Abstract
Ehrlichia chaffeensis is an obligately intracellular gram-negative bacterium and is the etiologic agent of human monocytic ehrlichiosis (HME). Although E. chaffeensis induces the generation of several cytokines and chemokines by leukocytes, E. chaffeensis lacks lipopolysaccharide and peptidoglycan. Bioinfomatic analysis of the E. chaffeensis genome, however, predicted genes encoding 15 lipoproteins and 3 posttranslational lipoprotein-processing enzymes. The present study showed that by use of multidimensional liquid chromatography followed by tandem mass spectrometry, all predicted lipoproteins as well as lipoprotein-processing enzymes were expressed by E. chaffeensis cultured in the human promyelocytic leukemia cell line HL-60. Consistent with this observation, a signal peptidase II inhibitor, globomycin, was found to inhibit E. chaffeensis infection and lipoprotein processing in HL-60 cell culture. To study in vivo E. chaffeensis lipoprotein expression and host immune responses to E. chaffeensis lipoproteins, 13 E. chaffeensis lipoprotein genes were cloned into a mammalian expression vector. When the DNA constructs were inoculated into naïve dogs, or when dogs were infected with E. chaffeensis, the animals developed delayed-type hypersensitivity reactions at cutaneous sites of the DNA construct deposition and serum antibodies to these lipoproteins. This is the first demonstration of lipoprotein expression and elicitation of immune responses by a member of the order Rickettsiales. Multiple lipoproteins expressed by E. chaffeensis in vitro and in vivo may play key roles in pathogenesis and immune responses in HME.
Human monocytic ehrlichiosis (HME) is an emerging tick-borne illness caused by infection of monocytes/macrophages with a gram-negative obligately intracellular bacterium, Ehrlichia chaffeensis (2, 7). Since its discovery in 1986 (32), HME has been increasingly diagnosed in the United States and other parts of the world (9, 35). HME is a systemic disease characterized by fever, headache, myalgia, anorexia, and chills and is frequently accompanied by leukopenia, thrombocytopenia, anemia, and elevated serum hepatic aminotransferase levels. Doxycycline is the drug of choice for treatment of HME; however, delayed initiation of therapy, the presence of underlying illness, and immunosuppression often lead to severe complications or death (35).
The relative paucity of bacteria detected in the blood and tissues of most patients infected with E. chaffeensis, even those with severe illnesses, suggests that clinical manifestations of HME may be mediated by host immune responses and possibly amplified by specific cytokine production (39, 40). In fact, the generation of several proinflammatory cytokines and chemokines, immunosuppressive cytokines, and interferons in E. chaffeensis-infected cells in vitro (25) and in animals experimentally infected with closely related Ehrlichia spp. (19, 48, 50) has been documented. Understanding of the bacterial factors potentially involved in HME pathogenesis and immune responses would help improve the therapy.
Pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides (LPS), peptidoglycan, and lipoproteins are double-edged swords: while they contribute to pathogenesis by inducing proinflammatory cytokines leading to inflammation, they stimulate innate immunity to confer initial host resistance to pathogens (13). The innate immune responses influence the nature of subsequent acquired immune responses, thereby, in combination with inflammation, ultimately affecting host morbidity and mortality. E. chaffeensis lacks all genes for the biosynthesis of LPS and most genes for the biosynthesis of peptidoglycan; thus, it does not produce LPS or peptidoglycan (29). However, little is known about the role of other PAMPs, such as lipoproteins, in E. chaffeensis.
The first objective of the present study was, therefore, to analyze the E. chaffeensis genome sequence for genes encoding putative lipoproteins and lipoprotein-processing enzymes, to analyze the expression of these proteins in cell culture, and to investigate the involvement of the lipoproteins in E. chaffeensis infection of host cells in vitro. A previous study showed transcription of the lspA gene, encoding the type II signal peptidase, and the lgt gene, encoding prolipoprotein diacylglyceryl transferase, by Rickettsia typhi in cell culture (37). However, expression of lipoproteins by members of the order Rickettsiales, including E. chaffeensis, has not been determined.
The second objective of this study was to determine lipoprotein expression by E. chaffeensis in infected mammals and immune responses to the lipoproteins. While immunocompetent mice clear E. chaffeensis infection within 2 weeks (53), dogs can be naturally and experimentally infected with E. chaffeensis for as long as several months (8, 51, 55). Using E. chaffeensis lipoprotein genes cloned into a mammalian expression vector, we determined lipoprotein expression by E. chaffeensis in infected dogs and investigated whether serum antibody and delayed-type hypersensitivity (DTH) reactions are developed against E. chaffeensis lipoproteins.
