Abstract
We evaluated the possibility of using Yersinia pseudotuberculosis as a live vaccine against plague because it shares high genetic identity with Y. pestis while being much less virulent, genetically much more stable, and deliverable orally. A total of 41 Y. pseudotuberculosis strains were screened by PCR for the absence of the high pathogenicity island, the superantigens YPM, and the type IV pilus and the presence of the pYV virulence plasmid. One strain (IP32680) fulfilled these criteria. This strain was avirulent in mice upon intragastric or subcutaneous inoculation and persisted for 2 months in the mouse intestine without clinical signs of disease. IP32680 reached the mesenteric lymph nodes, spleen, and liver without causing major histological lesions and was cleared after 13 days. The antibodies produced in vaccinated animals recognized both Y. pseudotuberculosis and Y. pestis antigens efficiently. After a subcutaneous challenge with Y. pestis CO92, bacteria were found in low amounts in the organs and rarely in the blood of vaccinated animals. One oral IP32680 inoculation protected 75% of the mice, and two inoculations induced much higher antibody titers and protected 88% of the mice. Our results thus validate the concept that an attenuated Y. pseudotuberculosis strain can be an efficient, inexpensive, safe, and easy-to-produce live vaccine for oral immunization against bubonic plague.
Yersinia pestis, the etiologic agent of bubonic and pneumonic plague, is a gram-negative bacterium with an extraordinary pathogenicity. Transmitted by infected fleas, the bacteria cause a hyperplasia of the draining lymph node (bubo), followed by septicemia and the patient's death within 1 week if an effective antibiotherapy is not administered on time. Although the bubonic form of the disease is by far the most common in the world (45), a primary bubonic infection sometimes develop into a secondary pneumonic infection highly contagious from human to human via the generation of aerosols. This form of the disease is almost systematically fatal within 3 days, and there is a concern that Y. pestis could be used this way as a weapon in bioterrorism (20). Additionally and of great public health concern was the recent identification of a Y. pestis strain naturally resistant to eight antibiotics, including those recommended for plague treatment and prophylaxis (16). The widespread diffusion of the transmissible plasmid conferring this resistance suggests that other resistant strains are very likely to appear (43). Against such multidrug resistance in Y. pestis, one of the only alternatives would be mass vaccination, but no safe and efficient vaccine against plague is currently available.
The live attenuated Y. pestis strain EV76 efficiently protected against bubonic plague and induced high titers of antibodies but did not confer long-lasting immunity and induced mild to severe side reactions (27). Furthermore, the immunogenicity and virulence of the EV76 vaccine preparations used in different countries were found to be highly variable, most likely because of the genetic drift of the bacteria used (27, 51). Indeed, a high genomic plasticity is observed in Y. pestis (5, 30) and results from frequent chromosomal rearrangements between the numerous copies of insertion sequences (IS) present in its genome. Killed whole-cell Y. pestis vaccines has been produced previously in the United States (USP) and in Australia (CSL). These vaccines are also known to be reactogenic in humans and induce a shorter and less effective protection than EV76 (27). The USP killed vaccine was recently discontinued in the United States.
A recent effort has been made to develop new recombinant subunit vaccines against human plague. Most of these subunit vaccines use Y. pestis-specific capsular antigen F1 and/or the low calcium response antigen V (LcrV) that is present in the three pathogenic Yersinia species. Such vaccines were found to efficiently protect mice against bubonic and pneumonic plague and are well tolerated in humans (3, 4, 18, 23, 37, 41, 47, 48). However, vaccines composed of a limited number of antigens may not be able to protect against F1-negative strains (49) or strains harboring LcrV variants (5). The technical expertise required for the production of recombinant vaccines is also an obstacle for developing countries, and injectable vaccines always raise the problem of contamination via used syringes.
Live vaccines offer several advantages over recombinant vaccines. Their high antigenic complexity guarantees a response against a broad range of antigenic targets. In live vaccines, antigens are present in their native forms with normal glycosylations, they are produced de novo as long as the bacteria persist, which provides a prolonged stimulation of the immune system, and there is no need for an adjuvant since bacterial antigens (lipopolysaccharide [LPS] and other pathogen-associated signatures) naturally stimulate the innate immune system. They generally induce both antibody production and a cell-mediated response, and once developed and validated, these vaccines can be produced locally, allowing more economically feasible mass production.
Yersinia pseudotuberculosis is the recent ancestor of Y. pestis (1) but is much less virulent and usually causes enteric diseases that are rarely fatal. The two species are very close with more than 95% genetic identity and virulence plasmids that share a conserved colinear backbone (8). The genome of Y. pseudotuberculosis is, however, much more stable than that of Y. pestis because it contains fewer IS copies (5, 8, 51). We report that oral inoculation with a Y. pseudotuberculosis strain, selected for its very low virulence, induces an efficient immunity against bubonic plague without causing adverse reactions. This demonstrates that a live attenuated Y. pseudotuberculosis can be a promising vaccine against bubonic plague.
MATERIALS AND METHODS
Yersinia strains and culture media.
The Y. pseudotuberculosis strains used in the present study are listed in Table 1. The fully virulent Y. pestis strain CO92 whose genome has been sequenced (30) was used for animal challenge, and the attenuated strain EV76 (27) was used as a control vaccine. Bacteria were usually grown at 28°C on Luria-Bertani agar plates supplemented with 0.2% hemin (LBH) for 48 h before use. A selective medium containing Irgasan (0.1 μg/ml; LBHI) was defined to quantify yersiniae in ex vivo samples. For inoculation in mice, bacteria grown at 28°C for 48 h on LBH plates were collected and resuspended in sterile saline for immediate use. Bacterial concentrations were evaluated by spectrometry at 600 nm and confirmed by CFU counts on LBH plates.
TABLE 1.
