Abstract
Mycoplasma genitalium is a sexually transmitted bacterial pathogen that causes nongonococcal chlamydia-negative urethritis, mucopurulent cervicitis, endometritis, pelvic inflammatory disease, and tubal factor infertility in humans. However, pathogenic agents that induce inflammatory responses have not been identified in M. genitalium. In this study, we examined the involvement of Toll-like receptors (TLRs) in activation of the immune response by a lipoprotein from M. genitalium and their active component responsible for NF-κB activation. The Triton X-114 detergent phase of M. genitalium was found to induce NF-κB through TLR2. The active component of the Triton X-114 detergent phase was a lipoprotein precursor, MG149. The activation of NF-κB by MG149 was inhibited by a dominant negative (DN) construct of TLR1 but not by a DN construct of TLR6. These results indicate that the activation of NF-κB by MG149 is dependent on TLR1 and TLR2. A synthetic lipopeptide derived from MG149 containing three acyl chains also induced NF-κB through TLR1 and TLR2. Thus, the results show that MG149, a triacylated lipoprotein from M. genitalium, activates NF-κB through TLR1 and TLR2.
Mycoplasma genitalium is an important, emerging sexually transmitted bacterial pathogen that is capable of eliciting a wide range of reproductive tract disease syndromes (3, 19, 20, 28). M. genitalium has been identified as a causative agent of nongonococcal, chlamydia-negative urethritis (8, 50, 51) in men and of mucopurulent cervicitis, endometritis, pelvic inflammatory disease, and tubal factor infertility in women (6, 7, 24, 33, 34, 45). However, pathogenic agents that induce an inflammatory response, such as endotoxin and exotoxin, have not been identified in M. genitalium.
Recently, it has been reported that Toll-like receptors (TLRs) with a pattern recognition receptor function play a critical role in early innate recognition and in the inflammatory responses by the host defense against invading microbes (1, 21). Of 10 TLR family members reported, TLR2, TLR4, TLR5, and TLR9 have been implicated in the recognition of different bacterial components. Peptidoglycan, lipoarabinomannan, zymosan, and lipoproteins from various microorganisms are recognized by TLR2 (2, 4, 22, 26, 46, 47, 49, 52). On the other hand, lipopolysaccharide, bacterial flagellin, and bacterial DNA are recognized by TLR4, TLR5, and TLR9, respectively (12, 13, 16, 35). These TLR family members have been shown to activate NF-κB via interleukin-1R-associated signal molecules, including myeloid differentiation protein (MyD88), interleukin-1R-activated kinase, tumor necrosis factor receptor-associated factor 6, and NF-κB-inducing kinase (27).
In this study, we examined the involvement of TLRs in activation of the immune response by lipoproteins from M. genitalium and their active components responsible for NF-κB activation. MG149, a triacylated lipoprotein, was found to activate NF-κB through TLR1 and TLR2.
MATERIALS AND METHODS
Cells.
Cells of a human kidney cell line, 293T, were cultured in Dulbecco modified Eagle medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin. To exclude the possibility of mycoplasma infections in the cell cultures, the cell suspensions were inoculated onto pleuropneumonialike organism agar medium once a month. To check the contamination of mycoplasma, cells were also stained with 4′,6-diamidino-2-phenylindole (DAPI) once a week.
Synthesis of lipopeptide.
Lipopeptide was synthesized by Bio-Synthesis (Lewisville, TX).
TX-114 phase partitioning.
M. genitalium G37 was cultured in SP-4 medium to the beginning of the stationary phase and then pelleted by centrifugation for 10 min at 12,000 × g. M. genitalium cells were inactivated by incubation at 60°C for 30 min. Triton X-114 (TX-114) phase partitioning was performed as previously described by Feng and Lo (9, 10). Briefly, a mycoplasma pellet was suspended in Tris-buffered saline containing 1 mM EDTA (TBSE) and solubilized by adding TX-114 to a final concentration of 1%, and then it was incubated at 4°C for 1 h. The lysate was incubated at 37°C for 10 min for phase separation. After centrifugation at 10,000 × g for 20 min, the upper aqueous phase was removed and replaced by the same volume of TBSE. The phase separation procedure was repeated twice. The final TX-114 detergent phase was resuspended in TBSE to the original volume, and 2.5 volumes of methanol was added to precipitate membrane components and incubated at −20°C overnight. After centrifugation, the pellet was suspended in dimethyl sulfoxide (DMSO). The aqueous phase was washed twice by adding TX-114 to a final concentration of 2%. The TX-114-insoluble pellet was dissolved in DMSO. The protein concentration of the suspension was determined by using the Coomassie protein assay reagent (Pierce, Rockford, IL).
Expression vectors.
