Abstract
Invasive aspergillosis is characterized by hyphal invasion of the blood vessels, which contributes to the pathogenesis of this disease. During this angioinvasion, Aspergillus fumigatus interacts with the endothelial cell lining of the blood vessels. We investigated the response of vascular endothelial cells to A. fumigatus infection in vitro and in mouse models of invasive pulmonary aspergillosis. Infection with hyphae, but not with conidia, stimulated endothelial cells to synthesize E-selectin, vascular cell adhesion molecule 1 (VCAM-1), interleukin 8, and tumor necrosis factor alpha (TNF-α) in vitro. Killed hyphae induced approximately 40% less stimulation than did live hyphae. Endothelial cell stimulation required contact between the hyphae and endothelial cells but not endocytosis of the organisms. Studies with ΔgliP and ΔstuA null mutants of A. fumigatus indicated that the extent of endothelial cell stimulation was not influenced by gliotoxin or other StuA-dependent factors synthesized by A. fumigatus. In neutropenic mice infected with wild-type A. fumigatus, increased pulmonary expression of E-selectin, cytokine-induced neutrophil chemoattractant (KC), and TNF-α occurred only when neutropenia had resolved. In nonneutropenic mice immunosuppressed with corticosteroids, A. fumigatus stimulated earlier pulmonary expression of E-selectin, VCAM-1, and KC, while expression of intercellular adhesion molecule 1 and TNF-α was suppressed. In both mouse models, expression of E-selectin and KC was associated with high pulmonary fungal burden, angioinvasion, and neutrophil adherence to endothelial cells. Therefore, the expression of leukocyte adhesion molecules and secretion of proinflammatory cytokines by endothelial cells in response to A. fumigatus could enhance the host defense against this organism by contributing to the recruitment of activated leukocytes to sites of angioinvasion.
Invasive aspergillosis is the most common mold infection in immunocompromised patients, with Aspergillus fumigatus accounting for the majority of these cases (13). A characteristic finding in invasive aspergillosis is invasion of the blood vessels by the fungus (2, 13). There are two major consequences of this angioinvasion. First, it results in pulmonary hemorrhage and vascular thrombosis at the site of the initial infection. Thrombosis of the pulmonary vasculature leads to tissue infarction, which permits fungi at the center of these infarcted regions to grow unchecked, as there is poor delivery of leukocytes as well as antifungal agents to these areas. Second, angioinvasion is the mechanism by which organisms disseminate via the bloodstream to other organs, a process that is associated with a dismal prognosis (28).
The key role of angioinvasion in the pathogenesis of invasive aspergillosis has prompted us to study the interactions of A. fumigatus with vascular endothelial cells in vitro. Endothelial cells endocytose both conidia and hyphae of A. fumigatus in vitro, a process that results in both endothelial cell damage and stimulation of tissue factor expression (11, 18, 29). It is probable that this expression of tissue factor contributes to the intravascular thrombosis that occurs at sites of A. fumigatus angioinvasion. Endothelial cells have the capacity to influence the local host response against microbial pathogens through expression of leukocyte adhesion molecules and secretion of proinflammatory cytokines (5). Other fungal pathogens, such as Candida albicans, stimulate endothelial cell expression of these proinflammatory mediators (4, 16, 21). However, the effect of A. fumigatus on the expression of leukocyte adhesion molecules and proinflammatory cytokines by endothelial cells has not been previously determined. In the present study, we investigated the capacity of A. fumigatus to stimulate the expression of these immunomodulatory factors by endothelial cells, both in vitro and in vivo.
MATERIALS AND METHODS
Strain and growth conditions.
The strains and source of A. fumigatus that were used in these experiments are listed in Table 1. Conidia and hyphae were grown and prepared as described previously (11). Briefly, all A. fumigatus strains, except for the ΔstuA null mutant and the ΔstuA::stuA complemented strain, were grown on Sabouraud dextrose agar (Becton Dickinson, Franklin Lakes, NJ) for at least 7 days at 35°C. Conidia were harvested by flooding plates with phosphate-buffered saline (Mediatech, Inc., Herndon, VA) containing 0.1% Tween 80 (vol/vol) (Sigma-Aldrich, St. Louis, MO). Hyphae were prepared by incubating the conidia in Sabouraud dextrose broth on 150-mm-diameter petri dishes at 37°C in 5% CO2 for approximately 6 h. The resulting germ tubes were 4 to 10 μm in length.
TABLE 1.
Strains of A. fumigatus used in the experiments
The ΔstuA mutant has a defect in conidiation (22) and produced insufficient conidia for the experiments when grown on Sabouraud agar. Therefore, this mutant was grown on YEPD agar (1% yeast extract, 2% peptone, 2% glucose, 2% agar) for 6 days prior to the harvesting of the conidia. Af293 (the wild type) and the ΔstuA::stuA complemented strain were grown similarly on YEPD agar for comparison in these experiments.
For experiments using killed organisms, hyphae were incubated in 0.02% thimerosal in Hanks balanced salt solution (Irvine Scientific, Santa Ana, CA) at room temperature overnight and then washed extensively before use (11).
Endothelial cells.
Endothelial cells were isolated from human umbilical cord veins by the method of Jaffe et al. (7) and cultured as previously described (21) in tissue culture medium consisting of M-199 medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 10% defined bovine calf serum (both from Gemini Bio-Products, Inc., West Sacramento, CA), and containing 2 mM l-glutamine with penicillin and streptomycin (Irvine Scientific, Santa Ana, CA). Endothelial cells were maintained in a humidified incubator at 37°C in 5% CO2 and used in the experiments after the second or third passage.
