Abstract
Severe experimental infections with Cryptosporidium parvum have been reported in immunocompromised animals such as SCID mice (mice without functional T cells and B cells). In a C. parvum infection with 1 × 106 oocysts/mouse in SCID beige (SCIDbg) mice (SCID mice lacking functional NK cells), oocyst shedding was first demonstrated 18 days after infection. However, shedding was shown as early as 3 days after the same infection in SCIDbgMN mice. All of the SCIDbgMN mice died within 16 days of C. parvum infection, while 100% of the SCIDbg mice exposed to the parasite survived. SCIDbgMN mice are SCIDbg mice depleted of functional macrophages (Mφ) and neutrophils (PMN), suggesting that the severity early after C. parvum infection is strongly influenced by the functions of Mφ and PMN. All SCIDbgMN mice orally infected with a lethal dose of C. parvum survived after they were inoculated with Mφ from SCIDbg mice exposed to C. parvum (CP-Mφ) or resident Mφ previously cultured with PMN from C. parvum-infected SCIDbg mice (CP-PMN). However, all SCIDbgMN mice inoculated with CP-PMN alone or resident Mφ alone died after C. parvum infection. CP-Mφ were identified as classically activated Mφ (M1Mφ), and CP-PMN were characterized as PMN-I. In in vitro studies, resident Mφ converted to M1Mφ after transwell cultivation with CP-PMN. These results indicate that the resistance of SCIDbg mice early after C. parvum infection is displayed through the function of M1Mφ which are converted from resident Mφ influenced by CP-PMN (PMN-I).
Cryptosporidium hominis (an anthroponotic pathogen) and Cryptosporidium parvum (a zoonotic pathogen) cause endemic and epidemic diarrheal disease in immunocompromised humans, such as AIDS patients (9, 26, 52). In this work, we designate the infection “chronic” when symptoms developed 3 to 8 weeks after Cryptosporidium infection and “acute” when the symptoms developed 4 days to 2 weeks after the infection. Immunocompetent hosts usually present with a transient diarrheal illness and are able to clear the infection spontaneously (7). However, as a common opportunistic pathogen in AIDS patients (or in severely immunocompromised hosts), C. parvum causes a severe diarrhea associated with significant mortality. Because CD4+ T-cell counts are dramatically decreased in AIDS patients, the role of CD4+ T cells in the host resistance of these patients against C. parvum infection has been studied (7, 34, 41, 48), and CD4+ T cells have shown to be key effector cells in the host resistance against C. parvum infection (7, 34, 48). The importance of gamma interferon (IFN-γ) in host anti-C. parvum resistance has also been demonstrated in IFN-γ gene knockout (GKO) mice (10, 23, 46, 56). In those studies, adult GKO mice manifested both acute and chronic C. parvum infections. In contrast, SCID mice manifested only chronic C. parvum infection, and wild-type mice did not manifest acute or chronic C. parvum infection. Interestingly, GKO neonatal mice manifested severe acute C. parvum infection and died within 8 days of C. parvum infection, while wild-type neonatal mice were able to clear acute and chronic C. parvum infections (22). Since the antigen presentation capacity of macrophages (Mφ) in neonatal mice is poor (25), these facts suggest that IFN-γ and Mφ are crucial in host resistance against acute C. parvum infection. In subsequent studies, CD4+ T cells were shown to be the major effector cells that produce IFN-γ in mice resistant to C. parvum infection. However, how C. parvum infection is controlled by IFN-γ in the host's antiprotozoan resistance remains unclear. The activation of Mφ (4), induction of antimicrobial peptides (6), and induction of chemokines that act as chemoattractants for immune cells to infected sites (23) possibly play a role in IFN-γ-associated host anti-C. parvum resistance.
In our current studies, acute C. parvum infection with a high mortality rate routinely developed in SCIDbgMN mice, while it was not demonstrated in SCID beige (SCIDbg) mice. SCIDbg mice are mice lacking functional T cells, B cells, and NK cells, and SCIDbgMN mice are SCIDbg mice depleted of functional Mφ and neutrophils (PMN) (47). Therefore, it is indicated that any immunocompetent cells remaining in SCIDbg mice (or immunocompetent cells that exist in SCIDbg mice and do not exist in SCIDbgMN mice) play a role in the host resistance against acute C. parvum infection. Thus, Mφ and PMN are indicated as cells that play a role in the host resistance against acute C. parvum infection.
Mφ have been well described as key cells responsible for host defense against invading intracellular pathogens (17, 30). Classically activated Mφ (M1Mφ) were described as effector cells for controlling infections with Leishmania and Toxoplasma parasites (27, 43). M1Mφ are characterized as Mφ with the abilities to (i) kill infected cells, (ii) express inducible nitric oxide synthase (iNOS), and (iii) secrete nitric oxide, proinflammatory cytokines, and Th1 response-associated cytokines (30). In our previous studies, an immunostimulating subset of PMN (PMN-I) was demonstrated in the peripheral blood of mice with mild burn injuries, and these PMN were characterized as Gr-1+ CD11b− CD49d+ IFN-γ-, CCL3-, and interleukin-12 (IL-12)-producing cells (47). When resident Mφ were cultured in dual-chamber transwells with PMN-I, Mφ conversion from resident Mφ to M1Mφ was demonstrated (47). During gastrointestinal infections, recruitment of Mφ and PMN from circulation to lamina propria has been well documented (5). Therefore, in the present study, a role of PMN and Mφ in the host resistance of SCIDbg mice early after C. parvum infection was investigated.
MATERIALS AND METHODS
Mice.
