Abstract
The journey of the Chagas' disease parasite Trypanosoma cruzi in the human body usually starts in the skin after an insect bite, when trypomastigotes get through the extracellular matrix to bind specific surface receptors in the epidermis and dermis to enter cells, where they differentiate and replicate. As the infection spreads to the heart, nervous system, and other parts of the body via the circulatory system, the parasite must also cope with additional receptors in the immune system and vascular endothelium. The molecular underpinnings that govern host cell receptor recognition by T. cruzi counterreceptors remain largely unknown. Here, we describe an immunoprecipitation strategy designed to concurrently identify host receptors and complementing parasite counterreceptors. Extracellular domains of growth factor receptors fused to human immunoglobulin G (IgG) Fc were incubated with parasite lysates, immunoprecipitated on protein G-Sepharose, and eluted with Laemmli sample buffer. Possible T. cruzi counterreceptors pulled down by the receptor-Fc bait were visualized on immunoblots probed with multispecific high-affinity IgG from chronic chagasic sera and on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels stained with silver or Coomassie blue. In screening receptors important for nervous system repair, this parasite counterreceptor immunoprecipitation (PcIP) assay identified 7 to 11 polypeptides (molecular masses, 14 kDa to 55 kDa) that bound to the coreceptors of glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) GFRα-1, -2, and -3. Binding was specific because the T. cruzi mimic of host GFLs, named TGFL, did not react with GFL coreceptor tyrosine kinase RET and with other neurotrophic receptors. The polypeptides were located on the parasite outer membrane and bound noncovalently to each other. TGFL eluted from the GFL receptor/protein G affinity column with 0.5 M NaCl, pH 7.5, and potently promoted neurite outgrowth and cell survival in a GFL-sensitive mouse pheochromocytoma cell line. Given that GFLs are neuron survival factors crucial for development and maintenance of central and peripheral nervous systems, it may be that T. cruzi mimicry of host GFLs helps in mutually beneficial host repair of infected and damaged nervous tissue. As there are >30 growth factor receptor-Fc chimeras commercially available, this PcIP assay can be readily adapted to identify receptors/counterreceptors in other T. cruzi invasion sites and in other infections such as Lyme disease, amebiasis, and schistosomiasis.
Parasite invasion of mammalian hosts depends on the interplay between parasite counterreceptors (ligands) and host receptors. Identifying and characterizing these interactions are critical to understand the mechanisms underlying disease pathogenesis and to design therapeutics and vaccines. Currently, systematic approaches to simultaneously identify host receptors and parasite counterreceptors are not available. And, as illustrated by three representative examples, once a receptor or ligand is discovered, it usually takes several years to identify the second, complementing component of the molecular pair (13, 42, 44).
The first example relates to the facultative intracellular gram-positive Listeria monocytogenes, a food-borne bacterial pathogen that can cause meningitis and abortions in pregnant women (17). It was discovered in 1997 that the surface protein internalin (InlB) promotes invasion of nonphagocytic cells (5) and, 3 years later, that it does so through the Met receptor tyrosine kinase (41). The Met receptor is normally bound and activated by hepatocyte growth factor (HGF) during embryogenesis and tissue regeneration (4). Because InlB and HGF are not homologous, the discovery that they share the same receptor was based not on structural comparisons but on the serendipitous observation that soluble InlB elicits a cellular response resembling that of HGF (33, 41).
Second, it was discovered in 2000 that the protozoan parasite Leishmania major, the etiological agent of cutaneous leishmaniasis and kala-azar, expresses a surface protein with leucine-rich repeat (LRR) motifs (31). Four years later, it was noticed that the LRR protein is a counterreceptor of complement receptor 3 (CR3), one of the many ligand/receptor pairs Leishmania uses to invade macrophages (23). The identification of CR3/LRR recognition was serendipitous, as it was based on a bank of monoclonal antibodies against macrophage surface antigens and not on structural similarities because the LRR protein is unrelated to complement iC3b, fibrinogen, glucan, and other CR3 ligands of mammalian origin (48).
And the third case is Trypanosoma cruzi, the agent of Chagas' disease, which afflicts millions of people in this hemisphere. This obligate intracellular parasite invades a variety of cells in the heart, nervous system, and other parts of the body and expresses a surface protein with neuraminidase (34) and sialyltransferase activities (40). In 2000, we found that the neuraminidase moonlights as a parasite-derived neurotrophic factor (PDNF) by activating two signaling pathways, the phosphoinositide 3-kinase/protein Akt kinase and mitogen-activated protein kinase pathways, to promote neurite outgrowth and survival of neurons and Schwann cells (7-9). However, the nature of the receptor underlying PDNF-dependent host cell survival was found to be the nerve growth factor (NGF) receptor TrkA 4 years later (10). And it took a few more years for us to realize that PDNF/TrkA binding leads to T. cruzi invasion (14). As with L. monocytogenes InlB and Leishmania LRR motif protein, chance played a major role in the discovery that PDNF is a functional mimic of NGF.
To find out whether T. cruzi expresses additional mimics of neurotrophic factors, we developed an assay that simultaneously screens for the interacting molecular pairs by taking advantage of commercially available mammalian receptors fused to the human immunoglobulin G (IgG) Fc domain. The assay consists of mixing the receptor-Fc chimera with T. cruzi lysates, immunoprecipitating the receptor-Fc chimera and the bound protein on protein G-Sepharose, staining sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels with silver or Coomassie blue, and/or immunoblotting with high-affinity multispecific chagasic IgG or sera. Using a set of receptors important for the maintenance of the nervous system, this parasite counterreceptor immunoprecipitation (PcIP) assay identified a novel and biologically active T. cruzi mimic of the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs) (1, 2, 39), named TGFL (T. cruzi mimic of GFLs). The PcIP assay can easily assist in the identification of receptors/counterreceptors in other infectious organisms.
MATERIALS AND METHODS
Parasite and cell lines.