Endothelial cell stimulation.
The effects of A. fumigatus on endothelial cell expression of leukocyte adhesion molecules were determined by a minor modification of our previously described method (16). Briefly, the endothelial cells were grown in fibronectin-coated 96-well tissue culture plates for 72 h prior to use. On the day of the experiment, the medium was aspirated and each well was then infected with 3.3 × 104 conidia or hyphae in 200 μl of tissue culture medium for 8 h at 37°C. Parallel wells were incubated with medium alone as a negative control, with tumor necrosis factor alpha (TNF-α) at 17 pg/ml for the E-selectin experiments, or with 85 pg TNF-α/ml for the vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) experiments as a positive control. At the end of the incubation period, the cells were rinsed, fixed with 2 to 4% paraformaldehyde, and then incubated with monoclonal antibodies to E-selectin, VCAM-1, or ICAM-1 (Biodesign International, Saco, ME) overnight. The cells were washed and incubated with horseradish peroxidase-conjugated sheep anti-mouse secondary antibody for 1 h. The plates were developed by addition of SigmaFast O-phenylenediamine (Sigma-Aldrich), and the optical density at 490 nm was determined.
Experiments designed to detect endothelial cell secretion of TNF-α and interleukin 8 (IL-8) were performed similarly to the leukocyte adhesion molecule experiments except that the endothelial cells were grown in 24-well tissue culture plates and the inoculum was 2 × 105 A. fumigatus conidia or hyphae in 0.5 ml of tissue culture medium. In the IL-8 experiments, TNF-α (1 ng/ml) was used as the positive control. After 8 h of incubation, the conditioned medium was collected and centrifuged at 1,000 × g to remove the cells. The concentrations of TNF-α and IL-8 in the supernatant were measured using commercial enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Inc., Minneapolis, MN, and Biosource International, Inc., Camarillo, CA, respectively).
To determine if the endocytosis of A. fumigatus was necessary for stimulation of endothelial cell E-selectin expression, the endothelial cells were infected with organisms in the presence of 70 nM of the microfilament inhibitor cytochalasin D. This concentration of cytochalasin D is sufficient to block endocytosis of A. fumigatus and has no detectable endothelial cell toxicity (11). Control endothelial cells were exposed to the diluent (dimethyl sulfoxide) alone.
To investigate whether soluble factors secreted by A. fumigatus influenced E-selectin expression, 3.3 × 104 hyphae were placed in tissue culture filter inserts (0.2-um pore size; Nunc, Rochester, NY), which were suspended approximately 1 mm above the endothelial cells. The infected endothelial cells were incubated for 8 h, after which cells were processed as described above for E-selectin stimulation.
All experiments were performed in duplicate or triplicate on at least three different occasions.
Endothelial cell damage.
The extent of endothelial cell damage caused by hyphae of the various strains of A. fumigatus was determined using a 51Cr release assay exactly as described previously (11). The endothelial cells were grown in 24-well tissue culture plates, and the inoculum, medium, and incubation time were the same as in the endothelial cell stimulation experiments. The experiments were performed in triplicate three times.
Mouse model of invasive pulmonary aspergillosis.
To determine the effect of A. fumigatus infection on the expression of leukocyte adhesion molecules and cytokines in vivo, we used our previously described mouse models of invasive pulmonary aspergillosis (24, 25). All studies with mice were approved by the Institutional Animal Use and Care Committee of the Los Angeles Biomedical Research Institute in accordance to the National Institutes of Health guidelines for the ethical treatment of animals. In the neutropenic mouse model, specific-pathogen-free male BALB/c mice weighing 17 to 20 g were immunosuppressed by administration of 250 mg/kg of body weight of both cyclophosphamide (Western Medical Supply, Arcadia, CA) and cortisone acetate (Sigma-Aldrich) 2 days prior to infection and with 200 mg/kg of cyclophosphamide and 250 mg/kg of cortisone acetate 3 days after infection. In the nonneutropenic, immunosuppressed mouse model, mice were given 10 mg of cortisone acetate every other day, from day −4 to day +4 relative to the day of infection, for a total of five doses. In both models, the mice were infected with A. fumigatus by placing them for 1 h in an inhalational chamber containing an aerosol generated from 12 ml of 1 × 109 conidia/ml. Control mice were immunosuppressed with the same regimens but were not infected. While the mice were neutropenic or immunosuppressed with steroids, they were given 5 mg ceftazidime subcutaneously once daily to prevent bacterial infection.
On days 2, 4, 6, and 8 after infection in the neutropenic model, and on days 2, 4, and 6 after infection in the nonneutropenic model, groups of at least eight infected and six uninfected mice were sacrificed. These time points were chosen so that unbiased comparison could be made between infected and uninfected groups, because there was >90% survival of all mice. The lungs were excised and rinsed in phosphate-buffered saline. Two sets of infected lungs were processed for histopathology (described below). The remaining lungs were weighed and homogenized in the presence of protease inhibitor cocktail (Sigma-Aldrich). Nonidet P-40 substitute (Sigma-Aldrich) was added to a final concentration of 1% and the mixture was incubated on ice for 20 min (8). Next, the homogenate was clarified by centrifugation at 1,000 × g at 4°C for 5 min, after which the supernatant was collected and stored at −80°C. The concentrations of murine soluble E-selectin (sE-selectin), sVCAM-1, sICAM-1, and cytokine-induced neutrophil chemoattractant (KC) were determined by use of an ELISA from R&D Systems. The concentration of TNF-α was determined by use of an ELISA from BioSource International.
