Abstract
Cryptosporidium parvum is an obligate intracellular protozoan capable of causing severe diarrheal disease in a wide variety of mammals, including humans. C. parvum infection has been associated with induction of apoptosis in exposed epithelial cells, and we now demonstrate that apoptosis is restricted to a subset of cells actively infected with C. parvum. Approximately 20% of the infected cells underwent apoptosis within 48 h of infection, suggesting that the majority of the infected cells are rescued from apoptosis. C. parvum infection resulted in low-level activation of multiple members of the caspase family, including caspase-2, -3, -4, -6, -8, and -9. The kinetics of caspase activation correlated with apoptosis over a 48-h time course. Pan caspase inhibitors reduced apoptosis of epithelial cells infected by C. parvum. Furthermore, C. parvum infection inhibited staurosporine-induced apoptosis and caspase-3/7 activation at 24 h and 48 h. Infection with C. parvum led to upregulation of genes encoding inhibitors of apoptosis proteins (IAPs), including c-IAP1, c-IAP2, XIAP, and survivin. Knockdown of survivin gene expression, but not that of c-IAP1, c-IAP2, or XIAP expression, increased caspase-3/7 activity as well as apoptosis of infected cells and decreased C. parvum 18S rRNA levels. These data suggest that the apoptotic response of infected intestinal epithelial cells is actively suppressed by C. parvum via upregulation of survivin, favoring parasite infection.
Apoptosis is programmed cell death that can occur in response to intracellular and extracellular signals (26, 62). This process eventually leads to the packaging of cell contents into apoptotic bodies that can be phagocytosed by other cells without induction of a strong inflammatory response. In contrast, necrosis is typified by rapid cell swelling and lysis and is a random pathological form of cell death due to acute cellular injury and which normally induces a strong immune response (26, 62). Apoptosis is an evolutionarily conserved mechanism orchestrated by the cell's genome-encoded proteins. The key morphological changes of apoptosis are mediated by caspases (54), a very conserved and tightly regulated family of intracellular cysteine proteases. Once an initiator caspase is activated, it processes downstream effector caspases that are responsible for apoptosis (17). Given that apoptosis signaling mediated by caspases is irreversible, procaspase activation and caspase activities must be precisely regulated in order to prevent the undesired death of host cells (21, 54). Direct inhibition of caspase activity by cellular inhibitor of apoptosis proteins (IAPs) is one of the most important mechanisms for apoptosis inhibition. Eight human IAP homologues have been identified, including NAIP, c-IAP1, c-IAP2, XIAP, survivin, Bruce, ILP-2, and livin (13, 60). It has been demonstrated that XIAP, c-IAP1, and c-IAP2 are able to directly inhibit caspase-3, -7, and -9 (50, 56), while survivin binds specifically to the effector cell death proteases caspase-3 and -7 but not to the initiator protease caspase-8 (15, 42, 55, 57).
By residing inside host cells, intracellular pathogens are protected from many host immune responses. However, host cells can employ apoptosis as a defense mechanism to impede replication of intracellular pathogens (5, 22, 26, 62). Since intracellular pathogens depend on the host cell machinery to complete their life cycle and at the same time require intact host cells to shield themselves from the host immune defense system, it is not surprising that many intracellular parasites are able to manipulate the apoptosis pathways of host cells. Viruses, such as adenovirus, poxvirus, baculoviruses, and hepatitis virus (32, 39, 49, 63), and bacteria, including Chlamydia, Shigella flexneri, Rickettsia, Brucella melitensis, and Ehrlichia (11, 20, 24, 25), have evolved a variety of strategies to block apoptosis at different stages within the apoptotic pathways. However, our understanding of apoptosis inhibition by intracellular protozoan parasites remains limited (5, 26).
Cryptosporidium parvum is an obligate intracellular parasite that causes severe acute gastrointestinal illness in animals, including humans. Experimental C. parvum infections of different human cell lines and neonatal mouse models have demonstrated apoptosis in epithelial cells and blunting of intestinal villi of the small intestine (3, 10, 18, 41, 52). Toxoplasma gondii, Trypanosoma cruzi, and Leishmania infections are accompanied by host cell apoptosis, which has been interpreted as an important defense strategy for the host (5, 26, 40, 46). C. parvum infection has been shown to induce caspase activation and Fas ligand-mediated apoptosis (8, 38, 41, 44). This process is associated with a dramatic reduction in the number of intracellular parasites (8, 38, 64), indicating that host cell apoptosis impairs C. parvum development in vitro. However, C. parvum is highly infectious, and as few as 1 to 30 oocysts can cause clinical disease in humans or animals (7, 16). Therefore, C. parvum may have evolved means to control host cell apoptosis to facilitate the establishment of infection in the newly exposed host.
Currently, the molecular mechanisms involved in regulating apoptosis during C. parvum infection remain unclear. We report here that apoptosis of intestinal epithelial cells occurs during C. parvum infection, but only in a minority of infected cells. C. parvum infection protected the HCT-8 cell cultures from staurosporine-induced apoptosis and was associated with the upregulation of genes encoding IAP family members, including c-IAP1, c-IAP2, XIAP, and survivin. Finally, knockdown of survivin by RNA interference (RNAi) increased caspase-3/7 activity and apoptosis of HCT-8 cells and decreased parasite load.
MATERIALS AND METHODS
C. parvum infection model.
C. parvum oocysts (Iowa strain) were purchased (Bunch Grass Farm, Drury, ID) and stored in antibiotics at 4°C. Before infecting the cells, oocysts were surface sterilized by treatment with a 33% Clorox bleach solution (1 ml/3 × 107 oocysts) on ice for 7 min, washed profusely with Hanks' buffered saline solution, and stored in Hanks' buffered saline solution at 4°C overnight. Monolayers of the human ileocecal adenocarcinoma (HCT-8, ATCC CCL-244; American Type Culture Collection, Manassas, VA) cell line were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in a humidified incubator at 37°C in an atmosphere containing 5% CO2 and infected as previously described (58). Briefly, sterilized oocysts were warmed to room temperature for 30 min and inoculated onto HCT-8 cell monolayers at a ratio of one oocyst per cell. Following a 2-h excystation period, unexcysted oocysts and free sporozoites were washed from monolayers with warm RPMI 1640 medium, and cultures were incubated in C. parvum infection medium (14, 53, 58) for specified time points at 37°C. The infection rate was 80% to 90% at 24 h, depending on the batch of oocysts and the storage period. Cells without infection were used for mock controls.
Gene silencing by RNAi.