To prepare TLR1, TLR2, and TLR6 expression vectors (pFLAG-TLR1, pFLAG-TLR2, and pFLAG-TLR6), the coding regions of TLR1, TLR2, and TLR6 minus the NH2-terminal signal sequences were amplified by PCR from a cDNA of THP-1 cells and cloned into the expression vector pFLAG-CMV1 (Sigma, St. Louis, MO), in which a preprotrypsin leader precedes an NH2-terminal FLAG epitope. Dominant negative (DN) TLR1 and DN TLR6 expression vectors were constructed by subcloning TIR (Toll and interleukin-1 receptor) homology domain-deleted TLR1 and TLR6 fragments into pFLAG-CMV1 (pFLAG-dTLR1 and pFLAG-dTLR6). An NF-κB cis-reporting system containing pNF-kB-luc was purchased from Stratagene (La Jolla, CA).
Transfection and luciferase assay.
Transient transfection was performed by using FuGENE6 (Roche, Basel, Switzerland) according to the manufacturer's instructions. A total of 1 × 105 293T cells were transfected with 0.1 μg of pFLAG-TLR2, 0.01 μg of pNF-kB-luc, 0.01 μg of the pRL-TK internal control plasmid (Promega, Madison, WI), and 0.2 μg of a DN TLR-expressing plasmid in 24-well plates. After 48 h, transfected cells were stimulated with the TX-114 detergent phase or purified proteins. After a further 8 h of incubation, the cells were lysed, and the luciferase activity was assayed using a dual-luciferase reporter assay system (Promega). Both firefly and Renilla luciferase activities were monitored with a Lumat LB9507 luminometer (Berthold, Wildbad, Germany). Normalized reporter activity was determined by dividing the firefly luciferase value by the Renilla luciferase value. Relative induction was calculated by dividing the normalized reporter activity of the test samples by the normalized reporter activity of the unstimulated samples.
Reversed-phase high-pressure liquid chromatography (HPLC).
The TX-114 detergent phase was dissolved in 6 M guanidine hydrochloride, and 100 μg of the TX-114 detergent phase was applied to a μBondasphere C18 300A column (Waters, Milford, MA). Elution was performed with a linear 0 to 100% water-2-propanol gradient. The flow rate was 1.0 ml/min for up to 70 min. Each fraction was dried in vacuo using a centrifugal concentrator (Tomy, Tokyo, Japan) at room temperature and dissolved in 100 μl of DMSO.
Purification from an acrylamide gel.
One hundred micrograms of the TX-114 detergent phase was fractionated by reversed-phase HPLC. The fraction that was eluted by HPLC was separated by 10% glycine- or 15% Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. The gel was stained with an MS silver stain kit (Wako, Osaka, Japan). Ten major proteins were eluted by homogenization with a 1% SDS solution. The homogenized gel was removed by centrifugation at 12,000 × g at room temperature. Five volumes of acetone was added to the supernatant, which was followed by incubation overnight. Proteins were pelleted by centrifugation at 12,000 × g at 4°C and dissolved in DMSO. The protein concentration was determined by using a Coomassie brilliant blue (CBB) protein assay solution (Nacalai Tesque, Kyoto, Japan).
PMF.
Peptide mass fingerprinting (PMF) was carried out using the method of Yoshino et al. (54). Briefly, the CBB- or silver-stained bands were excised from the Tricine-SDS-PAGE gel and sliced into small strips. To remove CBB, the strips were incubated in 50% methanol and 5% acetic acid for 1 h and washed twice in water. The strips were dehydrated by incubation with 100% acetonitrile. To alkylate the protein, the strips were incubated at 60°C for 1 h with 10 mM dithiothreitol in 100 mM ammonium hydrogen carbonate, which was followed by treatment at room temperature for 30 min with 55 mM iodoacetamide (Nacalai Tesque) in 100 mM ammonium hydrogen carbonate. In-gel trypsin digestion was carried out by incubation with 10 μg/ml trypsin (Promega). The digested peptides were eluted by using 5% formic acid (Wako). The peptides were dried in vacuo and dissolved in saturated α-cyano-4-hydroxycinnamic acid (Nacalai Tesque) in 50% acetonitrile and 0.1% trifluoroacetic acid. The molecular weights of the peptides were determined with Autoflex (Bruker Daltnics, Bremen, Germany). The database was searched by using MASCOT (Science Matrix, Boston, MA).
Macrophage stimulation.
To induce peritoneal macrophages, 0.1 mg of OK432 (Chugai Pharmaceutical, Tokyo, Japan) was injected into the peritoneal cavity of C57BL mice. Two days later, peritoneal exudate cells (macrophages) were harvested and centrifuged. The cell pellets were suspended in serum-free medium optimized for macrophage culture (Gibco Invitrogen, Carlsbad, CA). The cells were allowed to adhere on 96-well culture plates for 2 h at 37°C in the presence of 5% CO2. Nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and the remaining adherent cells were stimulated with the TX-114 detergent phase or purified proteins for 6 h. The production of tumor necrosis factor alpha (TNF-α) was measured using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
Statistical analysis.