The Silvio X-10/4 strain of T. cruzi was maintained in Vero cells in Dulbecco's modified Eagle medium (DMEM) containing 1% fetal calf serum (FCS) (Gibco Laboratories, Grand Island, NY) at 37°C in a 5% CO2 atmosphere as described previously (6). Trypomastigotes were collected 5 or 6 days after Vero cells were infected with T. cruzi. Epimastigotes were obtained from noncellular cultures grown at 28°C in liver infusion-tryptose medium supplemented with 10% FCS (36). In vitro extracellularly generated amastigotes were obtained exactly as described previously (27). Mouse pheochromocytoma (MPC) CRC1 10/9 cells, a gift from Arthur S. Tischler (Department of Pathology, Tufts Medical Center, Boston, MA), were grown in DMEM containing 10% FCS, 100 U/ml penicillin, and 100 U/ml streptomycin at 37°C in a 5% CO2 atmosphere (35).
PcIP assay.
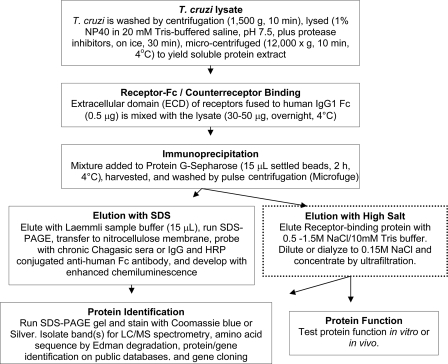
The procedure for the PcIP assay is schematically depicted in Fig. 1. T. cruzi was washed by centrifugation (1,500 × g, 10 min), lysed on ice for 30 min in lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mM EGTA, 1% NP-40, 2.5 mm sodium pyrophosphate, 1 mm glycerophosphate, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride plus a protease inhibitor cocktail; Roche Molecular Biochemicals, Indianapolis, IN), and centrifuged (Microfuge; 12,000 × g, 10 min, 4°C) to yield soluble protein lysate. The protein concentration in the lysate was determined by a Bradford method-based protein assay (Bio-Rad, Hercules, CA). T. cruzi lysate (30 to 50 μg), precleared on protein G-Sepharose (2 h, 4°C), was mixed overnight at 4°C with 0.5 μg extracellular domain of receptor fused to human IgG1 Fc (hereafter referred to as receptor or receptor-Fc) (R&D Systems, Minneapolis, MN), mixed with protein G-Sepharose (∼15 μl) (GE Healthcare, Piscataway, NJ), washed with lysis buffer, and harvested by pulse centrifugation.
FIG. 1.
Flow sheet for the isolation of TGFL. The dotted-line box (elution with high salt) denotes preparative scale purification, where the volume of reagents specified in the other boxes are increased proportionally.
Elution was with Laemmli sample buffer (15 μl) at room temperature, and the eluate was resolved by 7.5 to 15% SDS-PAGE, followed by nitrocellulose membrane blotting. The membrane was blocked with 5% nonfat milk in Tris-buffered saline plus 0.1% Tween 20 (TBST) (30 min, 37°C), probed with a 1:1,000 dilution of chronic Chagasic sera or 10 μg/ml IgG purified from Chagasic serum in 5% milk (overnight, 4°C), washed with TBST, incubated with horseradish peroxidase (HRP)-conjugated anti-human Fc antibody (Promega Corporation, Madison, WI) diluted 1:5,000 in 5% milk (1 h, room temperature), and developed with enhanced chemiluminescence (Perkin-Elmer, Life Sciences, Boston, MA).
For large-scale purification, the amounts of reagents were scaled up proportionally. However, here elution was with two column volumes of 0.5 M NaCl-10 mM Tris buffer, pH 7.5, and the eluate was concentrated by ultrafiltration in an Amicon ultracentrifugal filter device (10,000-molecular-weight cutoff) (Millipore Corporation, Billerica, MA). In some cases, the eluate (i.e., TGFL) was diluted to 0.15 M NaCl and reapplied to a new protein G-Sepharose column to remove residual receptor-Fc. The eluate was stored in Tris-buffered saline containing 30 to 40% glycerol or 1% bovine serum albumin (BSA) after sterilization with 0.22-μm filters. The protein was resolved by 7.5 to 15% SDS-PAGE and stained with Coomassie blue or silver. The protein concentration of TGFL was obtained in Coomassie blue-stained SDS-PAGE gels by comparison with standard curves generated with crystalline BSA followed by densitometry (CS-800 calibrated densitometer; Bio-Rad, Hercules, CA) using the Quantity One program, version 4.4.0, as described previously (15). The average yield of four different purifications was 1.0 μg TGFL/1.2 × 108 trypomastigotes (Silvio strain of T. cruzi).
Enzyme-linked immunosorbent assay (ELISA).
Microtiter (96-well) plates were coated with GDNF (500 ng/ml), neurturin (75 ng/ml), and artemin (300 ng/ml) (all from R&D Systems) and TGFL (95 ng/ml) (isolated as described above), in 50 mM carbonate buffer (pH 9.6) overnight at 4°C, washed three times with phosphate-buffered saline (PBS) plus 0.1% Tween-20 (PBST), blocked with 5% goat serum-PBST (1 h, 37°C), washed three times in PBST, and incubated with receptor-Fc in 5% BSA in PBST (2 h, 37°C). After a washing with PBST and incubation with alkaline phosphatase-labeled anti-human Fc antibody in 1% BSA-PBST (1 h, 37°C), the plates were washed before the addition of alkaline phosphatase substrate p-nitrophenylphosphate at room temperature, and the nitrophenol product was estimated ∼1 to 2 h afterwards in a microplate reader (measuring A405). To measure GFRα (GFL receptor α) binding to T. cruzi lysates, trypomastigotes, epimastigotes, and amastigotes were frozen (108/ml) at −70°C in PBS containing a cocktail of protease inhibitors and defrosted at 37°C three times and centrifuged (1,200 × g, 10 min) and the clear supernatant (equivalent to 5 × 105 parasites/well) was applied to microtiter wells in 50 mM carbonate buffer, pH 9.6, overnight at 4°C, blocked with 5% goat serum, and incubated with the Fc chimera of GFRα-1 or fibroblast growth factor receptor extracellular domain 1 (FGF-R1α) for 2 h at 37°C, followed by alkaline phosphatase-conjugated anti-human Fc antibody for 1 h at 37°C. The absorbance values were read at 405 nm after 1 h.