Seven- to 11-week-old male BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). SCIDbg mice (BALB/c origin) were purchased from Taconic (Petersburgh, NY). SCIDbg mice were defined as immunodeficient mice lacking functional T cells, B cells, and NK cells. SCIDbgMN mice were SCIDbg mice depleted of functional Mφ and PMN. SCIDbgMN mice were created from SCIDbg mice after treatment with carrageenan (0.4 mg/mouse, intravenously) and anti-Ly6G monoclonal antibody (MAb) (100 μg/mouse, intraperitoneally) plus whole-body X irradiation (4 Gy) (47). No functional Mφ were found in the reticuloendothelial systems of these mice at 3 to 7 days after the final treatment. Also, PMN were not recovered from these mice at 1 to 7 days after X irradiation, even when they were exposed to pathogens. When bone marrow cells or peripheral blood cells taken from these mice were tested morphologically for residual PMN after Wright-Giemsa and alkaline phosphatase staining, no PMN were detected until 7 days after the combination treatment (47). The Institutional Animal Care and Use Committee of The University of Texas Medical Branch at Galveston approved all procedures for the animal experiments developed in this study (approval number 02-09-066). Various groups of mice were infected with 1 × 106 C. parvum oocysts in 200 μl of phosphate-buffered saline (PBS) by gastric gavage. The dose of oocysts was chosen on the basis of prior experiments.
Parasites.
C. parvum oocysts (originally isolated from calf feces; obtained from James A. Harp, U.S. Department of Agriculture, Ames, IA) were utilized in this study. Oocysts were maintained in specific-pathogen-free C57BL/6 mice that were treated with dexamethasone, and oocysts were purified from the fecal material of these mice as previously described (37). Oocysts then were surface sterilized with 0.55% sodium hypochlorite solution for 10 min, washed three times with PBS (15), and kept at 4°C for up to 6 months prior to use. Sporozoites were prepared by oocyst excystation in Hanks balanced salt solution containing 0.4% sodium taurocholate and 0.25% trypsin for 60 min at 37°C (3). Small numbers of contaminating oocysts in the sporozoite preparation were removed after being passed through transwells with a 3-μm-pore-size polycarbonate filter under centrifugation (800 rpm, 3 s) (16).
Mφ preparations.
As sources of Mφ, peritoneal exudates of SCIDbg mice (resident Mφ) or SCIDbg mice 3 days after infection with 1 × 106 C. parvum oocysts (CP-Mφ) were utilized. To isolate Mφ, peritoneal exudate cells of these mice were adjusted to 5 × 106 cells/ml in MagCellect buffer (R&D Systems, Minneapolis, MN) and incubated with magnetic beads (Dynal, Great Neck, NY) coated with anti-F4/80 MAb at a ratio of one cell to five beads for 30 min at 4°C. F4/80+ cells (Mφ) were then magnetically harvested. The purity of monocytes isolated in this procedure was routinely more than 97%. Mφ were identified as M1Mφ when they showed an ability to produce CCL5 and to express iNOS mRNA. A standard M1Mφ preparation was generated from resident Mφ (1 × 106 cells/ml) after stimulation with a mixture of poly(I·C) (20 μg/ml) and CpG DNA (5 μg/ml) for 24 h (51). A standard M2aMφ/M2cMφ preparation was obtained from mice 2 days after severe flame burn injury (third degree, 25% total body surface area), and a standard M2bMφ preparation was obtained from mice 10 days after the same burn injury (21). RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) was used for the cultivation of Mφ. Three different subtypes of M2Mφ (M2aMφ, M2bMφ, and M2cMφ) have been described previously (30). These subsets of M2Mφ are discriminated from each other by their properties (11, 30, 31, 44). Thus, CCL17-producing Mφ with the FIZZ1 gene are identified as M2aMφ, CCL1-producing Mφ with the SPHK1 gene are classified as M2bMφ, and CXCL13-producing Mφ with the FIZZ1 gene are recognized as M2cMφ. All of the M2Mφ subtypes express the IL-10 gene (30). Therefore, in this study, CCL17, CCL1, and CXCL13 were used as biomarkers for M2aMφ, M2bMφ, and M2cMφ, respectively. We have already reported (21) that M2aMφ and M2cMφ are isolated from peritoneal cavities of mice 2 days after severe flame burn injury (third degree, 25% total body surface area burn) and that M2bMφ are isolated from peritoneal cavities of mice 10 to 21 days after the same burn injury. Therefore, peritoneal Mφ from mice 2 days after burn injury (third degree, 25% total body surface area) were utilized as the standard M2aMφ/M2cMφ preparation, and peritoneal Mφ isolated from mice 10 days after the same burn injury were utilized as the standard M2bMφ preparation.
PMN preparations.
As previously described, crude PMN were isolated from whole peripheral blood using Ficoll-Hypaque and dextran sedimentations (53). Briefly, peripheral blood was withdrawn from the hearts of mice with a heparinized syringe. The peripheral blood was centrifuged with Ficoll-Hypaque, and precipitates were obtained as a PMN-rich fraction. Precipitates were then suspended in 1% dextran (T-500; Pharmacia, Piscataway, NJ) and kept for 1 h at room temperature to allow the sedimentation of residual erythrocytes. The resulting fraction was treated with erythrocyte-lysing kits (R&D Systems) to eliminate small amounts of erythrocytes, and crude PMN preparations were obtained. For further purifications, PMN were negatively selected from this preparation using a mixture of biotin-conjugated anti-CD3 (T cells) and anti-F4/80 (monocytes/Mφ) MAbs for 30 min at 4°C. The obtained cells were then suspended in MagCellect buffer and incubated with magnetic beads coated with streptavidin at a ratio of one cell to five beads for 30 min at 4°C. The beads were magnetically separated to the side of the tube, and the remaining cells were harvested. The obtained cells were >96% viable and >98% pure PMN when analyzed by flow cytometry with fluorescein isothiocyanate-conjugated anti-Gr-1 MAb and Wright-Giemsa/alkaline phosphatase staining. The majority of the contaminating cells in the preparations were shown to be erythrocytes when analyzed by flow cytometry using phycoerythrin-conjugated anti-TER-119 MAb (specific for mouse erythrocytes). Monocytes and T cells were not detected in these PMN preparations when tested by flow cytometry using phycoerythrin-conjugated anti-CD3 or F4/80 MAb. RPMI 1640 medium supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics was used for the cultivation of PMN. Previously, we have described three different subsets of PMN (47). These PMN are distinguished from each other in the following ways: PMN-I (Gr-1+ CD11b− CD49d+) produce IL-12/CCL3 and express TLR2/TLR4/TLR5/TLR8; PMN-II (Gr-1+ CD11b+ CD49d−) produce IL-10/CCL2 and express TLR2/TLR4/TLR7/TLR9; and normal PMN (PMN-N) (Gr-1+ CD11b− CD49d−), which lack the ability to produce these cytokines, express TLR2/TLR4/TLR9 (47). Therefore, in this study IL-12/CCL3 and IL-10/CCL2 were utilized as biomarkers for PMN-I and PMN-II, respectively. A PMN preparation was considered to be PMN-N when it did not produce these soluble factors. PMN-II functioned to convert resident Mφ to M2Mφ, while PMN-I stimulated resident Mφ conversion to M1Mφ. PMN-N did not show any activities in the modification of Mφ.