Results expressed as means and standard deviations were compared using a one-way analysis of variance. The differences between groups were compared by using multiple comparisons (Bonferroni t test). A P value of <0.01 was considered significant.
RESULTS
TLR2-dependent activation of NF-κB by the TX-114 detergent phase of M. genitalium.
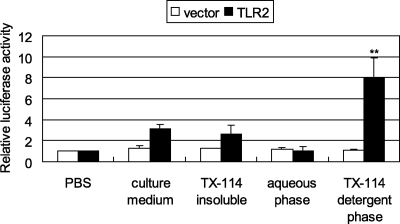
Mycoplasmas are wall-less bacteria that contain no lipopolysaccharide and peptidoglycan (ligands for TLR2 and TLR4, respectively) (53). M. genitalium also has no flagella (a ligand for TLR5) (11). Therefore, lipoproteins, which are ligands for TLR2, are possible candidates for an inflammation-inducing factor in M. genitalium. In fact, we previously demonstrated that lipoproteins derived from M. pneumoniae induce NF-κΒ through TLR2 (41, 44). To elucidate whether M. genitalium induces NF-κB through TLR2, we initially performed TX-114 phase partitioning of M. genitalium. 293T cells were transfected with both the TLR2 expression vector pFLAG-TLR2 and the reporter vector pNF-kB-luc, in which the luciferase reporter gene was fused to the NF-κB enhancer. When 293T cells transfected with pFLAG-TLR2 were stimulated with the TX-114 detergent phase of M. genitalium, the level of luciferase expression increased (Fig. 1). In contrast, upon stimulation with the mycoplasma culture medium, the TX-114-insoluble phase, and the aqueous phase, the levels of luciferase expression induced by these stimuli were as high as the unstimulated control level. When 293T cells were transfected with the empty vector pFLAG-CMV1, the level of luciferase expression was not increased (Fig. 1). The results suggest that the TX-114 detergent phase of M. genitalium induces NF-κB through TLR2.
FIG. 1.
TX-114 phase partitioning. 293T cells were transfected with 0.01 μg/ml of pFLAG-TLR2, 0.01 μg/ml of pNF-kB-luc, and 0.01 μg/ml of pRL-TK. The cells were stimulated with 1.0 μg/ml of mycoplasma culture medium, the insoluble fraction, the aqueous phase, and the TX-114 detergent phase of M. genitalium. The values are the means and standard deviations of three independent assays. **, P < 0.01 compared with PBS.
Purification of NF-κB-activating components of the TX-114 detergent phase.
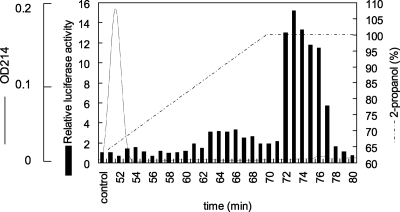
To examine the active components of the TX-114 detergent phase of M. genitalium, the TX-114 detergent phase was fractionated by reversed-phase HPLC with a linear 2-propanol gradient. To measure the ability of fractions to induce NF-κB, each fraction was added to 293T cells transfected with pFLAG-TLR2 and pNF-kB-luc. As shown in Fig. 2, the active components of the TX-114 detergent phase were eluted with 100% 2-propanol at 72 min. Below, the fraction containing the active components is referred to as f72.
FIG. 2.
Fractionation of the TX-114 detergent phase by reversed-phase HPLC. The TX-114 detergent phase was dissolved in 6 M guanidine hydrochloride, and 100 μg of the TX-114 detergent phase was separated by reversed-phase HPLC. Elution was performed with a linear 0 to 90% water-2-propanol gradient. 293T cells transfected with 0.01 μg/ml of pFLAG-TLR2 and 0.01 μg/ml of pNF-kB-luc were stimulated with 0.2% of each fraction. OD214, optical density at 214 nm.
Analysis of active components of the TX-114 detergent phase.
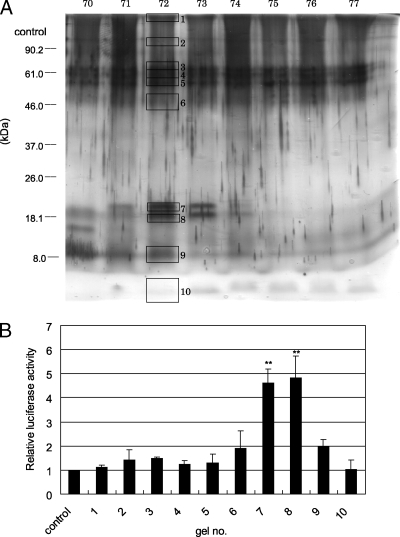
To determine the molecular weights of the active components of the TX-114 detergent phase, f72 was separated by glycine-SDS-PAGE, and the gel was cut into 10 pieces, which were numbered (Fig. 3A). Proteins extracted from each gel piece as described in Materials and Methods were incubated with 293T cells transfected with pFLAG-TLR2 and pNF-kB-luc. When the cells were incubated with the proteins extracted from each piece of the gel, the proteins with molecular masses around 18 kDa (P18) and 20 kDa (P20) were found to possess higher NF-κB-inducing activity than the other proteins (Fig. 3B).