Fluorescence microscopy.
Amastigotes, trypomastigotes, and epimastigotes were fixed with 4% paraformaldehyde, blocked with 10% goat sera, and incubated with 1 μg/ml GFRα-1-Fc or FGF-R1α for 1 h and anti-human IgG-594 Alexa for 1.5 h. Fluorescence was observed under an Olympus 1X70 fluorescence microscope equipped with a Spot camera and software.
T. cruzi pull-down assay.
The three stages of T. cruzi were incubated with GFRα-1-Fc or FGF-R1α-Fc at 4°C for 1 h and processed for immunoblotting as described earlier (14). See also the Fig. 4 legend.
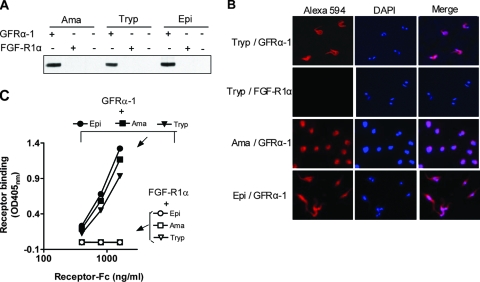
FIG. 4.
Binding of GFRα-1 to the surface of T. cruzi at different developmental stages. (A) Pull-down assay. Live T. cruzi amastigotes (Ama) trypomastigotes (Tryp), and epimastigotes (Epi) were incubated with GFRα-1 or FGF-R1α (1 μg/6 × 106 parasites) for 1 h at 4°C, washed by centrifugation, resuspended in 2% SDS, and analyzed by immunoblotting with anti-human IgG, which identified T. cruzi-bound GFRα-1, but not FGF-R1α. (B) Fluorescence microscopy showing visualization of GFRα-1 binding to T. cruzi. Tryp, Ama, and Epi were fixed in 4% paraformaldehyde and reacted with GFRα-1 or FGF-R1α, Alexa 594-labeled secondary antibody, and DAPI (4′,6-diamidino-2-phenylindole), followed by fluorescence microscopy. Note that GFRα-1 binds to the surface of T. cruzi at the three stages and that DAPI identifies the nucleus and kinetoplast for each Epi and Tryp parasite. FGF-R1α did not bind to Tryp (second row), Ama, or Epi (not shown). (C) ELISA. Microtiter wells were coated with freeze-thaw lysates of Epi, Ama, and Tryp and reacted with GFRα-1 or FGF-R1α followed by alkaline phosphatase-labeled secondary antibody. Note that GFRα-1 bound robustly to substratum-coated Epi, Ama, and Tryp, while FGF-R1α did not react.
Cell survival and neurite extension.
For neurite extension assays, MPC cells in DMEM-10% FCS were plated in microtiter wells (5 × 103/well, overnight) and treated with 100 μl of different concentrations of TGFL or GDNF (in DMEM-1.0% FCS plus 0.02% BSA) for 3 to 5 days. Cells were fixed with 4% paraformaldehyde in PBS (20 min, room temperature), washed with PBS, permeabilized with 0.2% Triton X-100-PBS (5 min, room temperature), blocked with 10% goat serum (2 h, room temperature), washed with PBS, incubated with anti-neurofilament NF200 diluted 1:500 in 2.5% goat serum (overnight, 4°C), washed, and incubated with Alexa 488-conjugated anti-rabbit IgG (Molecular Probes) (20 μg/ml in 2.5% goat serum, 1 h, room temperature). Images were analyzed by fluorescence microscopy with Spot camera software. At least 300 cells per well were randomly selected for measurement of the length of neurites, defined as an extension of more than the diameter of the cell body.
For cell survival experiments, medium was replaced with serum-free (0.1% FCS) medium for 4 to 5 days and the medium was changed to medium containing Hoechst 33342 (20 μg/ml) and propidium iodide (PI) (10 μg/ml). After 5 min, the cells were visualized under UV irradiation at 340 to 380 nm in an inverted fluorescence microscope equipped with a Spot digital camera. At least 300 cells per well were randomly selected for counting live and dead cells.
RESULTS
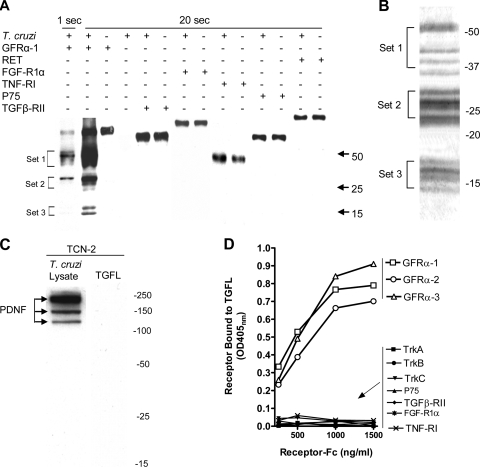
To screen for putative T. cruzi counterreceptors that might complement growth factor receptors important to nervous system repair, the extracellular domains of six relevant receptors fused to human IgG Fc (GFRα-1, RET [rearranged during transformation], transforming growth factor β receptor extracellular domain II [TGF-β-RII], FGF-R1α, tumor necrosis factor receptor extracellular domain 1 [TNF-R1], and p75) were incubated with nonionic detergent extracts of trypomastigotes, pulled down on protein G-Sepharose, washed to remove nonspecifically bound materials, eluted with Laemmli sample buffer under reducing conditions (2% β-mercaptoethanol), and analyzed by Western blotting using, as a probe, chagasic serum or IgG and HRP-conjugated secondary antibody for visualization by enhanced chemiluminescence (Fig. 1). Controls were (i) receptor-Fc, without preincubation with T. cruzi, pulled down on protein G-Sepharose and (ii) T. cruzi lysates directly adsorbed to protein G-Sepharose without premixing with receptor-Fc. This way, we found that GFRα-1 specifically pulls down a complex mixture of T. cruzi proteins (i.e., TGFL). Consistently, short exposure (1 s) of the immunoblots to the film revealed two sets of bands (sets 1 and 2; molecular masses, 31 to 55 kDa) (Fig. 2A, left lane [lane 1]), and longer exposures (20 s) showed an additional one (set 3; two bands; molecular mass ∼ 16 kDa) (Fig. 2A, lane 2). Because of the denaturing action of 2% SDS, Laemmli sample buffer always elutes Fc-receptor from the protein G-Sepharose regardless of whether the counterreceptor is pulled down (lanes 1 and 2) or not (all other lanes).