Screening by PCR of serotype II Y. pseudotuberculosis strains evaluated for use as a vaccine for the presence of virulence determinants
| Strain | Origin | pYV
|
Type IV pilus
|
HPI, int | Superantigen
|
|||
|---|---|---|---|---|---|---|---|---|
| yopK | yopM | pilN | pilR | ypmAC | ypmB | |||
| IP32554 | Human blood | - | - | - | - | - | - | - |
| IP32555 | Human blood | - | - | + | + | - | - | - |
| IP32576 | Human blood | - | - | - | - | - | - | - |
| IP32584 | Pig feces | - | - | - | - | - | - | - |
| IP32585 | Antelope lymph node | - | - | - | - | - | - | - |
| IP32589 | Human lymph node | - | - | - | - | - | - | - |
| IP32596 | Rabbit | - | - | + | + | - | - | - |
| IP32598 | Human blood | - | - | - | - | - | - | - |
| IP32621 | Not determined | + | + | - | - | - | - | - |
| IP32628 | Veterinary | - | - | + | + | - | - | - |
| IP32642 | Bird feces | + | + | + | + | - | - | - |
| IP32643 | Parrot | - | - | - | - | - | - | - |
| IP32661 | Veterinary | - | - | - | - | - | - | - |
| IP32672 | Hare | + | + | + | + | - | - | - |
| IP32678 | Hare | - | - | - | - | - | - | - |
| IP32679 | Hare | - | - | - | - | - | - | - |
| IP32680 | Hare | + | + | - | - | - | - | - |
| IP32681 | Hare | - | - | + | + | - | - | - |
| IP32692 | Hare | + | + | + | + | - | - | - |
| IP32721 | Hare | + | + | - | - | - | - | - |
| IP32828 | Human feces | + | + | + | + | - | - | - |
| IP32860 | Human blood | + | + | - | - | - | - | - |
| IP32870 | Hare liver | + | + | + | + | - | - | - |
| IP32873 | Hare liver | + | + | + | + | - | - | - |
| IP32874 | Hare liver | + | + | + | + | - | - | - |
| IP32876 | Hare blood | + | + | + | + | - | - | - |
| IP32892 | Monkey | - | - | - | - | - | - | - |
| IP32896 | Human | + | + | - | - | - | - | - |
| IP32908 | Bird liver | + | + | - | - | - | - | - |
| IP32913 | Monkey lung | - | - | + | + | - | - | - |
| IP32926 | Hare spleen | + | + | + | + | - | - | - |
| IP32934 | Hare liver | + | + | + | + | - | - | - |
| IP32969 | Monkey liver | - | - | - | - | - | - | - |
| IP33006 | Human blood | - | - | - | - | - | - | - |
| IP33047 | Hare | - | - | - | - | - | - | - |
| IP33054 | Human blood | - | - | - | - | - | - | - |
| IP33067 | Human lymph node | + | + | + | + | - | - | - |
| IP33098 | Human feces | + | + | - | - | - | - | - |
| IP33286 | Human blood | + | + | + | + | - | - | - |
| IP33293 | Human feces | - | - | - | - | - | - | - |
| IP33306 | Hare | - | - | - | - | - | - | - |
PCR.
DNA was obtained by thermolysis of bacteria in a solution containing 50 mM NaOH and 0.25% sodium dodecyl sulfate. The primers (obtained from Proligo France) amplified the int gene of the high pathogenicity island (HPI) (24), the yopM (33), and yopK (forward, 5′-CTGCTCATTTACTGAGAACG-3′; reverse, 5′-CTATAAGTAGAGAGTTTTTCGG-3′), genes of the pYV virulence plasmid, the pilR and pilN genes of the type IV pilus locus (10), and the ypmAC or ypmB genes coding for YPM superantigens (15). The housekeeping gene ureB encoding an urease subunit was used as an internal positive control for the PCRs. PCR amplification reaction mixtures contained 1 U of AmpliTaq polymerase (Applied Biosystems), 10 mM deoxynucleoside triphosphates, and 10 μM concentrations of each primer. A PCR program working for all gene fragments was defined, involving one step at 95°C for 5 min, followed by 30 cycles of amplification consisting in three steps each: (i) 94°C for 30 s, (ii) 55°C for 30 s, and (iii) 72°C for 90 s. Strains used as positive controls for the various PCR primers were as follows: IP32953 for int, yopM, and yopK; IP33053 for pilN and pilR; and IP32887 and IP31411 for ypmAC. These strains were taken from the National Reference Center strain collection except strain 1093 positive for ypmB, kindly provided by M. Simonet, Institut Pasteur of Lille. The amplification reactions were done in a PTC-100 thermocycler (Bio-Rad).
In vivo analyses.
Female OF1 mice, 6 to 8 weeks old, were purchased from Charles River (l'Arbresle, France). All animal care and experimentations were conducted according to the guidelines of the Institut Pasteur. Mouse infections with virulent bacteria were performed in a BSL3 animal facility. Inoculation of bacteria via the intragastric (i.g.) route (200 μl in saline) was done with a curved feeding needle, and subcutaneous (s.c.) inoculations (100 μl) were performed in the abdominal skin. Mice were not fasted before intragastric inoculation. Mortality, body weight, behavior, and fur appearance were recorded daily for 3 weeks.
To follow the kinetics of bacterial dissemination in vivo, groups of three mice per time point were humanely euthanized after one or two i.g. inoculations. Various organs (Peyer's patches, mesenteric lymph nodes, livers, and spleens), as well as feces (two fecal pellets taken in the large intestine), were collected aseptically and homogenized in sterile phosphate-buffered saline (PBS), using 3-mm glass beads and an electric mixer mill (Retsch, Germany). Serial dilutions of the homogenates were plated on LBH plates for counting.
For histological analysis, organs were fixed with buffered formaldehyde for 48 h, embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin-eosin or Giemsa stain. The severity of lesions caused to tissues by Y. pseudotuberculosis strains were observed on blinded histological sections and quantified by using a newly defined lesions score ranging from 0 to 10: 0, no inflammation; 1, minimal inflammation (congestion, microhemorrhages); 2, fewer than 2 abscesses in a given organ; 3, from 2 to 5 microabscesses; 4, from 5 to 10 microabscesses; 5, from 10 to 20 microabscesses; 6, from 20 to 50 microabscesses; 7, confluent intratissular necrosis zone; 8, organ destruction below 50%; 9, organ destruction from 50 to 80%; and 10, total destruction and very abundant polymorphonuclear cells. At least 10 fields per slide were read on two slides per mouse.
Vaccination using the IP32680 strain consisted of one i.g. inoculation with 2 × 109 CFU in saline or two inoculations with a 30-day interval. Vaccination using the Y. pestis EV76 strain consisted of a single s.c. injection of 107 CFU as described by Flashner et al. (14).
To obtain immune sera, mice were anesthetized, and blood was collected from the retro-orbital vein. To analyze immunoglobulin A (IgA) in intestinal lavage samples, mice were humanely euthanized, and the gut section extending from the stomach to the cecum was flushed with 10 ml of cold PBS containing protease inhibitors (Complete; Roche). After centrifugation, the supernatant was filtered and frozen until use.
