Abstract
Increased incidence of bovine tuberculosis (TB) in the United Kingdom caused by infection with Mycobacterium bovis is a cause of considerable economic loss to farmers and the government. The Eurasian badger (Meles meles) represents a wildlife source of recurrent M. bovis infections of cattle in the United Kingdom, and its vaccination against TB with M. bovis bacillus Calmette-Guérin (BCG) is an attractive disease control option. Delivery of BCG in oral bait holds the best prospect for vaccinating badgers over a wide geographical area. Using a guinea pig pulmonary challenge model, we evaluated the protective efficacy of candidate badger oral vaccines, based on broth-grown or ball-milled BCG, delivered either as aqueous suspensions or formulated in two lipids with differing fatty acid profiles (one being animal derived and the other being vegetable derived). Protection was determined in terms of increasing body weight after aerosol challenge with virulent M. bovis, reduced dissemination of M. bovis to the spleen, and, in the case of one oral formulation, restricted growth of M. bovis in the lungs. Only oral BCG formulated in lipid gave significant protection. These data point to the potential of the BCG-lipid formulation for further development as a tool for controlling tuberculosis in badgers.
There was an estimated 225% increase in the number of bovine tuberculosis (TB) incidents in Great Britain between the years 1996 and 2006 (7). This adversely affects animal health and welfare and causes considerable economic losses to farmers and the government. Although transmission of Mycobacterium bovis bacteria between cattle is an important factor in the spread of the disease (31), the Eurasian badger (Meles meles) represents an additional wildlife source of recurrent M. bovis infections of cattle in both the United Kingdom and Ireland (25, 26, 32). Badgers are afforded protection under the law in the United Kingdom, and their culling as a means to reduce bovine TB is both controversial (19) and demonstrated to have both positive and negative effects on the incidence of bovine TB (24, 25).
In this context, the vaccination of badgers against TB is an attractive option as a possible means to reduce the incidence of and control bovine TB. It has long been recognized that delivery of vaccine in oral bait holds the best prospect for vaccinating badgers over a wide geographical area (8), and this method is well attested for mass vaccination of other wildlife species (21). In the short-to-medium term, M. bovis bacillus Calmette-Guérin (BCG) represents the best available option for vaccination of wildlife against TB (8, 20). BCG has the advantage of a long history of safety and efficacy in a variety of animal species (8) but has the limitation of little to no efficacy when delivered in a nonviable state (9, 41). This is made more likely in the case of oral delivery by inactivation in the low-pH environment of the stomach (5, 11, 41).
Therefore, the success of an oral BCG vaccine for wildlife will depend in large part on the ability to formulate BCG so that it maintains a viable state for prolonged periods in bait and is delivered to the gastrointestinal tract (GIT) in such a way as to establish bacterial replication in the lymphoid tissues sufficient to maintain protective immunity (41). These issues have recently been addressed through the incorporation of BCG vaccine into a lipid-based matrix (1-4, 6, 12, 46). Use of the lipid matrix allowed BCG to be retained in a viable but static state for several weeks at an ambient temperature (6). For rodent models and brushtail possums, oral delivery of lipid formulations containing live BCG was shown to establish populations of viable, replicating BCG in the alimentary tract lymphatic system (1, 46), which in mice persisted for at least seven months postvaccination (3). Voluntary uptake of the vaccine (which can be readily induced following flavoring of the lipid matrix) was shown to confer protection against virulent M. bovis or M. tuberculosis aerosol challenge in mice (2, 3, 6) and against challenge to the pulmonary tract with virulent M. bovis in possums and cattle (4, 12). The durations of protection after oral vaccination were at least 7 months for mice and 12 months for possums (3, 10).
For a commercial vaccine to comply with European Union regulatory requirements, it is necessary for the raw materials to be of consistent quality and defined specifications. The production methodology also has to be validated and consistent. In the case of a licensed oral BCG-lipid vaccine for badgers, this must include sourcing BCG from a supplier compliant with good manufacturing practice guidelines (GMP) and the BCG-lipid formulation being manufactured in a facility compliant with GMP. Experimental vaccines used to generate data for regulatory submission should be shown to be representative of the proposed final product. With an aim to facilitate compliance with European Union regulatory requirements, we have evaluated a commercial source of BCG (Danish strain 1331; Statens Serum Institute, Denmark), as well as a pharmaceutical-grade version of lipid K, which has been previously reported as a lipid delivery matrix for oral BCG vaccination of brushtail possums (4).