FIG. 2.
Identification and specificity of TGFL. (A) PcIP. The indicated growth factor receptor-Fc chimeras (GFRα-1, RET, TGF-β-RII, FGF-R1α, TNF-RI, and P75) (each, 0.5 μg) were incubated overnight 4°C with T. cruzi trypomastigote lysate (∼25 to 30 μg), adsorbed to protein G-Sepharose, washed, eluted with 2% SDS, run on a nonreducing SDS-PAGE gel, blotted onto nitrocellulose, probed with chagasic sera followed by HRP-labeled secondary anti-human IgG antibody, and visualized with the ECL system for 1 second and 20 seconds. Numbers on the right represent molecular masses in kDa. The experiment was repeated more than 15 times with nearly identical results. Control lanes, lysate adsorbed to protein G-Sepharose without premixing with receptor (T. cruzi +) and receptor adsorbed to protein G-Sepharose without premixing with lysate (receptor +); adsorption of lysate-receptor mixtures to protein G-Sepharose is denoted by appropriate + and − signs in the corresponding lanes. (B) Coomassie blue staining of TGFL (1.9 μg, equivalent to 2.3 × 108 T. cruzi trypomastigotes, Silvio strain) twice purified on GFRα-1/protein G-Sepharose column after discontinuous 10 to 15% SDS-PAGE. Numbers on the right indicate molecular masses in kDa. The experiment was repeated three times with similar patterns. (C) Western blots of T. cruzi lysates (left) and TGFL (sample analogous to leftmost lane in panel A) (right) probed with PDNF/trans-sialidase-specific monoclonal antibody TCN-2. (D) ELISA. TGFL isolation was similar to that for panel A except that TGFL was eluted from GFRα-1-Fc/protein G-Sepharose beads with 0.5 M NaCl-10 mM Tris, pH 7.5, diluted to 0.15 M NaCl, and concentrated by ultrafiltration. TGFL was repurified and eluted as above, stored in 30% glycerol, and then used for an ELISA (microtiter plates were coated with 95 ng/ml TGFL overnight at 4°C, blocked with 5% goat serum for 1 h at 37°C followed by the indicated concentrations of Fc chimera receptors in 5% BSA-PBST, pH 7.2, for 2 h at 37°C, washed, and developed with alkaline phosphatase-labeled anti-human Fc antibody). Points are averages of triplicate points. The experiment was repeated three times with similar results.
TGFL visualized in the Western blot of Fig. 2A corresponded to 5 × 106 trypomastigotes, but this parasite load did not yield enough TGFL to be detectable on silver- or Coomassie blue-stained SDS-PAGE gels. We therefore sought ways to scale up the yield and increase the concentration of TGFL for visualization by Coomassie blue-stained gels. We found that high concentrations of NaCl at neutral to alkaline pH break the binding of the Fc-receptor to the counterreceptor but not to protein G. Preliminary experiments using various concentrations of NaCl (0.5 M to 1.5 M, pH 7.0 to 7.8) showed that TGFL, but not the Fc-receptor, is readily eluted from the protein G-Sepharose affinity column with 10 mM Tris-buffered 0.5 M NaCl, pH 7.5. This allowed pooling of eluates from various independent purifications or from larger-scale batches of reagents. Pools of eluates were concentrated and dialyzed by ultrafiltration and immediately used for SDS-PAGE for silver or Coomassie blue staining.
The banding pattern of Coomassie blue-stained TGFL, corresponding to ∼2.3 × 108 trypomastigotes, or ∼50 times that needed for visualization in Western blotting (Fig. 2A), was similar to that in immunoblots except for 2 additional bands in set 3, for a total of 11 bands (Fig. 2B). Silver-stained gels gave a similar pattern for Coomassie blue-stained gels (not shown). The banding pattern of TGFL separated under reducing conditions (Fig. 2B) remained unchanged when TGFL was run in the absence of reducing agents (not shown), indicating that the TGFL bands are not associated with each other by covalent (disulfide) bonds. Unlike what was found with the Laemmli buffer, TGFL eluted with 0.5 M NaCl does not contain GFRα-1-Fc-receptor because it remains bound to protein G-Sepharose (Fig. 2B), though it can be eluted with 2% SDS (Fig. 2A).
To determine whether PDNF, a T. cruzi-derived protein that promotes trophic actions on neurons through the activation of TrkA receptors (10), is a component of the TGFL complex, we probed TGFL with the PDNF-specific monoclonal antibody TCN-2 by Western blotting (36). TCN-2 did not bind TGFL (Fig. 2C) under conditions in which it did produce the classical multibanding pattern with T. cruzi lysates (Fig. 2A and B). Furthermore, trans-sialidase activity, an intrinsic property of PDNF (10), was not detected in various TGFL preparations (data not shown).
