Abstract
Staphylococcus aureus experimental endocarditis relies on sequential fibrinogen binding (for valve colonization) and fibronectin binding (for endothelial invasion) conferred by peptidoglycan-attached adhesins. Fibronectin-binding protein A (FnBPA) reconciles these two properties—as well as elastin binding—and promotes experimental endocarditis by itself. Here we attempted to delineate the minimal subdomain of FnBPA responsible for fibrinogen and fibronectin binding, cell invasion, and in vivo endocarditis. A large library of truncated constructs of FnBPA was expressed in Lactococcus lactis and tested in vitro and in animals. A 127-amino-acid subdomain spanning the hinge of the FnBPA fibrinogen-binding and fibronectin-binding regions appeared necessary and sufficient to confer the sum of these properties. Competition with synthetic peptides could not delineate specific fibrinogen- and fibronectin-binding sites, suggesting that dual binding arose from protein folding, irrespective of clearly defined binding domains. Moreover, coexpressing the 127-amino-acid subdomain with remote domains of FnBPA further increased fibrinogen binding by ≥10 times, confirming the importance of domain interactions for binding efficacy. In animals, fibrinogen binding (but not fibronectin binding) was significantly associated with endocarditis induction, whereas both fibrinogen binding and fibronectin binding were associated with disease severity. Moreover, fibrinogen binding also combined with fibronectin binding to synergize the invasion of cultured cell lines significantly, a feature correlating with endocarditis severity. Thus, while fibrinogen binding and fibronectin binding were believed to act sequentially in colonization and invasion, they appeared unexpectedly intertwined in terms of both functional anatomy and pathogenicity (in endocarditis). This unforeseen FnBPA subtlety might bear importance for the development of antiadhesin strategies.
Staphylococcus aureus is a major pathogen responsible for a wide variety of diseases, including skin and soft-tissue infections, bacteremia, osteomyelitis, and endocarditis (11). The first step in infection is host tissue colonization, which is mediated by a wealth of surface-bound adhesins, also referred to as MSCRAMMs (for microbial surface components recognizing adhesive matrix molecules) (15). Important staphylococcal MSCRAMMs were shown to bind matrix proteins as diverse as fibrinogen (13, 29), fibronectin (24), elastin (1, 20), collagen (15), and keratin (14). Moreover, the MSCRAMMs binding fibronectin, i.e., fibronectin-binding protein A (FnBPA) and FnBPB, were also shown to trigger active internalization into eukaryotic cells in vitro and in vivo (16, 19, 25).
Recent experiments demonstrated that binding to fibrinogen and binding to fibronectin via clumping factor A (ClfA) and FnBPA were essential to promote experimental endocarditis in rats (17, 19). The individual contributions of these properties were assessed by expressing truncated and chimeric ClfA and FnBPA in poorly pathogenic Lactococcus lactis (19). Fibrinogen binding promoted early valve colonization but not persistence and invasion. In contrast, fibronectin binding was unable to initiate infection but mediated aortic cell invasion and microbial persistence once the valve had been colonized. Thus, the two adherence functions were required for progressive infection.
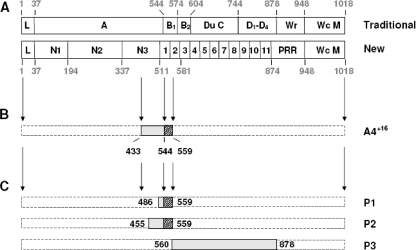
In fact, FnBPA is a multifunctional adhesin reunifying both fibrinogen- and fibronectin-binding capacities (22, 24, 29), as well as elastin binding (20). As a result, it can promote valve colonization and invasion by itself. However, whether these functions are partially redundant, additive, or synergistic for infection is not known. FnBPA has a symmetric functional anatomy (Fig. 1A). Its N-terminal part (called the A domain) is responsible for fibrinogen (29) and elastin binding (9, 20), whereas its C-terminal part (called B-DuC-D) binds to fibronectin (23). This traditional organization was recently refined into further subdomains which might cooperate with each other for binding (23). Residues in the N-terminal domain of the protein are predicted to fold into three subdomains (N1, N2, and N3), with N2 and N3 being involved in fibrinogen and elastin binding (9). Symmetrically, the C-terminal residues are responsible for fibronectin binding and are organized in 11 tandem repeats, each interacting with so-called type 1 modules of fibronectin through a tandem beta-zipper mechanism (23).
FIG. 1.
Schematic representation of FnBPA and major derived peptides used in the experiments described here. (A) Traditional (24) and new (23) layouts of the protein's domains from its N- to its C-terminal end (left and right sides, respectively). Major regions include the leader sequence (L), the fibrinogen- and elastin-binding domains (A), the fibronectin-binding repeats (B-DuC-D), the wall-spanning and -anchoring domains (Wr and Wc), and the membrane-spanning sequence (M). These regions are further divided into functional subdomains in the new layout of the protein. (B) Minimal FnBPA fragment (A4+16) reconciling all of its major properties involved in endocarditis pathogenesis, including fibrinogen and fibronectin binding, triggering of cell internalization, and colonizing of damaged valves. (C) Synthetic peptides used in competition assays against FnBPA and A4+16. Amino acid numbering is displayed around the schemes.