FIG. 3.
Molecular masses of the active components in f72. (A) The fraction that eluted at 72 min was separated by 10% glycine-SDS-PAGE under reducing conditions. Ten major protein bands were obtained in the gel and numbered. The protein was extracted as described in Materials and Methods. (B) 293T cells transfected with 0.01 μg/ml of pNF-kB-luc and 0.01 μg/ml of pFLAG-TLR2 were stimulated with eluted proteins at a final concentration of 100 ng/ml. Luciferase activity was measured as described in the text. The values are the means and standard deviations of three independent assays. **, P < 0.01 compared with the control.
Identification of NF-κB-activating components.
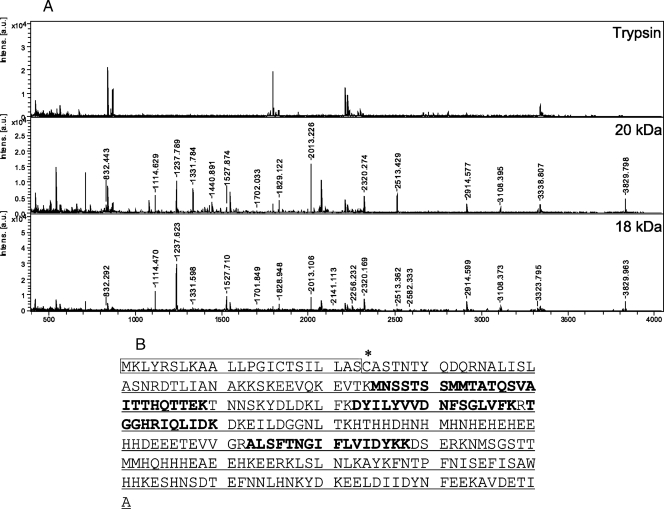
We next tried to identify P18 and P20 in f72. To this end, PMF was carried out. The P18 and P20 proteins showed similar PMF patterns (Fig. 4A). As a result of a search of the Swiss-Prot database using MASCOT, these proteins were matched with MG149 of M. genitalium (Fig. 4B). The protein score for MG149 was 48; scores greater than 47 are considered to be significant (P < 0.05). MG149 was predicted to have molecular masses of 32,426.9 and 29,981.9 Da for the native and processed forms, respectively. MG149 contains a signal peptide near the N terminus, followed by a predicted lipid-binding cysteine, and its N terminus is hydrophobic (11). These findings strongly suggest that MG149 is a lipoprotein.
FIG. 4.
PMF. (A) Comparison of acquired PMF patterns of the 18-kDa protein, the 20-kDa protein, and the trypsin control. a.u., arbitrary units. (B) Amino acid sequence of MG149. The box indicates the signal peptide, the asterisk indicates the lipid-binding cysteine, underlining indicates the mature protein, and bold type indicates peptides detected by mass spectrometry.
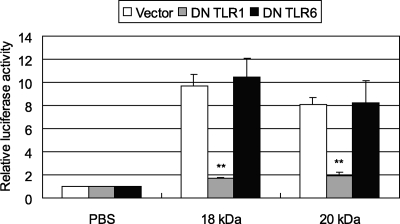
TLR6-independent NF-κB activation by P18 and P20.
Diacylated lipopeptides, including macrophage-activating lipopeptide 2, have been reported to be recognized by mouse TLR2 cooperatively with TLR6 (48). To investigate whether MG149 (P18 and P20) is also recognized by both TLR2 and TLR6 for NF-κB activation, we transfected a plasmid encoding DN TLR6 (pFLAG-dTLR6) into 293T cells with both pFLAG-TLR2 and pNF-kB-luc. Initially, the levels of expression of TLR1 and TLR6 on 293T cells were analyzed by reverse transcription-PCR. Both the TLR1 and TLR6 genes were found to be transcribed (data not shown) in 293T cells. The effect of DN TLR6 on the expression of TLR2 was also analyzed by flow cytometry. The level of TLR2 expression was almost constant irrespective of the expression of DN TLR6, as well as DN TLR1 (data not shown). When the cells transfected with the control vector were stimulated with P18 and P20, the levels of NF-κB activation were increased (Fig. 5). When the cells were transfected with DN TLR6, the levels of NF-κB activation were not strikingly decreased. These results suggest that NF-κB activation by P18 and P20 is independent of TLR6.
FIG. 5.