C. parvum infection.
Various groups of mice were infected orally with 106 oocysts/mouse of C. parvum. The level of cryptosporidiosis in these mice was assessed by (i) the number of oocysts in their daily fecal samples and (ii) their mortality rates (37). Also, weight change, appetite, and diarrhea were observed in these mice. To determine the oocyst shedding, each mouse was placed in a wire-floored cage with underlying moist paper towels (37). Freshly excreted fecal pellets from each mouse were collected and kept at 4°C in PBS until oocyst isolation. These fecal samples were collected once a day for up to 2 weeks and twice a week for up to 5 weeks. Each daily fecal specimen was passed through a metal sieve (pore size, approximately 250 μm) in order to remove large food particles, and the remaining suspension was collected in a 50-ml centrifuge tube. The sieved fecal suspension was centrifuged at 1,600 × g for 10 min, and the pellet was suspended in 15 ml of saturated NaCl solution and overlaid with 4 ml of water. After centrifugation at 1,600 × g for 20 min, oocysts were harvested from the layer between the saturated salt solution and water and washed once in 10 ml of water (37). The number of oocysts was counted using a hemocytometer, and oocyst shedding was expressed as the number of oocysts excreted per day per mouse. To determine the mortality rates, all mice were monitored every day for 8 weeks. The mortality rates in test groups were compared with those in control groups using the log rank test. All mice utilized in this study were housed in individually ventilated isolation cubicles. In these housing conditions, SCIDbg mice and SCIDbgMN mice X irradiated or not irradiated survive for more than 2 months after the irradiation, indicating that our X-irradiation protocol has a minimal influence on the mortality of SCIDbgMN mice infected with C. parvum. Diarrhea has been demonstrated in SCIDbgMN mice within 5 days of C. parvum infection. Heavy weight loss and suppression of appetite following diarrhea might be the cause of death in these mice.
Statistical analysis.
The results obtained were analyzed statistically using analysis of variance. Survival curves were analyzed using the Kaplan-Meier log rank test. The results were considered significant if the P value was lower than 0.05.
RESULTS
C. parvum infection in SCIDbgMN mice.
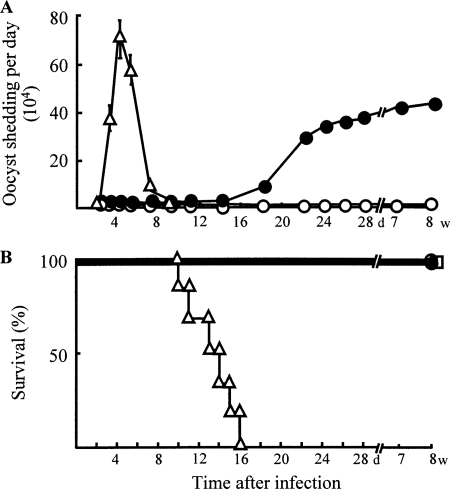
Normal mice, SCIDbg mice, and SCIDbgMN mice were orally infected with C. parvum at a dose of 1 × 106 oocysts/mouse, and the time course of daily oocyst shedding in each mouse group was compared. Oocysts were not demonstrated in daily fecal samples from normal mice infected with C. parvum. In SCIDbg mice, the oocyst excretion was first detected at 18 days after infection, and the number of oocysts shed by these mice increased up to 3.6 × 105 oocysts/mouse/day at 28 days after infection. Oocyst shedding was demonstrated in SCIDbg mice 8 weeks after C. parvum infection. Heavy weight loss and suppression of appetite were not observed in these mice. In contrast, oocyst shedding was observed within 3 days of infection in SCIDbgMN mice, and it peaked on day 4 (Fig. 1A). These mice exhibited heavy weight loss and suppression of appetite; they began dying 10 days after infection, and all of them died within 16 days of C. parvum infection (Fig. 1B). All of the SCIDbgMN mice not having C. parvum infection survived until the end of the experimental period. These results indicate that oral infection with C. parvum in SCIDbgMN mice causes acute cryptosporidiosis with high mortality rates, while it causes chronic infection without any mortality in SCIDbg mice.
FIG. 1.
Susceptibility of normal mice, SCIDbg mice, and SCIDbgMN mice to C. parvum infection. Normal mice (open circles), SCIDbg mice (closed circles), and SCIDbgMN mice (open triangles) (12 per group) were infected orally with 1 × 106 C. parvum oocysts per mouse. Fecal pellets were collected from each group of mice for oocyst count (oocyst shedding/mouse/day) (A). To determine the mortality rates (B), these infected mice and uninfected SCIDbgMN mice (open squares) were observed every 12 h for 8 weeks. These data are representative of three different experiments.
Anti-C. parvum effector cells for acute cryptosporidiosis.