Chemically synthesized small interfering RNA duplexes (siRNA) for c-IAP1 (5′GUUCAAGCCAGUUACCCUCTT), c-IAP2 (5′AACAGTGGATATTTCCGTGGC), XIAP (5′AAGTGGTAGTCCTGTTTCAGC), and survivin (5′GGACCACCGCAUCUCUACATT) and control (scrambled) siRNAs corresponding to sequences that did not match any human transcripts were purchased (Ambion, Austin, TX). Transfections were performed with HCT-8 cells cultured to approximately 70% confluence in 24-well plates using SiPORT Amine transfection agent (Ambion, Austin, TX) according to the manufacturer's instructions. Briefly, 2.5 × 105 HCT-8 cells were seeded in complete growth medium 1 day before transfection. For each transfection reaction, 3 μl SiPORT Amine was diluted into 46 μl Opti-MEM serum-free medium. siRNA-amine complexes were prepared by mixing 1.25 μl of siRNA (20 μM) with 49 μl Opti-MEM serum-free medium containing amine. The final concentration of the siRNA was 100 nM. Transfections were performed in 250 μl of serum-free medium for 24 h. Thereafter, 0.5 ml of fresh medium containing 10% (vol/vol) fetal bovine serum was added to achieve complete growth conditions. In each experiment, untreated controls were included. Cells were infected with C. parvum at 48 h after transfection. Total RNA was harvested from all cultures, performed in triplicate, at 24 and 48 h postinfection (p.i.) and prepared for quantitative reverse transcription-PCR (qRT-PCR). Data from three independent biological experiments are presented.
Immunofluorescence analysis of C. parvum and apoptosis.
Confluent HCT-8 cells grown in 24-well tissue culture plates were infected with C. parvum for different time periods. Cells were fixed in 2% paraformaldehyde, permeabilized with 0.2% (vol/vol) Triton X-100 in phosphate-buffered saline (pH 7.4), and immunostained for C. parvum, followed by DAPI (4′,6′-diamidino-2-phenylindole) treatment, as previously described (37). C. parvum was visualized by staining with the monoclonal antibody Cp-65.10, which binds to all C. parvum life stages (unpublished data), followed by a Cy3-labeled anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Nuclear staining was visualized using 5 ng/ml DAPI (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were assessed by immunofluorescence microscopy. Number of infected cells and nuclear condensation, an indicator of late apoptosis, were scored for each well at each time point. Over 1,000 cells were counted.
Colorimetric caspase activity assay.
Caspase activities from C. parvum-infected HCT-8 cells were measured with the Caspase Colorimetric Substrate Set II Plus kit (Lab Biovision, Palo Alto, CA) according to the manufacturer's instructions. In brief, protein extracts (100 μg) collected from infected and control cultures were added to a reaction buffer containing p-nitroanilide-labeled specific caspase substrate and incubated for 12 h at 37°C. Relative caspase activity was measured as optical density at 405 nm. Results are presented as the means of the three replicates from three independent experiments.
Apo-ONE homogeneous caspase-3/7 assay.
Caspase-3/7 activities were measured using the Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI) according to the manufacturer's suggestions. HCT-8 cells were plated in 24-well cell culture plates and transfected with siRNA complexes (c-IAP1, c-IAP2, XIAP, and survivin), followed by infection with C. parvum, as described above. At specified time points p.i., infected monolayers were washed once with C. parvum infection medium and then exposed to staurosporine (10 μM final concentration) in fresh C. parvum infection medium for an additional 2 h. A total of 80,000 cells from each sample were collected, lysed with lysis buffer containing caspase substrate Z-DEVD-R100, and incubated at room temperature for 12 h. Caspase-3/7 activities were measured as fluorescence released from Z-DEVD-R100 at 485/535 nm (excitation/emission) using a fluorescence microplate reader (BMG Lab Technologies, Durham, NC). Results are presented as the medians of the four replicates in one representative of three independent experiments.
Caspase inhibition and apoptosis induction.
The pan-caspase inhibitor peptide VAD-FMK and inactive analog Z-FA-FMK (Lab Biovision, Palo Alto, CA) were added to the culture medium at 50 μM at 1 h prior to infection and maintained in the medium for the duration of the experiments. Staurosporine was added to the culture medium at 10 μM and incubated for 2 h. These cultures were either processed for microscopy or harvested for protein extracts as described above. Apoptotic cells were scored from at least three fields of four replicates in one experiment.
Real-time PCR analysis.
Total RNA was prepared using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Purified RNA was treated with RNase-free DNase (Qiagen, Valencia, CA). Two micrograms of RNA was reverse transcribed in a 20-μl reaction mixture primed by random hexamers and catalyzed by Superscript II (200 units; Invitrogen, Carlsbad, CA). Real-time PCRs were performed on the MX3000P detection system (Stratagene, La Jolla, CA) using Sybr green QPCR master mix (Stratagene, La Jolla, CA) and 6 ng cDNA as the template. Gene-specific primers (Table 1) were added at a 50 nM final concentration and were designed to yield products of approximately 100 bp in length. The real-time PCR program included 50 cycles of amplification with alternating steps of 15 s of denaturation at 95°C and 1 min of annealing/extension at 60°C. Relative quantification of the product was done using the comparative threshold cycle (CT) method (35) with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression as a normalizer. The mean fold change of target gene expression was averaged for the three different biological samples.
TABLE 1.
Primer sequences used
| Gene | GenBank accession no. | Primer sequences |
|---|---|---|
| GAPDH | NM_002046 | Forward, ACAGTCAGCCGCATCTTCTT; reverse, ACGACCAAATCCGTTGACTC |
| Survivin (baculoviral IAP repeat-containing 5) | NM_001168 | Forward, GCCCAGTGTTTCTTCTGCTT reverse, GACAGAAAGGAAAGCGCAAC |
| XIAP (baculoviral IAP repeat-containing 4) | NM_001167 | Forward, GCTTGCAAGAGCTGGATTTT; reverse, TTGTTCCCAAGGGTCTTCAC |
| c-IAP1 (baculoviral IAP repeat-containing 2) | NM_001166 | Forward, AGCTTGCAAGTGCTGGTTTT; reverse, TCTCCAGATTCCCAACACCT |
| c-IAP2 (baculoviral IAP repeat-containing 3) | NM_001165 | Forward, CCAAGTGGTTTCCAAGGTGT; reverse, TGGGCTGTCTGATGTGGATA |
| C. parvum 18S rRNA | Forward, TAGAGATTGGAGGTTGTTCCT; reverse, CTCCACCAACTAAGAACGGCC |
Statistical analysis.
The data for real-time PCR and the colorimetric caspase activity assay are expressed as means ± standard errors of the means. For the colorimetric caspase activity assay, the significance of difference was determined by one-way analysis of variance (P < 0.05). All other results are medians ± ranges (from the minimum to the maximum value), and the significance of difference were analyzed by the nonparametric Mann-Whitney test.