Here we attempted to delineate the minimal region of FnBPA that is required for valve infection. A large library of L. lactis strains expressing different FnBPA domains was constructed and tested for in vitro and in vivo adherence and infectivity. A 127-amino-acid fragment which spanned part of fibrinogen-binding module N3, as well as the first fibronectin-binding tandem repeat, could bind both fibrinogen and fibronectin and was necessary and sufficient for the invasion of cultured cell lines in vitro and valve infection in animals. Domain shuffling and competition assays with synthetic peptides also indicated that physically distant regions of FnBPA could cooperate for its binding. Moreover, the dynamics of infection and a mathematical model of cell internalization showed that fibrinogen binding was likely to be the first step in in vivo infection and acted synergistically with fibronectin binding to trigger further cell invasion. This dual role of fibrinogen binding in disease induction and progression may provide an argument for making it a priority target in antiadherence strategies.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. lactis subsp. cremoris 1363 and Escherichia coli XL1-Blue (Stratagene) were used for cloning experiments by conventional and already described methods (18, 21). L. lactis was grown at 30°C in M17 medium (Oxoid) supplemented with 0.5% glucose. E. coli was grown at 37°C in Luria-Bertani medium. S. aureus Cowan and 8325-4 were grown at 37°C in Mueller-Hinton or tryptic soy broth (Difco). Whenever appropriate, antibiotics were added to the media at the following concentrations: erythromycin, 5 μg/ml for L. lactis and 500 μg/ml for E. coli; ampicillin, 75 μg/ml for E. coli. Bacterial stocks were kept at −70°C in liquid medium supplemented with 10% (vol/vol) glycerol.
DNA transformation and cloning experiments.
Plasmids expressing fragments of the S. aureus fnbA gene were generated from pOri23-fnbA (17) by an inverse PCR strategy described by Massey et al. (12). The Expand Long Template PCR system (Roche Diagnostics) was used as recommended in the manufacturer's instructions. The amplification conditions were 18 cycles of 95°C for 30 s, 60°C for 30 s, and 68°C for 10 min. Amplicons were purified with a QIAquick PCR purification kit (Qiagen), digested with NcoI or PvuI, respectively, and self-ligated with T4 DNA ligase.
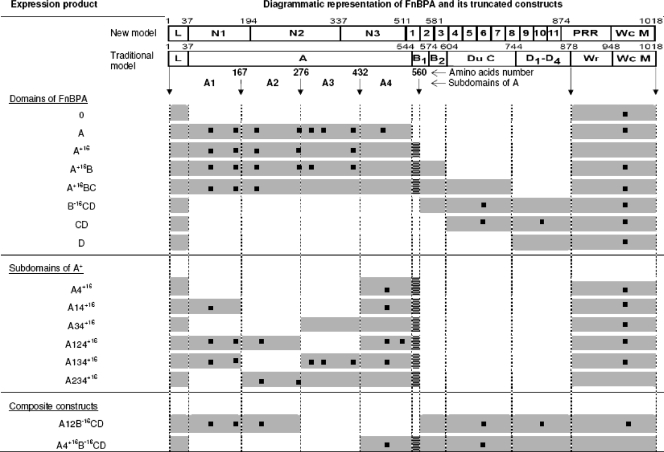
The maps of native FnBPA and its truncated derivatives expressed in lactococci are depicted in Fig. 1 and 2. For the primers selected to amplify the various domains or FnBPA subdomains, see Table S1 in the supplemental material. To facilitate amplicon religation, an NcoI or a PvuI restriction site was incorporated at the 5′ end of each primer. The constructs were named according to the traditional nomenclature of FnBPA domains. Domains Du and C were always together and were hence referred to as C for simplicity.
FIG. 2.
Recombinant FnBPA products expressed in lactococci and validation by LC-MS. FnBPA is represented at the top in its new and traditional views (see also Fig. 1). L, leader sequence; Wr and Wc, cell wall-spanning and -anchoring regions; M, membrane-spanning sequence. The different constructs expressed in L. lactis are shown below. Grey rectangles indicate the presence of the corresponding part of FnBPA, whereas a missing rectangle indicates that this part was removed during gene construction. Black squares represent the peptides identified by LC-MS, confirming the presence of the proteins on the cell surface.
In order to overlap the hinge between the described A and B domains of FnBPA, an extended A domain (named A+16) was generated which included the traditional A domain plus the first 16 N-terminal amino acids of the B domain. In symmetry, a B−16CD construct was generated which included the B domain minus its first 16 N-terminal amino acids, followed by the whole Du-C and D domains. To further dissect the functional regions of A+16, this domain was subdivided into four equivalent portions of ca. 130 amino acids each (named A1, A2, A3, and A4+16) which were expressed either individually or as fusions with other subdomains of FnBPA (Fig. 2).