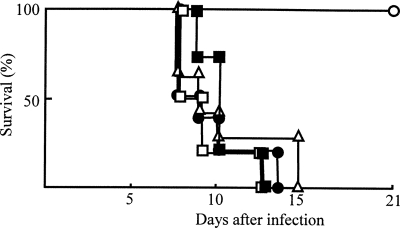
Acute cryptosporidiosis did not occur in SCIDbg mice orally infected with C. parvum. However, it occurred severely in SCIDbgMN mice exposed to the pathogen in the same fashion. Since the difference between SCIDbg mice and SCIDbgMN mice was the presence (SCIDbg mice) or lack (SCIDbgMN mice) of Mφ and PMN, these cells were strongly suggested as key effector cells in the host defense against acute cryptosporidiosis. Therefore, by reconstitution experiments we next examined the role of Mφ and PMN in host resistance of SCIDbgMN mice against acute infection with C. parvum. When Mφ from peritoneal cavities of SCIDbg mice 3 days after C. parvum infection (CP-Mφ) were adoptively transferred to SCIDbgMN mice and these mice were infected with 1 × 106 oocysts of C. parvum, all of these mice survived. Based on our preliminary studies, in these experiments 1 × 106 cells/mouse of Mφ were inoculated intravenously into SCIDbgMN mice. However, the same number of Mφ from uninfected SCIDbg mice (resident Mφ) did not protect SCIDbgMN mice exposed to the same C. parvum infection (Fig. 2). All of the SCIDbgMN mice not having C. parvum infection survived until the end of the experimental period. These results indicate that CP-Mφ (but not resident Mφ) play a role in the resistance of SCIDbgMN mice against C. parvum infection.
FIG. 2.
C. parvum infection in SCIDbgMN mice inoculated with CP-Mφ. SCIDbgMN mice were inoculated with 1 × 106 cells/mouse of CP-Mφ (open circles), resident Mφ (closed circles), CP-PMN (open squares), or PMN-N (closed squares). SCIDbgMN mice injected with PBS served as controls (open triangles). These mice were then infected orally with 1 × 106 C. parvum oocysts per mouse. To determine the mortality rates, these mice were observed every 12 h for 3 weeks. The data shown are representative of three experiments.
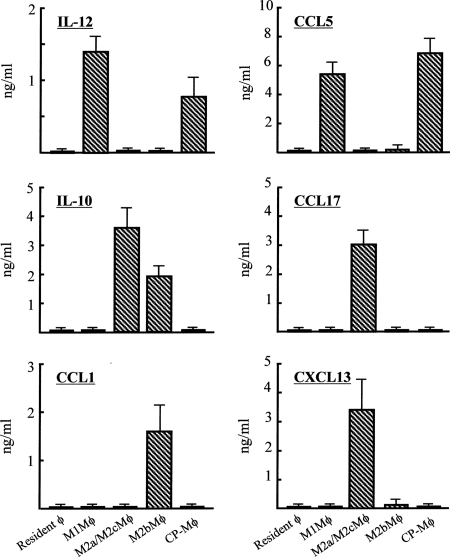
In the next experiments, CP-Mφ were characterized based on their cytokine/chemokine production profiles. CCL5 and IL-12 are biomarkers for M1Mφ. CCL5 and IL-12 were detected in culture fluids of M1Mφ and CP-Mφ. However, IL-10, CCL17, CCL1, and CXCL13 were not demonstrated in culture fluids of M1Mφ and CP-Mφ. Culture fluids of resident Mφ did not contain these soluble factors (Fig. 3). Furthermore, iNOS mRNA was expressed by M1Mφ and CP-Mφ, while this mRNA was not expressed by resident Mφ or any M2Mφ subpopulations (data not shown). From these results, CP-Mφ were identified as M1Mφ.
FIG. 3.
Cytokine and chemokine production by CP-Mφ. A total of 1 × 106 cells/ml of CP-Mφ and resident Mφ (Mφ from uninfected SCIDbg mice) were cultured for 48 h. As positive controls, standard M1Mφ, M2aMφ/M2cMφ, and M2bMφ preparations (21) were cultured under the same conditions. Harvested culture fluids were assayed for IL-12, CCL5, IL-10, CCL17, CCL1, and CXCL13 by enzyme-linked immunosorbent assay. Error bars indicate standard errors of the means.
Role of CP-PMN in host resistance against acute cryptosporidiosis.
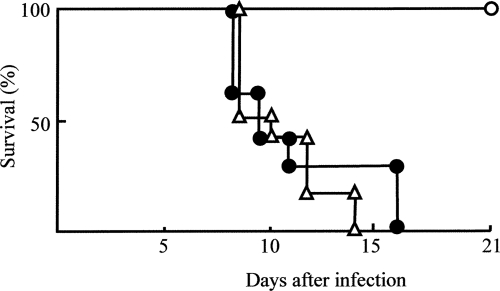
The effect of CP-PMN on the anti-C. parvum functions of Mφ was examined. SCIDbgMN mice were inoculated with resident Mφ alone, resident Mφ transwell cultured with PMN-N, or resident Mφ transwell cultured with CP-PMN and then were exposed to 1 × 106 C. parvum oocysts/mouse. Transwell cultivation between resident Mφ (1 × 106 cells/ml, lower chamber) and CP-PMN (1 × 105 cells/ml, upper chamber) was performed for 18 h. When SCIDbgMN mice inoculated with resident Mφ or with resident Mφ previously transwell cultured with PMN-N were exposed to C. parvum infection, all of them died within 16 days of the infection. However, all of the SCIDbgMN mice that were inoculated with resident Mφ previously transwell cultured with CP-PMN survived (Fig. 4). This indicates that CP-PMN have a function in resident Mφ conversion to anti-C. parvum Mφ.
FIG. 4.
Lethal C. parvum infection in SCIDbgMN mice inoculated with various preparations of resident Mφ. Resident Mφ (1 × 106 cells/ml, lower chamber) were cultured with CP-PMN (1 × 105 cells/ml, upper chamber) in a dual-chamber transwell for 18 h. As controls, resident Mφ were cultured with medium or PMN-N in the same fashion. These resident Mφ preparations cultured with CP-PMN (open circles), PMN-N (closed circles), or medium (open triangles) were harvested from the lower chamber and adoptively transferred to SCIDbgMN mice at 1 × 106 cells/mouse. These mice were then infected orally with 1 × 106 C. parvum oocysts per mouse. The mortality rates for these mice were observed every day for 3 weeks. The data shown are representative of three experiments.
Cooperation of Mφ and PMN in host anti-C. parvum resistance in SCIDbg mice.