The fungal burdens of the mice were evaluated by determining the pulmonary galactomannan content. The amount of galactomannan in the lung homogenates was measured using the Platelia Aspergillus enzyme immunoassay (Bio-Rad, Hercules, CA) according to a minor modification of the manufacturer's instructions as reported previously (23). Briefly, a 15-μl sample of each pulmonary homogenate was diluted in ultrapure water (from 1:20 to 1:300 for samples harvested on days 2 to 8, respectively) and processed according to the manufacturer's instructions. Optical densities were then compared to a standard curve made of serial dilutions of a standard pool of lung homogenates obtained from highly infected mice to determine the relative galactomannan content per gram of lung tissue.
At each time point, the lungs from two mice were used for histopathological examination. They were fixed in zinc-buffered formalin and then stored in 70% ethanol. They were subsequently embedded in paraffin, after which thin sections were prepared and stained with Gomori methenamine-silver (GMS) and periodic acid-Schiff (PAS) stains.
Statistical analysis.
Differences in adherence and the endothelial cell response to A. fumigatus in vitro were compared using analysis of variance. The results of the animal experiments were analyzed using the Wilcoxon rank sum test. P values of ≤0.05 were considered to be significant.
RESULTS
A. fumigatus hyphae, but not conidia, stimulated endothelial cells to express leukocyte adhesion molecules and secrete proinflammatory cytokines in vitro.
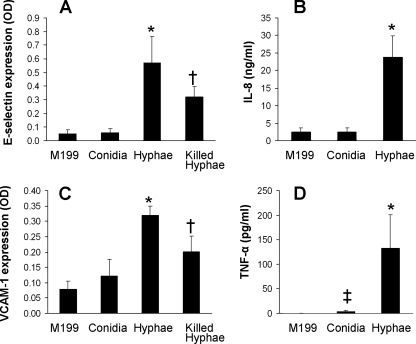
Endothelial cells were infected for 8 h with A. fumigatus hyphae or conidia, after which the endothelial cell surface expression of the leukocyte adhesion molecules E-selectin, VCAM-1, and ICAM-1 was measured. We found that hyphae stimulated endothelial cells to express E-selectin and VCAM-1, whereas conidia did not (Fig. 1A and C). Neither form of A. fumigatus stimulated endothelial cells to express ICAM-1 (data not shown). We also investigated whether the two different forms of A. fumigatus had different effects on the endothelial cell secretion of IL-8 and TNF-α. Hyphae also stimulated much greater secretion of both of these cytokines than did conidia (Fig. 1B and D).
FIG. 1.
A. fumigatus hyphae stimulate endothelial cells. Endothelial cells were exposed to the indicated conditions for 8 h, after which the surface expression of E-selectin (A) and VCAM-1 (C) was measured and the accumulation of IL-8 (B) and TNF-α (D) in the culture medium was determined. Results are the means ± standard deviations from three experiments, each performed in duplicate or triplicate. *, P < 0.0001 versus endothelial cells exposed to M199 or conidia; †, P < 0.009 versus live hyphae and P < 0.0001 versus M199; ‡, P < 0.0005 versus M199. OD, optical density.
Next, we investigated whether fungal viability influenced the response of endothelial cells to A. fumigatus hyphae. Hyphae were killed with the metabolic poison thimerosal to minimize changes to the cell surface. Although the killed hyphae stimulated approximately 40% less expression of E-selectin and VCAM-1 than did live hyphae, they still induced a significant increase in E-selectin and VCAM-1 expression compared to what was seen for unstimulated endothelial cells (Fig. 1A and C). These results indicate that a factor that is associated with killed hyphae is capable of stimulating endothelial cells. Also, live hyphae likely have a different cell surface and/or an ongoing metabolic process required for maximal endothelial stimulation.
Stimulation of E-selectin expression required direct contact between hyphae and endothelial cells.
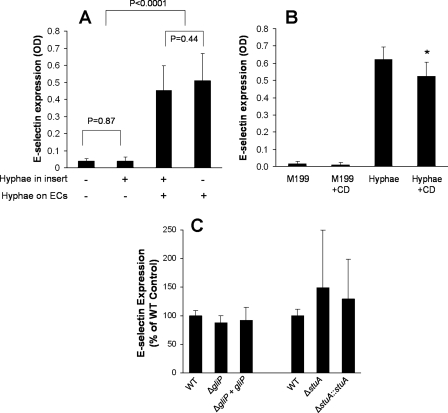
Next, the mechanisms by which A. fumigatus stimulates endothelial cells were investigated using E-selectin expression as an indicator of endothelial cell stimulation. The finding that A. fumigatus viability was required for maximal stimulation of endothelial cells suggested that viable A. fumigatus hyphae may secrete a soluble factor that stimulates endothelial cells. To investigate this possibility, we prevented contact between the organisms and endothelial cells by adding the hyphae to tissue culture inserts suspended over the endothelial cells. The presence of hyphae in the tissue culture insert had no effect on the levels of E-selectin expression by both uninfected and infected endothelial cells (Fig. 2A). Therefore, contact between A. fumigatus hyphae and endothelial cells is required for endothelial cell stimulation.
FIG. 2.
A. fumigatus stimulation of cell E-selectin expression requires direct endothelial cell contact but not endocytosis of the organism and is not influenced by gliotoxin or other stuA-dependent secondary metabolites. Endothelial cell expression of E-selectin was measured when the organisms were suspended above the endothelial cells (ECs) in filter inserts with 0.2-μm pores (A), after infection with wild-type A. fumigatus hyphae in the presence (+CD) or absence of cytochalasin D (B), and after infection with the indicated A. fumigatus mutants (C). Results are the means ± standard deviations from three experiments, each performed in triplicate. *, P < 0.035 compared to endothelial cells exposed to hyphae without cytochalasin D. OD, optical density; WT, wild type.