BCG crosses the intestinal barrier through the M cells of Peyer's patches (PP) (28), from which it can stimulate lymphocyte responses in the spleen, mesenteric lymph nodes (MLN), and PP themselves (1, 28, 34, 38). Therefore, one limitation on efficient uptake of BCG by the gut-associated lymphoid tissue (GALT) is likely to be particle size. From a variety of different experimental systems in rodents, it has emerged that uptake into the PP and passage to the liver and spleen via the MLN generally decreases with increasing particle size (23, 33, 37). In this context, previous work with BCG-lipid formulations has utilized broth-grown cultures of BCG, for both their high viability and their dispersion into single cells. While use of a preparation of BCG Danish strain 1331 created in compliance with GMP would facilitate the eventual licensing of an oral vaccine, the commercially available BCG vaccine from the Statens Serum Institute is a ball-milled preparation that contains considerably more aggregates of BCG than its broth-grown counterpart (data not shown). We therefore evaluated both forms of BCG Danish strain 1331 in order to establish whether the form of BCG would impact its efficacy when delivered orally.
Proof of concept for the oral BCG vaccine was evaluated in a guinea pig vaccination-challenge model, chosen because of the susceptibility of the guinea pig to M. bovis (17, 47) and the relevance of the challenge route for badger TB pathogenesis (18). Lipid-encapsulated BCG delivered to guinea pigs orally was immunogenic, and live bacilli were recovered from the mesenteric and cervical lymph nodes. Protection against aerosol challenge with virulent M. bovis was demonstrated for BCG only when delivered in lipid, and there was little difference between the ball-milled and broth-grown forms of BCG. These data point to the potential of the BCG-lipid formulation for the further development of a licensed oral TB vaccine for badgers.
MATERIALS AND METHODS
Bacteria and media.
Three preparations of BCG Danish strain 1331 were used: a lyophilized preparation, a ball-milled preparation in sodium glutamate solution, and a broth-grown preparation. Both the lyophilized and ball-milled preparations were obtained from the Statens Serum Institute, Copenhagen, Denmark. Broth-grown BCG was prepared from the lyophilized BCG by culture in Middlebrook 7H9 medium plus albumin-dextrose-catalase and 0.01% (vol/vol) Tween 80 to an optical density at 600 nm of approximately 0.2 (mid-log-phase growth). Virulent Mycobacterium bovis isolate AF2122/97 (29) was propagated in Middlebrook 7H9 medium and stored as frozen aliquots prior to aerosol infection of guinea pigs. For routine bacterial enumeration, BCG was plated onto modified Middlebrook 7H11 plus oleic acid-albumin-dextrose-catalase (OADC) and glycerol, and M. bovis was plated onto modified Middlebrook 7H11 plus OADC and pyruvate.
Lipid formulation of M. bovis BCG.
Two lipids with differing fatty acid profiles were used to encapsulate BCG for oral vaccination of guinea pigs. Lipid C consisted of an animal-derived fractionated lipid complex as described previously (4). Lipid PK consisted of a pharmaceutical-grade, nonhydrogenated derivative of lipid K (a vegetable-derived lipid containing a mixture of triglycerides of fatty acids as previously described [4]). Broth-grown or ball-milled BCG bacilli were encapsulated into lipid C or lipid PK as previously described (4).
Postvaccination BCG quantitative bacteriology and immune assessment.
In order to confirm that orally administered lipid formulations were effectively delivering BCG bacilli to guinea pigs, we undertook a bacteriological assessment of lymphoid tissues, excised from animals in the absence of M. bovis challenge. Guinea pigs (eight per group) were orally vaccinated with 2.6 × 107 CFU of broth-grown BCG in lipid C or 3.0 × 107 CFU of broth-grown BCG in lipid PK. Eight weeks later, the guinea pigs were euthanized by CO2 asphyxiation. Separate tissue homogenates were prepared in Dulbecco's modified Eagle's base medium (DMEM) from each animal for MLN, cervical-region lymph nodes (CLN), PP, and spleens, as described previously for the processing of murine tissues (1). Homogenates were centrifuged, resuspended in Middlebrook 7H9 broth, and plated in duplicate onto Middlebrook 7H11 agar; BCG bacilli were enumerated after 20 days of bacterial growth. Additionally, subsamples of spleen homogenates were washed in DMEM and resuspended to a concentration of 5 × 106 viable mononuclear leukocytes/ml in DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin-100 μg/ml streptomycin, and 5.5 × 10−5 M 2-mercaptoethanol. One-hundred-microliter aliquots of cell suspensions were plated, in triplicate, into 96-well tissue culture plates (Nunc, Denmark) and cultured in the presence or absence of 50 μg/ml M. bovis purified protein derivative (PPD-B; Prionics Inc., Switzerland) for a total of four days. Lymphocyte proliferation, due to antigen stimulation, was assessed by tritiated-thymidine incorporation over the final 18 h of culture, as described previously for murine cells (22). Data were expressed as stimulation indices as described previously (4, 6, 22), and responses of vaccinees were compared against those of a control group (n = 4) comprising nonvaccinated guinea pigs.
Vaccination of guinea pigs for evaluation of vaccine efficacy.