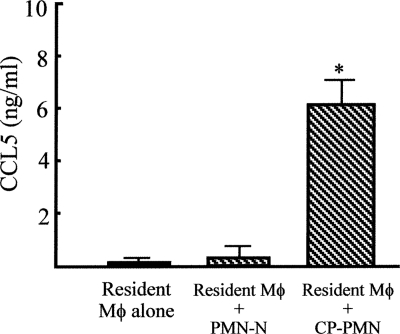
From experiments utilizing SCIDbg mice and SCIDbgMN mice, both Mφ and PMN were shown to be required for host defense against acute C. parvum infection. CP-Mφ, shown to be anti-C. parvum effector cells, were characterized as M1Mφ. Therefore, the role of CP-PMN in the anti-C. parvum resistance of Mφ was examined. When resident Mφ (lower chamber) were cultured with CP-PMN (upper chamber) in dual-chamber transwells, Mφ harvested from the lower chamber produced CCL5. However, this chemokine was not produced by resident Mφ alone or resident Mφ transwell cultured with PMN-N (Fig. 5). These results indicate that CP-PMN play a role in the Mφ conversion from resident Mφ to M1Mφ.
FIG. 5.
Production of CCL5 by resident Mφ transwell cultured with CP-PMN. Resident Mφ (1 × 106 cells/ml, lower chamber) were cultured in a dual-chamber transwell with medium, PMN-N, or CP-PMN (1 × 105 cells/ml, upper chamber). Eighteen hours after cultivation, Mφ were harvested from the lower chamber, and then they were recultured (1 × 106 cells/ml) for 24 h without any stimulation. The amounts of CCL5, a biomarker for M1Mφ, in the culture fluids were measured by enzyme-linked immunosorbent assay. Data are means ± standard errors of the means (n = 5).
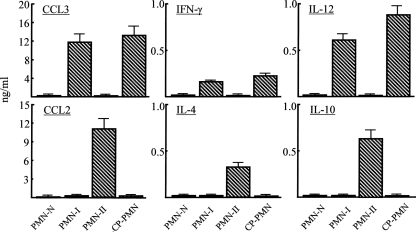
In the next experiments, CP-PMN were characterized based on their cytokine/chemokine production profiles. Standard preparations of PMN-I, PMN-II, and PMN-N were prepared as described previously (47). CCL3 and IL-12 are representative cytokines produced by PMN-I, and PMN-II have been characterized as CCL2- and IL-10-producing cells. PMN-N do not produce soluble factors. CCL3, IFN-γ, and IL-12 (but not CCL2, IL-4, and IL-10) were detected in culture fluids of CP-PMN and PMN-I, while CCL2, IL-4, and IL-10 (but not CCL3, IFN-γ, and IL-12) were demonstrated in culture fluids of PMN-II (Fig. 6). These cytokines were not detected in culture fluids of PMN-N. These results indicate that CP-PMN are PMN-I.
FIG. 6.
Production of cytokines and chemokines by CP-PMN. A total of 1 × 106 cells/ml of CP-PMN and PMN-N were cultured for 18 h without any stimulation. As controls, PMN-I and PMN-II preparations (see Materials and Methods) were cultured in the same conditions. Harvested culture fluids were assayed for CCL3, CCL2, IFN-γ, IL-4, IL-12, and IL-10 by enzyme-linked immunosorbent assay. Error bars indicate standard errors of the means.
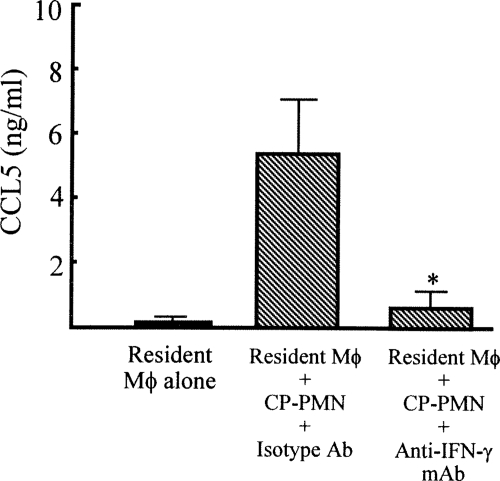
In the next experiments, we examined how CP-PMN induce Mφ conversion from resident Mφ to M1Mφ. Since Mφ conversion to M1Mφ was shown when resident Mφ were transwell cultured with CP-PMN, soluble factors released from CP-PMN were suggested to be involved in this Mφ conversion. IFN-γ was shown to be an effector molecule for CP-PMN-induced Mφ conversion to M1Mφ, because M1Mφ were not obtained when resident Mφ were transwell cultured with CP-PMN in the presence of anti-IFN-γ MAb (Fig. 7). These results indicate that Mφ conversion from resident Mφ to M1Mφ is mediated by IFN-γ released from CP-PMN. In our previous studies (47), IL-12 and CCL3 were also shown to be involved in Mφ conversion from resident Mφ to M1Mφ. CCL3 has been shown to be required when IL-12 is produced by Mφ (12), and IL-12 has been described as a key cytokine in the maximal IFN-γ production by Mφ (29). Since CCL3 and IL-12 are produced by CP-PMN (Fig. 6), these soluble factors may function additionally in Mφ conversion from resident Mφ to M1Mφ and/or IFN-γ production by CP-PMN themselves.
FIG. 7.
Effect of anti-IFN-γ MAb on M1Mφ generation stimulated with CP-PMN. Resident Mφ (1 × 106 cells/ml, lower chamber) were cultured in a dual-chamber transwell with CP-PMN (1 × 105 cells/ml, upper chamber) in the presence of anti-IFN-γ MAb (2 μg/ml). Eighteen hours after cultivation, Mφ were harvested from the lower chamber, and then they were recultured (1 × 106 cells/ml) for 24 h without any stimulation. The amounts of CCL5, a biomarker for M1Mφ, in the culture fluids were measured by ELISA. Data are means ± standard errors of the means (n = 5).
DISCUSSION
A variety of animal models have been used to study the nature and role of host immune responses to C. parvum infection, including neonatal mice (22), dexamethasone-treated mice (54), congenital immunodeficient (nude, RAG−/−, SCID) mice (1, 28, 33), and mice with targeted gene mutations for major histocompatibility complex class II, CD40, IFN-γ or T-cell receptor α (2, 19, 22, 50). From the studies performed with these models, the central importance of CD4+ T cells (chronic) and IFN-γ (acute and chronic) in the resistance to and recovery of animals from C. parvum infection have been proven. Thus, IFN-γ GKO mice manifested both acute and chronic infections (17), while chronic C. parvum infections developed in SCID mice or normal mice depleted of CD4+ T cells (48). Normal mice or normal mice depleted of CD8+ T cells did not manifest either acute or chronic infection (48).