The various constructs in pOri23 were propagated in E. coli XL1 (21), purified with the Wizard Plus Midipreps DNA purification system (Promega), and electroporated into L. lactis as described previously (18).
Detection of surface-expressed recombinant proteins.
The presence of the recombinant proteins expressed in L. lactis was determined by two different methods, Western immunoblotting and liquid chromatography coupled with mass spectrometry (LC-MS). Western immunoblotting was performed as previously described (7, 10), with antibodies recognizing residues 479 to 493 (IQNNKFEYKEDTIKE) of FnBPA (17). LC-MS can assess the correct expression of staphylococcal proteins in the cell wall of L. lactis recombinant bacteria, as well as the presence or absence of the desired internal fragments (A. Panchaud, E. Widmer, M. Kussmann, P. Moreillon, and M. Affolter, submitted for publication). Briefly, cells grown to an OD600 of 0.4 were harvested by centrifugation and resuspended in 40 ml phosphate-buffered saline (PBS), before the addition of 40 ml 8% boiling sodium dodecyl sulfate. The suspension was further boiled for 15 min. Boiled cells were washed several times and then resuspended in 1 ml water. Cell lysis was performed with a FastPrep apparatus (Qbiogene, Morgan Irvine, CA) with glass beads by three cycles of spinning for 45 s at 6.5 m/s. After ultracentrifugation, the pellet containing the cell walls was resuspended in 1.5 ml Milli-Q water, dried with a SpeedVac, and resuspended in 100 μl of 100 mM ammonium bicarbonate (NH4HCO3) at pH 7.8.
Proteins were reduced for 30 min at 50°C with 10 μl 45 mM 1,4-dithio-dl-threitol (Fluka, Buchs, Switzerland) and further alkylated for 60 min at room temperature in the dark with 10 μl of 100 mM iodoacetamide (Fluka). The resultant protein mixture was digested overnight at 37°C with trypsin (Promega, Madison, WI) at an enzyme-protein ratio of 1:50 (wt/wt). The tryptic peptides were cleaned on Sep-Pak tC18 cartridges (Waters, Milford, CT) and eluted with 2 × 1 ml 80% acetonitrile in two separate 1.5-ml centrifuge tubes. Peptides were diluted by adding 190 μl 0.1% formic acid and stored at −20°C until MS processing. The resulting peptides were separated by LC on a 5 to 50% acetonitrile-0.1% formic acid gradient and analyzed on an HCTultra ion-trap mass spectrometer (Bruker, Bremen, Germany) coupled on line to an Ultimate 3000 high-performance liquid chromatography system (Dionex, Sunnyvale, CA) equipped with an analytical Magic C18 reversed-phase column (100 by 0.075 mm, 5 μm; Spectronex, Basel, Switzerland) by gas phase fractionation. Proteins were identified by comparison to a database consisting of the L. lactis proteome plus the expected staphylococcal protein with Mascot (Matrix Science, London, England) (see Table S2 in the supplemental material).
In vitro adherence assays.
In vitro adherence to immobilized fibrinogen or fibronectin was assessed as previously described (2, 18), with 96-well plates (Nunc-Immuno Plate, MaxiSorp Surface; Nalge Nunc International) coated with serial twofold dilutions of purified fibrinogen or fibronectin (starting with 500 μg/ml). Each experiment was repeated on three or more independent occasions. Adherence titers were defined as the inverse of the highest dilution of ligand demonstrating adherence by optical density.
Competition experiments.
Three peptides were used as competitors in adherence assays (Fig. 1C). P1 (corresponding to amino acids 486 to 559 of FnBPA) and P2 (corresponding to amino acids 455 to 559 of FnBPA) were synthesized in vitro. P3 (corresponding to fragments 560 to 880, i.e., B−16CD) was expressed in E. coli with the six-histidine tag expression vector pQE-30 of Qiagen. Briefly, the B−16CD fragment of FnBPA (corresponding to nucleotides 1798 to 2760) amplified by PCR (forward primer, 5′-AGGGATCCGATGGTGGAGGTGGATA-3′; reverse primer, 5′-AGCCCGGGTGGCGTTGGTGGCACGATTGGAG) was cloned into pQE-30 between the BamHI and SmaI restriction sites (in bold) and propagated in E. coli. Recombinant proteins were expressed and purified by Ni2+ chelate chromatography by following the recommendations of the manufacturer.
For competition experiments, the wells of the microtiter plates were coated with constant ligand concentrations of 125 μg/ml fibrinogen and 31.25 μg/ml fibronectin. Coated plates were first incubated for 2 h at 37°C with serial twofold dilutions of either competitor (starting with 32 μg/ml) and washed twice with PBS before the addition of 5 × 107 CFU of the recombinant bacteria. Experiments were repeated on three or more independent occasions. Results were expressed as a percentage of the adhesion achieved without a competitor.
Bacterial internalization into cultured cell lines.