After A. fumigatus hyphae come in contact with endothelial cells, they induce their own endocytosis by these cells (11). We investigated whether the endothelial cell endocytosis of A. fumigatus hyphae was required for induction of leukocyte adhesion molecule expression. Endothelial cells were infected with the hyphae in the presence of the microfilament inhibitor cytochalasin D at a concentration that was sufficient to block endothelial cell endocytosis of A. fumigatus (11). Cytochalasin D had a minimal effect on the expression of E-selectin induced by A. fumigatus (Fig. 2B). Collectively, these results indicate that contact of A. fumigatus hyphae with endothelial cells in the absence of endocytosis is sufficient to induce endothelial cell stimulation.
A. fumigatus mutants with defects in the synthesis of gliotoxin and other putative secondary metabolites stimulated normal E-selectin expression.
The A. fumigatus genome contains 22 to 26 gene clusters that specify the enzymes necessary for the synthesis of a corresponding number of secondary metabolites (mycotoxins) (15, 20). These secondary metabolites include gliotoxin and aflatoxin and have the potential to significantly influence the response of endothelial cells to fungal infection (19, 25, 27). Although we found that soluble factors secreted by A. fumigatus were not sufficient to induce endothelial cell E-selectin expression, it remained possible that one or more of these secondary metabolites could modulate the endothelial cell response to A. fumigatus. To investigate this possibility, we analyzed the endothelial cell response to infection with ΔgliP and ΔstuA null mutant strains. These strains have defects in the synthesis of secondary metabolites. The ΔgliP mutant does not synthesize gliotoxin (25). The ΔstuA mutant has reduced expression of five secondary metabolite gene clusters, including the one responsible for the synthesis of gliotoxin (22). Infection with the ΔgliP and ΔstuA mutants stimulated the same level of E-selectin expression on endothelial cells as was induced by the wild-type strain (Fig. 2C). These results indicate that neither gliotoxin nor the secondary metabolites whose synthesis is governed by StuA influence endothelial cell E-selectin expression.
To interpret the stimulation results with the various A. fumigatus mutants, we also measured the extent of endothelial cell damage caused by these strains. After 8 h of incubation, hyphae of the wild-type and null mutant strains caused very little endothelial cell damage (<10% specific 51Cr release), and there was no difference in the amounts of damage caused by the different strains (data not shown).
Neutropenic mice with invasive aspergillosis had delayed expression of leukocyte adhesion molecules and proinflammatory cytokines.
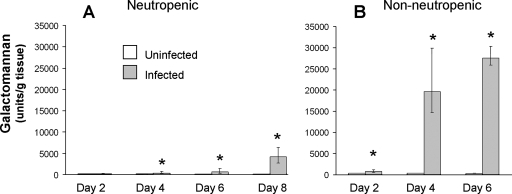
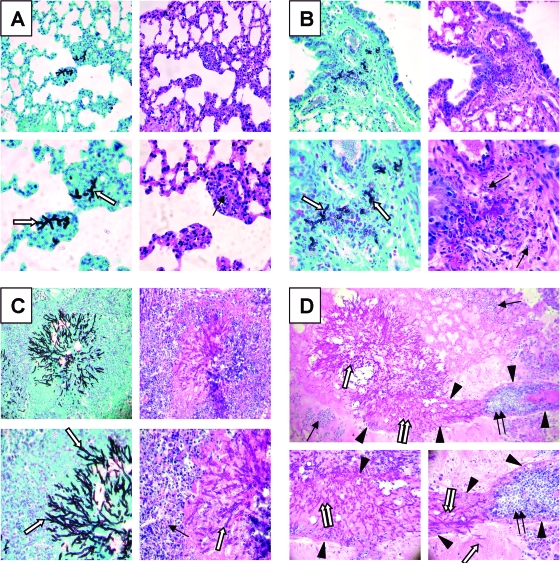
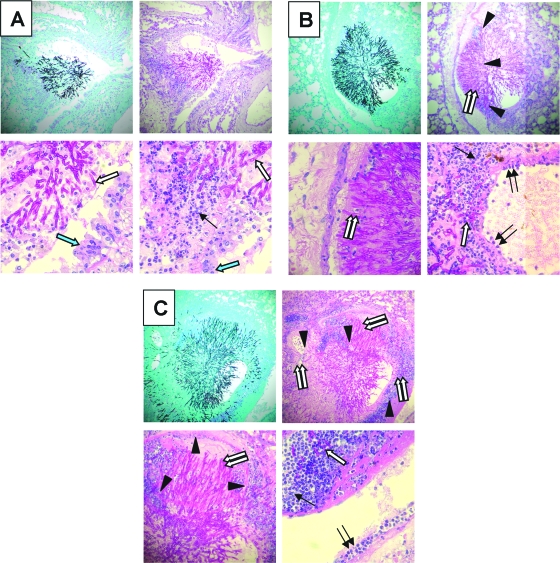
Next, we investigated the time course of pulmonary fungal burden, phagocyte accumulation, and expression of leukocyte adhesion molecules and proinflammatory cytokines in neutropenic mice with invasive pulmonary aspergillosis. The pulmonary fungal burden of the infected mice, as measured by whole-lung galactomannan content, increased exponentially over time (Fig. 3A). As expected, the galactomannan content of the lungs of the uninfected mice remained low at all time points. Histopathological examination of the lungs also demonstrated that the number of fungi and the size of the lesions increased progressively over time (Fig. 4). No fungi were visible in the lungs at day 2 of infection (data not shown). By day 4 of infection, rare foci of infection that contained a few hyphae were visible (Fig. 4A). As expected for neutropenic mice, there were very few leukocytes present at these foci, which were mainly mononuclear cells. By day 6 of infection, the foci of infection were larger and contained more hyphae. These hyphae were surrounded by a few leukocytes, which were fragmented (Fig. 4B). By day 8 of infection, the mice were no longer neutropenic (24). There were many foci of infection and they contained large numbers of hyphae. The hyphae were surrounded by an extensive inflammatory infiltrate consisting mainly of neutrophils (Fig. 4C). At this time point, angioinvasion was visible (Fig. 4D). Furthermore, neutrophils could be seen adhering to the walls of the invaded blood vessels. This neutrophil margination is evidence that these endothelial cells were expressing leukocyte adhesion molecules.
