Abstract
The protective immune response against liver stages of the malaria parasite critically requires CD8+ T cells. Although the nature of the effector mechanism utilized by these cells to repress parasite development remains unclear, a critical role for gamma interferon (IFN-γ) has been widely assumed based on circumstantial evidence. However, the requirement for CD8+ T-cell-mediated IFN-γ production in protective immunity to this pathogen has not been directly tested. In this report, we use an adoptive transfer strategy with circumsporozoite (CS) protein-specific transgenic T cells to examine the role of CD8+ T-cell-derived IFN-γ production in Plasmodium yoelii-infected mice. We show that despite a marginal reduction in the expansion of naive IFN-γ-deficient CS-specific transgenic T cells, their antiparasite activity remains intact. Further, adoptively transferred IFN-γ-deficient CD8+ T cells were as efficient as their wild-type counterparts in limiting parasite growth in naive mice. Taken together, these studies demonstrate that IFN-γ secretion by CS-specific CD8+ T cells is not essential to protect mice against live sporozoite challenge.
Studies in rodent malaria models have provided definitive evidence that CD8+ T-cell responses against the pre-erythrocytic stages of the malarial parasite Plasmodium play a role in protective immunity. The mechanism by which such a response mediates protection is, however, poorly understood. CD8+ T cells can deliver effector responses by a variety of pathways, including perforin/granzyme-mediated killing or the secretion of inflammatory cytokines such as gamma interferon (IFN-γ) or tumor necrosis factor alpha (TNF-α). Of these, a strong case has been made in the literature for the antiplasmodial activity of IFN-γ. Direct administration of human and mouse IFN-γ inhibits pre-erythrocytic parasite development in monkeys and mice, respectively (6), and recombinant IFN-γ prevents hepatic schizogony of Plasmodium falciparum (13) and P. berghei (22) in hepatocyte cultures.
IFN-γ-dependent protection against malaria liver stages can be elicited by immunization with sporozoites or subunit vaccines, as well as by adoptive transfer of parasite-specific T-cell clones (5, 19, 23-25). Other investigators have observed correlations between the requirement for IFN-γ and CD8+ T cells for protective immunity (10, 16, 24, 28). Mice adoptively transferred with a cytotoxic-T-cell clone (28) and immunized with the bite of P. berghei-infected mosquitoes (24) or needle injection of radiation-attenuated sporozoites (5) were not protected if IFN-γ was neutralized with antibodies during rechallenge. Finally, in two recent studies using genetically attenuated sporozoites, protection was linked to CD8+ T cells (10) and was abolished in IFN-γ-deficient and T-cell-deficient mice (16). This abundance of correlative data has led to the general acceptance that CD8+ T cells secrete IFN-γ in order to execute protective function against malaria liver-stage parasites.
However, the need for CD8+ T-cell-derived IFN-γ has never been formally demonstrated and, in fact, some studies have shown that CD8+ T-cell-mediated immunity against liver-stage parasites is independent of IFN-γ, both in vitro (8, 9) and in vivo (19). Thus, it is still unclear whether IFN-γ secretion by antiparasite CD8+ T cells is required for elimination of Plasmodium liver stages. We address this issue by using a mouse model system with Plasmodium yoelii where CD8+ T cells are the sole mediator of antisporozoite immunity. Unlike previous approaches that globally ablated IFN-γ using neutralizing antibodies or genetic knockouts, an adoptive transfer strategy allowed us to directly compare the competency of IFN-γ-deficient and normal effector CD8+ T cells with regard to their in vivo antiparasite activity.
MATERIALS AND METHODS
Mice.
Five- to eight-week-old female BALB/c mice were purchased from Taconic (Hudson, NY). Transgenic mice expressing a T-cell receptor (TCR) specific for the SYVPSAEQI epitope of the P. yoelii circumsporozoite (CS) protein were derived as previously described (20). Mice that have previously been backcrossed to the Thy1.1+ BALB/c background for >20 generations were used from our colony. BALB/c-Ifngtm1Ts mice homozygous for the Ifngtm1Ts targeted mutation were purchased from Jackson Laboratories. These were crossed to CS-specific TCR transgenic mice from our colony and F1 progeny positive for the TCR transgene were crossed back to BALB/c-Ifngtm1Ts mice to obtain TCR transgenic mice homozygous for the Ifngtm1Ts targeted mutation. Genotyping protocols were followed as outlined elsewhere (http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=228). All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Immunizations.
Generation of the recombinant vaccinia virus expressing the 9-mer SYVPSAEQI epitope (VV-CS) has been described previously (11, 18). VV-CS was diluted in Hanks balanced salt solution containing 1% heat-inactivated mouse serum to appropriate concentration and delivered intravenously in 200 μl.