MATERIALS AND METHODS
Bacterial strains.
E. chaffeensis Arkansas (7) and St. Vincent (36) (ATCC, Manassas, VA) were cultured in the canine macrophage cell line DH82 (52) in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (U.S. Biotechnologies, Parker Ford, PA) and 4 mM l-glutamine (Invitrogen) at 37°C under a humidified atmosphere of 5% CO2-95% air. Methylprednisolone (1 μM; Sigma, St. Louis, MO) was added for propagating E. chaffeensis St. Vincent. E. chaffeensis Arkansas was also cultured in HL-60 cells as previously described (33).
Analysis of E. chaffeensis lipoprotein genes.
E. chaffeensis open reading frames (ORFs) were predicted based on the E. chaffeensis genome sequence prior to annotation by The Institute for Genomic Research (10). All ORFs beginning with ATG and encoding more than 50 amino acids were predicted using the GeneQuest program from the Lasergene DNAStar package (DNAStar, Madison, WI). Deduced amino acid sequences were searched against the Prosite lipoprotein profile (PS00013) using the PS_Scan program from Alexandre Gattiker at the Swiss Institute of Bioinformatics (http://www.expasy.org/ftp/databases/prosite/tools/ps_scan). The search results were later combined with annotations of the E. chaffeensis genome (GenBank accession no. NC_007799).
Proteomic analysis.
E. chaffeensis Arkansas cultured in HL-60 cells was purified as described by Ge and Rikihisa (11). Tryptic digested peptides were used for peptide-level bottom-up proteomics, with three sample preparation methods: (i) global preparation from whole-cell lysates, (ii) soluble preparation, and (iii) insoluble preparation, as described by Adkins et al. (1). Strong cation-exchange fractionation and multidimensional capillary liquid chromatography-tandem mass spectrometry (LC-MS-MS) analysis were performed as previously described (1). Peptides were identified by using SEQUEST to search the mass spectra using the annotated E. chaffeensis Arkansas (NC_007799) protein data file. The data obtained with the three preparations were combined to increase proteome coverage and accuracy.
Effects of globomycin on E. chaffeensis infection and lipoprotein processing.
Globomycin (Sankyo Chemical Co., Tokyo, Japan) dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 1, 10, or 25 μg/ml, or the same volume of DMSO (no more than 0.5%, vol/vol), was added to E. chaffeensis infected HL-60 cells at 1 day postinfection (p.i.) and incubated at 37°C for 2 h to 1 day. The cells were harvested and stained with Diff-Quik, and numbers of E. chaffeensis organisms were scored in 100 HL-60 cells in triplicate culture wells (31). The images of Diff-Quik-stained cells were captured by a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI) coupled to a Nikon microscope (Nikon Instruments Inc., Melville, NY). Total RNA was extracted from an aliquot of samples with an RNeasy Protect minikit (Qiagen, Valencia, CA). cDNA was synthesized, and real-time reverse transcription-PCR (RT-PCR) was performed to analyze the expression of host cell glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (primers obtained from Clontech, Palo Alto, CA) as previously described (30).
Alternatively, ∼5 × 106 cells were collected and lysed in 0.5 ml of ice-cold radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) with freshly added protease inhibitor cocktail (Calbiochem, San Diego, CA). After incubation on ice for 20 min, cells were sonicated; and whole-cell lysates were collected by centrifugation at 10,000 × g for 10 min. Lysates were then subjected to Western blotting using custom-raised rabbit antisera against a 14-mer peptide of E. chaffeensis OmpA (amino acids 81 to 94, HTDTRGTDEYNLEL; EZBiolab, Westfield, IN) (28). This highly antigenic and hydrophilic 14-mer peptide fragment was chosen from the OmpA amino acid sequence based on the Protean program in the DNAStar software package. According to BLAST search results for short, nearly exactly matched sequences in the NCBI nonredundant database, the peptide sequence is unique and has little to no homology to any other known proteins (E > 10).
Canine GM-CSF construct.