RESULTS
C. parvum infection leads to cellular apoptosis.
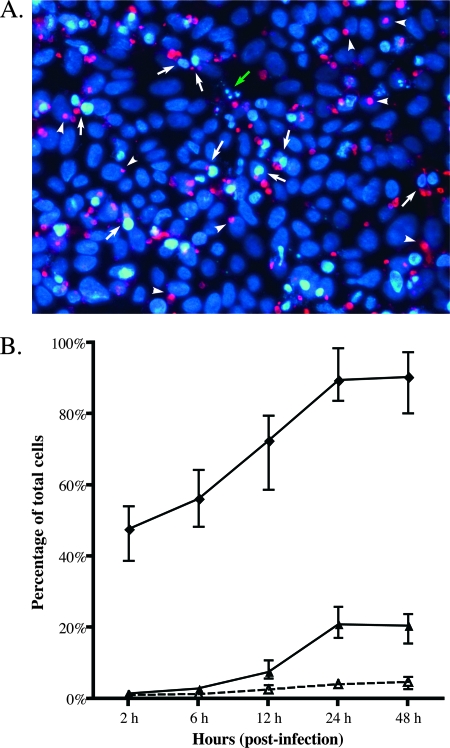
Previous C. parvum studies showing the occurrence of apoptosis during infection appear to be at odds with infection studies that demonstrate clinical disease occurring with extremely low doses of C. parvum oocysts. Conversely, one study reported clear evidence of apoptosis inhibition (37). To clarify, we reexamined C. parvum infection in an HCT-8 intestinal epithelial cell in vitro model. Confluent HCT-8 cells were infected at a parasite/cell ratio of 1:1, and cultures were stained for parasites and the appearance of chromatin condensation characteristic of apoptosis (Fig. 1A). As shown in Fig. 1B, apoptotic cells started to appear at around 12 h p.i., reached maximum of 20% at 24 h p.i., and remained static at 48 h p.i. This is in agreement with prior reports (37). By 24 h p.i., 91% of the cells were infected, indicating that apoptosis is not a major response by the host HCT-8 cells. Importantly, apoptosis colocalized with cells containing parasites, as we detected apoptosis in only 3% of the cells not infected by C. parvum, which is consistent with the background level of apoptotic cells in our cell culture system. Infection of HCT-8 cultures at a low multiplicity of infection (1:10) also did not reveal apoptosis in neighboring uninfected cells (data not shown). These observations suggest that apoptosis in infected cells or neighboring cells is not an effective response to limit intracellular replication of C. parvum, as only 20% of the infected cells are eliminated by apoptosis.
FIG. 1.
Apoptosis in HCT-8 cells infected with C. parvum. (A) Fluorescence micrograph of C. parvum-infected HCT-8 cells at 12 h p.i. Developing parasites were labeled using monoclonal antibody Cp1-65.10 (pan-C. parvum) and a Cy3-conjugated secondary antibody (red). Nuclei were stained with DAPI (blue). Arrows indicate infected cells undergoing apoptosis. The green arrow indicates the presence of apoptotic bodies. Arrowheads indicate nonapoptotic cells infected with C. parvum. (B) The C. parvum infectivity rate (in percent) and the percentage of cells exhibiting signs of apoptosis induction were enumerated over time. Infected cells were determined by scoring HCT-8 cells colocalized with parasites (⧫). Apoptotic cells among C. parvum-infected cells (▴) and among noninfected cells (▵) at different time points were estimated based on scoring HCT-8 cells showing signs of chromatin condensation by DNA staining. Data are medians ± ranges from four biologically different experiments. Apoptosis in uninfected cultures was less than 3% at all time points (data not shown).
C. parvum inhibits host cell apoptosis.
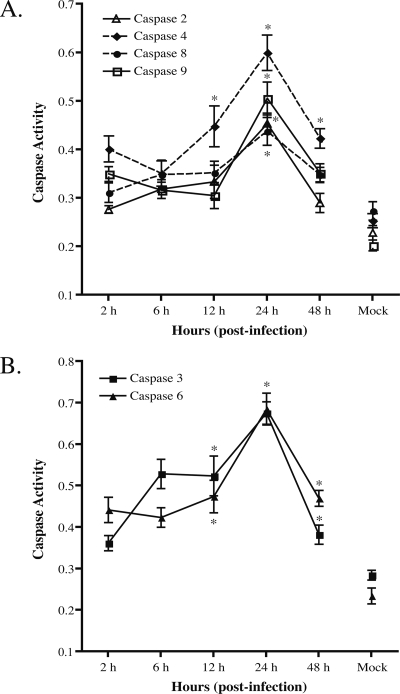
Caspase activation in cells infected with C. parvum correlated with the low fraction of apoptotic cells (Fig. 2). Compared to those in uninfected cultures, the activities of initiator caspase-4 (Fig. 2A) and effector caspase-3 and -6 (Fig. 2B) were increased by 2- to 2.5-fold at 24 h p.i., and they declined by 48 h. The activities of initiator caspase-2, -8, and -9 were observed to be only slightly elevated at 24 h (Fig. 2A). We did not find significant activation of caspase-1 and caspase-10 in the same infected cells that displayed caspase-3, -4, and -6 activation (data not shown). The kinetics of caspase activation correlated with the low level of 20% apoptosis observed in C. parvum-infected cell cultures. To assess whether apoptosis in C. parvum-infected intestinal epithelial cells requires caspase activation, we treated HCT-8 cells with pan-caspase inhibitors 1 h before and during C. parvum infection for 24 h and 48 h. As shown in Fig. 3, compared to infected cells without inhibitor treatment, pretreatment with pan-caspase inhibitor resulted in markedly reduced apoptosis in the infected HCT-8 monolayers. This result suggests that C. parvum-associated apoptosis of epithelial cells is mediated in large part by caspase activation.
FIG. 2.
Assessment of the cytosolic caspase activity of C. parvum-infected HCT-8 cells. Protein extracts harvested from C. parvum-infected HCT-8 cell time courses were used with a commercial caspase colorimetric substrate kit to determine specific caspase activities. (A) Measurement of initiator caspase activities in C. parvum-infected HCT-8 cells. (B) Measurement of effector caspase activities in C. parvum-infected HCT-8 cells. Note that all lysates assayed in the absence of caspase substrates showed values similar to those of control cell lysates incubated with caspase substrates (data not shown). Each point represents mean values ± standard errors from three biologically different experiments. *, P < 0.05 compared with mock infections at 48 h (one-way analysis of variance).
FIG. 3.
Effect of caspase inhibition on HCT-8 cell apoptosis. Monolayers of HCT-8 cells were treated with a pan-caspase inhibitor or an inactive analog peptide 1 h prior to infection with C. parvum and continuously cultured in itspresence thereafter. The percentage of all cells (infected or noninfected) that were apoptotic was measured by counting cells showing nuclei condensation from at least three fields of each sample. Shown are results for cultures infected with C. parvum only (▴), cultures treated with negative peptide only (⋄), cultures treated with negative peptide and C. parvum infected (⧫), and cultures treated with pan-caspase inhibitor and C. parvum infected (▪). Mock-infected cells and cells treated with the pan-caspase inhibitor had less than 3% apoptosis at all time points (data not shown). Results are the medians ± ranges from four replicates in one of two separate experiments. An asterisk indicates that the percentage of apoptosis is significantly different between cells treated with C. parvum alone and cells treated with pan-caspase inhibitor and C. parvum at indicated time points.