In this study, cryptosporidiosis was shown to develop quickly (within 3 days of C. parvum infection) and severely (a 100% mortality rate) in SCIDbgMN mice (SCIDbg mice depleted of functional Mφ and PMN) compared with that in SCIDbg mice (mice depleted of functional T, B, and NK cells). All of the SCIDbgMN mice died within 16 days after C. parvum infection, whereas all of the SCIDbg mice survived after the same infection. These facts indicate that Mφ and PMN play an important role in the host resistance of mice against acute C. parvum infection. The SCIDbgMN mice utilized in these experiments were created from SCIDbg mice after X irradiation (4 Gy) and other treatments (anti-Ly6G MAb and carrageenan). Since irradiation is known to damage the intestinal epithelium, there was a concern that intestinal epithelial cell-mediated innate immune responses (e.g., antimicrobial peptide production and cytokine/chemokine production) would be impaired in SCIDbgMN mice and the susceptibility of these mice to C. parvum infection would be increased. However, the resistance of SCIDbgMN mice to C. parvum infection was completely restored to the level shown by SCIDbg mice after inoculation with CP-Mφ and/or resident Mφ and CP-PMN. These results strongly suggest that the influence of intestinal epithelial cell damage caused by X irradiation on the decreased host resistance of SCIDbgMN mice to C. parvum infection is minimal. Plasmacytoid dendritic cells (DC) and eosinophils present in SCIDbg mice may be strongly influenced by treatment with anti-Ly6G MAb plus X irradiation. Although fewer than 1% of plasmacytoid DC and eosinophils are present in the peripheral blood cells of healthy SCIDbg mice (47), the accumulation of these cells at the inflammation site has been described (20, 55). So far, the active pathological role of these cells at the C. parvum infection site is not known. Analytical studies on the role of plasmacytoid DC and eosinophils in acute C. parvum infection in SCIDbg mice are needed.
However, the host resistance of SCIDbgMN mice to acute C. parvum infection was not restored to the level shown by SCIDbg mice, even when they were inoculated with CP-PMN (PMN from C. parvum-infected SCIDbg mice), N-PMN (PMN from uninfected SCIDbg mice), resident Mφ (Mφ from uninfected SCIDbg mice), or resident Mφ previously treated with PMN-N. SCIDbgMN mice resisted acute C. parvum infection when they were inoculated with CP-Mφ (Mφ from C. parvum-infected SCIDbg mice) or both resident Mφ and CP-PMN. Also, SCIDbgMN mice resisted acute C. parvum infection when they were inoculated with resident Mφ previously cultured with CP-PMN. In subsequent experiments, CP-PMN were identified as PMN-I, with the ability to produce IFN-γ. Also, CP-Mφ or resident Mφ previously cultured with CP-PMN were identified as M1Mφ, because these Mφ produced IL-12 and CCL5 and expressed iNOS mRNA. These results indicate that M1Mφ (or CP-Mφ) are generated from resident Mφ in cooperation with CP-PMN (or PMN-I), and IFN-γ released from CP-PMN may play a role in the Mφ conversion from resident Mφ to M1Mφ. M1Mφ may act as final effector cells in the host resistance of SCIDbg mice to acute C. parvum infection. Supporting this, SCIDbgMN mice inoculated with authentic M1Mφ were shown to be resistant against acute C. parvum infection. The beige mutation in SCID mice causes a defect in the cytotoxic function of NK cells. However, other functions of NK cells may be retained in SCIDbg mice, suggesting that NK cells retained in C. parvum-infected SCIDbg mice are involved as cells that produce IFN-γ. However, these mice did not resist C. parvum infection even when they were inoculated with resident Mφ. This suggests that in SCIDbg mice, the amounts of IFN-γ released from NK cells are not enough to convert resident Mφ to M1Mφ.
M1Mφ have been well described as major effector cells for the host's innate immunities (36, 40, 42). In general, M1Mφ exhibit high oxygen consumption, the ability to kill cells infected with intracellular pathogens, cytotoxicity against tumor cells, the ability to express iNOS, and the ability to secrete NO, proinflammatory cytokines, and Th1 response-associated cytokines (36, 40, 49). The effector molecules of M1Mφ that inhibit C. parvum replication remain unclear. Recent papers have shown an increase in the expression of cathelicidin-related antimicrobial peptide mRNA in M1Mφ (6) and that cathelicidins are cytotoxic against both sporozoites and oocysts of C. parvum (13). This suggests that antimicrobial peptides released from M1Mφ may play a role in the M1Mφ-associated host defense of SCIDbg mice acutely infected with C. parvum. As mentioned above, CP-PMN were shown to be stimulator cells for Mφ conversion from resident Mφ to M1Mφ. However, Mφ conversion from resident Mφ to M1Mφ did not occur when resident Mφ were transwell cultured with CP-PMN in the presence of anti-IFN-γ MAb (data not shown). These results strongly suggest that IFN-γ released from CP-PMN is an effector molecule in the CP-PMN-associated Mφ conversion from resident Mφ to M1Mφ.
An inhibitory effect of IFN-γ on C. parvum invasion and replication in cultures of intestinal epithelial cells has been reported (24, 38). Thus, parasite replication was diminished up to 50% when the cultures were performed with 50 ng/ml of IFN-γ. The minimum effective dose of IFN-γ for inhibitory activity against parasite replication was 10 ng/ml. In our experiments, 200 pg/ml of IFN-γ was detected in sera of SCIDbg mice exposed to 1 × 106 C. parvum oocysts. This indicates that the parasite replication in SCIDbg mice may not be directly influenced by this amount of IFN-γ. However, 200 pg/ml of IFN-γ has been shown to be enough for the conversion from resident Mφ to M1Mφ. These facts suggest that IFN-γ released from CP-PMN plays a role in the Mφ conversion from resident Mφ to M1Mφ.