DH82 cells were stimulated with 2 μM phorbol myristate acetate (Sigma) and 2 μM ionomycin (Sigma) for 3 h to induce granulocyte-macrophage colony-stimulating factor (GM-CSF) production (20). RT-PCR was performed to amplify GM-CSF cDNA from these cells. Oligo(dT) (Invitrogen) was used as the reverse transcription primer. PCR was performed using the high-fidelity DNA polymerase Pfu Turbo (Stratagene, La Jolla, CA). The GM-CSF primer pair was designed based on the canine GM-CSF cDNA sequence (GenBank accession no. S49738) with additional flanking restriction enzyme recognition sites, SalI and BamHI, respectively (see Table S1 in the supplemental material). The PCR product was cloned into pCR-XL-Topo (Invitrogen) according to the manufacturer's instructions. The recombinant DNAs were purified using the Concert Rapid Miniprep system (Invitrogen) and sequenced by a dideoxy termination method with a model 373 DNA sequencer (Applied Biosystems, Foster City, CA). GM-CSF was then excised from pCR-XL by digestion with SalI and BamHI and was subcloned into the mammalian expression vector VR1055 (Vical Incorporated, San Diego, CA), which had been treated with SalI and BamHI.
Lipoprotein constructs for mammalian expression.
We designed primers with flanking BamHI or BglII sites covering the predicted sequences of mature lipoproteins (with the signal peptide removed) (see Table S1 in the supplemental material). For mature lipoprotein DNA sequences missing either the BamHI or the BglII site, forward or reverse primers included sequences of the restriction enzyme not present in the mature lipoprotein DNA sequences (see Table S1 in the supplemental material). For mature lipoprotein DNA sequences lacking both BamHI and BglII sites, forward primers contained BamHI flanking sequences and reverse primers contained BglII flanking sequences (see Table S1 in the supplemental material). DNA sequences corresponding to mature lipoproteins were first amplified by PCR using Pfu Turbo and then cloned into the pBluntII vector using the pBlunt Topo kit (Invitrogen). Recombinants were screened by PCR using primers for amplification and BamHI and/or BglII digestion. Lipoprotein DNA sequences were first excised from recombinants using BamHI and/or BglII and then ligated to dephosphorylated VR1020 (Vical) digested with BamHI and/or BglII. Recombinants were screened by BamHI and/or BglII digestion and by PCR using a forward primer derived from VR1020 (VR1020-Seq-F, CGTGAATTTAAGGGACGCTG) and the appropriate reverse primer for each lipoprotein. All constructs were confirmed by sequencing using the primers shown in Table S2 in the supplemental material.
Dog immunization.
Seven specific-pathogen-free (SPF) beagle dogs (six females and one male; body weight, 7 to 10 kg) from Battelle (Columbus, OH) and one SPF beagle dog (female; body weight, 8 kg) from Perry Farm (Mt. Sterling, OH) were randomly divided into four groups (two dogs per group). All dogs tested free of E. chaffeensis infection based on immunofluorescence antibody labeling (41) and nested PCR targeting E. chaffeensis 16S rRNA (3). For the control group, consisting of dog 1 and dog 2, each dog received 500 μg of VR1020 DNA plus 250 μg of canine GM-CSF construct DNA. For the immunization groups, ECH_0482 (dog 3 and dog 4), ECH_0558 (dog 5 and dog 6), and ECH_0929 (dog 7 and dog 8), each dog received 500 μg of lipoprotein construct DNA plus 250 μg of canine GM-CSF construct DNA. All DNA constructs were administered with a Bioject 2000 gene gun (Bioject Medical Technologies, Tualatin, OR) to the quadriceps femoris muscles. All dogs were immunized three times, at 3-week intervals. Blood specimens were collected 1 week after the third immunization to check antibody titers. A cutaneous DTH test was performed 18 days after each immunization. One spot for each lipoprotein construct DNA per DTH test was administered to each dog. Skin thickness change and red zone diameter data for each group were combined for the analysis.
Experimental infection of dogs.