Cell isolation.
Single cell suspensions of splenocytes were obtained by mechanical disruption of spleens between two microscope glass slides and filtering through 100-μm-pore-size nylon mesh. To isolate intrahepatic lymphocytes, livers were disrupted mechanically, suspended in Hanks balanced salt solution with 2% fetal calf serum and 10 mM HEPES and filtered through a 150-μm-pore-size mesh. This cell suspension was centrifuged, and the pellet was resuspended in a solution of Percoll (Amersham Biosciences) as described previously (12), centrifuged at 500 × g, and washed extensively before use. Where necessary, purified populations of CD8+ T cells were obtained by using the mouse CD8+ T-cell isolation kit according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany).
Adoptive transfers.
For adoptive transfer experiments using naive cells, mice received 106 naive TCR-Tg CD8+ T cells isolated from the spleens of TCR-Tg wild-type (WT) or IFN-γ knockout (IFNγKO) mice. The proportion of tetramer+ CD8+ T cells was calculated by flow cytometry, and 106 transgenic cells were transferred into congenic recipient mice. For experiments involving transfer of activated/effector CD8+ T cells, we first transferred 106 naive transgenic cells into congenic mice, which were then immunized with 5 × 106 PFU recombinant vaccinia virus carrying the SYVPSAEQI epitope (VV-CS). Fourteen days later, activated CD8 T cells were isolated from the spleens of these mice as described above, the number of tetramer+ cells was quantified, and 2 × 106 cells were transferred into recipient mice.
Tetramer and ELISPOT analysis.
Enzyme-linked immunospot (ELISPOT) assays to measure SYVPSAEQI-specific interleukin-2 (IL-2)-secreting cells were performed essentially as described previously (2) with modifications to exclude IL-2 addition to the medium during peptide stimulation in vitro.
Parasite quantification in the liver.
In challenge experiments, live P. yoelii (17XNL) sporozoites were inoculated intravenously, and 40 h later the livers were harvested, the total RNA was isolated, and the parasite burden in the liver was quantified by using reverse transcription (RT), followed by real-time PCR as outlined previously (1).
Data acquisition and analysis.
Fluorescence-activated cell sorting (FACS) data was acquired on a FACSCalibur machine and analyzed by using CellQuest software (Becton Dickinson). ELISPOT plates were read with an immunospot plate reader, and the data were analyzed by using immunospot software (Cellular Technology, Ltd., Cleveland, OH). Graphs were prepared and analyzed by using GraphPad software (GraphPad Software, Inc., San Diego, CA). A Student t test was used for pairwise comparisons to determine statistical significance.
RESULTS
Naive IFN-γ-deficient CD8+ T cells are primed against CS.
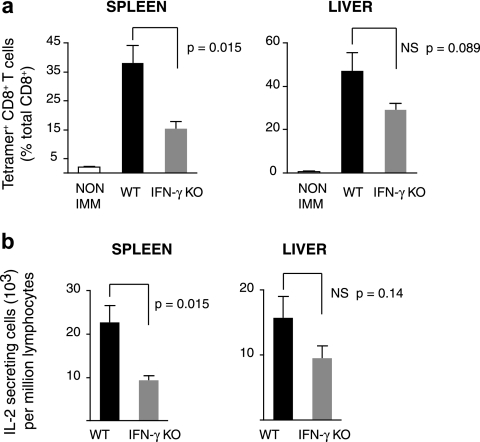
To avoid ambiguities regarding the specific mechanisms that may be involved in generating an effective immune response against malaria liver stages, we generated effector CD8+ T cells by immunization with a vaccinia virus that expresses the SYVPSAEQI epitope of the P. yoelii CS protein (i.e., VV-CS). Therefore, in this experimental model, CD8+T cells against this epitope are the only effector cells that can possibly recognize parasite antigens after challenge of immunized mice. In previous studies we have shown that vaccinia virus expressing the CS epitope alone—and not WT virus—activates the SYVPSAEQI-specific CD8+ T cells (18). To facilitate the tracking of antigen-specific T cells, WT and IFNγKO TCR-transgenic CD8+ T cells specific for the major histocompatibility complex (MHC) class I-restricted SYVPSAEQI epitope of the P. yoelii CS protein (TCR-Tg) were used. We first evaluated the induction phase of the immune response and compared the capacity of normal and IFN-γ-deficient CD8+ T cells to mount a response after immunization. BALB/c mice received WT or IFNγKO CD8+ T cells and were immunized with VV-CS. Eight days later, lymphocytes were isolated from the spleens and livers, and the CD8+ T-cell response was evaluated by FACS using tetramer staining and an ELISPOT assay to detect IL-2-secreting cells since IFN-γ production could not be used to evaluate T-cell activation. In response to immunization with VV-CS, there was a major increase in the number of both WT and IFNγKO CD8+ T cells as detected by tetramer staining (Fig. 1a). However, the clonal burst of the IFN-γ deficient CD8+ T cells was diminished compared to WT cells: the percentages of IFNγKO TCR-Tg cells on day 8 were 39% of the WT in the spleen and 61% in the liver. Similar reductions were observed in the number of IL-2-producing cells, with the response of IFNγKO cells being lower than their WT counterparts in the spleen (40%) and liver (60%) (Fig. 1b). Thus, in this model, naive IFN-γ-deficient CD8+ T cells can be efficiently primed, although the response is less robust than that of naive IFN-γ-sufficient T cells.