Cooperation of TLR1, TLR6, and TLR2 for NF-κB induction by MG149. 293T cells were transfected with 0.2 μg of pFLAG-dTLR6 or pFLAG-dTLR1, 0.01 μg/ml of pFLAG-TLR2, 0.01 μg/ml of pNF-kB-luc, and 0.01 μg/ml of pRL-TK. The cells were stimulated with 100 ng/ml of the 18- and 20-kDa proteins. The values are the means and standard deviations of three independent assays. **, P < 0.01 compared with the vector.
TLR1-dependent NF-κB activation by P18 and P20.
We next determined whether P18 and P20 are recognized by both TLR1 and TLR2 for NF-κB activation. We transfected a plasmid encoding DN TLR1 (pFLAG-dTLR1) into 293T cells with both pFLAG-TLR2 and pNF-kB-luc. When the cells were transfected with the control vector, stimulation with P18 and P20 increased the levels of NF-κB induction. In contrast, upon transfection with DN TLR1, stimulation failed to augment NF-κB induction (Fig. 5). These results indicate that the cooperation of TLR1 is required for NF-κB activation by P18 and P20. Triacylated bacterial lipopeptides, such as Pam3CSK4, have been reported to be recognized by murine TLR1 in association with TLR2 (49). Taken together, these results suggest that P18 and P20 are triacylated lipoproteins.
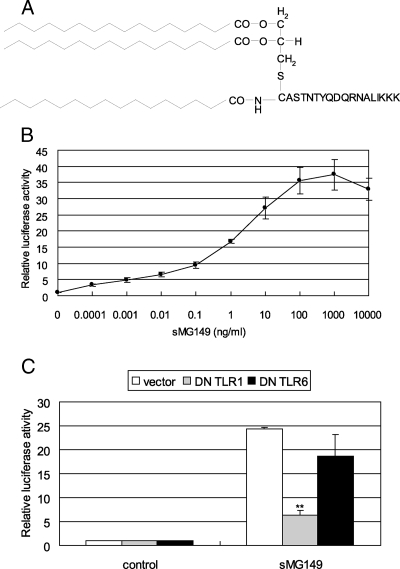
NF-κB-inducing activity of synthetic lipopeptide.
To confirm that MG149 has NF-κB-inducing activity, a lipopeptide with 15 N-terminal amino acid residues of MG149 was synthesized (sMG149). Three acyl chains of palmitic acid were coupled to the N-terminal cysteine residue, since the acyl chains of FoF1-ATPase derived from M. pneumoniae, macrophage-activating lipopeptide 2 from M. fermentans, and FSL-1 from Mycoplasma salivarium have been shown to be palmitic acids (29, 40, 41). In fact, an initially synthesized peptide was completely insoluble in any solvent. To solubilize the peptide, three lysine residues were attached to the C terminus of the peptide (Fig. 6A). To clarify that the synthetic lipopeptide has NF-κB-inducing activity, 293T cells were transfected with pFLAG-TLR2 and pNF-kB-luc and then stimulated with various concentrations of the lipopeptide. When the cells were stimulated with sMG149, the levels of NF-κB activation increased in a dose-dependent manner (Fig. 6B). These results show that the synthetic lipopeptide derived from MG149 also has NF-κB-inducing activity.
FIG. 6.
Synthetic lipopeptide sMG149. (A) Structure of synthetic lipopeptide sMG149. S-(2,3-Dihydroxypropyl)-cysteinyl residues were acylated with a third amide-linked palmitic acid. (B) Induction of NF-κB by sMG149. 293T cells were transfected with 0.01 μg/ml pFLAG-TLR2, 0.01 μg/ml of pNF-kB-luc, and 0.01 μg/ml of pRL-TK. The cells were stimulated with the indicated concentrations of sMG149. The values are the means ± standard deviations of three assays. (C) Cooperation of TLR1 and TLR2 for NF-κB induction by sMG149. 293T cells were transfected with 0.2 μg/ml of pFLAG-dTLR6 or pFLAG-dTLR1, 0.01 μg/ml of pFLAG-TLR2, 0.01 μg/ml of pNF-kB-luc, and 0.01 μg/ml of pRL-TK. The cells were stimulated with 10 ng/ml of sMG149. The values are the means and standard deviations of three independent assays. **, P < 0.01 compared with the vector.
TLR1-dependent NF-κB activation by synthetic lipopeptide.
Next, we addressed whether the synthetic lipopeptide induces NF-κB activation through TLR1 or TLR6. 293T cells were transfected with pFLAG-TLR2, pNF-kB-luc, pFLAG-dTLR1, or pFLAG-dTLR6, followed by incubation with the synthetic lipopeptide sMG149. When the cells were stimulated with sMG149, NF-κB activation was decreased by transfection with DN TLR1 but not by transfection with DN TLR6 (Fig. 6C). These results indicate that the synthetic lipopeptide containing three acyl chains activates NF-κB through TLR1- and TLR2-dependent but TLR6-independent pathways.