Eight separate groups of vaccinated guinea pigs were used to evaluate vaccine efficacy. Outbred female Dunkin Hartley guinea pigs (weighing between 250 and 300 g) free from intercurrent infection were obtained from Harlan, United Kingdom, and were vaccinated in groups of eight animals. Two groups received broth-grown or ball-milled BCG without formulation in lipid. Oral vaccines were preloaded in 1-ml syringes and delivered at the back of the mouth slowly to ensure the full dose was swallowed. The efficacy of each oral vaccine was evaluated against that of the lyophilized BCG vaccine as the positive control. A single vial of lyophilized BCG vaccine was reconstituted with 1 ml Sauton diluent supplied by the Statens Serum Institute with the vaccine and delivered subcutaneously (s.c.) in a volume of 0.1 ml in the nape of the neck. The delivered dose of BCG was determined by plating an aliquot of the vaccine preparation. The delivered doses were 2 × 105 to 8 × 105 CFU for s.c. BCG, 1.7 × 108 CFU for broth-grown BCG (or 9.2 × 107 CFU when in lipid), and 7.9 × 107 CFU for ball-milled BCG.
Aerosol challenge with M. bovis.
Twelve weeks after vaccination, all animals were challenged via the aerosol route with M. bovis strain AF2122/97 by use of a fully contained nose-only-exposure Henderson apparatus as previously described (16, 47). A fine-particle aerosol with a mean diameter range of 2 μm (diameter range, 0.5 to 7 μm) (36) was generated with a saline suspension containing 1 × 106 CFU/ml in order to obtain an estimated retained inhaled dose of approximately 10 to 20 CFU delivered to the lungs of each animal. The Henderson apparatus allows a controlled delivery of aerosols to the animals, and the reproducibility of the system and relationship between numbers of lesions and concentration of bacilli in the Collison nebulizer have been described previously (17, 47).
Monitoring of animals postchallenge and necropsy.
Following aerosol challenge, the guinea pigs were housed at Advisory Committee on Dangerous Pathogens (ACDP) containment level 3 and weighed regularly for a marker of disease progression. The animals were euthanized by peritoneal overdoses of sodium pentobarbitone five weeks after challenge or when an individual weighed 20% less than its maximal body weight (humane endpoint). The spleen and lungs were aseptically removed and the lungs weighed after removal of the trachea and lung-associated lymphoid tissues. Spleen and lungs were placed into separate sterile tubes for storage at −20°C until processed for bacteriology. Frozen tissues were thawed and homogenized in sterile deionized water by use of a rotating-blade macerator system. Viable counts were performed by preparing decimal dilutions in sterile deionized water and plating 100-μl aliquots onto Middlebrook 7H11 agar plus OADC and pyruvate. Plates were incubated at 37°C for 5 weeks before counting the number of M. bovis colonies (CFU).
RESULTS
BCG delivery and vaccine immunogenicity using lipid formulations.
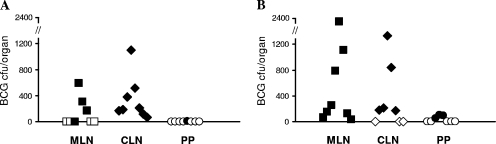
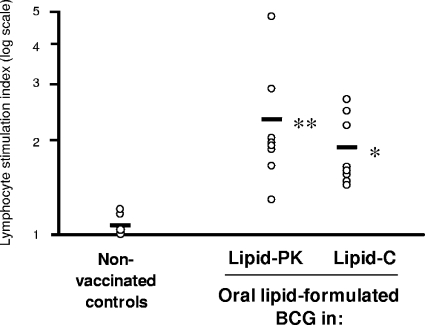
Given that this was the first time the lipid-BCG formulations had been evaluated with the guinea pig, we first conducted a preliminary experiment to establish the delivery and immunogenicity of lipid-BCG to guinea pigs. Eight weeks postvaccination, all guinea pigs which had been orally vaccinated with broth-grown BCG in lipid were culture positive for BCG in the alimentary tract lymphatic system (Fig. 1); no viable BCG bacilli were isolated from splenic tissues of any animal. Average total BCG loads per animal were 1,006 and 479 CFU for lipid C and lipid PK vaccinees, respectively. Distribution profiles indicated that BCG had successfully colonized the CLN tissues in all guinea pigs receiving BCG in lipid PK (Fig. 1A), whereas all guinea pigs receiving BCG in lipid C had BCG persistent in their MLN tissues (Fig. 1B). However, there was no statistically significant difference between the proportions of animals with BCG cultured from the MLN compared with the CLN for either lipid (Fisher's exact test). BCG was recovered only in low numbers from the PP of one animal that received BCG in lipid PK and from three that received BCG in lipid C. Broth-grown BCG delivered in either lipid matrix invoked a proliferative response among guinea pig splenic lymphocytes cultured in the presence of PPD-B antigen (Fig. 2). There was no statistically significant difference between the two vaccinated groups.
FIG. 1.
BCG distribution profiles in alimentary tract lymphatic tissues following oral vaccination of guinea pigs with BCG formulated in lipid PK (A) or lipid C (B). Data represent BCG CFU counts per total organ homogenate for MLN, CLN, and PP. Filled symbols represent viable BCG cultured from the tissue; open symbols represent culture-negative tissue.