FIG. 1.
(a) BALB/c mice received TCR-Tg cells obtained from WT or IFNγKO transgenic mice and were immunized with 2 × 106 vaccinia virus. Eight days later, the number of tetramer+CD8+ cells was determined by FACS analysis and is plotted as a proportion of total CD8+ cells within splenocytes and hepatic lymphocyte populations. (b) IL-2-secreting cells within the same populations were identified by ELISPOT assay. Histograms represent means ± the standard errors of the mean (n = 4).
Effector IFN-γ-deficient CD8+ T cells are protective.
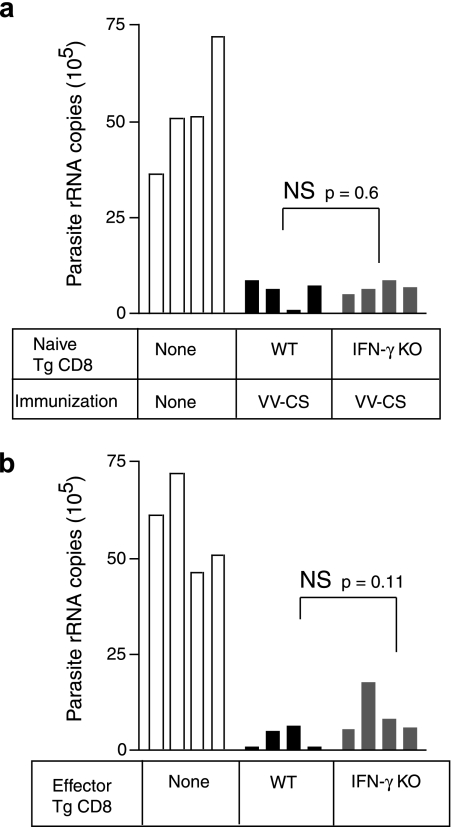
We next evaluated the antiparasite activity exerted by IFN-γ deficient CD8+ T cells compared to WT cells. Similar to the previous experiment, WT and IFNγKO CD8+ T cells were transferred into naive BALB/c mice, which were then immunized with VV-CS. Eight days later, the mice were challenged with live P. yoelii sporozoites, and the parasite load in the liver was evaluated by RT-PCR 40 h thereafter. Unexpectedly, the parasite load was reduced to similar extents in immunized mice harboring either WT or IFNγKO CD8+ T cells (Fig. 2a). Thus, in spite of a quantitatively reduced response of IFNγKO CD8+ T cells during immunization, these cells limited parasite development in the liver to a degree equal to that of WT cells. Any difference in parasite growth between mice harboring WT or IFNγKO CD8+ T cells was statistically insignificant.
FIG. 2.
(a) BALB/c mice received TCR-Tg CD8+ T cells isolated from WT or IFNγKO mice and were immunized with 2 × 106 PFU VV-CS. Eight days later, mice were challenged with 3.5 × 104 P. yoelii sporozoites and parasite-specific rRNA levels in the liver determined by quantitative RT-PCR. (b) Mice received WT or IFNγKO TCR-Tg CD8+ T cells and were immunized as described for panel a. Eight days later, 2 × 106 purified TCR-Tg CD8+ spleen cells were recovered and retransferred into secondary naive BALB/c hosts that were subsequently challenged with 3.5 × 104 P. yoelii sporozoites. The parasite load in the liver was evaluated as for panel a. Each bar represents a single mouse. A Student t test was used to evaluate statistical significance.