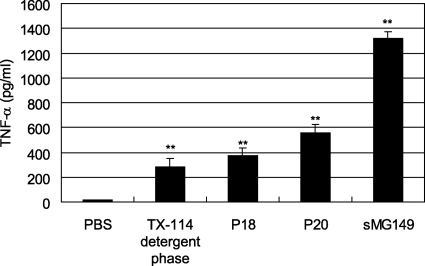
Induction of TNF-α by the TX-114 detergent phase, P18, P20, and sMG149.
To investigate whether the components derived from M. genitalium actually induce inflammatory cytokines in macrophages, the levels of TNF-α production by mouse peritoneal macrophages stimulated with the TX-114 detergent phase, P18, P20, or sMG149 were determined by an ELISA. Peritoneal macrophages treated with PBS did not produce TNF-α. On the other hand, the levels of TNF-α production were increased by the TX-114 detergent phase, P18, P20, and sMG149 (Fig. 7). These results suggest that the TX-114 detergent phase, P18, and P20 possess activity that induces inflammatory cytokines, including TNF-α.
FIG. 7.
TNF-α induction by the TX-114 detergent phase, P18, P20, and sMG149. A total of 3 × 105 peritoneal macrophages/well obtained from C57BL mice were stimulated with 1.0 μg/ml of the TX-114 detergent phase, 100 ng/ml of P18, 100 ng/ml of P20, or 100 ng/ml of sMG149 in a 96-well plate for 6 h. The levels of TNF-α in the culture supernatants were determined using ELISA kits. The values are the means and standard deviations of three independent assays. **, P < 0.01 compared with PBS.
DISCUSSION
In this study, we demonstrated that the TX-114-soluble phase of M. genitalium activates NF-κB. The active components were found to be 18- and 20-kDa proteins (P18 and P20, respectively). The PMF results showed that both of these proteins are the MG149 lipoprotein of M. genitalium. The predicted molecular masses of MG149 were 32,426.9 and 29,981.9 Da for the native and processed forms, respectively. These results indicate that the purified P18 and P20 proteins may be the differentially processed forms of MG149. However, we could not rule out the possibility that the different migration patterns of the same protein were due to the considerable manipulation that was used, such as denaturation of the protein. Both P18 and P20 induced NF-κB through TLR1- and TLR2-dependent but TLR6-independent pathways. The experiments with a synthetic lipopeptide (part of MG149) containing three palmitic acids showed that the activation of NF-κB by the synthetic lipopeptide was TLR1 and TLR2 dependent. A previous report showed that triacylated bacterial lipopeptides, including Pam3CSK4, are recognized by TLR1 in association with TLR2 (49). These findings led us to believe that MG149 separated from M. genitalium is a triacylated lipoprotein.
Curiously, it remains unclear whether mycoplasmas have triacylated lipoproteins. In triacylated lipoproteins, three fatty acids are bound to the N-terminal cysteine residue; two of the fatty acids are in a diacylglyceride that is linked through a thioether bond to the sulfhydryl group, and one is bound to the amine group (N acylation). Chemically identified lipoproteins from M. fermentans, M. hyorhinis, M. salivarium, and M. gallisepticum are not N acylated (17, 29, 30, 40). The N-acyltransferase gene responsible for N acylation has not been detected in the M. pneumoniae, M. genitalium, and M. penetrans genomes (11, 14, 39) either. However, a study of the ratio of N-amide and O-ester bonds in M. gallisepticum and M. mycoides suggested that such mycoplasmas possess diacylated and triacylated lipoproteins (18). Furthermore, the resistance to Edman degradation of proteins from M. mycoides also indicates the presence of N acylation (5). We previously showed that lipoproteins from M. fermentans enhance human immunodeficiency virus replication through TLR2 and TLR6 but lipoproteins from M. penetrans facilitate human immunodeficiency virus replication through TLR1, TLR2, and TLR6 (42). We also reported that the lipoproteins isolated from M. pneumoniae, as well as the synthetic lipopeptides containing three acyl chains, induce NF-κB through TLR1- and TLR2-dependent but TLR6-independent pathways (44). Taken together, these findings support the idea that triacylated lipoproteins are present in mycoplasma species.