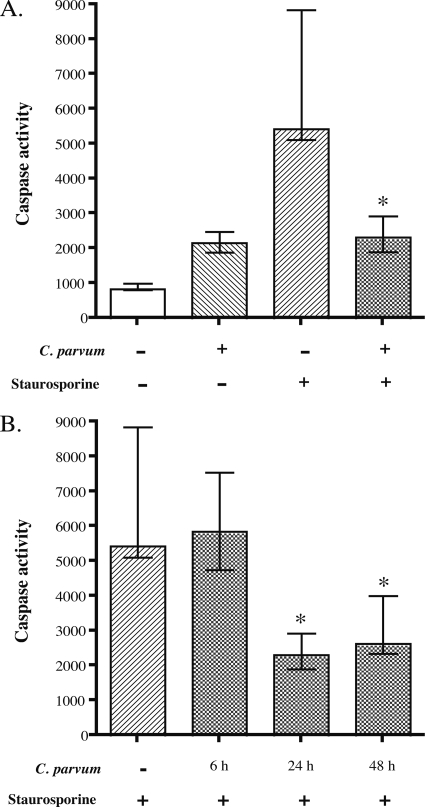
To determine whether C. parvum-infected HCT-8 cell are able to undergo apoptosis, we induced apoptosis with staurosporine and measured caspase activity. Staurosporine (10 μM final concentration) treatment for 2 h was sufficient to induce 60% apoptosis in HCT-8 cells (data not shown) and induced a sevenfold increase in caspase-3/7 activity (Fig. 4A). However, only a 2.5-fold increase in caspase-3/7 activity compared to that in control cultures was detected in cultures infected with C. parvum for 24 h. Treatment of infected cells (24 h p.i.) with staurosporine for 2 h did not increase caspase-3/7 activity above that observed for infection alone (Fig. 4A). These results demonstrate that C. parvum infection actively inhibits staurosporine-induced caspase-3/7 activity. Caspase-3/7 activity was significantly lower in cells infected with C. parvum for 24 h and 48 h prior to treatment with staurosporine for 2 h than in control uninfected cells. However, caspase-3/7 activity induced by staurosporine was not affected in cells treated at 6 h p.i. (Fig. 4B). These results suggest that C. parvum actively inhibits caspase activation in intestinal epithelial cells, but this does not occur immediately after attachment of sporozoites to the host cell.
FIG. 4.
Effect of C. parvum infection on staurosporine-induced caspase-3/7 activity in HCT-8 cells. Monolayers were either infected or mock infected with C. parvum for indicated period of time, followed by treatment of paired cultures with staurosporine (10 μM) for 2 h. Protein extracts of each sample were incubated with a caspase binding substrate for 12 h, followed by quantification via fluorescence analysis. (A) Staurosporine-induced caspase-3/7 activity inhibition by C. parvum. Cultures were infected for 24 h prior to a 2-h treatment with staurosporine. Data are representative for one of three biologically independent experiments and are presented as the medians and ranges from four technical replicates. The asterisk denotes a significant difference (P < 0.05) from cells treated with staurosporine alone. (B) Time course of the effect of C. parvum infection on staurosporine-induced caspase-3/7 activation. HCT-8 cells were infected for the times shown, followed by treatment with staurosporine for another 2 h. The resulting protein extracts were assayed as described above. Results are presented as the medians and ranges from four technical replicates from one of three biologically independent experiments. An asterisk indicates a significant difference (P < 0.05) from cells treated with staurosporine alone.
C. parvum infection increases levels of transcripts of genes encoding IAPs.
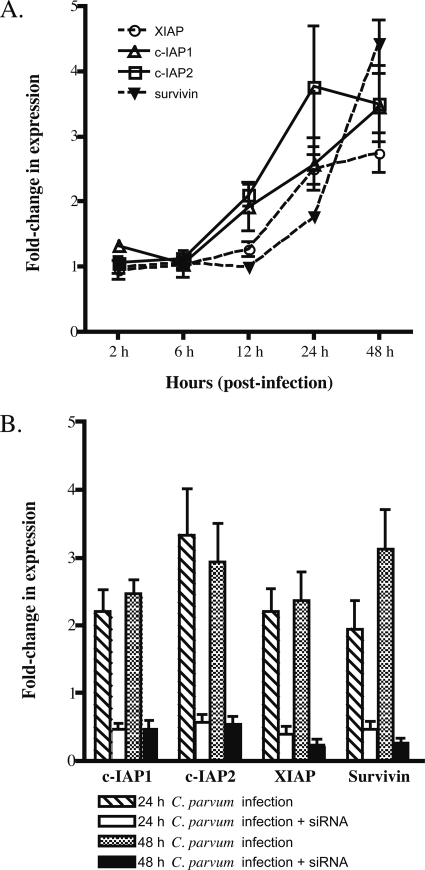
C. parvum may inhibit apoptosis in part by suppressing full activation of effector caspases. In addition, prior expression microarray work from our lab suggested that induction of IAP genes occurs during HCT-8 infection by C. parvum (J. Liu et al., unpublished data). We confirmed microarray results for c-IAP1, c-IAP2, XIAP, survivin, livin, and NAIP (Fig. 5A) by qRT-PCR. Transcripts for c-IAP1 and c-IAP2 were twofold higher than those in uninfected cells at 12 h and remained elevated until 48 h. XAIP transcript levels were also increased over twofold at 24 h and 48 h. Expression of survivin was delayed compared to that of the other IAPs and reached a 4.3-fold increase at 48 h p.i. NAIP and livin transcript levels were too low to be detected in our experimental system (data not shown). Thus, c-IAP1, c-IAP2, XIAP, and survivin expression was upregulated in C. parvum-infected cells, albeit with different kinetics. Increased expression of genes that disrupt caspase activities is consistent with C. parvum infection actively inhibiting apoptosis of host cells.
FIG. 5.
Expression of IAPs in infected cultures. (A) qRT-PCR analysis of static mRNA transcript levels for c-IAP1, c-IAP2, XIAP, and survivin genes of C. parvum-infected HCT-8 cells. Relative quantification of specific transcript expression was calculated using GAPDH transcripts to normalize by the comparative CT method. The mean fold change and standard error of target gene expression were calculated from three independent biological replicates. (B) Effect of siRNA silencing on specific IAP genes during C. parvum infection. HCT-8 cells were transfected with specific siRNAs for 48 h, followed by infection with C. parvum for an additional 24 or 48 h. Specific transcript levels were quantified by real-time PCR by the comparative CT method. Results are expressed relative to normalized GAPDH mRNA levels in cultures treated with scramble siRNA controls. The mean fold change of target gene expression was averaged for the three different biological samples. Bars indicate standard errors.
Knockdown of cellular survivin increases apoptosis and caspase-3/7 activity of infected epithelial cells.