GFLs constitute a family of four ligands, GDNF, neurturin, artemin, and persephin, that are most specific for GFRα-1, GFRα-2, GFRα-3, and GFRα-4, respectively (1, 2). We used ELISA to determine whether TGFL binds GDNF receptor GFRα-1 preferentially; for this, various concentrations of Fc-GFRα-1, -2, and -3 (Fc-GFRα-4 is not, as of now, commercially available) were added to TGFL-coated microtiter wells, followed by alkaline phosphatase-labeled secondary antibody. As shown in Fig. 2D, the three GFRα receptors bound robustly to TGFL, unlike TrkA, TrkB, and TrkC, the tyrosine kinase receptors of neurotrophins NGF, brain-derived neurotrophic factor, and neurotrophin-3. In addition, TGFL did not react with the pan-neurotrophin receptor p75 or with TGFβ-RII, FGF-R1, or TNF-RI (Fig. 2D), confirming the findings of the PcIP assay (Fig. 2A).
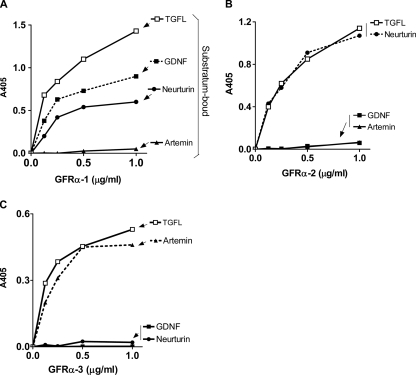
To independently confirm that TGFL did not discriminate among the three GFRα receptors (Fig. 2D), we used an ELISA designed to ascertain receptor/ligand specificity (38). We coated microtiter wells with GDNF, neurturin, artemin, and TGFL and determined the specificity of the binding of soluble GFRα-Fc chimeras to the solid-state ligands. The results showed that GFRα-1 bound to GDNF and to neurturin but not to artemin (Fig. 3A), GFRα-2 bound to neurturin but not GDNF and artemin (Fig. 3B), and GFRα-3 bound only to artemin (Fig. 3C), in agreement with previous reports (1-3, 38). In contrast, all three GFRα receptors bound robustly to TGFL, with binding curves analogous to those of bona fide endogenous receptors (Fig. 3A, B, and C) and with an affinity of ∼4 nM, similar to that of GDNF/GFRα-1 and GFRα-3/artemin (3).
FIG. 3.
Binding of GFRα-Fc-receptors to TGFL. Microtiter wells were coated with TGFL (stored in 30% glycerol) (95 ng/ml) and GDNF (500 ng/ml), neurturin (75 ng/ml), and artemin (300 ng/ml) (all stored at 30% glycerol), blocked with 5% goat serum, and reacted with the indicated concentrations of GFRα-1 (A), GFRα-2 (B), and GFRα-3 (C), followed by alkaline phosphatase-conjugated anti-human IgG antibody. Values are averages of triplicate wells. The experiment was repeated four times with similar results.
The molecular mass of GDNF, neurturin, and artemin monomers by SDS-PAGE is ∼15 kDa (1, 2). Thus, the possibility exists that TGFL set 3 (molecular mass ∼ 16 kDa) might contain structural analogs of GDNF. This, however, does not appear to be the case because antibodies to GDNF, neurturin, and artemin did not cross-react with TGFL in ELISAs (data not shown). Based on an assay similar to that in Fig. 3A, chagasic sera did not react with the GFLs either (data not shown), suggesting that trypanosome TGFL is structurally unrelated to the mammalian GFLs.
To test whether TGFL is located on the surface of T. cruzi (trypomastigotes, epimastigotes, and amastigotes), we took advantage of a recently described pull-down assay that has been used to characterize TrkA binding to the T. cruzi outer membrane (14). In this assay, live parasites are incubated at 4°C (to minimize endocytosis) with Fc-receptors for 60 min and washed and the amount of parasite-bound Fc-receptor is determined by Western blotting using anti-human IgG antibody and scanning densitometry. The results showed that live trypomastigotes, epimastigotes, and amastigotes effectively pulled down GFRα-1 but not FGF-R1α (Fig. 4A). The conclusion that GFRα-1 binds to the surface of T. cruzi was further evaluated by fluorescence microscopy, which revealed the binding of the receptor, but not of FGF-R1α, to the surface of paraformaldehyde-fixed trypomastigotes, epimastigotes, and amastigotes (Fig. 4B). PcIP analysis of T. cruzi trypomastigote surface proteins labeled with membrane-impermeable N-hydroxysuccinimide-biotin (22) further confirmed the surface location of TGFL (data not shown). Furthermore, coating of microtiter wells with freeze-thaw T. cruzi lysates followed by GFRα-1-Fc and alkaline phosphatase-labeled anti-human Fc antibody confirmed the specific binding of GFRα to the three stages of T. cruzi (Fig. 4C). However, we could not detect TGFL in T. cruzi-conditioned supernatants under conditions in which PDNF/trans-sialidase is readily identified (i.e., in the cell-free medium obtained after harvesting parasites from Vero cells and after incubating parasites (108/ml) in RPMI medium-1% BSA for 4 h at 37°C; furthermore, TGFL was not released into the supernatant obtained by treating T. cruzi with 2 M NaCl (data not shown). These results indicate that TGFL is tightly bound to the T. cruzi outer membrane but that it that can be readily solubilized with the nonionic detergent NP-40 or by freezing and thawing.
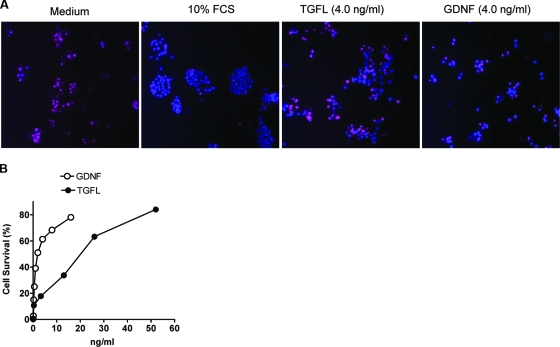
Neuroblastoma cell lines are derived from peripheral sympathetic neuroblast tumors, and many of them express GFR; consequently, the cell lines respond to GDNF and other GDNF family members (18). We used a GDNF-sensitive MPC cell line (35) to test the possibility that TGFL mimics GFLs in promoting differentiation and survival of neuronal cells. Like GDNF, TGFL induced a robust neurite outgrowth in the MPC cells (Fig. 5A), which was dose dependent and saturable (Fig. 5B). To determine whether TGFL supports cell survival, MPC cells were grown in a medium containing a low concentration of FCS (0.1%) for 3 days, a condition that promotes apoptosis, visualized by staining with PI (which detects dead nuclei) and Hoechst 33342 (which stains live and dead nuclei) (Fig. 6A, medium), as a dose-dependent and saturable response (Fig. 6B), with a 50% inhibitory concentration of 18 ng/ml, compared to 1.5 ng/ml for GDNF. Not surprisingly, cells grown in 10% FCS survived much better than cells grown in starvation medium containing TGFL or GDNF, though some apoptotic cells were nevertheless observed (Fig. 6A).