FIG. 3.
Time course of pulmonary fungal burden in mice with invasive pulmonary aspergillosis. Mice were immunosuppressed either with cortisone acetate and cyclophosphamide (A) or with high-dose cortisone acetate alone (B). They were infected with A. fumigatus conidia in an aerosol chamber. Control mice were immunosuppressed but not infected. At the indicated time points, the mice were sacrificed, after which the galactomannan content of their lungs was determined. Results are the medians ± interquartile ranges for six to nine mice per group. *, P < 0.009 compared to uninfected mice at the same time point.
FIG. 4.
Histopathology of the lungs of neutropenic mice with invasive aspergillosis. (A) Day 4 results showing a small focus of hyphae surrounded by a mononuclear infiltrate. (B) Day 6 results showing a larger focus of infection with more hyphae surrounded by leukocytes with apoptotic nuclei. (C and D) Day 8 results showing extensive hyphae surrounded by numerous neutrophils (C) and angioinvasion, disruption of endothelial cells, and adjacent neutrophil margination (D). In panels A to C, GMS staining is shown on the left and PAS staining on the right, while the tops are shown at a magnification of ×200 and the bottoms at ×400. (D) PAS stain; top-panel magnification, ×100; bottom-panel magnification, ×400. Single white arrow, fungi; black arrows, leukocytes; arrowheads, blood vessel walls; double white arrows, hyphae within the blood vessel lumen; double black arrows, neutrophils adherent to endothelial cells.
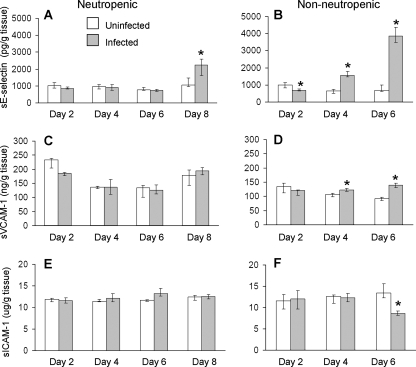
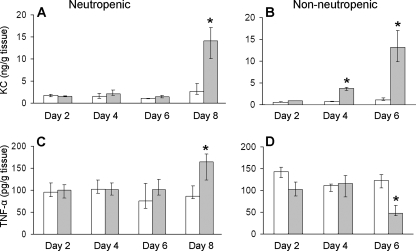
The profile of whole-lung leukocyte adhesion molecule expression and proinflammatory cytokine content paralleled the time course of leukocyte recruitment. During the first 6 days of infection, there was no significant increase in the expression E-selectin, VCAM-1, KC, and TNF-α compared to what was seen for immunosuppressed, uninfected mice at the same time points (Fig. 5 and 6). The expression of E-selectin, KC, and TNF-α did not increase significantly until day 8 of infection. There was no significant change in the expression of ICAM-1 or VCAM-1 at any of the time points tested. The increase in E-selectin, KC, and TNF-α corresponded to the resolution of neutropenia and the recruitment of large numbers of neutrophils to the foci of infection. These results suggest that there is minimal host response to A. fumigatus during the first 6 days of infection in these highly immunocompromised mice. They further indicate that significant expression of leukocyte adhesion molecules and proinflammatory cytokines does not occur until immunosuppression wanes.
FIG. 5.
Time course of pulmonary leukocyte adhesion molecule expression during invasive aspergillosis of neutropenic and nonneutropenic immunosuppressed mice. Concentrations of sE-selectin (A and B), sVCAM-1 (C and D), and sICAM-1 (E and F) in lung homogenates at the indicated time points. Results are the medians ± interquartile ranges of at least six mice per time point. *, P < 0.03 compared to uninfected mice at the same time point.
FIG. 6.
Time course for pulmonary cytokine expression during invasive aspergillosis in neutropenic and nonneutropenic immunosuppressed mice. Concentrations of KC (A and B) and TNF-α (C and D) in lung homogenates at the indicated time points. Results are the medians ± interquartile ranges of at least six mice per time point. *, P < 0.01 compared to uninfected mice at the same time point.
Invasive pulmonary aspergillosis in nonneutropenic mice immunosuppressed with cortisone acetate alone had more-rapid progression of disease and earlier expression of leukocyte adhesion molecules and cytokines.
The results with the neutropenic mice suggested that the presence of neutrophils significantly influenced the endothelial cell response to A. fumigatus. Therefore, we investigated the host response to invasive pulmonary aspergillosis in nonneutropenic mice that were immunosuppressed with cortisone acetate alone. In these mice, the pulmonary fungal burden increased earlier and more rapidly than in the neutropenic mice despite the fact that the initial infectious doses were identical in both models (Fig. 3B).