Immunoassays.
To produce the Y. pseudotuberculosis or Y. pestis antigenic solutions used for coating in the enzyme-linked immunosorbent assays (ELISAs), either strain IP32680 or CO92 was grown at 37°C on LB agar for 48 h, centrifuged, resuspended in cold PBS containing protease inhibitors, and sonicated (Branson sonicator). Centrifuged supernatant was sterilized by filtration (0.22-μm pore size), and its protein content was measured by using a Bradford assay (Bio-Rad). ELISA plates (Nunc) were coated overnight with the antigenic solution (5 μg/ml) in PBS. The recombinant LcrV fusion protein for LcrV-specific ELISA was produced by fusion of the Y. pestis CO92 lcrV gene with the Schistosoma japonicum glutathione S-transferase gene (39). Briefly, the lcrV gene amplified by PCR was cloned in frame with the glutathione S-transferase gene into the pGEX B expression vector under control of the lactose promoter. Escherichia coli strain BL21 was transformed with the vector and expression of the recombinant protein was induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma). The recombinant protein was purified from the E. coli bacterial lysate by using glutathione-agarose beads (Sigma). Serum and intestinal lavage samples were serially diluted in PBS containing 0.1% bovine serum albumin for determination of antibody titers by ELISA. Secondary rat antibodies directed against mouse IgG, IgG1, or IgG2a were from Becton Dickinson Pharmingen. Secondary goat antibodies directed against mouse IgG2b, IgG3, or IgA were from Caltag Laboratories. All secondary antibodies were coupled to horseradish peroxidase used with TMB substrate (OptiEIA; BD Pharmingen). Titers were determined by reading the sample dilution giving half of the maximal signal on the titration curve (generally, an optical density at 450 nm of 1). The risk that the very low IgA titers obtained could result from a defect of the horseradish peroxidase-coupled anti-IgA second antibody was excluded by demonstrating that this reagent works in an ELISA designed to quantify antigen-specific IgA in bronchoalveolar lavage samples of Bacillus anthracis-vaccinated mice (data not shown; courtesy of P. Goossens, Institut Pasteur).
Evaluation of mouse protection against a challenge with Y. pestis.
The fully virulent Y. pestis strain CO92 was grown at 28°C and a suspension in saline containing 1,000 CFU (≥100 times the 50% lethal dose [LD50]) was injected s.c. in the linea alba of vaccinated and unvaccinated mice 30 days after the last vaccine dose. Animal survival was monitored for 21 days. Y. pestis dissemination in the body and septicemia were assessed 2 days after the challenge with CO92 by homogenizing various organs and plating serial dilutions of organ samples or blood.
Statistical analysis.
The Spearman's rank test was used to evaluate the correlation between antibody titers against Y. pestis and Y. pseudotuberculosis. The Fisher exact test was used to evaluate the protection in mice, and the Student t test was used to compare bacterial loads in challenged mouse groups.
RESULTS
Selection of avirulent Y. pseudotuberculosis strains.
Our criteria to select a Y. pseudotuberculosis strain suitable for use as a vaccine were that this strain should be: (i) avirulent, (ii) able to persist for at least one week in the intestine without causing severe lesions, and (iii) capable of inducing an adaptative immune response. A total of 41 Y. pseudotuberculosis strains of serotype II (Table 1), known to lack the HPI (12), were tested for the presence of major virulence factors. The absence of the HPI was confirmed by PCR amplification of the HPI-borne gene int (24). The absence of other known virulence factors such as the superantigens YPM A/B/C (15), the type IV pilus encoded by the Yersinia adhesion pathogenicity island (9), and the presence of the pYV virulence plasmid was checked by PCR. Strains had to possess the pYV plasmid to be selected because this element is essential for the bacterial persistence in the host and encodes antigens of interest for vaccination (41). Among 41 strains tested by PCR (Table 1), seven (listed in Table 2) met our criteria.
TABLE 2.
Comparison of virulence in mice of the seven selected Y. pseudotuberculosis strains and the virulent strain IP32953
| Strain | Serotype | Mortalitya after:
|
|
|---|---|---|---|
| s.c. inoculationb | i.g. administrationc | ||
| IP32953 | I | 8/10 | 9/10 |
| IP32896 | II | 2/5 | 2/5 |
| IP33098 | II | 0/5 | 5/5 |
| IP32908 | II | 1/5 | 3/5 |
| IP32621 | II | 2/5 | 0/5 |
| IP32860 | II | 0/5 | 2/5 |
| IP32721 | II | 0/10 | 0/10 |
| IP32680 | II | 0/10 | 0/30 |
Mortality (indicated as the number of dead mice/total number of mice tested) was monitored daily for 3 weeks. For strains killing no mice in both conditions, the experiments were reproduced to confirm the absence of virulence, and the results were pooled.
A total of 109 CFU were inoculated s.c. in ventral skin.
A total of 2 × 109 CFU were given i.g. by using a feeding needle.
To eliminate virulent isolates, high doses of bacteria were inoculated s.c. (109 CFU) or i.g. (2 × 109 CFU) to groups of five mice. Strains IP32680 and IP32721 were the only isolates completely avirulent at these doses (Table 2).
The persistence of these two strains in the intestine of infected mice was checked at different time points (days 1, 2, 3, 6, and 13) after inoculation. The fully virulent Y. pseudotuberculosis strain IP32953 (8) was used as positive control. While IP32721 was rapidly cleared from the intestinal tract (data not shown), IP32680 was able to persist over the 13-day survey period in the feces of infected mice (Fig. 1A) and was detectable for up to 2 months in some mice (data not shown). Because of its avirulence and prolonged persistence, strain IP32680 was selected for further analyses.
FIG. 1.
Y. pseudotuberculosis IP32680 colonizes the digestive tract and transiently penetrates into deep organs. Strains IP32680 or IP32953 (108 CFU) were inoculated i.g. to groups of three mice that were sacrificed at different time points. Bacteria recovered from either feces (A), the intestinal tract (B), or deep organs (C) were counted. Given are the CFU per two fecal pellets (feces), per gram (liver), per whole organ (spleen and mesenteric lymph nodes), or per two Peyers' patches. In a second set of experiments (vaccination protocol), mice received twice 2 × 109 bacteria i.g. at a 30-day interval (arrows), and bacterial counts were performed similarly (D). Shown are the means of log CFU + the standard error of the mean from three mice per point of one representative experiment out of two. nd, not detected.
