin. Microbiol. 45:3270-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klebanoff, S. J., S. L. Hilier, D. A. Eschenbach, and A. M. Waltersdorph. 1991. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J. Infect. Dis. 164:94-100. [DOI] [PubMed] [Google Scholar]
- 10.Moreira, J. L., R. M. Mota, M. F. Horta, S. M. Teixeira, E. Neumann, J. R. Nicoli, and A. C. Nunes. 2005. Identification to the species level of Lactobacillus isolated in probiotic prospecting studies of human, animal or food origin by 16S-23S rRNA restriction profiling. BMC Microbiol. 5:15. doi: 10.1186/1471-2180-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual, L. M., M. B. Daniele, C. Pájaro, and L. Barberis. 2006. Lactobacillus species isolated from the vagina: identification, hydrogen peroxide production and nonoxynol-9 resistance. Contraception 73:78-81. [DOI] [PubMed] [Google Scholar]
- 13.Sobel, J. D. 2007. Vulvovaginal candidosis. Lancet 368:1961-1971. [DOI] [PubMed] [Google Scholar]
- 14.Sobel, J. D., and W. Chaim. 1996. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J. Clin. Microbiol. 34:2497-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ström, K., J. Sjögren, A. Broberg, and J. Schnürer. 2002. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-trans-4-OH-l-Pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 68:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilsala-Timisjärvi, A., and T. Alatossava. 1997. Development of oligonucleotide primers from 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int. J. Food Microbiol. 35:49-56. [DOI] [PubMed] [Google Scholar]
- 17.Trama, J. P., K. E. Pascal, J. Zimmerman, M. J. Self, E. Mordechai, and M. E. Adelson. 2008. Rapid detection of Atopobium vaginae and association with organisms implicated in bacterial vaginosis. Mol. Cell. Probes 22:96-102. [DOI] [PubMed] [Google Scholar]
- 18.Vásquez, A., T. Jakobsson, S. Ahrné, U. Forsum, and G. Molin. 2002. Vaginal Lactobacillus flora of healthy Swedish women. J. Clin. Microbiol. 40:2746-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter, J., G. W. Tannock, A. Tilsala-Timsjarvi, S. Rodtong, D. M. Loach, K. Munro, and T. Alatossava. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilks, M., R. Wiggins, A. Whiley, E. Hennessy, S. Warwick, H. Porter, A. Corfield, and M. Millar. 2004. Identification and H2O2 production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J. Clin. Microbiol. 42:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zdolsek, B., D. Hellberg, G. Fröman, S. Nilsson, and P. A. Märdh. 1995. Vaginal microbiological flora and sexually transmitted diseases in women with recurrent or current vulvovaginal candidiasis. Infection 23:81-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.