SPF beagle dog A (female; body weight, 9 kg; Marshall Farms, New Rose, NY) and SPF dog B (body weight, 11 kg; Perry Farm), which tested free of E. chaffeensis infection as described above, were experimentally infected by intravenous injection of 5 × 106 DH82 cells infected with E. chaffeensis Arkansas or St. Vincent (nearly 100% of cells infected), respectively, in Dulbecco's modified Eagle medium. Dog A was reinoculated with 5 × 106 E. chaffeensis Arkansas-infected DH82 cells on days 4 and 24 after the first inoculation. Dog B was reinoculated with 5 × 106 E. chaffeensis St. Vincent-infected DH82 cells on days 28 and 72 after the first inoculation. DTH tests were performed on infected dogs A and B and on a control, dog C (female; body weight, 14 kg; Perry Farm) inoculated with uninfected DH82 cells. For dog A, DTH tests were performed after the third E. chaffeensis inoculation on days 43, 58, and 67. Each lipoprotein construct DNA was administered intradermally to dog A at one of five sites (one site was used for the first DTH test, two sites were used for the second test, and two sites were used for the third test). Each stimulant was administered to dog B at one of four sites after the third E. chaffeensis inoculation on day 53 only. Each stimulant was administered to uninfected dog C at two sites for DTH tests on day 53 only.
DTH analysis.
The hair on the caudal aspect of the back of each dog was shaved. The shaved area was divided according to the stimulants to be administered. Skin thickness was measured at the center of each premarked area using a caliper with 0.1-mm grades (General Hardware Manufacturing Co., New York, NY). Mammalian lipoprotein expression vector constructs, canine GM-CSF DNA, and VR1020 DNA were purified from plasmid-transformed Escherichia coli using the EndoFree Mega plasmid kit to prevent endotoxin contamination (Qiagen). DNAs were dissolved in saline solution (0.85% NaCl) and passed through a 0.22-μm-pore-size filter. Concentrations of purified plasmids were determined by A260 spectrometry using the GeneQuant II RNA and DNA calculator (Pharmacia Biotech Inc., Cambridge, England). Either VR1020 DNA or lipoprotein construct DNA (50 μg each) plus 25 μg canine GM-CSF DNA in 50 to 100 μl of saline were injected intradermally into the center of each premarked skin area. Phytohemagglutinin (PHA; Calbiochem; 50 μl at 2.5 mg/ml) in phosphate-buffered saline (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4 [pH 7.2]) was used as a positive control. At 72 h postinjection, skin thickness and the diameter of the red zone formed at each injection site were measured, and color photographs for injection sites were taken. Changes in skin thickness and the size of the red zone were used as indicators of DTH reactions. For dog A, which had very clean skin and slow hair growth, the color density for each spot was quantified using Multi Gauge software (FujiFilm Medical Systems, Stamford, CT). In brief, the background was subtracted for each spot, and color density was normalized using the negative control (VR1020 plus GM-CSF construct) for each experiment.
Recombinant lipoprotein expression.
Table S1 in the supplemental material shows primers flanked with an NdeI site on the forward primer and an XhoI site on the reverse primer, covering the predicted mature sequence (without the signal peptide) for lipoprotein ECH_0482 and full-length ORFs (including the signal peptide) for ECH_0558 and ECH_0929. With these primers, lipoprotein DNA fragments were amplified from purified E. chaffeensis DNA using Pfu Turbo polymerase. PCR products were cloned into pCR-Blunt II-topo (Invitrogen) according to the manufacturer's instructions. Inserts excised from pCR-Blunt II were directionally subcloned into E. coli expression vector pET29a(+) (Novagen, San Diego, CA) digested with NdeI and XhoI. The recombinant plasmids were amplified in E. coli NovaBlue (Novagen). Recombinant lipoproteins were expressed in E. coli BL21(DE3). BL21(DE3) cells transformed with lipoprotein constructs were propagated in liquid LB medium in the presence of 50 μg/ml kanamycin (Invitrogen) overnight at 37°C and then subcultured at a 1:200 dilution. When the optical density at 600 nm of the E. coli culture reached 0.6, isopropyl-β-d-thiogalactopyranoside (Gold Bio Technology, St. Louis, MO) was added at a final concentration of 1 mM. The cells were cultured for another 4 h before harvesting by centrifugation at 3,000 × g for 5 min. The expression level of recombinant ECH_0482 in the transformed-E. coli whole-cell lysate was too low to be used as antigen directly, and therefore this protein was affinity purified by His-Select (Sigma) column chromatography. Recombinant lipoproteins ECH_0558 and ECH_0929 were obtained directly from transformed E. coli whole-cell lysates and used as antigens for Western blot analysis.
Western blot analysis.