Although the previous experiment demonstrates that primed IFNγKO cells have intact antiparasite activity, the experimental design resulted in an unequal expansion of WT and IFNγKO effector cells (Fig. 1). Therefore, we designed a second experiment to accurately control cell numbers and also discount other unidentified factors in immunized mice that could bias protective immunity. For this purpose, we purified activated CD8+ cells from the spleens of mice that had previously received either WT or IFNγKO CD8+ T cells and were immunized as described above with VV-CS. Equal numbers of TCR-Tg cells (2 × 106) were then transferred into new naive hosts, which were subsequently challenged with live parasites. In previous studies, we have determined that 2 × 106 effector cells is the minimal number necessary to provide protection in naive mice (14). The results of these experiments showed that both WT and IFNγKO effector CD8+ T cells were able to eliminate liver stage parasites with equal efficiency (Fig. 2b). Taken together, our data demonstrate that IFN-γ is not a critical mediator of protective cytotoxic T cells to reduce the parasite load in the liver.
DISCUSSION
The primary finding of our study is that protection afforded by CS-specific CD8+ T cells against viable P. yoelii liver stages is independent of their own production of IFN-γ. Our conclusions contrast with previous studies (5, 10, 16, 24, 28), which suggest that IFN-γ was critical for CD8+ T-cell-mediated protection. This apparent disagreement may be due to the involvement of other IFN-γ-secreting cells in protective immunity, such as CD4+ T cells, which can produce IFN-γ and are known to directly inhibit the development of liver stages (4, 26). Moreover, IFN-γ is a pleiotropic cytokine that is known to modulate several aspects of antigen processing and MHC class I presentation (7, 21), and it also impacts on other cytokine networks (27), such that IFN-γ-deficient mice used in other studies might exhibit altered protective responses even though CD8+ T cells themselves may not be the source of this cytokine. Indeed, we demonstrated here that CD8+ T-cell responses themselves are significantly reduced when these cells are unable to produce IFN-γ (Fig. 1a).
The specific antiparasitic determinant produced by CD8+ T cells in this process still remains to be identified. A previous study using the same transgenic system reported here used TCR-Tg CD8+ cells deficient in perforin or FasL or both and found that the protective effector mechanism was independent of these molecules (15). This was in agreement with studies showing that global ablation of perforin, Fas, and granzyme B did not affect protective immunity induced by attenuated sporozoites (5, 17). Other candidate effector molecules still remain to be evaluated, such as TNF-α, although studies have demonstrated that the neutralization of TNF-α does not alter protection (19). It is nonetheless clear that this inhibition of parasite development in our model is highly specific, as shown in our earlier studies where adoptively transferred TCR-Tg CD8+ T cells inhibited parasite development only after being activated by immunization with P. yoelii sporozoites or VV-CS and not the WT virus (20). Moreover, our recent studies using bone marrow chimeric mice demonstrate that adoptively transferred activated CD8+ TCR-Tg cells inhibit parasite development only when parenchymal liver cells express the appropriate class I MHC (H-2Kd), regardless of the MHC haplotype on bone marrow-derived cells (3).
It is possible that unconventional mechanisms of protection may be operating in this antimalaria model. It is also possible that this antiparasite effect is mediated by multiple redundant mechanisms, and thus an approach based on the elimination or neutralization of a single factor may not be informative. Finally, while our results indicate that IFN-γ is not necessary as an effector molecule mediating the direct protective effects of CD8+ T cells, our study cannot discount the possibility that IFN-γ is involved in complex cellular interactions where secretion by other cell types facilitates CD8+ T-cell-mediated elimination of liver-stage parasites.