In vitro bacterial internalization into bovine aortic endothelial cells (BAEC) was assessed with a previously described antibiotic protection assay (19, 25). Confluent endothelial cells were incubated with 105 CFU of bacteria for 90 min at 37°C and washed three times with PBS before the addition of 100 μg/ml gentamicin to kill noninternalized bacteria. Cells were then washed three times with PBS and osmotically lysed by the addition of 1 ml of H2O. Lysates were finally plated for colony counts. Results are expressed as the percentage of surviving bacteria compared with the inoculum.
Experimental endocarditis in rats.
Sterile aortic vegetations were produced in female Wistar rats as previously described (5). Twenty-four hours after catheterization, groups of 8 to 10 rats were challenged intravenously with 7.5 log10 CFU, an inoculum lower than the minimal inoculum of the parent L. lactis subsp. cremoris strain infecting ≥90% of the animals (90% infective dose or ID90) (17). Recombinants producing endocarditis at this low inoculum demonstrated increased infectivity compared to the parent. Rats were killed 24 h later. Quantitative valve and spleen cultures were prepared as previously described (17). Bacterial densities in the vegetations were expressed as the mean log10 number of CFU per gram of tissue. The limit of detection was ≥2 log10 CFU/g of tissue. Rats with sterile valve cultures were considered uninfected.
Statistical analysis.
Chi-square and nonparametric Wilcoxon tests were used for comparison wherever appropriate. Fibronectin- and fibrinogen-binding titers and cell internalization percentages of the different truncated parts of FnBPA were first globally compared by using analysis of variance, and then post hoc tests (Fisher's PLSD test) were used, allowing direct comparisons between L. lactis expressing truncated parts of FnBPA with (i) L. lactis expressing all of FnBPA (positive control) and (ii) L. lactis 0 expressing only the signal sequence and cell wall-anchoring domain (negative control). The association between the different domains and subdomains of FnBPA and infection of valves or spleen, evaluated as a qualitative variable, was adjusted for by using logistic regression with backward stepping. The association between fibronectin or fibrinogen binding with BAEC internalization, evaluated as a continuous variable, was first tested by using simple regression and then adjusted for by using multiple linear regression with backward stepping. The association between fibronectin- or fibrinogen-binding and bacterial titers in vegetations and spleens was also first evaluated by using simple regression and then adjusted for by using multiple linear regression with backward stepping. Final models included all variables significantly and independently associated with the dependent variables, including adjustments for possible variations in inoculum size. For all tests, a P value of <0.05 was considered significant.
RESULTS
L. lactis constructs expressing various FnBPA fragments.
Figure 2 presents all of the FnBPA constructs expressed in L. lactis. Full expression and anchoring of all of the constructs in the cell wall of the surrogate bacteria were confirmed both by ligand-blotting experiments (data not shown) and by LC-MS. For each construct tested, Fig. 2 shows the positions of the different fragments recognized by LC-MS (black squares). These results confirm that all recombinant lactococci expressed the desired FnBPA fragments on their surface.
In vitro adherence and cell internalization.
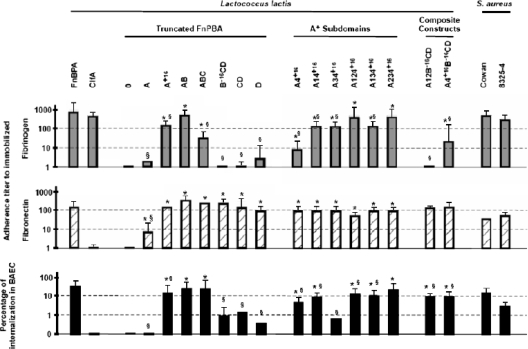
Figure 3 shows that (from left to right and top to bottom) (i) FnBPA-expressing lactococci could bind both fibrinogen and fibronectin, as well as trigger cell internalization, whereas ClfA-expressing recombinants bound only fibrinogen, as expected from previous results (19); (ii) truncated forms of FnBPA could bind both fibrinogen and fibronectin, as well as trigger cell internalization, only if they contained the extended A+16 domain depicted in Fig. 2; (iii) the minimal subdomain of A+16 that could confer all of these three properties was A4+16 (depicted in Fig. 1 and 2), yet combining it with other subdomains of A (i.e., A1, A2, and A3) increased its fibrinogen-binding capacity by up to 10 times; and (iv) deletion of minimal A4+16 from FnBPA ablated fibrinogen binding (see composite construct A12B−16CD), while adding it restored fibrinogen binding (see composite construct A4+16B−16CD). Thus, A4+16 was the minimal active subdomain conferring all three FnBPA properties, and various other subdomains of the protein could cooperate with it for ligand binding.
FIG. 3.