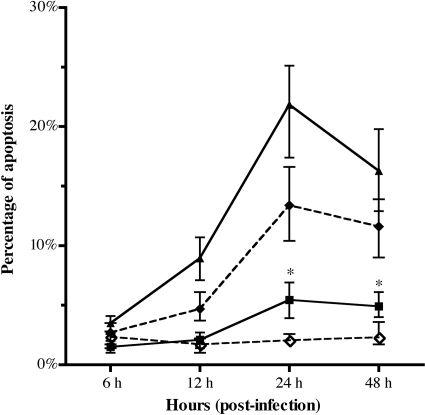
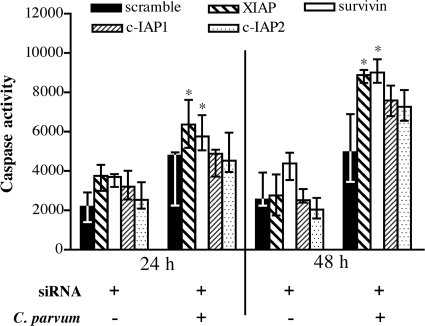
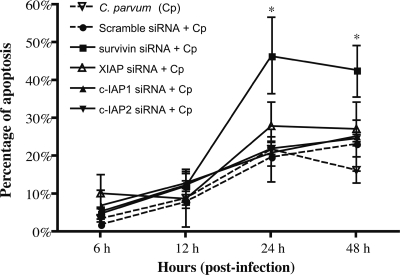
To examine whether IAPs are required for inhibition of caspase activation in C. parvum-infected cells, siRNA silencing was performed. siRNA treatment reduced transcript levels for c-IAP1, c-IAP2, XIAP, and survivin by ∼80% compared to those in untreated cells at 24 h and 48 h after siRNA transfection (Fig. 5B) but had no affect on uninfected cell viability (data not shown). siRNA knockdown of survivin or XIAP transcripts caused a statistically significant (P < 0.05) increase in caspase-3/7 activity in infected cells at 24 h and 48 h (Fig. 6). However, siRNA knockdown only of survivin, and not that of c-IAP1, c-IAP2, or XIAP, caused a significant increase in apoptosis at 24 and 48 h (Fig. 7). These data suggest that both XIAP and survivin are involved in blocking caspase activity but that survivin induction is required for blocking apoptosis in HCT-8 cells infected with C. parvum.
FIG. 6.
Caspase-3/7 activity in C. parvum-infected cells following RNAi-mediated inhibition of IAP gene expression. HCT-8 cells were transfected with functional siRNAs or a nonbinding control (scramble) for 48 h, followed by infection with C. parvum for another 24 h and 48 h. Protein extracts of each sample were analyzed for caspase-3/7 activity by incubation with a caspase binding substrate for 12 h, followed by quantification via fluorescence. Data are the medians and ranges from four technical replicates from one of two independent experiments. An asterisk indicates a significant difference (P < 0.05) from cells treated with the scrambled siRNA control at the indicated time points.
FIG. 7.
Apoptosis induction in C. parvum-infected HCT-8 cells following RNAi-mediated inhibition of IAP transcripts. HCT-8 cells were transfected with functional siRNAs against survivin, XIAP, c-IAP1, c-IAP2, or a nonbinding control (scramble), or left untreated, for 48 h, followed by infection with C. parvum for an additional 6, 12, 24, or 48 h. Apoptotic cells were scored based on chromatin condensation observed by DNA staining using DAPI. Cells from four fields in each sample were counted. Data points represent medians ± ranges from four replicates in one of two separate experiments. An asterisk indicates that the percentage of apoptosis is significantly different (P < 0.05) from that of cells treated with C. parvum and scramble siRNA at the indicated time points.
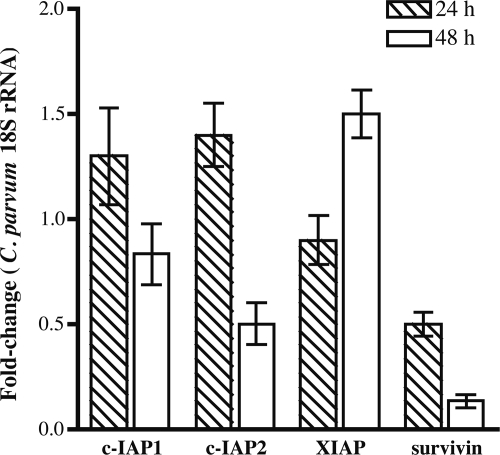
Knockdown of survivin by RNAi decreases C. parvum 18S rRNA expression.
The impact of downregulation of host cellular IAPs on C. parvum infection was measured by expression of C. parvum 18S rRNA at 24 h and 48 h p.i. Previous work (4) has shown that 18S rRNA gene expression correlates well with the number of live C. parvum parasites. C. parvum 18S rRNA transcripts in cultures treated with survivin siRNA were decreased by 2-fold at 24 h and by 10-fold at 48 h (Fig. 8). Knockdown of c-IAP1, c-IAP2, or XIAP by siRNA did not significantly affect C. parvum 18S rRNA expression at 24 h p.i. However, inhibition of c-IAP2 transcripts decreased parasite 18S rRNA levels twofold at 48 h. Our results suggest that upregulation of survivin during parasite infection is required to block cellular apoptosis and that the inhibition of host cell apoptosis promotes C. parvum infection and development.
FIG. 8.
Quantification of C. parvum infection in HCT-8 cells knocked down for specific IAP genes by siRNA. C. parvum 18S rRNA were measured by qRT-PCR analysis. HCT-8 cells were transfected with functional siRNAs against IAPs for 48 h, followed by infection with C. parvum for an additional 24 h and 48 h. RNA harvested from these samples was analyzed by normalization to GAPDH transcripts. Fold change results are expressed relative to normalized C. parvum 18S rRNA expression levels in C. parvum-infected cells treated with scramble siRNA controls at each time point. The mean fold change and standard error of C. parvum 18S rRNA gene expression were calculated for the three different biological samples.
DISCUSSION
Apoptosis of infected host cells is widely recognized as an immediate defense response to limit the spread of certain infections (23, 26). Cell death is thought to help contain the spread of infection by impeding replication of the intracellular parasite. Moreover, cell removal by apoptosis is a normal ongoing process for the intestinal epithelial cell at the end of its 3-day life span. Apoptosis ensures the integrity of the epithelial barrier and prevents unwarranted activation of inflammatory responses. Previous studies have implicated apoptosis as an important defense mechanism against C. parvum infection (3, 18, 38, 41). However, C. parvum is extraordinarily infectious and requires few oocysts to initiate disease (28). C. parvum appears to have evolved the ability to neutralize the immediate cellular apoptosis response in order to establish infection and to permit life cycle progression (37, 64).