We previously purified MPN162 as NF-κB-activating lipoprotein 2 (N-ALP2) from M. pneumoniae (44). Interestingly, as a result of a BLAST search, MG149 was found to be a homologue of N-ALP2. N-ALP2 was thought to be a triacylated lipoprotein that induces NF-κB through TLR1 and TLR2. N-ALP2 showed inflammation-inducing activity in the lungs of mice (43). These results suggest that MG149 also induces inflammatory responses in vivo. Furthermore, the diacylated lipoprotein MPN602 (known as subunit b of FoF1-type ATPase [FoF1-ATPase]) of M. pneumoniae was purified as an NF-κB-inducing protein. The FoF1-ATPase induced NF-κB through TLR1, TLR2, and TLR6 (41). M. genitalium has a homologue of M. pneumoniae FoF1-ATPase, but the NF-κB-inducing activity of the homologue was not detected in this study. The differences in the amino acid sequences might affect the NF-κB-inducing activity. Although M. genitalium is phylogenetically close to M. pneumoniae (25), these mycoplasmas are known to induce inflammatory responses in different organs. The distinct adherence-mediating epitopes in the adhesin of M. pneumoniae and M. genitalium (P1 and MgPa, respectively) are considered to be important for organ- and tissue-specific infection (32). Interestingly, we found that in the lungs of mice, the ability of diacylated FoF1-ATPase to induce an inflammatory response is much greater than that of triacylated N-ALP2, indicating that the levels of TLR expression are different in various tissues and cellular subsets (15, 31). These results suggest that the acylation of lipoproteins or the levels of expression of TLRs in organs contribute to the organ-specific inflammatory response induced by mycoplasmas.
M. genitalium is known to cause nongonococcal, chlamydia-negative urethritis (8, 50, 51) in men and mucopurulent cervicitis, endometritis, pelvic inflammatory disease, and tubal factor infertility in women (6, 7, 24, 33, 34, 45). However, apparent virulence factors associated with the pathogenesis of M. genitalium have not been identified. In this study, we identified the MG149 lipoprotein that induces an immune response. To our knowledge, this is the first report of a virulence factor of M. genitalium that can induce an inflammatory response.
Considering the interaction between TLRs and lipoproteins such as MG149, inhibition of the interaction may result in a reduction in the inflammatory response induced by M. genitalium. Accordingly, our data suggest that such lipoproteins are potential therapeutic targets. For example, lipoproteins and their derivatives might be suitable candidates as antigens for vaccination, causing the induction of specific antibodies capable of inhibiting TLR signaling. Alternatively, the derivatives may work as antagonists inhibiting TLR signaling. Furthermore, several reports have indicated that lipoproteins possess immune modulation and adjuvant abilities (23, 36-38). Thus, lipoproteins that are capable of interacting with TLRs and are derived from M. genitalium are potential molecules for use in the development of new weapons against M. genitalium infection.
Acknowledgments
We thank T. Ishikawa (Fukuoka Industrial Technology Center) for his technical support regarding PMF.
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285736-739. [DOI] [PubMed] [Google Scholar]
- 3.Bjornelius, E., P. Lidbrink, and J. S. Jensen. 2000. Mycoplasma genitalium in non-gonococcal urethritis—a study in Swedish male STD patients. Int. J. STD AIDS 11292-296. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285732-736. [DOI] [PubMed] [Google Scholar]
- 5.Chambaud, I., H. Wroblewski, and A. Blanchard. 1999. Interactions between mycoplasma lipoproteins and the host immune system. Trends Microbiol. 7493-499. [DOI] [PubMed] [Google Scholar]
- 6.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 161866-1874. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, C. R., L. E. Manhart, E. A. Bukusi, S. Astete, R. C. Brunham, K. K. Holmes, S. K. Sinei, J. J. Bwayo, and P. A. Totten. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359765-766. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi, T., and S. Maeda. 2002. Mycoplasma genitalium: another important pathogen of nongonococcal urethritis. J. Urol. 1671210-1217. [DOI] [PubMed] [Google Scholar]
- 9.Feng, S. H., and S. C. Lo. 1994. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect. Immun. 623916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, S. H., and S. C. Lo. 1999. Lipid extract of Mycoplasma penetrans proteinase K-digested lipid-associated membrane proteins rapidly activates NF-κB and activator protein 1. Infect. Immun. 672951-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270397-403. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 14.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 244420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 1684531-4537. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 17.Jan, G., C. Brenner, and H. Wroblewski. 1996. Purification of Mycoplasma gallisepticum membrane proteins p52, p67 (pMGA), and p77 by high-performance liquid chromatography. Protein Expr. Purif. 7160-166. [DOI] [PubMed] [Google Scholar]
- 18.Jan, G., C. Fontenelle, F. Verrier, M. Le Henaff, and H. Wroblewski. 1996. Selective acylation of plasma membrane proteins of Mycoplasma mycoides subsp. mycoides SC, the contagious bovine pleuropneumonia agent. Curr. Microbiol. 3238-42. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 181-11. [DOI] [PubMed] [Google Scholar]
- 20.Jensen, J. S., E. Bjornelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 1113-18. [DOI] [PubMed] [Google Scholar]
- 22.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 27433419-33425. [DOI] [PubMed] [Google Scholar]
- 23.Link, C., R. Gavioli, T. Ebensen, A. Canella, E. Reinhard, and C. A. Guzman. 2004. The Toll-like receptor ligand MALP-2 stimulates dendritic cell maturation and modulates proteasome composition and activity. Eur. J. Immunol. 34899-907. [DOI] [PubMed] [Google Scholar]
- 24.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187650-657. [DOI] [PubMed] [Google Scholar]
- 25.Maniloff, J. 1992. Phylogeny of Mycoplasma, p. 549-559. In J. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, DC.