Serum specimens from dogs 1 to 8 were tested by Western blotting for the presence of antibodies to the recombinant lipoproteins ECH_0482, ECH_0558, and ECH_0929, and an E. coli lysate transformed with the vector alone was used as a negative control. The dog A serum specimen, which was drawn on day 31 after the third inoculation of E. chaffeensis-infected DH82 cells prior to DTH testing, was tested for the presence of antibodies to E. chaffeensis lipoproteins. To verify the antibody specificity and the absence of immuno-cross-reactivity among lipoproteins, the dog A serum (1:500 dilution in 5% nonfat milk in Tris-buffered saline, pH 7.6, with 0.5% Tween 20) was preabsorbed with the lysate of E. coli transformed with the control vector pET29a(+) and then with each one of the recombinant lipoproteins, which had been separated by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane, at 4°C overnight with gentle rotation. The dog A serum specimens, with or without preabsorption with one of the recombinant lipoproteins, were then analyzed by Western blotting against the recombinant lipoproteins ECH_0482, ECH_0558, ECH_0929, and ECH_0498 (W. Bao and Y. Rikihisa, unpublished data). Peroxidase-conjugated goat anti-dog immunoglobulin G (1:1,000; Kirkegaard & Perry Laboratories, Gaithersburg, MD) was used as the secondary antibody, and reacting bands were visualized by enhanced chemiluminescence (ECL; GE Healthcare, Piscataway, NJ). Images were then captured using the LAS3000 image documentation system (FujiFilm Medical Systems) at the same setting and exposure time, and band intensities were quantified using Multi Gauge software (FujiFilm Medical Systems).
Statistical analysis.
Statistical analysis was carried out by one-way analysis of variance (ANOVA) or Student's t test, and a P value of <0.05 was considered significant.
RESULTS
Proteomic detection of E. chaffeensis lipoproteins and lipoprotein-processing enzymes.
Bioinformatic analysis of the E. chaffeensis genome predicted 15 putative lipoproteins (Table 1), among which 7 (ECH_0128, ECH_0482, ECH_0558, ECH_0625, ECH_0721, ECH_0929, and ECH_1037) were annotated as putative lipoproteins or hypothetical proteins without assigned functions and 8 (ECH_0378, ECH_0462, ECH_0497, ECH_0498, ECH_0561, ECH_0944, ECH_1072, and ECH_1119) were annotated with functions based on protein sequence homology (GenBank accession no. NC_007799) (10). At the protein level, all 15 predicted lipoproteins were expressed by E. chaffeensis cultured in HL-60 cells as identified by multidimensional LC-MS-MS (Table 1; see also Table S3 in the supplemental material).
TABLE 1.
Proteomic detection of lipoprotein-processing enzymes and putative lipoproteins in the E. chaffeensis genome
| Locus ID | Protein name | Accession no. | No. of amino acids | Total/unique peptide count | Sequence coverage (%) | Lipobox sequencea |
|---|---|---|---|---|---|---|
| Lipoprotein-processing enzymes | ||||||
| ECH_1101 | Prolipoprotein diacylglyceryl transferase (Lgt) | YP_507885 | 280 | 46/7 | 29.6 | N/A |
| ECH_1060 | Signal peptidase II (LspA) | YP_507845 | 146 | 4/2 | 15.8 | N/A |
| ECH_0178 | Apolipoprotein N-acyltransferase (CutE) | YP_507003 | 501 | 40/15 | 29.7 | N/A |
| Lipoproteins | ||||||
| ECH_0128 | Putative lipoprotein | YP_506956 | 258 | 49/18 | 58.1 | WFFMMLITTS.CSIC |
| ECH_0378 | Carbamoyl-phosphate synthase, large subunit | YP_507198 | 1,076 | 192/61 | 49.7 | GAGPIVIGQA.CEFD |