Many intracellular pathogens target caspase signaling as a means to impede apoptosis of the infected host cell. We show in this study that C. parvum inhibits apoptosis of infected human intestinal epithelial cells. C. parvum infection of HCT-8 cells in vitro induced only a minority (∼20%) of the infected cells to undergo apoptosis, and apoptosis was most apparent late in the infection. Correspondingly, only a low level of caspase activation was detectable in C. parvum-infected cultures. The attenuated apoptosis was not due to a defect in the host cell machinery, as staurosporine induced a strong caspase activation and apoptosis in approximately 60% of the HCT-8 cells. Rather, our data support the report by McCole et al. (37) that C. parvum actively inhibits host cell apoptosis. Caspase activation induced by staurosporine was significantly reduced in infected cultures, and C. parvum infection increased mRNA levels for the c-IAP1, c-IAP2, XIAP, and survivin antiapoptotic genes at between 12 and 48 h p.i. Experimental siRNA depletion of either survivin or XIAP resulted in increased effector caspase-3/7 activity, whereas only depletion of survivin both increased cellular apoptosis and reduced parasite growth. In the course of normal infection, both caspase activity and apoptosis peaked at 24 h and then decreased slightly through 48 h (Fig. 1B and 2). These kinetics correspond to the induction of transcripts for survivin and IAPs, which continued to increase between 24 and 48 h (Fig. 5A). Conversely, when survivin expression was inhibited by siRNA treatment, apoptosis remained elevated after 24 h, and caspase activity continued to increase (Fig. 6 and 7). Together these data show the importance of survivin in preventing apoptosis during later stages of C. parvum infection.
c-IAP1, c-IAP2, and XIAP promote cellular survival by inhibiting apoptosis in many infection models (30, 48, 61, 66). It is likely that several pathways contribute to the overall blockade of cellular apoptosis and that cell death or survival teeters on the net balance of these competing pathways. Toward this end, Bcl-2 expression is induced during C. parvum infection (38) and may contribute to the blockade of apoptosis early in infection. It has been shown that NF-κB activation is associated with C. parvum infection of epithelial cells and that inhibiting NF-κB activation promotes apoptosis (9, 37). However, it is not clear which NF-κB-regulated host genes are responsible for inhibiting apoptosis during C. parvum infection, and the key components in the rescue from apoptosis may be different depending on the cell type and the pathogen in question. Expression of both IAPs and survivin can be regulated by NF-κB activation. siRNA depletion experiments suggested that IAPs have some role in blocking apoptosis but that survivin is most critical after 24 h. Infection of HeLa cells with Chlamydia trachomatis upregulated cellular c-IAP2 and survivin levels (48). In contrast to what we observed for C. parvum, depletion of c-IAP1, c-IAP2, or XIAP, but not survivin, sensitized C. trachomatis-infected cells for apoptosis. c-IAP1, c-IAP2, and XIAP form heteromeric complexes in C. trachomatis-infected cells, and depletion of any one IAP caused disintegration of IAP complexes, leading to increased apoptosis (48). The interactions between c-IAP1, c-IAP2, and XIAP are essential for recruitment of caspases. Survivin is not part of these complexes (48), yet our work showed that C. parvum required survivin to inhibit apoptosis of infected cells. Survivin is required to preserve viability of dividing cells and is specifically involved in S phase and G2/M phase cell cycle progression (1, 6, 34). It is interesting to note that C. parvum infection and development have a significant preference for mitotic cells in S/G2/M phase cells (65). Inhibition of caspase activation was sensitive to both XIAP and survivin gene expression knockdown. IAP complexes (XIAP-IAP1-IAP2) and survivin appear to act independently to inhibit caspase activities and apoptosis. C. parvum has a greater requirement for survivin to inhibit host cell apoptosis than C. trachomatis, which requires formation of a XIAP-IAP1-IAP2 complex (48). Inhibition of caspases likely contributes to host cell survival, but it is unclear at this time how survivin contributes to blockade of apoptosis. Its role in cell cycle regulation must be considered.
Survivin has been shown to prevent cellular apoptosis in other infection models (36, 47). The hepatitis B virus HBX protein interacts with the cellular survivin and the HBXIP complex to suppress caspase activation and apoptosis in a survivin-dependent manner (47). Infection with the polyomavirus JC virus induces strong cellular survivin expression in glial cell cultures and oligodendrocytes which protected infected cells from apoptosis and advanced JC virus infection (36, 47). Similarly, depletion of survivin significantly decreased C. parvum 18S rRNA accumulation, indicating that progression through the C. parvum life cycle requires survivin expression within the infected host cell. It is likely, but not proven, that the dependency for survivin is its role in preventing apoptosis, which allows the parasite time to progress through its complicated life cycle, rather than a direct requirement of C. parvum for survivin. It is also completely unknown which C. parvum proteins or factors are involved in regulating cellular antiapoptotic gene expression.
The HCT-8 cell line mimics the cell cycling and relatively undifferentiated state of the intestinal crypt cells, permitting the complete parasite development from sporozoites to sporulated oocysts for both human and cattle genotypes (27). In vivo, intestinal epithelial cells are governed by the Wnt gradient, which forms undifferentiated cells at the crypt and fully differentiated and dying cells at the villus tip (51, 59). In vivo, C. parvum appears to replicate in the basal crypt cells (52), and parasite development has a significant preference for mitotic cells in S/G2/M phase cells (65). Survivin also has a role in cell cycle progression (1, 6, 34), and its expression is controlled by cell cycle pathway components such as Myc and PTEN that are active only in the undifferentiated cells (12, 19). If C. parvum can turn on survivin to manage apoptosis, it is also possible that this same mechanism operates to lock the host cell in a relatively undifferentiated state. Many viruses are known to usurp both the cell cycle and apoptosis (2, 29, 31, 33, 43, 45). It would be very interesting to determine whether induction of epithelial cell differentiation has an effect on C. parvum infection and apoptosis.
This study demonstrated the essential role of survivin in preventing apoptosis of HCT-8 epithelial cells during C. parvum infection in a process associated with decreased caspase activation. Taken together, these data are consistent with a model in which C. parvum actively inhibits apoptosis of host cells by upregulation of the c-IAP1, c-IAP2, XIAP, and survivin genes. Our studies demonstrate for the first time that the marked resistance to apoptosis and suppression of caspase activation observed in C. parvum-infected cells were due to the infection-induced upregulation of survivin that specifically blocked the activation of effector caspase-3 and -7. However, a delicate and dynamic balance of pro- and antiapoptotic signals determines the fate of a cell. While survivin appears to be a key requirement for rescue of the C. parvum-infected cell from apoptosis, it is probable that other antiapoptotic genes such as Bcl-2 family member contribute to this interaction, particularly at different stages of infection.
Acknowledgments
We thank Peter Southern for critical reading of the manuscript. We thank the University of Minnesota Imaging Center for their technical help in conducting fluorescence microscopy.