Infectivity of strain IP32680 for the mouse.
In a first set of experiments, the infectivity of strain IP32680 given orally was analyzed by comparison with the highly virulent IP32953 strain that was given at the same dose. Since the LD50 of IP32953 via the i.g. route is 1.6 × 107 CFU, a dose of 108 CFU was injected to induce high bacterial loads and lesions in the organs without immediately killing all mice. Mice that received IP32680 i.g. did not develop any clinical signs of infection (normal fur and behavior, no body weight loss) up to day 60, as opposed to those infected with IP32953 (Fig. 2), which were sick and often died.
FIG. 2.
Evolution of mouse weight after i.g. inoculation of yersiniae. Strains IP32680 or IP32953 (108 CFU) or saline (control group) were inoculated i.g. in groups of eight mice, and their individual weights were measured daily. Shown are means ± the standard deviation of eight mice per group for IP32680 and saline. Because IP32953 killed some mice, the mean is not given, and only three curves from individual animals are shown as examples, with death indicated by crosses. The results of one representative experiment out of two are shown.
To quantify tissue colonization, groups of mice infected i.g. with IP32680 or IP32953 were sacrificed at various time points postinfection, and the bacterial contents of their Peyer's patches, mesenteric lymph nodes, livers, and spleens were measured. IP32680 was present and persisted in the Peyer's patches and mesenteric lymph nodes longer than the 2-week survey period (Fig. 1B), showing bacterial colonization of these tissues. In these intestinal lymphoid tissues, the number of IP32680 CFU at the initial phase of infection (first week) was lower than that of IP32953, although the number of CFU became similar at later stages (but only survivors of IP32953 infection could then be examined). In contrast, IP32680 invaded deep organs much less efficiently than IP32953 (Fig. 1C). Bacterial counts of both strains reached a peak between days 3 and 6, and yet the number of IP32680 CFU in the spleen and liver of infected animals was 100 to 1,000 times lower than that of IP32953. Furthermore, the spleens and livers of the animals infected with IP32680 were cleared of the infection at day 15, while the spleens of the mice surviving infection with IP32953 still contained live bacteria (Fig. 1C).
Histological analysis showed a massive necrosis at day 6 (peak of infection) in the Peyer's patches of IP32953-infected mice, whereas only some marginal polymorphonuclear cells were present in those infected with IP32680 (Fig. 3A). Similarly, IP32953 induced numerous abscesses and abundant inflammatory polymorphonuclear infiltration in the spleen and liver, whereas IP32680 caused only rare microabscesses (Fig. 3A). To quantify the extent of organ lesions, samples from six mice per group were assessed by using a numeric scoring system. The mean scores indicated that lesions caused by IP32680 were very mild (Fig. 3B), whereas, as a comparison, mouse infection with 10 times less IP32953 resulted in much higher scores in surviving animals (Fig. 3B).
FIG. 3.
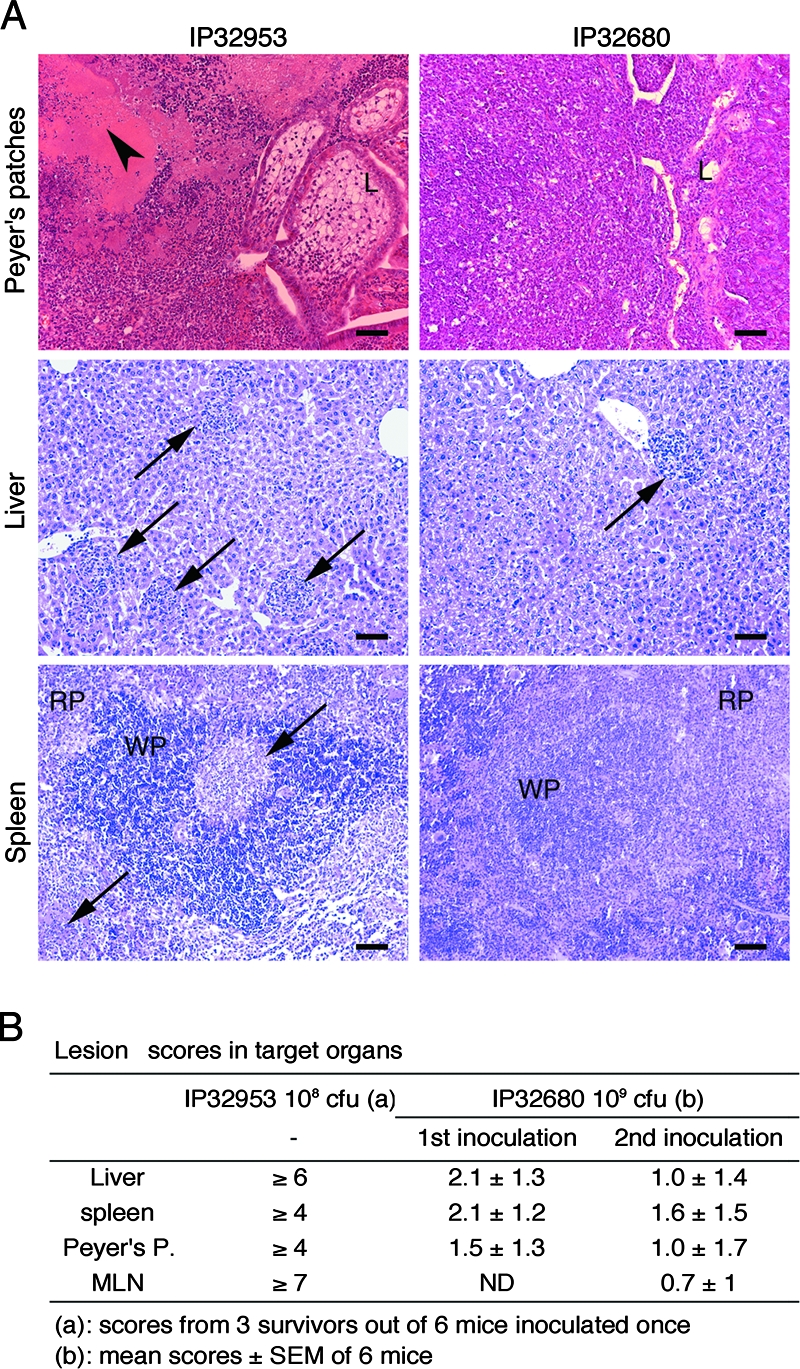
Intragastric inoculation of IP32680 causes minor lesions in mouse organs. (A) Groups of six mice from two pooled experiments were treated as described in legend of Fig. 1, and organs taken at day 6 (maximal bacterial load) were stained with hematoxylin-eosin and May-Grünwald-Giemsa stains. Shown are representative pictures of the Peyer's patches, livers, and spleens of animals inoculated with IP32953 or IP32680. Arrowheads indicate necroses, and arrows indicate abscesses. L, gut lumen; WP, white pulp; RP, red pulp. Scale bar, 100 μm. (B) Tissue lesion scores recorded at day 6 after the first or second oral inoculations (vaccination protocol) of IP32680 to six mice (mean ± the standard deviation). As a comparison, scores observed for mice having survived a single IP32953 inoculation (three out of six) are given. MLN, mesenteric lymph nodes.