The pulmonary histopathology of the nonneutropenic mice also demonstrated accelerated disease progression. After only 2 days of infection, foci of hyphal elements were visible (Fig. 7). These lesions increased in size over time and were much larger than the lesions seen at the corresponding time points for the neutropenic mice. Interestingly, for the nonneutropenic mice, most of the foci of infection appeared to originate from the bronchioles rather than the alveoli, while in neutropenic mice the infection appeared to originate predominantly from the alveoli. The inflammatory infiltrates surrounding the fungi were composed of neutrophils at all time points. By day 4 of infection, angioinvasion was visible. Also, some neutrophils were adherent to the vascular endothelium, indicating that the endothelial cells were expressing leukocyte adhesion molecules at this time point. On day 6 of infection, there was extensive angioinvasion and increased neutrophil adherence to the endothelium adjacent to the foci of infection.
FIG. 7.
Histopathology of the lungs of nonneutropenic mice with invasive aspergillosis. (A) Day 2 results showing a focus of hyphae in the bronchial epithelium that is invading into the adjacent lung tissue and is surrounded by a neutrophilic infiltrate. (B) Day 4 results showing a larger focus of infection with more hyphae surrounded by neutrophils. The hyphae have invaded into a blood vessel. Another lesion shows neutrophil margination. (C) Day 6 results showing extensive hyphae surrounded by numerous neutrophils, with invasion of three blood vessels and adjacent neutrophil margination. GMS staining is shown in the upper left panels and PAS staining in the other panels; top-panel magnification, ×100 (note the difference in magnification from that in Fig. 4.); bottom-panel magnification, ×400. Single white arrow, fungi; black arrows, leukocytes; arrowheads, blood vessel walls; double white arrows, hyphae within the blood vessel lumen; double black arrows, neutrophils adherent to endothelial cells; single blue arrow, bronchial epithelium.
As predicted by the histopathological findings, the lungs of the nonneutropenic mice had significantly increased expression of E-selectin, VCAM-1, and KC by day 4 and even greater expression by day 6 (Fig. 5 and 6). Surprisingly, the pulmonary expression of ICAM-1 and TNF-α in infected mice was significantly lower than that in the uninfected, immunosuppressed control mice, even on day 6, despite the high fungal burden of the infected mice. These results demonstrate that in the lungs of nonneutropenic mice, A. fumigatus infection has both immunostimulatory and immunosuppressive effects.
DISCUSSION
A. fumigatus conidia are important for the initiation of invasive aspergillosis, as they interact with pulmonary epithelial cells in the alveoli. However, only hyphae are visible at foci of established invasive and disseminated aspergillosis, suggesting that this form of the organism is most important for tissue invasion and hematogenous dissemination. Consistent with this observation, our data demonstrate that A. fumigatus hyphae and conidia induce different endothelial cell responses in vitro. Hyphae stimulated endothelial cells to express the leukocyte adhesion molecules E-selectin and VCAM-1 and secrete the proinflammatory cytokines TNF-α and IL-8. In contrast, conidia did not stimulate production of any leukocyte adhesion molecule or IL-8 and induced only minimal secretion of TNF-α. Previously, we found that A. fumigatus hyphae also induce endothelial cells to express tissue factor, while conidia do not (11). Thus, the mechanisms by which hyphae induce proinflammatory and procoagulant responses by endothelial cells may be similar. A. fumigatus hyphae have similarly been reported to induce a stronger proinflammatory response by other types of host cells. For example, both alveolar and peritoneal macrophages from mice secrete significantly more TNF-α in response to hyphae than in response to conidia (6, 26). It is known that the composition of the cell surface of A. fumigatus changes significantly as resting conidia swell and then germinate. However, it is not yet known which of these surface changes are responsible for the high host cell stimulation induced by hyphae compared to that induced by conidia.
The data also indicated that A. fumigatus stimulation of endothelial cells requires direct contact between the hyphae and endothelial cells. However, the organism does not need to be endocytosed by the endothelial cells to induce expression of E-selectin. Interestingly, killed hyphae still stimulated significant E-selectin expression. This result suggests that a factor associated with the cell wall of A. fumigatus is sufficient to stimulate endothelial cells. The identity of this factor is currently unknown. However, the fact that the ΔgliP and ΔstuA mutants stimulated the same amount of E-selectin as did the wild-type strain indicate that neither gliotoxin nor the other factors whose synthesis is governed by StuA influence endothelial cell stimulation under the conditions tested. Because StuA governs the expression of only a subset of secondary metabolite biosynthetic pathways, it still remains possible that one or more StuA-independent secondary metabolites alter the endothelial cell response to A. fumigatus.
The current results demonstrate that A. fumigatus stimulates endothelial cells significantly differently than does another fungal pathogen, C. albicans. Although C. albicans stimulates endothelial cells to express E-selectin, VCAM-1, IL-8, and TNF-α in vitro, it also induces the expression of ICAM-1, whereas A. fumigatus does not (4, 16, 21). We found that A. fumigatus did not stimulate endothelial cell ICAM-1 expression after 8 h of infection. Therefore, it remains possible that A. fumigatus might stimulate ICAM-1 expression at other time points. However, this possibility seems unlikely, as we found that A. fumigatus did not induce ICAM-1 expression in the lungs of either neutropenic or nonneutropenic mice at all time points tested. The lack of ICAM-1 stimulation could potentially be due to the production of gliotoxin, which is known to inhibit NF-κB-dependent expression of ICAM-1 (17). Like A. fumigatus, C. albicans must form hyphae to stimulate endothelial cells. Unlike what is seen for A. fumigatus, C. albicans stimulation of endothelial cells is completely blocked by cytochalasin D (4). Also, killed C. albicans hyphae do not stimulate endothelial cells (4), whereas killed A. fumigatus hyphae still induce significant endothelial cell stimulation.