This work was supported in part by grants R01-AI065246-02 from the National Institute of Health and 99 35204-8614 from the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Altieri, D. C. 2008. Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer 861-70. [DOI] [PubMed] [Google Scholar]
- 2.Andrus, L., P. Szabo, R. W. Grady, A. R. Hanauske, T. Huima-Byron, B. Slowinska, S. Zagulska, and H. M. Hanauske-Abel. 1998. Antiretroviral effects of deoxyhypusyl hydroxylase inhibitors: a hypusine-dependent host cell mechanism for replication of human immunodeficiency virus type 1 (HIV-1). Biochem. Pharmacol. 551807-1818. [DOI] [PubMed] [Google Scholar]
- 3.Buret, A. G., A. C. Chin, and K. G. Scott. 2003. Infection of human and bovine epithelial cells with Cryptosporidium andersoni induces apoptosis and disrupts tight junctional ZO-1: effects of epidermal growth factor. Int. J. Parasitol. 331363-1371. [DOI] [PubMed] [Google Scholar]
- 4.Cai, X., K. M. Woods, S. J. Upton, and G. Zhu. 2005. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob. Agents Chemother. 494437-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmen, J. C., and A. P. Sinai. 2007. Suicide prevention: disruption of apoptotic pathways by protozoan parasites. Mol. Microbiol. 64904-916. [DOI] [PubMed] [Google Scholar]
- 6.Castedo, M., J. L. Perfettini, T. Roumier, K. Andreau, R. Medema, and G. Kroemer. 2004. Cell death by mitotic catastrophe: a molecular definition. Oncogene 232825-2837. [DOI] [PubMed] [Google Scholar]
- 7.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, C. Wang, W. Jakubowski, and H. L. Dupont. 1999. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am. J. Trop. Med. Hyg. 60157-164. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X. M., G. J. Gores, C. V. Paya, and N. F. LaRusso. 1999. Cryptosporidium parvum induces apoptosis in biliary epithelia by a Fas/Fas ligand-dependent mechanism. Am. J. Physiol. 277G599-G608. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X. M., S. A. Levine, P. L. Splinter, P. S. Tietz, A. L. Ganong, C. Jobin, G. J. Gores, C. V. Paya, and N. F. LaRusso. 2001. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology 1201774-1783. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X. M., S. A. Levine, P. Tietz, E. Krueger, M. A. McNiven, D. M. Jefferson, M. Mahle, and N. F. LaRusso. 1998. Cryptosporidium parvum is cytopathic for cultured human biliary epithelia via an apoptotic mechanism. Hepatology 28906-913. [DOI] [PubMed] [Google Scholar]
- 11.Clark, C. S., and A. T. Maurelli. 2007. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect. Immun. 752531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosgrave, N., A. D. Hill, and L. S. Young. 2006. Growth factor-dependent regulation of survivin by c-myc in human breast cancer. J. Mol. Endocrinol. 37377-390. [DOI] [PubMed] [Google Scholar]
- 13.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116205-219. [DOI] [PubMed] [Google Scholar]
- 14.Deng, M., C. A. Lancto, and M. S. Abrahamsen. 2004. Cryptosporidium parvum regulation of human epithelial cell gene expression. Int. J. Parasitol. 3473-82. [DOI] [PubMed] [Google Scholar]
- 15.Dohi, T., E. Beltrami, N. R. Wall, J. Plescia, and D. C. Altieri. 2004. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J. Clin. Investig. 1141117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332855-859. [DOI] [PubMed] [Google Scholar]
- 17.Earnshaw, W. C., L. M. Martins, and S. H. Kaufmann. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68383-424. [DOI] [PubMed] [Google Scholar]
- 18.Elliott, D. A., and D. P. Clark. 2003. Host cell fate on Cryptosporidium parvum egress from MDCK cells. Infect. Immun. 715422-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erkanli, S., F. Kayaselcuk, E. Kuscu, T. Bagis, F. Bolat, A. Haberal, and B. Demirhan. 2006. Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int. J. Gynecol. Cancer 161412-1418. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, S. F., J. Vier, S. Kirschnek, A. Klos, S. Hess, S. Ying, and G. Hacker. 2004. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J. Exp. Med. 200905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal, L. 2001. Cell death inhibition: keeping caspases in check. Cell 104805-808. [DOI] [PubMed] [Google Scholar]
- 22.Grassme, H., V. Jendrossek, and E. Gulbins. 2001. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 6441-445. [DOI] [PubMed] [Google Scholar]
- 23.Green, D. R. 2005. Apoptotic pathways: ten minutes to dead. Cell 121671-674. [DOI] [PubMed] [Google Scholar]
- 24.Hacker, G., and S. F. Fischer. 2002. Bacterial anti-apoptotic activities. FEMS Microbiol. Lett. 2111-6. [DOI] [PubMed] [Google Scholar]
- 25.He, Y., S. Reichow, S. Ramamoorthy, X. Ding, R. Lathigra, J. C. Craig, B. W. Sobral, G. G. Schurig, N. Sriranganathan, and S. M. Boyle. 2006. Brucella melitensis triggers time-dependent modulation of apoptosis and down-regulation of mitochondrion-associated gene expression in mouse macrophages. Infect. Immun. 745035-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heussler, V. T., P. Kuenzi, and S. Rottenberg. 2001. Inhibition of apoptosis by intracellular protozoan parasites. Int. J. Parasitol. 311166-1176. [DOI] [PubMed] [Google Scholar]
- 27.Hijjawi, N. S., B. P. Meloni, U. M. Morgan, and R. C. Thompson. 2001. Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. Int. J. Parasitol. 311048-1055. [DOI] [PubMed] [Google Scholar]
- 28.Howe, A. D., S. Forster, S. Morton, R. Marshall, K. S. Osborn, P. Wright, and P. R. Hunter. 2002. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 8619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumiya, Y., S. F. Lin, T. J. Ellison, A. M. Levy, G. L. Mayeur, C. Izumiya, and H. J. Kung. 2003. Cell cycle regulation by Kaposi's sarcoma-associated herpesvirus K-bZIP: direct interaction with cyclin-CDK2 and induction of G1 growth arrest. J. Virol. 779652-9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James, M. A., J. H. Lee, and A. J. Klingelhutz. 2006. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 805301-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston, J. B., G. Wang, J. W. Barrett, S. H. Nazarian, K. Colwill, M. Moran, and G. McFadden. 2005. Myxoma virus M-T5 protects infected cells from the stress of cell cycle arrest through its interaction with host cell cullin-1. J. Virol. 7910750-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lannan, E., R. Vandergaast, and P. D. Friesen. 2007. Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J. Virol. 819319-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavia, P., A. M. Mileo, A. Giordano, and M. G. Paggi. 2003. Emerging roles of DNA tumor viruses in cell proliferation: new insights into genomic instability. Oncogene 226508-6516. [DOI] [PubMed] [Google Scholar]