IFN-γ released from CD4+ T lymphocytes has been described as an important molecule for the host defense of individuals infected with Cryptosporidium parvum. In a pediatric case report (14), it has been reported that in response to Cryptosporidium antigen, peripheral blood mononuclear cells from a patient with cryptosporidiosis had the ability to produce IL-10 (but not IFN-γ). However, peripheral blood mononuclear cells from a donor who had recovered from cryptosporidiosis had the ability to produce IFN-γ. These results suggest that IFN-γ plays a role in the recovery of individuals from cryptosporidiosis. In addition to IFN-γ, in this study, M1Mφ were shown to play a role in a host resistance against acute Cryptosporidium infection. Although cryptosporidiosis in humans with dysfunctional Mφ and PMN has not been cleared, patients with a predominance of M2Mφ, such as patients with severe burn injuries (unpublished data) and certain cancer patients (49), may be very susceptible to C. parvum infection. M2Mφ have been reported to be inhibitory to the generation of PMN-I and M1Mφ.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 2 June 2008.
REFERENCES
- 1.Adjei, A. A., J. T. Jones, M. W. Riggs, and F. J. Enriquez. 1999. Evidence of thymus-independent local and systemic antibody responses to Cryptosporidium parvum infection in nude mice. Infect. Immun. 673947-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre, S. A., P. H. Mason, and L. E. Perryman. 1994. Susceptibility of major histocompatibility complex (MHC) class I- and MHC class II-deficient mice to Cryptosporidium parvum infection. Infect. Immun. 62697-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowood, M. J. 2002. In vitro cultivation of Cryptosporidium species. Clin. Microbiol. Rev. 15390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15749-795. [DOI] [PubMed] [Google Scholar]
- 5.Boher, Y., I. Perez-Schael, G. Caceres-Dittmar, G. Urbina, R. Gonzalez, G. Kraal, and F. J. Tapia. 1994. Enumeration of selected leukocytes in the small intestine of BALB/c mice infected with Cryptosporidium parvum. Am. J. Trop. Med. Hyg. 50145-151. [PubMed] [Google Scholar]
- 6.Bu, H. F., X. Wang, Y. Q. Zhu, R. Y. Williams, W. Hsueh, X. Zheng, R. A. Rozenfeld, X. L. Zuo, and X. D. Tan. 2006. Lysozyme-modified probiotic components protect rats against polymicrobial sepsis: role of macrophages and cathelicidin-related innate immunity. J. Immunol. 1778767-8776. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., J. H. Harp, and A. G. Harmsen. 1993. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect. Immun. 613928-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 3461723-1731. [DOI] [PubMed] [Google Scholar]
- 9.Clark, D. P. 1999. New insights into human cryptosporidiosis. Clin. Microbiol. Rev. 12554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culshaw, R. J., G. J. Bancroft, and V. McDonald. 1997. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect. Immun. 653074-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, J. P., X. Zhang, K. A. Frauwirth, and D. M. Mosser. 2006. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 801298-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita, K., M. Kobayashi, D. N. Herndon, R. B. Pollard, and F. Suzuki. 2001. Macrophage inflammatory protein-1α (MIP-1α) induces the IL-12 production and improves the polarization of Th1 responses in thermally injured mice. J. Allergy Clin. Immunol. 107S81. (Abstract.) [Google Scholar]
- 13.Giacometti, A., O. Cirioni, M. S. Del Prete, B. Skerlavaj, R. Circo, M. Zanetti, and G. Scalise. 2003. In vitro effect on Cryptosporidium parvum of short-term exposure to cathelicidin peptides. J. Antimicrob. Chemother. 51843-847. [DOI] [PubMed] [Google Scholar]
- 14.Gomez Morales, M. A., C. M. Ausiello, A. Guarino, F. Urbani, M. I. Spagnuolo, C. Pignata, and E. Pozio. 1996. Severe, protracted intestinal cryptosporidiosis associated with interferon gamma deficiency: pediatric case report. Clin. Infect. Dis. 22848-850. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths, J. K., R. Moore, S. Dooley, G. T. Keusch, and S. Tzipori. 1994. Cryptosporidium parvum infection of Caco-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect. Immun. 624506-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gut, J., C. Petersen, R. Nelson, and J. Leech. 1991. Cryptosporidium parvum: in vitro cultivation in Madin-Darby canine kidney cells. J. Protozool. 3872S-73S. [PubMed] [Google Scholar]
- 17.Handman, E., and D. V. Bullen. 2002. Interaction of Leishmania with the host macrophage. Trends Parasitol. 18332-334. [DOI] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Hayward, A. R., M. Cosyns, M. Jones, and E. M. Ponnuraj. 2001. Marrow-derived CD40-positive cells are required for mice to clear Cryptosporidium parvum infection. Infect. Immun. 691630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan, S. P., and M. E. Rothenberg. 2006. Eosinophil function in eosinophil-associated gastrointestinal disorders. Curr. Allergy Asthma Rep. 665-71. [DOI] [PubMed] [Google Scholar]
- 21.Katakura, T., M. Miyazaki, M. Kobayashi, D. N. Herndon, and F. Suzuki. 2004. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J. Immunol. 1721407-1413. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix, S., R. Mancassola, M. Naciri, and F. Laurent. 2001. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect. Immun. 691635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacroix-Lamandé, S., R. Mancassola, M. Naciri, and F. Laurent. 2002. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 702090-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lean, I. S., S. A. McDonald, M. Bajaj-Elliott, R. C. Pollok, M. J. Farthing, and V. McDonald. 2003. Interleukin-4 and transforming growth factor beta have opposing regulatory effects on gamma interferon-mediated inhibition of Cryptosporidium parvum reproduction. Infect. Immun. 714580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, P. T., P. G. Holt, and A. S. McWilliam. 2001. Failure of MHC class II expression in neonatal alveolar macrophages: potential role of class II transactivator. Eur. J. Immunol. 312347-2356. [DOI] [PubMed] [Google Scholar]
- 26.Leoni, F., C. Amar, G. Nichols, S. Pedraza-Díaz, and J. McLauchlin. 2006. Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000. J. Med. Microbiol. 55703-707. [DOI] [PubMed] [Google Scholar]
- 27.Liew, F. Y., D. Xu, and W. L. Chan. 1999. Immune effector mechanism in parasitic infections. Immunol. Lett. 65101-104. [DOI] [PubMed] [Google Scholar]
- 28.Lukin, K., M. Cosyns, T. Mitchell, M. Saffry, and A. Hayward. 2000. Eradication of Cryptosporidium parvum infection by mice with ovalbumin-specific T cells. Infect. Immun. 682663-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, X., M. Aste-Amezaga, G. Gri, F. Gerosa, and G. Trinchieri. 1997. Immunomodulatory functions and molecular regulation of IL-12. Chem. Immunol. 681-22. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani, A., A. Sica, S. Sozzani, P. Allavena, A. Vecchi, and M. Locati. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25677-686. [DOI] [PubMed] [Google Scholar]
- 31.Martinez, F. O., S. Gordon, M. Locati, and A. Mantovani. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 1777303-7311. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.McDonald, V., and G. J. Bancroft. 1994. Mechanisms of innate and acquired resistance to Cryptosporidium parvum infection in SCID mice. Parasite Immunol. 16315-320. [DOI] [PubMed] [Google Scholar]
- 34.McDonald, V., H. A. Robinson, J. P. Kelly, and G. J. Bancroft. 1994. Cryptosporidium muris in adult mice: adoptive transfer of immunity and protective roles of CD4 versus CD8 cells. Infect. Immun. 622289-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reference deleted.