FIG. 2.
Splenic lymphocyte-proliferative responses to PPD-B stimulation among guinea pigs vaccinated orally with lipid-formulated BCG. PPD-B-induced stimulation indices for individual animals are shown with the group means as bars. The raw stimulation index data were nonnormally distributed (Anderson-Darling normality test) and were subsequently normalized by log10 transformation prior to statistical analysis (one-way analysis of variance and Dunnett's post hoc test) and graphical presentation. Asterisks refer to mean SI responses of vaccinated guinea pigs that were significantly higher than those of nonvaccinated (control) animals (*, P = 0.018; **, P = 0.003).
BCG-lipid formulations protect against weight loss following challenge with M. bovis.
Having established that broth-grown BCG in lipid was delivered successfully to the alimentary tract lymphatics of guinea pigs, resulting in a systemic immune response, separate groups of guinea pigs were vaccinated orally with BCG alone or BCG formulated with lipid in order to evaluate vaccine efficacy against aerosol challenge with virulent M. bovis. Additional groups received either lipid PK orally or BCG s.c. as negative or positive controls, respectively. Two different preparations (ball milled or broth grown) of BCG Danish strain 1331 were used to see if the type of preparation had a bearing on protective efficacy.
Following challenge, all guinea pigs survived to the end of the experiment (five weeks postchallenge), but there were differences in disease progression between groups. Groups of animals that received BCG in lipid showed an increase in body weight over the period of study. In contrast, the two groups that received BCG orally without lipid showed little overall change in body weight. Animals in the group that received lipid alone lost on average 11% of their body weight over the last four weeks of study (data not shown).
All BCG-lipid formulations conferred equivalent levels of protection to the spleen following challenge with M. bovis.
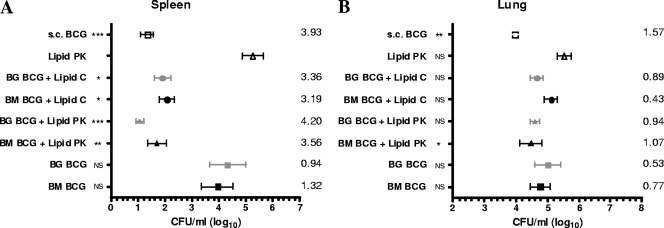
Figure 3A shows the mean concentrations of M. bovis in the spleens from each group five weeks after aerosol challenge with a low dose of M. bovis. All BCG-lipid formulations conferred significant protection to the spleen in comparison with the level of protection conferred by the nonvaccine control (lipid PK alone). BCG (whether broth grown or ball milled) failed to give significant protection when given orally in the absence of lipid, although the mean bacterial load was lowered approximately 10-fold.
FIG. 3.
Influence of vaccination on bacterial load of M. bovis in the spleens (A) and lungs (B) of guinea pigs infected by the aerosol route five weeks earlier. The means ± standard errors of the means are shown for each group. Significant differences compared with values from the lipid PK group are represented by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) and were derived using Dunn's multiple comparisons test (for the spleen data) and Dunnett's multiple comparisons test (for the lung data) following nonparametric and parametric analyses of variance, respectively. NS, not significant; BG, broth-grown; BM, ball-milled. The log10 differences in group means compared with the means of the lipid PK group are shown numerically.
A single BCG-lipid formulation confers protection to the lung following challenge with M. bovis.
Compared to that conferred to the spleen, the level of protection conferred to the lung by vaccination was considerably lower. Figure 3B shows the mean concentrations of M. bovis in the lungs from each group. Parenterally administered BCG (s.c. BCG) conferred 1.57 log10 protection to the lungs, approximately 40% of that achieved in the spleen. This was statistically significant (P = 0.01). For the oral vaccines, neither BCG without lipid nor BCG administered in lipid C gave statistically significant protection to the lung (P values of 0.576 and 0.132, respectively), whereas ball-milled BCG in lipid PK conferred significant protection (1.07 log10 reduction; P = 0.045). Results for broth-grown BCG in lipid PK were very similar (0.94 log10 reduction), with this formulation only just failing to achieve statistical significance (P = 0.078).
DISCUSSION
The present study demonstrates that oral vaccination of guinea pigs with BCG in a lipid matrix can give significant protection against aerosol infection with M. bovis. As the respiratory route is considered the principal route of exposure to natural M. bovis infection in the badger (18) and guinea pigs are particularly susceptible to aerosolized M. bovis (17, 47), this was considered a relevant and stringent animal model with which to evaluate the efficacies of oral vaccines for protection against M. bovis colonization of the lungs and subsequent dissemination to the spleen. This is the first report of the evaluation of the BCG-lipid vaccine in the guinea pig challenge model. Our study extends the growing body of literature on the utility of the lipid matrix for the oral delivery of BCG (1-4, 6, 12, 46). Protection was seen for oral BCG only when formulated in lipid, and in this study, protection was expressed in terms of increasing body weight after challenge with virulent M. bovis, reduced dissemination of M. bovis to the spleen, and, in the case of ball-milled BCG in lipid PK, restricted growth of M. bovis in the lungs.