Acknowledgments
This study was supported by National Institutes of Health grant number AI44375.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 311499-1502. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho, L. H., J. C. Hafalla, and F. Zavala. 2001. ELISPOT assay to measure antigen-specific murine CD8+ T-cell responses. J. Immunol. Methods 252207-218. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarty, S., I. A. Cockburn, S. Kuk, M. G. Overstreet, J. B. Sacci, and F. Zavala. 2007. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 131035-1041. [DOI] [PubMed] [Google Scholar]
- 4.Del Giudice, G., D. Grillot, L. Renia, I. Muller, G. Corradin, J. A. Louis, D. Mazier, and P. H. Lambert. 1990. Peptide-primed CD4+ cells and malaria sporozoites. Immunol. Lett. 2559-63. [DOI] [PubMed] [Google Scholar]
- 5.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 1651453-1462. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira, A., L. Schofield, V. Enea, H. Schellekens, P. van der Meide, W. E. Collins, R. S. Nussenzweig, and V. Nussenzweig. 1986. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science 232881-884. [DOI] [PubMed] [Google Scholar]
- 7.Fischer, A., S. Latour, and G. de Saint Basile. 2007. Genetic defects affecting lymphocyte cytotoxicity. Curr. Opin. Immunol. 19348-353. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman, S. L., D. Isenbarger, G. W. Long, M. Sedegah, A. Szarfman, S. Mellouk, and W. R. Ballou. 1990. T lymphocytes from mice immunized with irradiated sporozoites eliminate malaria from hepatocytes. Bull. W. H. O. 68(Suppl.)132-137. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman, S. L., D. Isenbarger, G. W. Long, M. Sedegah, A. Szarfman, L. Waters, M. R. Hollingdale, P. H. van der Meide, D. S. Finbloom, and W. R. Ballou. 1989. Sporozoite vaccine induces genetically restricted T-cell elimination of malaria from hepatocytes. Science 2441078-1081. [DOI] [PubMed] [Google Scholar]
- 10.Jobe, O., J. Lumsden, A. K. Mueller, J. Williams, H. Silva-Rivera, S. H. Kappe, R. J. Schwenk, K. Matuschewski, and U. Krzych. 2007. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I-dependent interferon-gamma-producing CD8+ T cells. J. Infect. Dis. 196599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 905214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 2912413-2417. [DOI] [PubMed] [Google Scholar]
- 13.Mellouk, S., R. K. Maheshwari, A. Rhodes-Feuillette, R. L. Beaudoin, N. Berbiguier, H. Matile, F. Miltgen, I. Landau, S. Pied, J. P. Chigot, et al. 1987. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J. Immunol. 1394192-4195. [PubMed] [Google Scholar]
- 14.Morrot, A., I. A. Cockburn, M. Overstreet, D. Rodriguez, and F. Zavala. 2006. Protective CD8+ T cells induced by malaria sporozoites do not undergo modulation of interleukin-7 receptor expression. Infect. Immun. 742495-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrot, A., and F. Zavala. 2004. Effector and memory CD8+ T cells as seen in immunity to malaria. Immunol. Rev. 201291-303. [DOI] [PubMed] [Google Scholar]
- 16.Mueller, A. K., M. Deckert, K. Heiss, K. Goetz, K. Matuschewski, and D. Schluter. 2007. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am. J. Pathol. 171107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renggli, J., M. Hahne, H. Matile, B. Betschart, J. Tschopp, and G. Corradin. 1997. Elimination of Plasmodium berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 19145-148. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J. R. Rodriguez, I. Bacik, J. R. Bennink, J. W. Yewdell, A. Garcia-Sastre, R. S. Nussenzweig, et al. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B-cell epitopes: comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 1534636-4648. [PubMed] [Google Scholar]
- 19.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T-cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3579-585. [DOI] [PubMed] [Google Scholar]
- 20.Sano, G., J. C. Hafalla, A. Morrot, R. Abe, J. J. Lafaille, and F. Zavala. 2001. Swift development of protective effector functions in naive CD8+ T cells against malaria liver stages. J. Exp. Med. 194173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenborn, J. R., and C. B. Wilson. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 9641-101. [DOI] [PubMed] [Google Scholar]
- 22.Schofield, L., A. Ferreira, R. Altszuler, V. Nussenzweig, and R. S. Nussenzweig. 1987. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J. Immunol. 1392020-2025. [PubMed] [Google Scholar]
- 23.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells, and antibodies required for immunity to malaria sporozoites. Nature 330664-666. [DOI] [PubMed] [Google Scholar]
- 24.Seguin, M. C., F. W. Klotz, I. Schneider, J. P. Weir, M. Goodbary, M. Slayter, J. J. Raney, J. U. Aniagolu, and S. J. Green. 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei-infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 180353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji, M., Y. Miyahira, R. S. Nussenzweig, M. Aguet, M. Reichel, and F. Zavala. 1995. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J. Immunol. 1545338-5344. [PubMed] [Google Scholar]
- 26.Tsuji, M., P. Romero, R. S. Nussenzweig, and F. Zavala. 1990. CD4+ cytolytic T cell clone confers protection against murine malaria. J. Exp. Med. 1721353-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Boxel-Dezaire, A. H., and G. R. Stark. 2007. Cell type-specific signaling in response to interferon-gamma. Curr. Top. Microbiol. Immunol. 316119-154. [DOI] [PubMed] [Google Scholar]
- 28.Weiss, W. R., J. A. Berzofsky, R. A. Houghten, M. Sedegah, M. Hollindale, and S. L. Hoffman. 1992. A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei. J. Immunol. 1492103-2109. [PubMed] [Google Scholar]