- 34.Li, F., G. Ambrosini, E. Y. Chu, J. Plescia, S. Tognin, P. C. Marchisio, and D. C. Altieri. 1998. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396580-584. [DOI] [PubMed] [Google Scholar]
- 35.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 36.Marusawa, H., S. Matsuzawa, K. Welsh, H. Zou, R. Armstrong, I. Tamm, and J. C. Reed. 2003. HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO J. 222729-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCole, D. F., L. Eckmann, F. Laurent, and M. F. Kagnoff. 2000. Intestinal epithelial cell apoptosis following Cryptosporidium parvum infection. Infect. Immun. 681710-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mele, R., M. A. Gomez Morales, F. Tosini, and E. Pozio. 2004. Cryptosporidium parvum at different developmental stages modulates host cell apoptosis in vitro. Infect. Immun. 726061-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moise, A. R., J. R. Grant, T. Z. Vitalis, and W. A. Jefferies. 2002. Adenovirus E3-6.7K maintains calcium homeostasis and prevents apoptosis and arachidonic acid release. J. Virol. 761578-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosser, D. M., and A. Brittingham. 1997. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology 115(Suppl.)S9-S23. [DOI] [PubMed] [Google Scholar]
- 41.Motta, I., M. Gissot, J. M. Kanellopoulos, and D. M. Ojcius. 2002. Absence of weight loss during Cryptosporidium infection in susceptible mice deficient in Fas-mediated apoptosis. Microbes Infect. 4821-827. [DOI] [PubMed] [Google Scholar]
- 42.Nawrocki, S. T., J. S. Carew, L. Douglas, J. L. Cleveland, R. Humphreys, and J. A. Houghton. 2007. Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res. 676987-6994. [DOI] [PubMed] [Google Scholar]
- 43.Ojala, P. M., K. Yamamoto, E. Castanos-Velez, P. Biberfeld, S. J. Korsmeyer, and T. P. Makela. 2000. The apoptotic v-cyclin-CDK6 complex phosphorylates and inactivates Bcl-2. Nat. Cell Biol. 2819-825. [DOI] [PubMed] [Google Scholar]
- 44.Ojcius, D. M., J. L. Perfettini, A. Bonnin, and F. Laurent. 1999. Caspase-dependent apoptosis during infection with Cryptosporidium parvum. Microbes Infect. 11163-1168. [DOI] [PubMed] [Google Scholar]
- 45.Oster, B., B. Bundgaard, and P. Hollsberg. 2005. Human herpesvirus 6B induces cell cycle arrest concomitant with p53 phosphorylation and accumulation in T cells. J. Virol. 791961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne, T. M., R. E. Molestina, and A. P. Sinai. 2003. Inhibition of caspase activation and a requirement for NF-kappaB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J. Cell Sci. 1164345-4358. [DOI] [PubMed] [Google Scholar]
- 47.Pina-Oviedo, S., K. Urbanska, S. Radhakrishnan, T. Sweet, K. Reiss, K. Khalili, and L. Del Valle. 2007. Effects of JC virus infection on anti-apoptotic protein survivin in progressive multifocal leukoencephalopathy. Am. J. Pathol. 1701291-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajalingam, K., M. Sharma, N. Paland, R. Hurwitz, O. Thieck, M. Oswald, N. Machuy, and T. Rudel. 2006. IAP-IAP complexes required for apoptosis resistance of C. trachomatis-infected cells. PLoS Pathog. 2e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito, K., K. Meyer, R. Warner, A. Basu, R. B. Ray, and R. Ray. 2006. Hepatitis C virus core protein inhibits tumor necrosis factor alpha-mediated apoptosis by a protective effect involving cellular FLICE inhibitory protein. J. Virol. 804372-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvesen, G. S., and C. S. Duckett. 2002. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell Biol. 3401-410. [DOI] [PubMed] [Google Scholar]
- 51.Sancho, E., E. Batlle, and H. Clevers. 2003. Live and let die in the intestinal epithelium. Curr. Opin. Cell Biol. 15763-770. [DOI] [PubMed] [Google Scholar]
- 52.Sasahara, T., H. Maruyama, M. Aoki, R. Kikuno, T. Sekiguchi, A. Takahashi, Y. Satoh, H. Kitasato, Y. Takayama, and M. Inoue. 2003. Apoptosis of intestinal crypt epithelium after Cryptosporidium parvum infection. J. Infect. Chemother. 9278-281. [DOI] [PubMed] [Google Scholar]
- 53.Schroeder, A. A., A. M. Brown, and M. S. Abrahamsen. 1998. Identification and cloning of a developmentally regulated Cryptosporidium parvum gene by differential mRNA display PCR. Gene 216327-334. [DOI] [PubMed] [Google Scholar]
- 54.Shi, Y. 2004. Caspase activation: revisiting the induced proximity model. Cell 117855-858. [DOI] [PubMed] [Google Scholar]
- 55.Shin, S., B. J. Sung, Y. S. Cho, H. J. Kim, N. C. Ha, J. I. Hwang, C. W. Chung, Y. K. Jung, and B. H. Oh. 2001. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 401117-1123. [DOI] [PubMed] [Google Scholar]
- 56.Silke, J., and D. L. Vaux. 2001. Two kinds of BIR-containing protein—inhibitors of apoptosis, or required for mitosis. J. Cell Sci. 1141821-1827. [DOI] [PubMed] [Google Scholar]
- 57.Tamm, I., Y. Wang, E. Sausville, D. A. Scudiero, N. Vigna, T. Oltersdorf, and J. C. Reed. 1998. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 585315-5320. [PubMed] [Google Scholar]
- 58.Upton, S. J., M. Tilley, and D. B. Brillhart. 1995. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111241-250. [DOI] [PubMed] [Google Scholar]
- 60.Vaux, D. L., and J. Silke. 2005. IAPs, RINGs and ubiquitylation. Nat. Rev. Mol. Cell Biol. 6287-297. [DOI] [PubMed] [Google Scholar]
- 61.Waldele, K., K. Silbermann, G. Schneider, T. Ruckes, B. R. Cullen, and R. Grassmann. 2006. Requirement of the human T-cell leukemia virus (HTLV-1) tax-stimulated HIAP-1 gene for the survival of transformed lymphocytes. Blood 1074491-4499. [DOI] [PubMed] [Google Scholar]
- 62.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53155-187. [DOI] [PubMed] [Google Scholar]
- 63.Westphal, D., E. C. Ledgerwood, M. H. Hibma, S. B. Fleming, E. M. Whelan, and A. A. Mercer. 2007. A novel Bcl-2-like inhibitor of apoptosis is encoded by the parapoxvirus ORF virus. J. Virol. 817178-7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widmer, G., E. A. Corey, B. Stein, J. K. Griffiths, and S. Tzipori. 2000. Host cell apoptosis impairs Cryptosporidium parvum development in vitro. J. Parasitol. 86922-928. [DOI] [PubMed] [Google Scholar]
- 65.Widmer, G., Y. L. Yang, R. Bonilla, S. Tanriverdi, and K. M. Ciociola. 2006. Preferential infection of dividing cells by Cryptosporidium parvum. Parasitology 133131-138. [DOI] [PubMed] [Google Scholar]
- 66.Zou, P., J. Kawada, L. Pesnicak, and J. I. Cohen. 2007. Bortezomib induces apoptosis of Epstein-Barr virus (EBV)-transformed B cells and prolongs survival of mice inoculated with EBV-transformed B cells. J. Virol. 8110029-10036. [DOI] [PMC free article] [PubMed] [Google Scholar]