In a second set of experiments, the infectivity of IP32680 was evaluated by using a vaccination protocol (two i.g. inoculations of 2 × 109 CFU of IP32680 at a 30-day interval). As previously observed, mice did not show signs of stress and did not loose weight (data not shown). Similarly, upon the first inoculation, IP32680 colonized the gut and reached the deep organs (Fig. 1D). After the second inoculation, colonization of the gut and Peyer's patches was similar to that observed after the first inoculation. Bacteria were, however, recovered in lower numbers from the mesenteric lymph nodes and the liver and were absent from the spleen. Lesions generated after a second inoculation of IP32680 (Fig. 3B) were significantly milder in the liver (P = 0.02) and not significantly different in the other organs tested. At least during the 60-day observation period, the bacteria could be found in feces, showing that mice develop a carrier state.
Antibody response elicited by IP32680 against Y. pseudotuberculosis and Y. pestis.
The capacity of strain IP32680 to induce an antibody response after i.g. administration either once or twice at a 30-day interval (prime-boost strategy; 2 × 109 CFU per dose) was evaluated. Blood was collected 30 days after the last inoculation, and serum antibody titers against Y. pseudotuberculosis antigens were analyzed by ELISA. After a single inoculation of IP32680 (Fig. 4A), moderate titers (<103) of IgG were detectable. The second IP32680 inoculation induced a marked increase (10 times) of these antibody titers. The IgG2a and IgG2b isotypes predominated after both the first and the second inoculations, suggesting a type 1 immune response (13). Immunization via the intestinal route was also expected to induce a mucosal type of immune response, characterized by high amounts of IgA released in mucosal secretions and subsequently by the presence of IgA in blood. Unexpectedly, Y. pseudotuberculosis-specific IgA production was low (titers of <50) in both the intestinal secretions and the sera of mice infected once with IP32680. The IgA titers increased moderately (titers of ≤100) in the double-inoculation regimen (data not shown).
FIG. 4.
Humoral immune response of IP32680-inoculated mice against Y. pseudotuberculosis and Y. pestis. (A) Serum antibody titers against IP32680 in mice inoculated i.g. once or twice at a 30-day interval with 2 × 109 CFU. Blood samples were collected 30 days after each inoculation. A filtered sonicate of the IP32680 strain was used for coating in ELISA plates. Shown are mean immunoglobulin titers ± the standard deviation from 12 mice per group, pooled from two independent experiments. (B) Correlation between the IgG titers against IP32680 and CO92 extracts in the sera of mice inoculated once or twice with IP32680. Shown are individual titers from eight mice per group. The linear regression analysis line is drawn, and the rho value obtained by using the Spearman's rank test is given (P = 0.0002).
Since the pYV-encoded LcrV antigen has previously been shown to induce an immune response and a protection against plague (3, 41), we examined whether antibodies were raised against LcrV in sera of mice vaccinated with IP32680 given once. IgG titers varied over a large range from animal to animal, with values from more than 8,000 to undetectable (data not shown). Because the number of vaccinated animals dying upon plague challenge was low, it was not possible to analyze statistically the relationship between the anti-LcrV titer and survival. However, the fact that several mice in which very low anti-LcrV titers were recorded survived the Y. pestis challenge suggests that anti-LcrV IgG might not be an essential component of the protective immune response raised, which is in agreement with the high number of possible antigenic targets present on live bacteria.
The antibodies elicited by IP32680 also strongly recognized Y. pestis antigens (Fig. 4B), and titers directed against the two species were closely correlated (Spearman's rho = 0.94; P = 0.0002), confirming the extensive antigenic identity between Y. pestis and Y. pseudotuberculosis.
Protection conferred by IP32680 against bubonic plague.
Mice vaccinated orally once or twice at a 30-day interval with IP32680 were exposed to an s.c. lethal challenge (1,500 CFU = 150 LD50) with the fully virulent Y. pestis strain CO92 30 days after the last immunization. Negative controls included groups of unvaccinated mice and positive controls groups of mice vaccinated with the EV76 live vaccine (14, 27). While all unvaccinated mice died, 75% of animals vaccinated with a single inoculation of IP32680 survived (P = 0.007). The protection conferred by two inoculations of IP32680 was superior (88% survival, P ≤ 0.001; Fig. 5A), although not statistically different from the protection conferred by one inoculation. In contrast, the protection conferred by the conventional EV76 vaccine strain was limited, with a mortality of 50% (Fig. 5A).
FIG. 5.
Protection against bubonic plague conferred by oral vaccination with IP32680. (A) Groups of eight mice received either saline (unvaccinated), 1 s.c. inoculation of 107 CFU of EV76 (positive control group), or 1 or 2 i.g. inoculations of 2 × 109 CFU of IP32680 at a 30-day interval. All mice were challenged s.c. with a lethal dose of 103 CFU of Y. pestis CO92 at day 30 (mice with EV76 or IP32680 given once) or day 60 (mice with IP32680 given twice), and mortality was monitored thereafter daily for 3 weeks. ***, P ≤ 0.005; **, P ≤ 0.01; ns, not significant. The results of one representative experiment out of two are shown. (B) Enumeration of CO92 bacteria in organs of challenged mice. Organs and blood from unvaccinated mice and from mice having received the IP32680 strain twice were taken to determine the bacterial load 2 days after a Y. pestis challenge performed as in panel A. Given are the CFU per gram (liver), per whole organ (spleen and two inguinal lymph nodes), or per milliliter of blood. The results from two independent experiments were pooled, and the means of log CFU ± the standard error of the mean of a total of 16 vaccinated mice and 21 unvaccinated mice are shown. ND, not detected. ****, P ≤ 0.00003.