In vitro adherence to immobilized fibrinogen and fibronectin and internalization into cultured endothelial cells. L. lactis expressing various FnBPA fragments were tested first for in vitro adherence to immobilized fibrinogen (upper panel) and fibronectin (middle panel) and second for internalization into cultured BAEC (lower panel). S. aureus Cowan and 8325-4 were used as wild-type controls, L. lactis 0 (i.e., expressing only the signal sequence and cell wall-anchoring domain) served as a negative control, and L. lactis FnBPA+ was used as a positive control. Binding titers were defined as the inverse of the highest dilution of ligand demonstrating adherence by optical density. Internalization results are expressed as the percentage of surviving bacteria compared to the initial inoculum. All data represent the median of three or more separate experiments. Intra- and interrun variations were ≤20%. For all L. lactis strains expressing truncated FnBPA or A+16 subdomains, significant differences (analysis of variance and Fisher's post hoc PLSD test, P < 0.05) from L. lactis 0 (*) and L. lactis FnBPA+ (§) are indicated.
Capacity of the different FnBPA domains to induce experimental endocarditis.
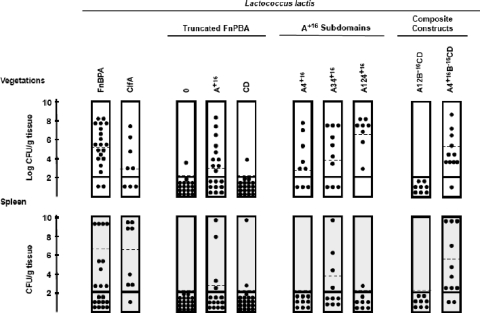
The various constructs were then tested in vivo for the capacity to induce endocarditis. Groups of rats with catheter-induced aortic vegetations were challenged with 7.5 log10 CFU of the different Lactococcus mutants, a number below the expected ID90 of the parent Lactococcus strain (Fig. 4). Only recombinants expressing whole FnBPA, domain A+16, subdomain A4+16, or any other constructs containing subdomain A4+16 were able to induce infective endocarditis (P < 0.01). Indeed, L. lactis expressing subdomain A4+16 alone or in combination infected 59/77 rats (76.6%), in contrast to those missing this specific domain (2/45 or 4.4% of the animals, P < 0.0001, chi-square test). A multivariate analysis further confirmed that A4+16 was the only FnBPA subdomain significantly associated with endocarditis (odds ratio = 23; 95% confidence interval, 8.7 to 62; P < 0.0001).
FIG. 4.
Results of experimental endocarditis. Groups of 8 to 10 animals with catheter-induced aortic vegetations were challenged with a bacterial inoculum (ca. 7.5 log10 CFU) below the expected ID90 of the L. lactis parent strain and killed 24 h later. Each dot indicates the bacterial density in the vegetations (upper panel) or the spleen (lower panel) of one single animal. Dashed lines represent mean bacterial concentrations. Bacterial densities in the tissues were expressed as the mean log10 number of CFU per gram of tissue (vegetations) or the mean number of CFU per gram of tissue (spleen). The limit of detection was ≥2 log10 CFU/g of tissue. Rats with sterile cultures were considered uninfected.
Relationships among fibrinogen and fibronectin binding, endocarditis induction, endocarditis severity, and invasion of cultured cell lines.
We further tested whether the magnitude of in vitro binding to fibrinogen or fibronectin (Fig. 3) correlated with the in vivo infectivity of the recombinants (Fig. 4). Only in vitro binding avidity for immobilized fibrinogen, but not for fibronectin, was statistically significantly associated with the in vivo induction of endocarditis (odds ratio per difference in dilution titer = 1.7; 95% confidence interval, 1.4 to 2.0; P < 0.0001, logistic regression). This confirmed that fibrinogen binding was strongly associated with endocarditis induction, whereas binding to fibronectin was not (19).
We next evaluated the importance of fibrinogen and fibronectin binding for disease severity, as assessed by vegetation bacterial titers. In contrast to endocarditis induction, for which fibrinogen binding was the only associated factor, disease severity correlated with both fibrinogen and fibronectin binding (coefficients of 0.428 ± 0.044 and 0.208 ± 0.081, P < 0.0001 and P = 0.01, respectively).
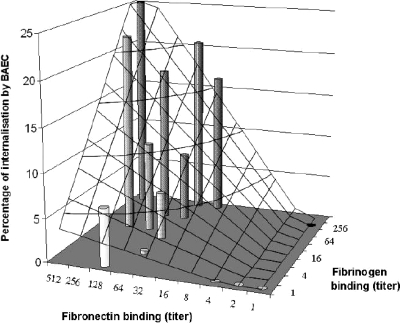
Since we previously showed that the capacity to invade endothelial cells in vitro was associated with progressive experimental endocarditis in animals (19), we further asked whether fibrinogen and fibronectin binding could synergize the invasion of cultured cell lines. Figure 3 confirms that fibronectin binding was a prerequisite for cell internalization. In addition, however, when fibrinogen binding was simultaneously present with fibronectin binding, it significantly promoted the rate of internalization compared to fibronectin binding alone (16.4% versus 4.6%, respectively; P < 0.0001, Wilcoxon test). Thus, at a given fibronectin-binding titer, the avidity for fibrinogen synergistically enhanced cell invasion (Fig. 5). This synergism was further confirmed by multivariate analyses where the intensities of fibrinogen binding and fibrinogen binding also correlated with the percentage of internalization into cultured endothelial cells (coefficients of 1.438 ± 0.228 and 1.734 ± 0.160, respectively; P < 0.0001). Hence, the fibrinogen-binding property of FnBPA was not only required for infection initiation but also combined with fibronectin binding to synergize bacterial uptake by endothelial cells, a feature correlating with disease severity in vivo (19).