There were several-hundred-fold more BCG bacilli delivered orally than were administered s.c. and, in all cases but one, they still failed to confer statistically significant protection to the lungs. Even with the gastric protection provided to BCG by the lipid, it is evident that BCG administered orally will need to be given at a higher titer than BCG administered parenterally in order to achieve comparable levels of protection. Whether this proves to be a serious constraint on the application of oral BCG for use in wildlife remains to be seen and will depend on studies in the target species to define the minimal oral dose required for protection, together with studies in vitro to define the formulated dose of BCG required to achieve this in the field.
Recent work demonstrated the association between the establishment and persistence of BCG in the GALT of mice following oral delivery in lipid and the persistence of effector immunity (22), and, by inference, protection from challenge with virulent mycobacteria (3). In the present study, BCG administered orally in lipid resulted in colonization of the upper (CLN) and lower (MLN) alimentary tract lymphatic tissues and to a lesser extent the PP also. Although we did not determine whether BCG bacilli were still present in the GALT at the time of challenge with M. bovis, BCG has been reported to persist in the murine GALT for at least seven months after oral delivery in lipid (3).
In the non-M. bovis-challenged guinea pigs, BCG was recovered from the alimentary tract lymphatic tissues of all animals which had received broth-grown BCG-lipid formulation, indicating regular lymphatic colonization. Among these animals, BCG was isolated at different amounts from the different lymphoid sites, although MLN and CLN tissues were the main sites of BCG replication. It was interesting to note that viable bacilli were recovered from 100% of the MLN tissues from animals vaccinated with BCG-lipid C, similar to what has been reported previously in murine studies (1, 3). In contrast, 100% colonization of CLN tissues occurred in animals which had received BCG-lipid PK. The same associations have been observed in equivalent studies with mice (F. E. Aldwell, unpublished observations): voluntary consumption of BCG in lipid PK most frequently results in replicating bacterial populations in upper alimentary tract lymph nodes (i.e., those draining the oropharyngeal region), in contrast to consumption of BCG in lipid C, for which replicating BCG bacilli are most frequently recovered from the lower alimentary tract lymph nodes (i.e., those draining the small intestine region). Thus, although the two lipid matrices appear to favor BCG tropism at different lymphatic sites, results from the present study suggest that the magnitudes of the ensuing cell-mediated immune response and the subsequent degrees of protection against M. bovis infection are equivalent between the two matrices. This is in accordance with a study in which a high dose of BCG given to mice either orally (so that BCG colonized the CLN) or intragastrically (thereby resulting in uptake exclusively by the MLN and PP) resulted in equivalent splenic T-cell responses and comparable levels of protection against intravenous challenge with M. tuberculosis (34).
Although the precise mechanism by which the lipid matrix enhances the efficacy of oral BCG is unknown, it is clear from the present study that the lipid plays a significant role in protecting BCG during transit through the GIT and/or augmenting delivery to the GALT. In regard to the latter mechanism, lipid delivery systems have been studied for some time for their ability to enhance the oral bioavailability of poorly absorbed compounds (14; this was recently reviewed in reference 40), although there are likely to be significant differences between the way the GIT handles drugs and how it handles live BCG. Nonetheless, it is interesting that the mixing of polystyrene nanoparticles (0.5 μm) with 6% lecithin (a mixture of glycolipids, triglycerides, and phospholipids) (43) was shown to significantly enhance the uptake of the nanoparticles into rat MLN compared with oral delivery of the microparticles in saline or 6% oleic acid (45).
Previous work with BCG-lipid formulations has utilized broth-grown cultures of BCG both for their high viability and their dispersion into single cells. With an aim to facilitate eventual licensing, we evaluated a ball-milled preparation of BCG Danish strain 1331 obtained from the Statens Serum Institute, Denmark. The bacteria are first grown as a surface pellicle and then ball milled in a solution of sodium glutamate to aid dispersion. Nonetheless, the ball-milled preparation contains considerably more aggregates of BCG than its broth-grown counterpart (data not shown). The fact that we found lipid-formulated ball-milled BCG to be as protective as broth-grown BCG suggests that the ball-milled BCG preparation contains sufficient singlet bacilli for effective delivery to the GALT. Interestingly, other studies have shown that although a greater quantity of smaller (2-μm) particles may be taken up by the GIT, larger (6-μm) particles appear to be more efficiently translocated to lymph nodes (15) and can even reach the lung (35). In a detailed study of mice, ovalbumin was delivered orally using microspheres over a 0.6-to-26.0-μm size range. The quantity of microspheres taken up into PP and subsequently translocated to the spleen was determined in relation to particle size, and the influence this had on systemic and mucosal antibody responses to ovalbumin was evaluated. It was concluded that the body distribution pattern of microspheres following PP uptake was a key factor in determining the induction of systemic or mucosal immune responses (44). Besides particle size, other factors affecting uptake of microparticles in the GIT include surface charge and hydrophobicity (27). Especially in the case of lipid-based delivery systems, the surface charge (30) or other characteristics of the lipid (39) may be more significant than the size of the particle droplet.