Y. pestis CO92 bacteria were found in high amounts in the liver, spleen, inguinal lymph nodes, and blood of the unvaccinated mice at day 2 postinjection (Fig. 5B), and the animals started to die at day 4. In contrast, Y. pestis was only rarely found in the organs and blood of IP32680-vaccinated mice, and in most of these animals there was a complete absence of bacteria in the organs (Fig. 5B). The difference in bacterial loads was statistically highly significant (P ≤ 0.00003) in all organs tested.
Therefore, i.g. inoculation of IP32680 conferred a protection against plague superior to that obtained with the conventional Y. pestis strain EV76, and two inoculations further increased this protection.
DISCUSSION
This study demonstrates that vaccination against bubonic plague can be obtained using an attenuated Y. pseudotuberculosis strain given orally. A live Y. pseudotuberculosis could thus be an interesting alternative to the live attenuated Y. pestis strain EV76, which causes severe side effects (27) and is genetically unstable. Vaccination with IP32680 given once or twice did not cause any visible symptoms in the vaccinated animals, and protection against bubonic plague induced by one (75%) or two (88%) inoculations of IP32680 (88%) was higher than that induced by EV76 (50%). An avirulent Y. pseudotuberculosis is therefore an interesting alternative to the use of a live attenuated Y. pestis, such as EV76, against bubonic plague.
Two ways of producing live vaccines have historically been used: attenuation of the original pathogen (for example, development of the BCG tuberculosis vaccine) or selection of a natural avirulent strain that is closely related to the pathogen (for example, the vaccinia virus strain used in smallpox vaccines). The option of attenuating Y. pestis by genetic engineering has been used by several laboratories, for instance by deletion of the yopH or pcm genes (6, 14) or by the addition of an E. coli lpxL gene to modify the LPS (28). All of these mutations led to a strong attenuation of virulence and a good protection against bubonic plague. Still, the use of Y. pestis presents a number of drawbacks. The major one is its genome instability (5, 30) caused by the high number of IS present in its genome, which are known to favor genetic rearrangements. Y. pseudotuberculosis has a much more stable genome due to its small number of IS (5, 8, 51). Finally, a Y. pseudotuberculosis live attenuated vaccine would be less problematic than a Y. pestis if accidentally released in the environment.
The main pitfall with a live vaccine is its ability to cause adverse reactions. IP32680 did not cause any visible clinical symptoms in the vaccinated mice. According to the concept of “danger” proposed by Matzinger (26), self or commensal material is tolerated because it does not cause inflammation, and vaccines must be able to trigger a certain level of inflammation in order for an immune response to start. The fact that IP32680 was able to colonize deep organs, to persist in situ for some weeks, and to induce mild lesions was most likely a key factor in the development of an effective immune response. Whereas an adjuvant is added to particulate vaccines to induce this inflammation, bacterial signatures such as LPS are likely to play this adjuvant role in our live vaccine.
Immunity against bubonic plague has previously been obtained in the mouse by sc inoculation of live Yersinia enterocolitica serotype O3 (2), which infects humans but is not very virulent in the mouse. Y. pseudotuberculosis is genetically closer to Y. pestis than Y. enterocolitica (1, 8); therefore, the immune response raised against Y. pseudotuberculosis is expected to be more efficient against Y. pestis than that induced by Y. enterocolitica. Vaccination using live virulent Y. pseudotuberculosis has been reported in the 1950s by Wake et al. (42), who vaccinated mice with a strain of Y. pseudotuberculosis (102 CFU) delivered via the s.c. route. The weaker protection obtained against bubonic plague (50%) was possibly due to the low dose of bacteria given, imposed by the virulence of the strain. Very recently, efficient protection against bubonic plague was also obtained using a dam mutant of Y. pseudotuberculosis IP32953 (clone C18) delivered orally (40). The isolate used was cured of the virulence plasmid pYV because the dam mutation resulted in a high rate of loss of the pYV plasmid, indicating that protective immunity can be raised against chromosome-encoded targets. Their observation that the pYV plasmid is not required is intriguing because pYV-encoded YopE and YopH toxins are required for survival in the intestinal tissue (25). However, it is possible that the HPI present in the dam-mutated pYV-cured C18 clone (and not in IP32680)—or other virulence factors to be identified—confers this ability by a different mechanism. Previously, Simonet et al. had reported that intravenous injection of a Y. pseudotuberculosis strain, attenuated by loss of pYV, provided a 50% protection against bubonic plague (36).
Attenuated live bacterial vaccines mimic natural infection and are expected to provide sustained exposure to target antigens, resulting in a stronger immune response. The vaccinia vaccine against smallpox and the BCG against tuberculosis are the most famous of attenuated vaccines, and other such vaccines recently evaluated in humans include Salmonella, Shigella, Vibrio cholerae, and Listeria vaccines (22). Compared to subunit vaccines of limited antigenic complexity like those using the LcrV and F1 antigens, a live Yersinia vaccine is expected to elicit an immune response against a wide array of antigens, thus conferring protection against various natural or genetically modified variants of Y. pestis. For example the LcrV antigen contains a region whose hypervariability may allow it to escape the immune response (35). Y. pestis strains lacking the F1 antigen have a similar virulence as F1-positive strains (44) and would escape an anti-F1 immune recognition. The risk that a mutated strain bypasses the immunity stimulated by a live vaccine is, on the contrary, very unlikely due to the high number of alternative targets. Protection induced by IP32680 can be ascribed to the efficient recognition of Y. pestis antigens, in agreement with genetic identity between Y. pestis and its ancestor.