FIG. 5.
TGFL promotes neurite outgrowth in MPC cells. (A) Cells were plated on microtiter wells and cultured overnight in DMEM containing 10% FCS (growth medium) and for 4 days in DMEM containing 1.0% FCS (starvation medium) without (medium) or with TGFL and GDNF. Cells were washed, fixed with 4% paraformaldehyde, blocked with 10% goat serum, permeabilized with 0.2% Triton X-100, and reacted with anti-NF200 antibody followed by Alexa-488-conjugated secondary anti-rabbit antibody. Original magnification, ×20. Cells grown in the absence of TGFL or GDNF (medium) were round and detached from the substratum after wash, while cells treated with TGFL or GDNF remained attached and displayed robust neurite outgrowth. (B) Quantitation of TGFL stimulation of neurite outgrowth (>300 cells per dose) in assays similar to those for panel A. The dashed line represents GDNF stimulation at 2.0 ng/ml.
FIG. 6.
TGFL promotes survival of MPC cells. (A) Cells were plated in microtiter wells, cultured overnight in 10% FCS and for 5 days in 10% FCS or 0.5% FCS without (medium) or with TGFL (4 ng/ml) and GDNF (4.0 ng/ml). The microtiter plate was centrifuged at 500 × g for 10 min to increase cell attachment to the substratum. Cell survival was visualized by staining with PI, which stains dead cells red, and Hoechst 33342, which stains live and dead cells blue. (B) TGFL and GDNF promotes cells survival in a dose-dependent manner. The protocol was similar to that for panel A. Cell viability (%) was calculated as 100 × (1 − number of PI-stained cells/total number of cells). Results represent averages of more that 300 cells per point, each in triplicate. This experiment was repeated three times with similar results.
DISCUSSION
We describe here a simple and easy-to-perform PcIP strategy that identified a novel T. cruzi counterreceptor for the GDNF family of mammalian neurotrophic receptors. This assay uses readily accessible reagents and depends on laboratory procedures used worldwide. The single-step assay consists of mixing a human IgG Fc chimera of growth factor receptors (>30 available commercially) with parasite lysates, adsorption to protein G-Sepharose, and elution with Laemmli sample buffer for rapid identification of putative counterreceptors by Western blotting and silver or Coomassie blue staining. Elution with relatively mild conditions (0.5 to 1.5 M NaCl, pH 7.0 to 7.8) can provide enough material for visualization with silver or Coomassie blue staining and for biological assays. Western blotting, to be of value, depends on high-affinity multispecific antibodies, but these are promptly found in the sera of relevant chronically infected individuals or experimentally infected animals.
Neurotrophic factors regulate many important aspects of the development and maintenance of the nervous system by playing essential roles in intercellular communication; they comprise neurotrophins (NGF, brain-derived neurotrophic factor, and neurotrophin-3), neurokines (ciliary neurotrophic factor, leukemia inhibitory factor), GFLs (GDNF, neurturin, artemin, and persephin) (1, 2, 20, 21), and others. NGF was identified more than half a century ago as the molecule that controls survival and maturation of developing neurons in the peripheral nervous system (26).
The GFL family, whose founding member, GDNF, was discovered relatively recently (28), belongs to the TGF-β superfamily of growth factors. Much attention is currently being paid to the therapeutic potential of GDNF family members, as exemplified by GDNF and artemin, which have been used in clinical trials of Parkinson's disease (43) and in the systemic treatment of neuropathic pain (16), respectively. GFLs are disulfide-linked dimers that react with GFRα receptors to form nonsignaling heterotetramers; this reaction represents the initiating step in the formation of a ternary signaling complex that contains the shared tyrosine kinase receptor RET. This ternary complex, through RET, directly activates several intracellular signaling cascades to regulate migration, chemotaxis, branching, morphogenesis, neurite outgrowth, and synaptic plasticity (1, 2, 47). Such ligand/receptor composition is unique in the TGF-β superfamily because TGF-β and other members such as activins and bone morphogenetic protein directly bind and activate two different types of signaling serine/threonine receptor kinases (30). Interestingly, TGFL, like counterpart GFLs, bound to GFRα but not to RET receptors in the PcIP assay (Fig. 2A).
To bind surface GFRα receptors, TGFL is strategically located on the surface of trypomastigotes (and other stages of T. cruzi) for proper trypanosome/host cell interaction. Interestingly, TGFL does not discriminate among the GFRα receptors (Fig. 3), in contrast to GDNF, neurturin, and artemin, which are selective for GFRα-1, -2, and -3 (Fig. 3), respectively (1, 2). The versatility of TGFL in recognizing GFRα receptors might very well be useful in the cross talk between T. cruzi and the central and peripheral nervous systems.
What might GFL mimicry mean to T. cruzi infection? We showed earlier that PDNF/trans-sialidase triggers T. cruzi-induced differentiation and survival of neurons and Schwann cells through the binding and activating of NGF receptor TrkA, and these findings were the motivation for designing a method to simultaneously detect novel ligand/receptor interactions (7, 9). Such neuroprotective responses were thought to be a mechanism used by T. cruzi to help the host repair damaged infected nervous tissues (9, 14).