In conclusion, we have demonstrated for the first time with guinea pigs that oral vaccination with BCG in lipid is both immunogenic and able to confer a level of protection against aerosol challenge with virulent M. bovis. This supports previous observations with other animal hosts (1-4, 6, 12, 46) and points to the potential of the BCG-lipid formulation for the further development of an oral vaccine for use for badgers in the United Kingdom. A previous study using a parenteral route of BCG vaccination in badgers demonstrated enhanced cell-mediated immunity, prolonged survival following intradermal M. bovis challenge, and delayed excretion of the organism (42). The reductions in M. bovis loads in the lungs and in bacterial dissemination to the spleen in the present study using oral BCG are encouraging in terms of the effect of BCG vaccination required for the badger, in which a reduction in excretion of M. bovis may be sufficient to break ongoing transmission to other badgers and cattle (13). Since BCG is stable and the lipid is solid at temperatures most likely to be encountered in the environment (4), this formulation also has the potential to serve as the bait delivery system itself, as has proven successful in delivery to captive possums in New Zealand (4, 10, 46), although further work is required to evaluate the palatability and uptake of a lipid bait to wild badgers.
Acknowledgments
This work was funded by the Department for Environment, Food and Rural Affairs, Great Britain. All animal procedures were carried out under licenses issued according to the U.K. Animals (Scientific Procedures) Act 1986 following local ethical review and to the New Zealand University of Otago Animal Ethics Committee.
The technical assistance of Matt Lambeth and Yvonne Coughlan (University of Otago) is gratefully acknowledged. We also thank the Statens Serum Institute for the supply of and advice regarding the BCG vaccine and S. Houghton, Veterinary Vaccines Consultancy, for advice on vaccine licensing.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Aldwell, F. E., M. A. Baird, C. E. Fitzpatrick, A. D. McLellan, M. L. Cross, M. R. Lambeth, and G. S. Buchan. 2005. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: anatomical sites of bacterial replication and immune activity. Immunol. Cell Biol. 83549-553. [DOI] [PubMed] [Google Scholar]
- 2.Aldwell, F. E., L. Brandt, C. Fitzpatrick, and I. M. Orme. 2005. Mice fed lipid-encapsulated Mycobacterium bovis BCG are protected against aerosol challenge with Mycobacterium tuberculosis. Infect. Immun. 731903-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldwell, F. E., M. L. Cross, C. E. Fitzpatrick, M. R. Lambeth, G. W. de Lisle, and B. M. Buddle. 2006. Oral delivery of lipid-encapsulated Mycobacterium bovis BCG extends survival of the bacillus in vivo and induces a long-term protective immune response against tuberculosis. Vaccine 242071-2078. [DOI] [PubMed] [Google Scholar]
- 4.Aldwell, F. E., D. L. Keen, N. A. Parlane, M. A. Skinner, G. W. de Lisle, and B. M. Buddle. 2003. Oral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine 2270-76. [DOI] [PubMed] [Google Scholar]
- 5.Aldwell, F. E., D. L. Keen, V. C. Stent, A. Thomson, G. F. Yates, G. W. de Lisle, and B. M. Buddle. 1995. Route of BCG administration in possums affects protection against bovine tuberculosis. N. Z. Vet. J. 43356-359. [DOI] [PubMed] [Google Scholar]
- 6.Aldwell, F. E., I. G. Tucker, G. W. de Lisle, and B. M. Buddle. 2003. Oral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect. Immun. 71101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. 2007. Animal Health 2006. The report of the Chief Veterinary Officer. Department for Environment, Food and Rural Affairs, London, United Kingdom.
- 8.Anonymous. 2003. Development of vaccines for bovine tuberculosis. Towards a sustainable policy to control TB in cattle. Department for Environment, Food and Rural Affairs, London, United Kingdom.