We used an inhalational neutropenic murine model of invasive aspergillosis to study the profile of leukocyte adhesion molecules and proinflammatory cytokines that was induced by A. fumigatus infection in vivo. A striking finding was that in these highly immunosuppressed mice, there was essentially no detectable increase in pulmonary leukocyte adhesion molecule and cytokine content during the first 6 days of infection. It was not until day 8 that there was significant evidence of an inflammatory response, with large increases of E-selectin, KC, and TNF-α expression. This response coincided with waning of immunosuppression, as evidenced by resolution of neutropenia. It was also accompanied by a substantial rise in pulmonary galactomannan content, increased size of fungal lesions, and histopathological evidence of invasion of blood vessels. Although KC and TNF-α are produced by multiple types of host cells, E-selectin is produced only by endothelial cells. Therefore, the significant increase in whole-lung E-selectin content at day 8 is compelling evidence of endothelial cell activation and provides a mechanism for the prominent neutrophil margination that was seen at this time point.
There are several nonexclusive possibilities for the lack of an early proinflammatory response to A. fumigatus in neutropenic mice. One possibility is that there was an increase in the production of proinflammatory mediators at the early time points but that this increase occurred only in the small foci of infection. Therefore, because these foci represented such a small fraction of the total lung tissue, no increase in cytokines and leukocyte adhesion molecules was detectable when their levels were assayed in whole-lung homogenates. By day 8, the dramatically increased pulmonary fungal burden and extensive angioinvasion were sufficient to cause a detectable increase in the level of proinflammatory mediators. Another possibility is that immunosuppression with cortisone acetate and cyclophosphamide inhibited the activation of endothelial cells and other cell types within the lung. Finally, it is also possible that in vivo, leukocytes are required to induce and/or amplify the host proinflammatory response to A. fumigatus.
To address some of these possibilities, we analyzed the host response to A. fumigatus in nonneutropenic mice that were immunosuppressed with cortisone acetate alone. In these mice, there was early stimulation of E-selectin, VCAM-1, and KC expression compared to what was seen for the neutropenic mice. This accelerated proinflammatory response in the nonneutropenic mice was associated with increased pulmonary fungal burden and more-rapid angioinvasion. It is likely that the increased expression of E-selectin, VCAM-1, and KC in the nonneutropenic mice was due to the presence of neutrophils at the foci of infection. In addition, it is probable that the greater pulmonary fungal burden and angioinvasion that was present at the early time points in these mice also contributed to the enhanced proinflammatory response.
A paradoxical finding was that after 6 days of infection, the expression of ICAM-1 and TNF-α was significantly lower than that in the uninfected control mice. We speculate that this suppression of ICAM-1 and TNF-α expression was due to the release of immunosuppressive secondary metabolites, such as gliotoxin, by A. fumigatus (17, 25, 27). It is clear that the synthesis of TNF-α and ICAM-1 must be governed by signal transduction mechanisms different from those for the synthesis of E-selectin, VCAM-1, and KC, because the synthesis of these other inflammatory mediators progressively increased during infection.
It was notable that the pulmonary fungal burden of the nonneutropenic mice was from 4-fold (day 2) to over 40-fold (days 4 and 6) higher than that of the neutropenic mice, even though both sets of mice received the same inoculum of A. fumigatus. Consistent with these results, we have found previously that nonneutropenic mice immunosuppressed with high doses of cortisone acetate have higher mortality than neutropenic mice that receive a lower dose of cortisone acetate (23, 25). A key difference between the nonneutropenic and neutropenic mouse models is that the total amount of cortisone acetate administered to the nonneutropenic mice was approximately fivefold greater than that given to the neutropenic mice. There are at least two reasons why high-dose corticosteroids worsen the course of invasive pulmonary aspergillosis. First, corticosteroids are broadly immunosuppressive. They suppress the secretion of proinflammatory cytokines by multiple types of host cells and inhibit the fungicidal activities of phagocytic cells (3, 10). Second, corticosteroids enhance the growth of A. fumigatus even in the absence of host cells (14). Our finding that treatment with cortisone acetate alone is sufficient to make mice susceptible to invasive aspergillosis is similar to the situation for humans, in whom the receipt of high-dose corticosteroids is a significant risk factor for developing this disease, even in the absence of neutropenia (1, 9, 12).
In summary, endothelial cells respond to hyphae but not conidia by expressing leukocyte adhesion molecules and proinflammatory cytokines in vitro. Endothelial cell stimulation also occurs in both neutropenic and nonneutropenic immunosuppressed mice with invasive pulmonary aspergillosis. In the neutropenic mouse model, this stimulation is not detectable until relatively late in the course of disease. In both mouse models, endothelial stimulation is associated with increasing fungal burden and angioinvasion. Additional investigation of the mechanisms by which A. fumigatus hyphae stimulate endothelial cells will likely provide new insights into the process of angioinvasion and the vascular inflammatory response, which is central to the pathogenesis of invasive and disseminated aspergillosis.