Thus, the functional mimicry of GDNF family ligands by T. cruzi supports the hypothesis that the parasite evolved mechanisms to help nervous tissue regeneration. This should be facilitated by the relatively high level of TGFL expression, ∼1.0 μg TGFL per 108 trypomastigotes or ∼10 ng TGFL/ml blood (parasitemia of ∼106/ml and bloodstream trypomastigote TGFL expression analogous to culture-derived counterpart), which is much above the threshold for a robust TGFL trophic response in vitro (5 ng/ml or 0.5 ng/well) (Fig. 5 and 6). The idea of T. cruzi evolving mechanisms through PDNF, TGFL, and perhaps other molecules to help the host repair infected tissues to maintain mutually beneficial structural and functional integrity of infected tissues is consistent with a similar emerging view in toxoplasmosis pathogenesis (24).
Neuroprotection produced by both PDNF (8-12, 14) and TGFL (this communication) may relate to several features of Chagas' disease progression. For example, many individuals with acute Chagas' disease have parasites in their spinal fluid but do not present symptoms such as severe headache, vomiting, nuchal rigidity, and visual phobia (19), which normally accompany infections of the brain parenchyma and meninges (37, 46). Furthermore, the majority of chronic chagasic patients remain asymptomatic for life and present an age-dependent increase in gastrointestinal and cardiac ganglia, a trend reverse to that of nonchagasic individuals (25). TGFL could participate in this process by activating GFRα, as this receptor is pivotal in the regulation of the survival and differentiation of several populations of neurons and glial cells in the enteric nervous system. On the other hand, disturbances in GFRα and RET signaling can lead to megacolon (Hirschsprung's disease) (29, 32). And this abnormality, together with megaesophagus and cardiomegaly, is a prominent feature of chronic symptomatic Chagas' disease (25, 45). Thus, one can envision TGFL participating in neuroregenerative and/or neurodegenerative events of Chagas' disease.
Acknowledgments
We thank Marina Chuenkova and Craig Weinkauf for helpful discussions and Jacqueline Sharon for help with the manuscript.
This work was supported by NIH NS40574 and NS429660.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Airaksinen, M. S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3383-394. [DOI] [PubMed] [Google Scholar]
- 2.Baloh, R. H., H. Enomoto, E. M. Johnson, Jr., and J. Milbrandt. 2000. The GDNF family ligands and receptors—implications for neural development. Curr. Opin. Neurobiol. 10103-110. [DOI] [PubMed] [Google Scholar]
- 3.Baloh, R. H., M. G. Tansey, P. A. Lampe, T. J. Fahrner, H. Enomoto, K. S. Simburger, M. L. Leitner, T. Araki, E. M. Johnson, Jr., and J. Milbrandt. 1998. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 211291-1302. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4915-925. [DOI] [PubMed] [Google Scholar]
- 5.Braun, L., S. Dramsi, P. Dehoux, H. Bierne, G. Lindahl, and P. Cossart. 1997. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol. 25285-294. [DOI] [PubMed] [Google Scholar]
- 6.Chuenkova, M., and M. E. Pereira. 1995. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas' disease. J. Exp. Med. 1811693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuenkova, M. V., F. B. Furnari, W. K. Cavenee, and M. A. Pereira. 2001. Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 989936-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuenkova, M. V., and M. A. Pereira. 2003. PDNF, a human parasite-derived mimic of neurotrophic factors, prevents caspase activation, free radical formation, and death of dopaminergic cells exposed to the Parkinsonism-inducing neurotoxin MPP+. Brain Res. Mol. Brain Res. 11950-61. [DOI] [PubMed] [Google Scholar]
- 9.Chuenkova, M. V., and M. A. Pereira. 2000. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell 111487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuenkova, M. V., and M. PereiraPerrin. 2004. Chagas' disease parasite promotes neuron survival and differentiation through TrkA nerve growth factor receptor. J. Neurochem. 91385-394. [DOI] [PubMed] [Google Scholar]
- 11.Chuenkova, M. V., and M. PereiraPerrin. 2006. Enhancement of tyrosine hydroxylase expression and activity by Trypanosoma cruzi parasite-derived neurotrophic factor. Brain Res. 1099167-175. [DOI] [PubMed] [Google Scholar]
- 12.Chuenkova, M. V., and M. PereiraPerrin. 2005. A synthetic peptide modeled on PDNF, Chagas' disease parasite neurotrophic factor, promotes survival and differentiation of neuronal cells through TrkA receptor. Biochemistry 4415685-15694. [DOI] [PubMed] [Google Scholar]
- 13.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304242-248. [DOI] [PubMed] [Google Scholar]
- 14.de Melo-Jorge, M., and M. PereiraPerrin. 2007. The Chagas' disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe 1251-261. [DOI] [PubMed] [Google Scholar]
- 15.Gao, W., and M. A. Pereira. 2001. Trypanosoma cruzi trans-sialidase potentiates T cell activation through antigen-presenting cells: role of IL-6 and Bruton's tyrosine kinase. Eur. J. Immunol. 311503-1512. [DOI] [PubMed] [Google Scholar]
- 16.Gardell, L. R., R. Wang, C. Ehrenfels, M. H. Ossipov, A. J. Rossomando, S. Miller, C. Buckley, A. K. Cai, A. Tse, S. F. Foley, B. Gong, L. Walus, P. Carmillo, D. Worley, C. Huang, T. Engber, B. Pepinsky, R. L. Cate, T. W. Vanderah, J. Lai, D. W. Sah, and F. Porreca. 2003. Multiple actions of systemic artemin in experimental neuropathy. Nat. Med. 91383-1389. [DOI] [PubMed] [Google Scholar]
- 17.Gellin, B. G., and C. V. Broome. 1989. Listeriosis. JAMA 2611313-1320. [PubMed] [Google Scholar]