- 36.Paulnock, D. M., and S. P. Coller. 2001. Analysis of macrophage activation in African trypanosomiasis. J. Leukoc. Biol. 69685-690. [PubMed] [Google Scholar]
- 37.Petry, F., H. A. Robinson, and V. McDonald. 1995. Murine infection model for maintenance and amplification of Cryptosporidium parvum oocysts. J. Clin. Microbiol. 331922-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollok, R. C., M. J. Farthing, M. Bajaj-Elliott, I. L. Sanderson, and V. McDonald. 2001. Interferon gamma induces enterocyte resistance against infection by the intracellular pathogen Cryptosporidium parvum. Gastroenterology 12099-107. [DOI] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Rothfuchs, A. G., D. Gigliotti, K. Palmblad, U. Andersson, H. Wigzell, and M. E. Rottenberg. 2001. IFN-α/β-dependent, IFN-γ secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 1676453-6461. [DOI] [PubMed] [Google Scholar]
- 41.Sadraei, J., M. A. Rizvi, and U. K. Baveja. 2005. Diarrhea, CD4+ cell counts and opportunistic protozoa in Indian HIV-infected patients. Parasitol. Res. 97270-273. [DOI] [PubMed] [Google Scholar]
- 42.Sester, D. P., K. J. Stacey, M. J. Sweet, S. J. Beasley, S. L. Cronau, and D. A. Hume. 1999. The actions of bacterial DNA on murine macrophages. J. Leukoc. Biol. 66542-548. [DOI] [PubMed] [Google Scholar]
- 43.Sher, A., C. Collazzo, C. Scanga, D. Jankovic, G. Yap, and J. Aliberti. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27521-528. [DOI] [PubMed] [Google Scholar]
- 44.Sironi, M., F. O. Martinez, D. D'Ambrosio, M. Gattorno, N. Polentarutti, M. Locati, A. Gregorio, A. Iellem, M. A. Cassatella, J. Van Damme, S. Sozzani, A. Martini, F. Sinigaglia, A. Vecchi, and A. Mantovani. 2006. Differential regulation of chemokine production by Fcγ receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J. Leukoc. Biol. 80342-349. [DOI] [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.Theodos, C. M., K. L. Sullivan, J. K. Griffiths, and S. Tzipori. 1997. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect. Immun. 654761-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuda, Y., H. Takahashi, M. Kobayashi, T. Hanafusa, D. N. Herndon, and F. Suzuki. 2004. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21215-226. [DOI] [PubMed] [Google Scholar]
- 48.Ungar, B. L., T. C. Kao, J. A. Burris, and F. D. Finkelman. 1991. Cryptosporidium infection in an adult mouse model. Independent roles for IFN-γ and CD4+ T lymphocytes in protective immunity. J. Immunol. 1471014-1022. [PubMed] [Google Scholar]
- 49.Van Ginderachter, J. A., K. Movahedi, G. Hassanzadeh, S. Meerschaut, A. Beschin, G. Raes, and P. De Baetselier. 2006. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211487-501. [DOI] [PubMed] [Google Scholar]
- 50.Waters, W. R., and J. A. Harp. 1996. Cryptosporidium parvum infection in T-cell receptor (TCR)-α- and TCR-δ-deficient mice. Infect. Immun. 641854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitmore, M. M., M. J. DeVeer, A. Edling, R. K. Oates, B. Simons, D. Lindner, and B. R. Williams. 2004. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 645850-5860. [DOI] [PubMed] [Google Scholar]
- 52.Xiao, L., R. Fayer, U. Ryan, and S. J. Upton. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 1772-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashiro, S., H. Kamohara, and T. Yoshimura. 1999. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-α plays a role in maximal MCP-1 mRNA expression. J. Leukoc. Biol. 65671-679. [DOI] [PubMed] [Google Scholar]
- 54.Yang, S., M. C. Healey, and C. Du. 1996. Infectivity of preserved Cryptosporidium parvum oocysts for immunosuppressed adult mice. FEMS Immunol. Med. Microbiol. 13141-145. [DOI] [PubMed] [Google Scholar]
- 55.Yoneyama, H., K. Matsuno, Y. Zhang, T. Nishiwaki, M. Kitabatake, S. Ueha, S. Narumi, S. Morikawa, T. Ezaki, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2004. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16915-928. [DOI] [PubMed] [Google Scholar]
- 56.You, X., and J. R. Mead. 1998. Characterization of experimental Cryptosporidium parvum infection in IFN-γ knockout mice. Parasitology 117525-531. [DOI] [PubMed] [Google Scholar]