- 9.Bloch, H., and W. Segal. 1955. Viability and multiplication of vaccines in immunization against tuberculosis. Am. Rev. Tuberc. Pulm. Dis. 71228-248. [DOI] [PubMed] [Google Scholar]
- 10.Buddle, B. M., F. E. Aldwell, D. L. Keen, N. A. Parlane, K. L. Hamel, and G. W. de Lisle. 2006. Oral vaccination of brushtail possums with BCG: Investigation into factors that may influence vaccine efficacy and determination of duration of protection. N. Z. Vet. J. 54224-230. [DOI] [PubMed] [Google Scholar]
- 11.Buddle, B. M., F. E. Aldwell, D. L. Keen, N. A. Parlane, G. Yates, and G. W. de Lisle. 1997. Intraduodenal vaccination of brushtail possums with bacille Calmette-Guerin enhances immune responses and protection against Mycobacterium bovis infection. Int. J. Tuberc. Lung Dis. 1377-383. [PubMed] [Google Scholar]
- 12.Buddle, B. M., F. E. Aldwell, M. A. Skinner, G. W. de Lisle, M. Denis, H. M. Vordermeier, R. G. Hewinson, and D. N. Wedlock. 2005. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine 233581-3589. [DOI] [PubMed] [Google Scholar]
- 13.Buddle, B. M., D. N. Wedlock, and M. Denis. 2006. Progress in the development of tuberculosis vaccines for cattle and wildlife. Vet. Microbiol. 112191-200. [DOI] [PubMed] [Google Scholar]
- 14.Bummer, P. M. 2004. Physical chemical considerations of lipid-based oral drug delivery—solid lipid nanoparticles. Crit. Rev. Ther. Drug Carrier Syst. 211-20. [PubMed] [Google Scholar]
- 15.Carr, K. E., R. A. Hazzard, S. Reid, and G. M. Hodges. 1996. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm. Res. 131205-1209. [DOI] [PubMed] [Google Scholar]
- 16.Chambers, M. A., A. Williams, D. Gavier-Widen, A. Whelan, G. Hall, P. D. Marsh, B. R. Bloom, W. R. Jacobs, and R. G. Hewinson. 2000. Identification of a Mycobacterium bovis BCG auxotrophic mutant that protects guinea pigs against M. bovis and hematogenous spread of Mycobacterium tuberculosis without sensitization to tuberculin. Infect. Immun. 687094-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers, M. A., A. Williams, D. Gavier-Widen, A. Whelan, C. Hughes, G. Hall, M. S. Lever, P. D. Marsh, and R. G. Hewinson. 2001. A guinea pig model of low-dose Mycobacterium bovis aerogenic infection. Vet. Microbiol. 80213-226. [DOI] [PubMed] [Google Scholar]
- 18.Clifton-Hadley, R. S., J. W. Wilesmith, and F. A. Stuart. 1993. Mycobacterium bovis in the European badger (Meles meles): epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiol. Infect. 1119-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coles, G. 2007. Attitudes to badger culling. Vet. Rec. 160416. [DOI] [PubMed] [Google Scholar]
- 20.Cross, M. L., and F. Aldwell. 2007. Oral vaccination against bovine tuberculosis with Mycobacterium bovis BCG. Expert Rev. Vaccines 6323-331. [DOI] [PubMed] [Google Scholar]
- 21.Cross, M. L., B. M. Buddle, and F. E. Aldwell. 2007. The potential of oral vaccines for disease control in wildlife species. Vet. J. 174472-480. [DOI] [PubMed] [Google Scholar]
- 22.Cross, M. L., M. R. Lambeth, Y. Coughlan, F. E. Aldwell, C. E. Fitzpatrick, G. W. de Lisle, B. M. Buddle, M. A. Baird, A. D. McLellan, and G. S. Buchan. 2007. Oral vaccination of mice with lipid-encapsulated Mycobacterium bovis BCG: effect of reducing or eliminating BCG load on cell-mediated immunity. Vaccine 251297-1303. [DOI] [PubMed] [Google Scholar]
- 23.Desai, M. P., V. Labhasetwar, G. L. Amidon, and R. J. Levy. 1996. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 131838-1845. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly, C. A., G. Wei, W. T. Johnston, D. R. Cox, R. Woodroffe, F. J. Bourne, C. L. Cheeseman, R. S. Clifton-Hadley, G. Gettinby, P. Gilks, H. E. Jenkins, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2007. Impacts of widespread badger culling on cattle tuberculosis: concluding analyses from a large-scale field trial. Int. J. Infect. Dis. 11300-308. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly, C. A., R. Woodroffe, D. R. Cox, F. J. Bourne, C. L. Cheeseman, R. S. Clifton-Hadley, G. Wei, G. Gettinby, P. Gilks, H. Jenkins, W. T. Johnston, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439843-846. [DOI] [PubMed] [Google Scholar]
- 26.Donnelly, C. A., R. Woodroffe, D. R. Cox, J. Bourne, G. Gettinby, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2003. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature 426834-837. [DOI] [PubMed] [Google Scholar]
- 27.Florence, A. T. 1997. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm. Res. 14259-266. [DOI] [PubMed] [Google Scholar]