- 18.Hishiki, T., Y. Nimura, E. Isogai, K. Kondo, S. Ichimiya, Y. Nakamura, T. Ozaki, S. Sakiyama, M. Hirose, N. Seki, H. Takahashi, N. Ohnuma, M. Tanabe, and A. Nakagawara. 1998. Glial cell line-derived neurotrophic factor/neurturin-induced differentiation and its enhancement by retinoic acid in primary human neuroblastomas expressing c-Ret, GFR alpha-1, and GFR alpha-2. Cancer Res. 582158-2165. [PubMed] [Google Scholar]
- 19.Hoff, R., R. S. Teixeira, J. S. Carvalho, and K. E. Mott. 1978. Trypanosoma cruzi in the cerebrospinal fluid during the acute stage of Chagas' disease. N. Engl. J. Med. 298604-606. [DOI] [PubMed] [Google Scholar]
- 20.Huang, E. J., and L. F. Reichardt. 2001. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24677-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, E. J., and L. F. Reichardt. 2003. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72609-642. [DOI] [PubMed] [Google Scholar]
- 22.Jullien, J., V. Guili, L. F. Reichardt, and B. B. Rudkin. 2002. Molecular kinetics of nerve growth factor receptor trafficking and activation. J. Biol. Chem. 27738700-38708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kedzierski, L., J. Montgomery, D. Bullen, J. Curtis, E. Gardiner, A. Jimenez-Ruiz, and E. Handman. 2004. A leucine-rich repeat motif of Leishmania parasite surface antigen 2 binds to macrophages through the complement receptor 3. J. Immunol. 1724902-4906. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S. K., and J. C. Boothroyd. 2005. Stage-specific expression of surface antigens by Toxoplasma gondii as a mechanism to facilitate parasite persistence. J. Immunol. 1748038-8048. [DOI] [PubMed] [Google Scholar]
- 25.Koberle, F. 1968. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 663-116. [DOI] [PubMed] [Google Scholar]
- 26.Levi-Montalcini, R. 1987. The nerve growth factor 35 years later. Science 2371154-1162. [DOI] [PubMed] [Google Scholar]
- 27.Ley, V., N. W. Andrews, E. S. Robbins, and V. Nussenzweig. 1988. Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells. J. Exp. Med. 168649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, L. F., D. Mismer, J. D. Lile, L. G. Armes, E. T. Butler III, J. L. Vannice, and F. Collins. 1989. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science 2461023-1025. [DOI] [PubMed] [Google Scholar]
- 29.Lui, V. C., E. T. Samy, M. H. Sham, L. M. Mulligan, and P. K. Tam. 2002. Glial cell line-derived neurotrophic factor family receptors are abnormally expressed in aganglionic bowel of a subpopulation of patients with Hirschsprung's disease. Lab. Investig. 82703-712. [DOI] [PubMed] [Google Scholar]
- 30.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14627-644. [PubMed] [Google Scholar]
- 31.Montgomery, J., T. Ilg, J. K. Thompson, B. Kobe, and E. Handman. 2000. Identification and predicted structure of a leucine-rich repeat motif shared by Leishmania major proteophosphoglycan and parasite surface antigen 2. Mol. Biochem. Parasitol. 107289-295. [DOI] [PubMed] [Google Scholar]
- 32.Newgreen, D., and H. M. Young. 2002. Enteric nervous system: development and developmental disturbances—part 2. Pediatr. Dev. Pathol. 5329-349. [DOI] [PubMed] [Google Scholar]
- 33.Niemann, H. H., V. Jager, P. J. Butler, J. van den Heuvel, S. Schmidt, D. Ferraris, E. Gherardi, and D. W. Heinz. 2007. Structure of the human receptor tyrosine kinase met in complex with the Listeria invasion protein InlB. Cell 130235-246. [DOI] [PubMed] [Google Scholar]
- 34.Pereira, M. E. 1983. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J. Immunol. Methods 6325-34. [DOI] [PubMed] [Google Scholar]
- 35.Powers, J. F., M. J. Evinger, P. Tsokas, S. Bedri, J. Alroy, M. Shahsavari, and A. S. Tischler. 2000. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 302309-320. [DOI] [PubMed] [Google Scholar]
- 36.Prioli, R. P., J. S. Mejia, and M. E. Pereira. 1990. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J. Immunol. 1444384-4391. [PubMed] [Google Scholar]
- 37.Rock, R. B., and P. K. Peterson. 2006. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J. Neuroimmune Pharmacol. 1117-126. [DOI] [PubMed] [Google Scholar]
- 38.Sanicola, M., C. Hession, D. Worley, P. Carmillo, C. Ehrenfels, L. Walus, S. Robinson, G. Jaworski, H. Wei, R. Tizard, A. Whitty, R. B. Pepinsky, and R. L. Cate. 1997. Glial cell line-derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc. Natl. Acad. Sci. USA 946238-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sariola, H., and M. Saarma. 2003. Novel functions and signalling pathways for GDNF. J. Cell Sci. 1163855-3862. [DOI] [PubMed] [Google Scholar]
- 40.Schenkman, S., D. Eichinger, M. E. Pereira, and V. Nussenzweig. 1994. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 48499-523. [DOI] [PubMed] [Google Scholar]
- 41.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103501-510. [DOI] [PubMed] [Google Scholar]
- 42.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304248-253. [DOI] [PubMed] [Google Scholar]
- 43.Slevin, J. T., G. A. Gerhardt, C. D. Smith, D. M. Gash, R. Kryscio, and B. Young. 2005. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 102216-222. [DOI] [PubMed] [Google Scholar]
- 44.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell. Microbiol. 5143-153. [DOI] [PubMed] [Google Scholar]
- 45.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend, G. C., and W. M. Scheld. 1998. Infections of the central nervous system. Adv. Intern. Med. 43403-447. [PubMed] [Google Scholar]
- 47.Wang, X., R. H. Baloh, J. Milbrandt, and K. C. Garcia. 2006. Structure of artemin complexed with its receptor GFRα3: convergent recognition of glial cell line-derived neurotrophic factors. Structure 141083-1092. [DOI] [PubMed] [Google Scholar]
- 48.Yefenof, E. 2000. Complement receptor 3 (CR3): a public transducer of innate immunity signals in macrophages. Adv. Exp. Med. Biol. 47915-25. [DOI] [PubMed] [Google Scholar]