Acknowledgments
We thank Norma Solis and Q. Trang Phan for expert assistance with tissue culture and the perinatal nurses of the Harbor-UCLA General Clinical Research Center for collection of umbilical cords. We are grateful for the assistance of Thomas Doedt, Norma Solis, and Daniele Ejzykowicz with the animal studies. The assistance of Samuel W. French for histopathological interpretation is appreciated.
This work was supported in part by grants M01RR00425, R21AI064511, and R01AI073829 and by contract no. N01-AI-30041 from the National Institutes of Health. D.C.S. was supported in part by a Canadian Institutes of Health operating grant. L.Y.C. was supported in part by a postdoctoral fellowship grant from the American Heart Association, Western States Affiliates.
Editor: A. Casadevall
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Cornillet, A., C. Camus, S. Nimubona, V. Gandemer, P. Tattevin, C. Belleguic, S. Chevrier, C. Meunier, C. Lebert, M. Aupee, S. Caulet-Maugendre, M. Faucheux, B. Lelong, E. Leray, C. Guiguen, and J. P. Gangneux. 2006. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin. Infect. Dis. 43577-584. [DOI] [PubMed] [Google Scholar]
- 2.Denning, D. W. 2000. Aspergillus species, p. 2674-2684. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingston, Philadelphia, PA.
- 3.Duong, M., N. Ouellet, M. Simard, Y. Bergeron, M. Olivier, and M. G. Bergeron. 1998. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J. Infect. Dis. 1781472-1482. [DOI] [PubMed] [Google Scholar]
- 4.Filler, S. G., A. S. Pfunder, B. J. Spellberg, J. P. Spellberg, and J. E. Edwards, Jr. 1996. Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect. Immun. 642609-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galley, H. F., and N. R. Webster. 2004. Physiology of the endothelium. Br. J. Anaesth. 93105-113. [DOI] [PubMed] [Google Scholar]
- 6.Gersuk, G. M., D. M. Underhill, L. Zhu, and K. A. Marr. 2006. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 1763717-3724. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 522745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laudes, I. J., R. F. Guo, N. C. Riedemann, C. Speyer, R. Craig, J. V. Sarma, and P. A. Ward. 2004. Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am. J. Pathol. 1641435-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32358-366. [DOI] [PubMed] [Google Scholar]
- 10.Lionakis, M. S., and D. P. Kontoyiannis. 2003. Glucocorticoids and invasive fungal infections. Lancet 3621828-1838. [DOI] [PubMed] [Google Scholar]
- 11.Lopes Bezerra, L. M., and S. G. Filler. 2004. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood 1032143-2149. [DOI] [PubMed] [Google Scholar]
- 12.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 1004358-4366. [DOI] [PubMed] [Google Scholar]
- 13.Marr, K. A., T. Patterson, and D. Denning. 2002. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 16875-894, vi. [DOI] [PubMed] [Google Scholar]
- 14.Ng, T. T., G. D. Robson, and D. W. Denning. 1994. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology 1402475-2479. [DOI] [PubMed] [Google Scholar]
- 15.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 4381151-1156. [DOI] [PubMed] [Google Scholar]
- 16.Orozco, A. S., X. Zhou, and S. G. Filler. 2000. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect. Immun. 681134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahl, H. L., B. Krauss, K. Schulze-Osthoff, T. Decker, E. B. Traenckner, M. Vogt, C. Myers, T. Parks, P. Warring, A. Muhlbacher, A. P. Czernilofsky, and P. A. Baeuerle. 1996. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J. Exp. Med. 1831829-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paris, S., E. Boisvieux-Ulrich, B. Crestani, O. Houcine, D. Taramelli, L. Lombardi, and J. P. Latge. 1997. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 651510-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepeljnjak, S., Z. Slobodnjak, M. Segvic, M. Peraica, and M. Pavlovic. 2004. The ability of fungal isolates from human lung aspergilloma to produce mycotoxins. Hum. Exp. Toxicol. 2315-19. [DOI] [PubMed] [Google Scholar]
- 20.Perrin, R. M., N. D. Fedorova, J. W. Bok, R. A. Cramer, J. R. Wortman, H. S. Kim, W. C. Nierman, and N. P. Keller. 2007. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 3e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan, Q. T., P. H. Belanger, and S. G. Filler. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 683485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard, D. C., T. Doedt, L. Y. Chiang, H. S. Kim, D. Chen, W. C. Nierman, and S. G. Filler. 2005. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol. Biol. Cell 165866-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheppard, D. C., K. A. Marr, D. N. Fredricks, L. Y. Chiang, T. Doedt, and S. G. Filler. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin. Microbiol. Infect. 12376-380. [DOI] [PubMed] [Google Scholar]
- 24.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 481908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spikes, S., R. Xu, C. K. Nguyen, G. Chamilos, D. P. Kontoyiannis, R. H. Jacobson, D. E. Ejzykowicz, L. Y. Chiang, S. G. Filler, and G. S. May. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197479-486. [DOI] [PubMed] [Google Scholar]
- 26.Steele, C., R. R. Rapaka, A. Metz, S. M. Pop, D. L. Williams, S. Gordon, J. K. Kolls, and G. D. Brown. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugui, J. A., J. Pardo, Y. C. Chang, K. A. Zarember, G. Nardone, E. M. Galvez, A. Mullbacher, J. I. Gallin, M. M. Simon, and K. J. Kwon-Chung. 2007. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 61562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upton, A., K. A. Kirby, P. Carpenter, M. Boeckh, and K. A. Marr. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44531-540. [DOI] [PubMed] [Google Scholar]
- 29.Wasylnka, J. A., and M. M. Moore. 2002. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect. Immun. 703156-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]