FIG. 5.
Synergy between fibrinogen and fibronectin binding for internalization into endothelial cells. The graph represents the predicted percentage of in vitro bacterial internalization into BAEC (vertical axis and latticework) according to the fibrinogen- and fibronectin-binding titers (horizontal axes). This representation was drawn from experimental data (cylinders) by multiple linear regression. It shows that at a given fibronectin-binding titer, the avidity for fibrinogen synergistically and dramatically enhanced internalization.
Attempt to outcompete A4+16 and FnBPA binding with synthetic peptides.
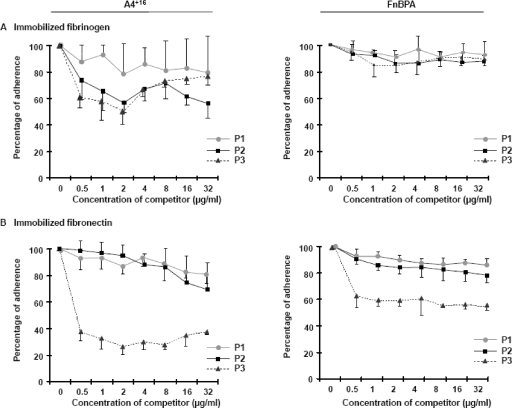
Because the minimal A4+16 subdomain conferred three major FnBPA properties involved in experimental endocarditis (i.e., fibrinogen binding, fibronectin binding, and endothelial cell invasion), we sought to determine whether synthetic peptides derived from it could compete with in vitro binding by itself or by whole FnBPA. Figure 1C maps competing peptides P1 and P2, encompassing parts of A4+16, and control peptide P3, encompassing fibronectin-binding domains B−16CD. Figure 6 depicts the results. Regarding fibrinogen binding, only peptide P2, which spanned almost the entire length of A4+16, demonstrated some competition with A4+16, but not with whole FnBPA. Control peptide P3 also interfered marginally with fibrinogen binding, although it contained only fibronectin-binding repeats. This might relate to the fact that the last 16 amino acids of A4+16 also overlapped one fibronectin-binding repeat, which itself also appeared indispensable to confer both fibrinogen and fibronectin binding on the fragment (Fig. 1 and 4). Thus, some kind of cross-interference could have occurred at this level.
FIG. 6.
Competition experiments. Synthetic peptides P1, P2, and P3, corresponding to either parts of the A4+16 fragment (P1 [•] and P2 [▪]) or the B−16CD fragment (P3 [▴]) (for precise localizations, see Fig. 1), were used to compete for the adhesion of either A4+16 (left panels) or FnBPA (right panels) to immobilized fibrinogen (A) or fibronectin (B). Coated plates were first incubated with serial twofold dilutions of either competitor (starting at 32 μg/ml) before the addition of 5 × 107 CFU of the recombinant bacteria. Experiments were done at least in triplicate. Results are expressed with the standard deviation as a percentage of the adhesion achieved without a competitor.
With regard to fibronectin binding, control peptide P3 could clearly compete for binding by A4+16 but competed only partially with binding by whole FnBPA. In contrast, P1 and P2 had no effect at the concentrations used in this study. Hence, competition under these conditions was only partial and less pronounced against whole FnBPA than against A4+16. This stresses the potential importance of the FnBPA domain redundancy.
DISCUSSION
Using ClfA and FnBPA as model proteins, we previously showed that fibrinogen binding is necessary and sufficient for endocarditis induction but not sufficient for disease progression and that fibronectin binding is necessary and sufficient for cell invasion and disease progression but not sufficient for disease induction (17, 19). This suggested a two-step disease process in which the two binding functions cooperate sequentially.