An attractive rationale for choosing oral inoculation is the avoidance of syringes, which are a major source of disease transmission, a problem frequently mentioned by the World Health Organization (WHO) in its vaccination guidelines (46). Oral vaccination with Y. pseudotuberculosis takes advantage of its capacity to colonize and persist in the gut for at least a few weeks, exerting a prolonged stimulation of the immune system. This local stimulation of mucosal immunity was expected to result in a protection against infection starting at other mucosal surfaces (19) and thus to potentially protect against pneumonic plague. However, our preliminary experiments of Y. pestis infection by aerosols in mice inoculated twice with IP32680 revealed a poor protection against pneumonic plague. Only 30% of the animals survived a nose-only exposure to aerosolized CO92 (8,000 CFU found in lungs 1 h after exposure; data not shown). The low IgA production observed in vaccinated mice may contribute to explain the low mucosal protection against pneumonic plague. Little information regarding IgA production in response to Y. pseudotuberculosis infection in the mouse is available. Whereas mouse immunization using an oral Salmonella-F1/V vaccine (29), as well as several active “probiotic” or commensal bacteria, succeeded in inducing IgA production in the gut (11), intestinal infection with virulent Y. enterocolitica O:8 failed to induce IgA production in either BALB/c or C57BL/6 mice (7). This low IgA response to live yersiniae could result from the fact that pathogenic yersiniae use their type 3 secretion system to induce apoptosis of antigen-presenting cells in Peyer's patches, as reported for macrophages (reviewed by Zhang and Bliska [50]). Whereas the fast elimination of IP32680 from the spleen after the second inoculation may be ascribed to a successful systemic immune response, the low IgA response in the gut may explain how the bacteria persist for several weeks in the gut as detected in feces, resulting in a “carrier” state. It also suggests that the immune response induced by IP32680 did not occur in the Peyer's patches and thus was mainly systemic and not mucosal.
The serum immunoglobulin isotype profile (dominance of IgG2a and IgG2b) found in mice vaccinated with IP32680 suggest that the immune response was oriented toward the Th1 type (13). This type of response is characterized by the production of cytokines such as gamma interferon and tumor necrosis factor alpha, which support phagocyte activation, an event favorable to the capture and destruction of bacteria. Peyer's patches instead favor the development of a Th2- or regulatory T-cell response (21). Lymphocytes and antibodies most likely work together to eliminate bacteria by providing help to phagocytes (38) because transfer of either lymphocytes (31) or antibodies (17) from immunized mice can protect against pneumonic plague. The protection against bubonic but not pneumonic plague reported here may therefore reflect the presence of IgG and the lack of IgA to collaborate with cells (32).
The present study demonstrates the potential of using a live attenuated strain of Y. pseudotuberculosis as an oral vaccine against bubonic plague. Bubonic plague is by far the most frequent form of the disease, and naturally occurring pneumonic plague usually starts from a primary bubonic plague case (34, 45). Vaccination against bubonic plague should thus reduce the frequency of both forms of the disease in countries where it is endemic. Strain IP32680 cannot be used for human vaccination because the causes of its attenuation are not known and the possibility of its reversion to full pathogenicity cannot be excluded. Therefore, development of live attenuated strains of Y. pseudotuberculosis harboring defined, irreversible mutations is necessary. Also, addition of Y. pestis-specific antigens to the Y. pseudotuberculosis vaccine strain could improve its efficiency against plague in both its bubonic and pneumonic forms. The most obvious antigen in that regard is F1, which is the main component of the pseudocapsule and therefore the most abundant antigen in Y. pestis in vivo. Indeed, the caf-1 operon that encodes for F1 has been previously transferred efficiently in a Salmonella strain (29). Such a genetically engineered oral Y. pseudotuberculosis plague vaccine would represent a promising approach for mass vaccination in countries where this disease is endemic.
Acknowledgments
This study was funded by an Action Concertée Incitative Microbiologie 2003 fund from the French Ministry of Health.
We thank L. Martin for her technical help with Y. pseudotuberculosis strains.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 27 May 2008.
REFERENCES
- 1.Achtman, M., K. Zurth, C. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 9614043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, J. M., A. Joseph-Francois, D. Mazigh, H. Bercovier, and H. H. Mollaret. 1978. Résistance à la peste de souris expérimentalement infectées par Yersinia enterocolitica. Ann. Microbiol. Inst. Pasteur 129b203-207. [PubMed] [Google Scholar]
- 3.Anderson, G. W., D. G. Heath, C. R. Bolt, S. L. Welkos, and A. M. Friedlander. 1998. Short- and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. Am. J. Trop. Med. Hyg. 58793-799. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, G. P., D. G. Heath, G. W. Anderson, S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 642180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anisimov, A. P., L. E. Lindler, and G. B. Pier. 2004. Intraspecific diversity of Yersinia pestis. Clin. Microbiol. Rev. 17434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubeck, S. S., and P. H. Dube. 2007. Yersinia pestis CO92 delta yopH is a potent live, attenuated plague vaccine. Clin. Vaccine Immunol. 141235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cangiani, E. E., F. R. Guiraldi, S. E. Pinto, and B. M. de Medeiros. 2007. Kinetics of B-cell response during Yersinia enterocolitica infection in resistant and susceptible strains of mice. Immunol. Investig. 36387-402. [DOI] [PubMed] [Google Scholar]
- 8.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 10113826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collyn, F., A. Billault, C. Mullet, M. Simonet, and M. Marceau. 2004. YAPI, a new Yersinia pseudotuberculosis pathogenicity island. Infect. Immun. 724784-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collyn, F., M. A. Lety, S. Nair, V. Escuyer, A. Ben Younes, M. Simonet, and M. Marceau. 2002. Yersinia pseudotuberculosis harbors a type IV pilus gene cluster that contributes to pathogenicity. Infect. Immun. 706196-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corthesy, B., H. R. Gaskins, and A. Mercenier. 2007. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 137781S-790S. [DOI] [PubMed] [Google Scholar]
- 12.De Almeida, A. M., A. Guiyoule, I. Guilvout, I. Iteman, G. Baranton, and E. Carniel. 1993. Chromosomal irp2 gene in Yersinia: distribution, expression, deletion and impact on virulence. Microb. Pathog. 149-21. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman, F. D., J. Holmes, I. M. Katona, J. F. Urban, Jr., M. P. Beckmann, L. S. Park, K. A. Schooley, R. L. Coffman, T. R. Mosmann, and W. E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8303-333. [DOI] [PubMed] [Google Scholar]