- 28.Fujimura, Y. 1986. Functional morphology of microfold cells (M cells) in Peyer's patches—phagocytosis and transport of BCG by M cells into rabbit Peyer's patches. Gastroenterol. Jpn. 21325-335. [PubMed] [Google Scholar]
- 29.Garnier, T., K. Eiglmeier, J. C. Camus, N. Medina, H. Mansoor, M. Pryor, S. Duthoy, S. Grondin, C. Lacroix, C. Monsempe, S. Simon, B. Harris, R. Atkin, J. Doggett, R. Mayes, L. Keating, P. R. Wheeler, J. Parkhill, B. G. Barrell, S. T. Cole, S. V. Gordon, and R. G. Hewinson. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 1007877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gershanik, T., S. Benzeno, and S. Benita. 1998. Interaction of a self-emulsifying lipid drug delivery system with the everted rat intestinal mucosa as a function of droplet size and surface charge. Pharm. Res. 15863-869. [DOI] [PubMed] [Google Scholar]
- 31.Goodchild, A. V., and R. S. Clifton-Hadley. 2001. Cattle-to-cattle transmission of Mycobacterium bovis. Tuberculosis (Edinburgh) 8123-41. [DOI] [PubMed] [Google Scholar]
- 32.Griffin, J. M., D. H. Williams, G. E. Kelly, T. A. Clegg, I. O'Boyle, J. D. Collins, and S. J. More. 2005. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev. Vet. Med. 67237-266. [DOI] [PubMed] [Google Scholar]
- 33.Kofler, N., C. Ruedl, C. Rieser, G. Wick, and H. Wolf. 1997. Oral immunization with poly-(d,l-lactide-co-glycolide) and poly-(l-lactic acid) microspheres containing pneumotropic bacterial antigens. Int. Arch. Allergy Immunol. 113424-431. [DOI] [PubMed] [Google Scholar]
- 34.Lagranderie, M., P. Chavarot, A. M. Balazuc, and G. Marchal. 2000. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral or intragastric administration in mice. Vaccine 181186-1195. [DOI] [PubMed] [Google Scholar]
- 35.LeFevre, M. E., D. C. Hancock, and D. D. Joel. 1980. Intestinal barrier to large particulates in mice. J. Toxicol. Environ. Health 6691-704. [DOI] [PubMed] [Google Scholar]
- 36.Lever, M. S., A. Williams, and A. M. Bennett. 2000. Survival of mycobacterial species in aerosols generated from artificial saliva. Lett. Appl. Microbiol. 31238-241. [DOI] [PubMed] [Google Scholar]
- 37.Mladenovska, K., I. Janevik, D. Glavas, F. Kumbaradzi, and K. Goracinova. 2002. Biodistribution studies of BSA loaded gelatin microspheres after peroral application. Int. J. Pharm. 242251-253. [DOI] [PubMed] [Google Scholar]
- 38.Müller-Schoop, J. W., and R. A. Good. 1975. Functional studies of Peyer's patches: evidence for their participation in intestinal immune responses. J. Immunol. 1141757-1760. [PubMed] [Google Scholar]
- 39.Porter, C. J., A. M. Kaukonen, B. J. Boyd, G. A. Edwards, and W. N. Charman. 2004. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm. Res. 211405-1412. [DOI] [PubMed] [Google Scholar]
- 40.Porter, C. J., N. L. Trevaskis, and W. N. Charman. 2007. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 6231-248. [DOI] [PubMed] [Google Scholar]
- 41.Skinner, M. A., D. L. Keen, N. A. Parlane, K. L. Hamel, G. F. Yates, and B. M. Buddle. 2005. Improving protective efficacy of BCG vaccination for wildlife against bovine tuberculosis. Res. Vet. Sci. 78231-236. [DOI] [PubMed] [Google Scholar]
- 42.Stuart, F. A., K. H. Mahmood, J. L. Stanford, and D. G. Pritchard. 1988. Development of diagnostic tests for, and vaccination against, tuberculosis in badgers. Mamm. Rev. 1874-75. [Google Scholar]
- 43.Szuhaj, B. F. 1985. Lecithins (AOCS monograph, 12), vol. 12. American Oil Chemists Society, Urbana, IL.
- 44.Tabata, Y., Y. Inoue, and Y. Ikada. 1996. Size effect on systemic and mucosal immune responses induced by oral administration of biodegradable microspheres. Vaccine 141677-1685. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, N. W., P. G. Jenkins, K. A. Howard, M. W. Smith, E. C. Lavelle, J. Holland, and S. S. Davis. 1996. Particle uptake and translocation across epithelial membranes. J. Anat. 189487-490. [PMC free article] [PubMed] [Google Scholar]
- 46.Wedlock, D. N., F. E. Aldwell, D. Keen, M. A. Skinner, and B. M. Buddle. 2005. Oral vaccination of brushtail possums (Trichosurus vulpecula) with BCG: immune responses, persistence of BCG in lymphoid organs and excretion in faeces. N. Z. Vet. J. 53301-306. [DOI] [PubMed] [Google Scholar]
- 47.Williams, A., A. Davies, P. D. Marsh, M. A. Chambers, and R. G. Hewinson. 2000. Comparison of the protective efficacy of bacille Calmette-Guerin vaccination against aerosol challenge with Mycobacterium tuberculosis and Mycobacterium bovis. Clin. Infect. Dis. 30(Suppl. 3)S299-S301. [DOI] [PubMed] [Google Scholar]