The present report shows that the system is more intertwined than originally thought—at least for FnBPA—in terms of both functional anatomy and pathogenic mechanisms. First, at the level of functional anatomy, the smallest subdomain of FnBPA reunifying all of its relevant functions in endocarditis pathogenesis was A4+16. This was in agreement with current knowledge on the fibronectin binding of FnBPA, since this subdomain encompasses 1 of the 11 fibronectin-binding repeats of FnBPA, which has been shown to be sufficient for fibronectin binding. Conversely, this was totally unexpected for fibrinogen binding since A4+16 spanned only part of the fibrinogen-binding N3 module and since we failed to observe significant fibrinogen binding when the A domain (encompassing the N1, N2, and N3 modules) was expressed alone, by contrast with previous studies (3, 8, 29). Adding only 16 amino acids (544 to 559) to domain A or A4 (leading to A+16 or A4+16, respectively) dramatically increased the binding affinity for both fibrinogen and fibronectin, whereas peptides containing the same 16 amino acids (e.g., P1 and P2 in Fig. 1C and 6) could barely outcompete such binding. Likewise, binding of A4+16 to fibrinogen was enhanced ≥10 times when the fragment was associated with upstream portions of FnBPA (e.g., A1 to A3), which did not efficiently bind fibrinogen by themselves (for instance, see domain A4+16 versus domain A34+16 in Fig. 3). Eventually, fibrinogen binding was affected to some degree by synthetic peptide B−16CD, which carried fibronectin-binding repeats that did not bind to fibrinogen. Such subtle functional folding was recently suggested from in silico and in vitro data indicating that FnBPA binds fibrinogen via a dock-lock-latch mechanism of binding (8). Indeed, whereas isolated modules N2 and N3 were not able to bind fibrinogen, C-terminal residues of domain N3, when associated with N2, are likely to undergo a conformational change to bind adjacent to domain N2, trapping the fibrinogen in its groove between N2 and N3 and locking it in place. Neither the N-terminal N1 domain nor amino acids 512 to 544 were needed for this binding. Since domain A4+16 does not encompass the N2 module and the N-terminal part of N3, it is likely that another mechanism is involved. Moreover, its specific regions for fibrinogen and fibronectin binding could not be separated by competition with synthetic peptides, suggesting that some kind of folding of the fragment afforded its dual capacity, irrespective of clear boundaries between individual binding domains.
Thus, this functional versatility may be partly related to the location of the protein; i.e., the in vivo conformation of surface-bound FnBPA may be different from that of unbound recombinant proteins. It is also compatible with the induced-fitness dynamics of FnBPA binding, where binding modules are disordered in the absence of fibronectin and undergo a disordered-to-ordered transition upon ligand binding (6). Moreover, the present results identify a region of dual binding at the hinge of the major fibrinogen- and fibronectin-binding regions of FnBPA. This provides a functional rationale for the redundant structure of FnBPA, which might have evolved a minimal (necessary and sufficient) intertwined core A4+16 reinforced by multiple binding modules. This redundancy may not only increase but also guarantee binding abilities in the case of blockage of some of these modules.
Second, at the level of pathogenic mechanisms, the primary role of fibrinogen binding in the induction of experimental endocarditis was statistically confirmed (17, 19). Indeed, platelet activation is an important initial step in the pathogenesis of infectious endocarditis, via a fibrinogen bridge between FnBPA and the platelet integrin GPIIb/IIIa. Since it has been shown that platelets in vitro can also be similarly activated by FnBPA fibronectin-binding domains only (3), it appears that the risk of in vivo endocarditis induction is mainly correlated with the avidity of fibrinogen binding. On the other hand, there is a shared responsibility of fibrinogen and fibronectin binding in disease severity. The synergistic effect of fibrinogen and fibronectin binding was clearly apparent in endothelial cell invasion, a property that is associated with in vivo invasion and persistence (17, 19). This synergy was unexpected, because fibronectin binding was previously shown to be sufficient (and necessary) for internalization into eukaryotic cells (25, 26). A contribution of fibrinogen binding in this situation had never been studied previously in S. aureus. However, it has already been shown in other pathogens such as Streptococcus agalactiae that fibrinogen binding may promote adherence, leading to the invasion of endothelial cells (4, 28). Thus, while fibronectin binding is sufficient for attachment to and internalization into eukaryotic cells, fibrinogen binding could enhance both steps, either by favoring attachment or by stabilizing the structure of the various fibronectin-binding motifs.
Staphylococcal FnBPA thus appears as a sophisticated multifunctional adhesin whose structure results from the association of various domains that are responsible for virulence. These domains act both sequentially (fibrinogen binding for disease initiation and fibronectin binding for endothelial cell invasion and disease progression) and synergistically, with fibrinogen binding enhancing endothelial cell bacterium uptake triggered by fibronectin binding. Moreover, fibrinogen binding is likely to operate at an early step in the infection process, whereas fibronectin binding, while pivotal in the pathogenic process of infectious endocarditis, is not the primum movens of valve infection but acts subsequently by triggering cell invasion.
Finally, the present observations also carry two practical messages. First, blocking the binding functions of FnBPA with peptides or antibodies targeting only part of it is likely to fail or to succeed only partially. This finds a concrete demonstration both in the present competition experiments (Fig. 6) and in previous work showing that antibodies directed against FnBPA could not block adherence and even increased it by stabilizing the fibronectin-FnBPA complex (27). Thus, the protein should be targeted extensively to ensure binding inhibition. Second, if one binding function were to be a priority target, then it would fibrinogen binding because it is directly responsible for disease initiation and also combines synergistically with fibronectin binding for further progression and persistence. While this assumption awaits experimental confirmation, it will be critical to test whether other fibrinogen-binding proteins of S. aureus also produce the same synergism with fibronectin binding.
Supplementary Material
Acknowledgments
We thank Marlyse Giddey and Jacques Vouillamoz for outstanding technical assistance.