- 26.Means, T. K., E. Lien, A. Yoshimura, S. Wang, D. T. Golenbock, and M. J. Fenton. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 1636748-6755. [PubMed] [Google Scholar]
- 27.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2253-258. [DOI] [PubMed] [Google Scholar]
- 28.Mena, L., X. Wang, T. F. Mroczkowski, and D. H. Martin. 2002. Mycoplasma genitalium infections in asymptomatic men and men with urethritis attending a sexually transmitted diseases clinic in New Orleans. Clin. Infect. Dis. 351167-1173. [DOI] [PubMed] [Google Scholar]
- 29.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 1851951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1998. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 664804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura, M., and S. Naito. 2005. Tissue-specific mRNA expression profiles of human Toll-like receptors and related genes. Biol. Pharm. Bull. 28886-892. [DOI] [PubMed] [Google Scholar]
- 32.Opitz, O., and E. Jacobs. 1992. Adherence epitopes of Mycoplasma genitalium adhesin. J. Gen. Microbiol. 1381785-1790. [DOI] [PubMed] [Google Scholar]
- 33.Palmer, H. M., C. B. Gilroy, E. J. Claydon, and D. Taylor-Robinson. 1991. Detection of Mycoplasma genitalium in the genitourinary tract of women by the polymerase chain reaction. Int. J. STD AIDS 2261-263. [DOI] [PubMed] [Google Scholar]
- 34.Pepin, J., A. C. Labbe, N. Khonde, S. Deslandes, M. Alary, A. Dzokoto, C. Asamoah-Adu, H. Meda, and E. Frost. 2005. Mycoplasma genitalium: an organism commonly associated with cervicitis among west African sex workers. Sex Transm. Infect. 8167-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 36.Rharbaoui, F., B. Drabner, S. Borsutzky, U. Winckler, M. Morr, B. Ensoli, P. F. Muhlradt, and C. A. Guzman. 2002. The Mycoplasma-derived lipopeptide MALP-2 is a potent mucosal adjuvant. Eur. J. Immunol. 322857-2865. [DOI] [PubMed] [Google Scholar]
- 37.Rharbaoui, F., A. Westendorf, C. Link, S. Felk, J. Buer, M. Gunzer, and C. A. Guzman. 2004. The Mycoplasma-derived macrophage-activating 2-kilodalton lipopeptide triggers global immune activation on nasal mucosa-associated lymphoid tissues. Infect. Immun. 726978-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero, F., E. Moreno, A. Ruiz-Bravo, and M. Jimenez-Valera. 2004. In vivo immunomodulation by Mycoplasma fermentans membrane lipoprotein. Curr. Microbiol. 48237-239. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki, Y., J. Ishikawa, A. Yamashita, K. Oshima, T. Kenri, K. Furuya, C. Yoshino, A. Horino, T. Shiba, T. Sasaki, and M. Hattori. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 305293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata, K., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 1656538-6544. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu, T., Y. Kida, and K. Kuwano. 2005. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J. Immunol. 1754641-4646. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu, T., Y. Kida, and K. Kuwano. 2004. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology 113121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu, T., Y. Kida, and K. Kuwano. 2008. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect. Immun. 76270-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu, T., Y. Kida, and K. Kuwano. 2007. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-κB through Toll-like receptors 1 and 2. Immunology 121473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simms, I., K. Eastick, H. Mallinson, K. Thomas, R. Gokhale, P. Hay, A. Herring, and P. A. Rogers. 2003. Associations between Mycoplasma genitalium, Chlamydia trachomatis and pelvic inflammatory disease. J. Clin. Pathol. 56616-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi, O., A. Kaufmann, K. Grote, T. Kawai, K. Hoshino, M. Morr, P. F. Muhlradt, and S. Akira. 2000. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 164554-557. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13933-940. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 16910-14. [DOI] [PubMed] [Google Scholar]
- 50.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183269-276. [DOI] [PubMed] [Google Scholar]
- 51.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i1288-1291. [DOI] [PubMed] [Google Scholar]
- 52.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401811-815. [DOI] [PubMed] [Google Scholar]
- 53.Weisburg, W. G., J. G. Tully, D. L. Rose, J. P. Petzel, H. Oyaizu, D. Yang, L. Mandelco, J. Sechrest, T. G. Lawrence, and J. Van Etten. 1989. A phylogenetic analysis of the mycoplasmas: basis for their classification. J. Bacteriol. 1716455-6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshino, K., N. Oshiro, C. Tokunaga, and K. Yonezawa. 2004. Mass spectrometry-based protein identification by correlation with sequence database. J. Mass Spectrom. Soc. Jpn. 52106-129. [Google Scholar]