- 14.Flashner, Y., E. Mamroud, A. Tidhar, R. Ber, M. Aftalion, D. Gur, S. Lazar, A. Zvi, T. Bino, N. Ariel, B. Velan, A. Shafferman, and S. Cohen. 2004. Generation of Yersinia pestis attenuated strains by signature-tagged mutagenesis in search of novel vaccine candidates. Infect. Immun. 72908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukushima, H., Y. Matsuda, R. Seki, M. Tsubokura, N. Takeda, F. N. Shubin, I. K. Paik, and X. B. Zheng. 2001. Geographical heterogeneity between Far Eastern and Western countries in prevalence of the virulence plasmid, the superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-pathogenicity island among Yersinia pseudotuberculosis strains. J. Clin. Microbiol. 393541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galimand, M., A. Guiyoule, G. Gerbaud, B. Rasoamanana, S. Chanteau, E. Carniel, and P. Courvalin. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337677-680. [DOI] [PubMed] [Google Scholar]
- 17.Green, M., D. Rogers, P. Russell, A. J. Stagg, D. L. Bell, S. M. Eley, R. W. Titball, and E. D. Williamson. 1999. The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol. Med. Microbiol. 23107-113. [DOI] [PubMed] [Google Scholar]
- 18.Heath, D. G., G. W. Anderson, J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 161131-1137. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11S45-S53. [DOI] [PubMed] [Google Scholar]
- 20.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, and K. Tonat. 2000. Plague as a biological weapon: medical and public health management. JAMA 2832281-2290. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki, A., and B. L. Kelsall. 2001. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J. Immunol. 1664884-4890. [DOI] [PubMed] [Google Scholar]
- 22.Kotton, C. N., and E. L. Hohmann. 2004. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect. Immun. 725535-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 632854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesic, B., and E. Carniel. 2005. Horizontal transfer of the high-pathogenicity island of Yersinia pseudotuberculosis. J. Bacteriol. 1873352-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logsdon, L. K., and J. Mecsas. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect. Immun. 714595-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzinger, P. 2002. The danger model: a renewed sense of self. Science 296301-305. [DOI] [PubMed] [Google Scholar]
- 27.Meyer, K. F., D. C. Cavanaugh, P. J. Bartelloni, and J. D. Marshall, Jr. 1974. Plague immunization. I. Past and present trends. J. Infect. Dis. 129(Suppl.)S13-S18. [DOI] [PubMed] [Google Scholar]
- 28.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 71066-1073. [DOI] [PubMed] [Google Scholar]
- 29.Oyston, P. C. F., E. D. Williamson, S. E. C. Leary, S. M. Eley, K. F. Griffin, and R. W. Titball. 1995. Immunization with live recombinant Salmonella typhimurium aroA producing F1 antigen protects against plague. Infect. Immun. 63563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. G. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, et al. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413523-527. [DOI] [PubMed] [Google Scholar]
- 31.Philipovskiy, A. V., and S. T. Smiley. 2007. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect. Immun. 75878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilette, C., Y. Ouadrhiri, V. Godding, J. P. Vaerman, and Y. Sibille. 2001. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18571-588. [DOI] [PubMed] [Google Scholar]
- 33.Pouillot, F., A. Derbise, M. Kukkonen, J. Foulon, T. K. Korhonen, and E. Carniel. 2005. Evaluation of O-antigen inactivation on Pla activity and virulence of Yersinia pseudotuberculosis harbouring the pPla plasmid. Microbiology 1513759-3768. [DOI] [PubMed] [Google Scholar]
- 34.Ratsitorahina, M., S. Chanteau, L. Rahalison, L. Ratsifasoamanana, and P. Boisier. 2000. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet 355111-113. [DOI] [PubMed] [Google Scholar]
- 35.Roggenkamp, A., A. M. Geiger, L. Leitritz, A. Kessler, and J. Heesemann. 1997. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect. Immun. 65446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonet, M., P. Berche, D. Mazigh, and M. Veron. 1985. Protection against Yersinia infection induced by non-virulence-plasmid-encoded antigens. J. Med. Microbiol. 20225-231. [DOI] [PubMed] [Google Scholar]
- 37.Simpson, W. J., R. E. Thomas, and T. G. Schwan. 1990. Recombinant capsular antigen (Fraction-1) from Yersinia pestis induces a protective antibody response in BALB/c mice. Am. J. Trop. Med. Hyg. 43389-396. [DOI] [PubMed] [Google Scholar]
- 38.Smiley, S. T. 2008. Current challenges in the development of vaccines for pneumonic plague. Expert Rev. Vaccines 7209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, D. B., and K. S. Johnson. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 6731-40. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, V. L., R. W. Titball, and P. C. Oyston. 2005. Oral immunization with a dam mutant of Yersinia pseudotuberculosis protects against plague. Microbiology 1511919-1926. [DOI] [PubMed] [Google Scholar]
- 41.Une, T., and R. R. Brubaker. 1984. Roles of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 1332226-2230. [PubMed] [Google Scholar]
- 42.Wake, A., H. Morita, and M. Wake. 1978. Mechanisms of long and short term immunity to plague. Immunology 341045-1052. [PMC free article] [PubMed] [Google Scholar]
- 43.Welch, T. J., W. F. Fricke, P. F. McDermott, D. G. White, M. L. Rosso, D. A. Rasko, M. K. Mammel, M. Eppinger, M. J. Rosovitz, D. Wagner, L. Rahalison, J. E. Leclerc, J. M. Hinshaw, L. E. Lindler, T. A. Cebula, E. Carniel, and J. Ravel. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS ONE 2e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welkos, S. L., K. M. Davis, L. M. Pitt, P. L. Worsham, and A. M. Freidlander. 1995. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib. Microbiol. Immunol. 13299-305. [PubMed] [Google Scholar]
- 45.WHO. 2004. Human plague in 2002 and 2003. Wkly. Epidemiol. Rec. 79301-306. [PubMed] [Google Scholar]
- 46.WHO. 2003. WHO-UNICEF-UNFPA joint statement on the use of auto-disable syringes in immunization services. World Health Organization, Geneva, Switzerland.
- 47.Williamson, E. D., S. M. Eley, K. F. Griffin, M. Green, P. Russell, S. E. C. Leary, P. C. F. Oyston, T. Easterbrook, K. M. Reddin, A. Robinson, and R. W. Titball. 1995. A new improved subunit vaccine for plague: the basis of protection. FEMS Immunol. Med. Microbiol. 12223-230. [DOI] [PubMed] [Google Scholar]
- 48.Williamson, E. D., H. C. Flick-Smith, C. LeButt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 733598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull. Org. Mond. Santé/Bull. W. H. O. 23408-409. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, Y., and J. B. Bliska. 2005. Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr. Top. Microbiol. Immunol. 289151-173. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, D., Y. Han, E. Dai, Y. Song, D. Pei, J. Zhai, Z. Du, J. Wang, Z. Guo, and R. Yang. 2004. Defining the genome content of live plague vaccines by use of whole-genome DNA microarray. Vaccine 223367-3374. [DOI] [PubMed] [Google Scholar]