This work was supported by grants 32-44099.96/2 and 3200-065371.01/1 from the Swiss National Fund and by a grant from the Fondation pour la Recherche Médicale to Lionel Piroth.
Editor: A. Camilli
Footnotes
Published ahead of print on 9 June 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Downer, R., F. Roche, P. W. Park, R. P. Mecham, and T. J. Foster. 2002. The elastin-binding protein of Staphylococcus aureus (EbpS) is expressed at the cell surface as an integral membrane protein and not as a cell wall-associated protein. J. Biol. Chem. 277243-250. [DOI] [PubMed] [Google Scholar]
- 2.Entenza, J. M., T. J. Foster, D. Ni Eidhin, P. Vaudaux, P. Francioli, and P. Moreillon. 2000. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 685443-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald, J. R., A. Loughman, F. Keane, M. Brennan, M. Knobel, J. Higgins, L. Visai, P. Speziale, D. Cox, and T. J. Foster. 2006. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcγRIIa receptor. Mol. Microbiol. 59212-230. [DOI] [PubMed] [Google Scholar]
- 4.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 723495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Héraïef, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.House-Pompeo, K., Y. Xu, D. Joh, P. Speziale, and M. Hook. 1996. Conformational changes in the fibronectin binding MSCRAMMs are induced by ligand binding. J. Biol. Chem. 2711379-1384. [DOI] [PubMed] [Google Scholar]
- 7.Hussain, M., K. Becker, C. von Eiff, J. Schrenzel, G. Peters, and M. Herrmann. 2001. Identification and characterization of a novel 38.5-kilodalton cell surface protein of Staphylococcus aureus with extended-spectrum binding activity for extracellular matrix and plasma proteins. J. Bacteriol. 1836778-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane, F., A. Loughman, V. Valtunila, M. Brennan, P. Speziale, and T. J. Foster. 2007. Fibrinogen and elastin bind to the same region within the A domain of fibronectin binding protein A, an MSCRAMM of Staphylococcus aureus. Mol. Microbiol. 63711-723. [DOI] [PubMed] [Google Scholar]
- 9.Keane, F. M., A. W. Clarke, T. J. Foster, and A. S. Weiss. 2007. The N-terminal A domain of Staphylococcus aureus fibronectin-binding protein A binds to tropoelastin. Biochemistry 467226-7232. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 11.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 12.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. J. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Höök, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3839-851. [DOI] [PubMed] [Google Scholar]
- 13.McDevitt, D., P. François, P. Vaudaux, and T. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11237-248. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4759-770. [DOI] [PubMed] [Google Scholar]
- 15.Patti, J., and M. Hook. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6752-758. [DOI] [PubMed] [Google Scholar]
- 16.Peacock, S., T. Foster, B. Cameron, and A. Berendt. 1999. Bacterial fibronectin binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 1453477-3486. [DOI] [PubMed] [Google Scholar]
- 17.Que, Y., P. François, J. Haefliger, J. Entenza, P. Vaudaux, and P. Moreillon. 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 696296-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Que, Y., J. Haefliger, P. Francioli, and P. Moreillon. 2000. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect. Immun. 683516-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Que, Y. A., J. A. Haefliger, L. Piroth, P. Francois, E. Widmer, J. M. Entenza, B. Sinha, M. Herrmann, P. Francioli, P. Vaudaux, and P. Moreillon. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 2011627-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roche, F. M., R. Downer, F. Keane, P. Speziale, P. W. Park, and T. J. Foster. 2004. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J. Biol. Chem. 27938433-38440. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Schwarz-Linek, U., M. Hook, and J. R. Potts. 2006. Fibronectin-binding proteins of gram-positive cocci. Microbes Infect. 82291-2298. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz-Linek, U., J. M. Werner, A. R. Pickford, S. Gurusiddappa, J. H. Kim, E. S. Pilka, J. A. Briggs, T. S. Gough, M. Hook, I. D. Campbell, and J. R. Potts. 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423177-181. [DOI] [PubMed] [Google Scholar]
- 24.Signäs, C., G. Raucci, K. Jönsson, P. Lindgren, G. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha, B., P. François, O. Nuesse, M. Foti, O. Hartford, P. Vaudaux, T. J. Foster, D. Lew, M. Herrmann, and K. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5-β1. Cell. Microbiol. 1101-117. [DOI] [PubMed] [Google Scholar]
- 26.Sinha, B., P. François, Y. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. Krause, G. Peters, and M. Herrmann. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 686871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speziale, P., D. Joh, L. Visai, S. Bozzini, K. House-Pompeo, M. Lindberg, and M. Hook. 1996. A monoclonal antibody enhances ligand binding of fibronectin MSCRAMM (adhesin) from Streptococcus dysgalactiae. J. Biol. Chem. 2711371-1378. [DOI] [PubMed] [Google Scholar]
- 28.Tenenbaum, T., C. Bloier, R. Adam, D. J. Reinscheid, and H. Schroten. 2005. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 734404-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wann, E., S. Gurusiddappa, and M. Höök. 2000. The fibronectin-binding MSRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 27513863-13871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.