| ECH_0462 | OmpA family protein | YP_507279 | 175 | 131/26 | 93.1 | FMLLCLILSS.CKTT |
| ECH_0482 | Putative lipoprotein | YP_507298 | 324 | 48/14 | 37.3 | FIPCTSVSVG.CVRYb |
| ECH_0497 | Type IV secretion system protein, VirB6 family | YP_507312 | 922 | 105/38 | 37.7 | LIISIFLLSS.CGYHb |
| ECH_0498 | Type IV secretion system protein,VirB6 family | YP_507313 | 1,468 | 264/60 | 40.1 | IILLLFFVTG.CGVN |
| ECH_0558 | Putative lipoprotein | YP_507370 | 583 | 88/33 | 68.8 | LVLIFCFVIS.CSNK |
| ECH_0561 | AcrB/AcrD/AcrF family protein | YP_507373 | 1,032 | 188/51 | 41.1 | FLLMVILIFG.CYSYb |
| ECH_0625 | Putative lipoprotein | YP_507435 | 133 | 8/4 | 27.8 | IYIALFLLSS.CHTIb |
| ECH_0721 | Putative lipoprotein | YP_507524 | 182 | 3/2 | 18.7 | TASVVGLALS.CVNMb |
| ECH_0929 | Putative lipoprotein | YP_507719 | 272 | 79/17 | 51.5 | FFLLPFSLMA.CVTVb |
| ECH_0944 | Na+/H+ ion antiporter family protein | YP_507733 | 163 | 5/4 | 33.7 | LFFITSGITS.CIITb |
| ECH_1037 | Hypothetical protein | YP_507822 | 1,231 | 173/61 | 50.9 | VVFPYVSLSA.CVDG |
| ECH_1072 | Outer membrane protein, OmpH family | YP_507857 | 182 | 214/41 | 85.2 | AIFILLFGLS.CQASb |
| ECH_1119 | Major outer membrane protein OMP-1M | YP_507903 | 298 | 50/18 | 58.1 | IVLSLVTLFA.CNVF |
Putative lipoproteins were predicted by LipoP, version 1.0. Dots indicate the predicted signal peptidase II cleavage site. The lipobox sequences of ECH_0128, ECH_0378, ECH_0462, ECH_0498, ECH_0558, ECH_1037, and ECH_1119 were detected by less than 2% of total peptides. Detailed peptide sequences detected are shown in Table S3 in the supplemental material. N/A, not applicable.
Lipobox sequences of ECH_0482, ECH_0497, ECH_0561, ECH_0625, ECH_0721, ECH_0929, ECH_0944, and ECH_1072 were not detected by multidimensional LC-MS-MS proteomics analysis.
The proper localization of bacterial lipoproteins on the membrane involves three lipoprotein-processing enzymes. First, the thiol group of the conserved cysteine residue, which is located in a stretch of amino acids referred to as the lipobox [consensus, Leu-(Ala/Ser)-(Gly/Ala)∧Cys], is modified with a diacylglyceryl group attached through a thioether linkage by prolipoprotein diacylglyceryl transferase (Lgt) (37). The prolipoproteins are then cleaved by signal peptidase II (SPase II; LspA), a specific lipoprotein-processing protease that cleaves before the conserved cysteine residue at the lipobox (indicated by ∧) (37). Acylation (the addition of lipid to the amine of the newly amino-terminal cysteine) occurs after cleavage of the signal peptide and is catalyzed by apolipoprotein N-acyltransferase (Lnt/CutE) (12). The E. chaffeensis genome encodes three genes required for lipoprotein processing: lspA (ECH_1060), lgt (ECH_1101), and cutE (ECH_0178) (10). Expression of the LspA, Lgt, and CutE proteins by E. chaffeensis was detected by multidimensional LC-MS-MS (Table 1). The predicted signal peptides of eight lipoproteins (ECH_0482, ECH_0497, ECH_0561, ECH_0625, ECH_0721, ECH_0929, ECH_0944, and ECH_1072) were not detected by proteomics analysis, whereas those of the remaining seven lipoproteins were detected at low levels, with less than 2% of total peptides identified in the respective proteins (Table 1; see also Table S3 in the supplemental material), supporting posttranslational processing of E. chaffeensis lipoproteins.
Globomycin inhibits E. chaffeensis lipoprotein OmpA cleavage and infection in HL-60 cells.
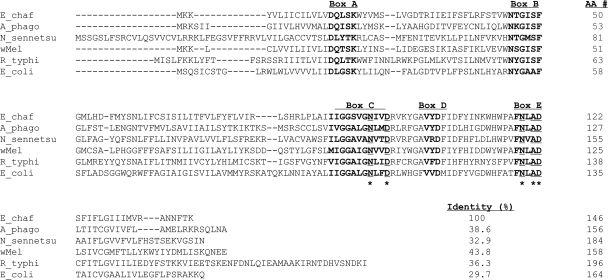
Bacterial SPase II cleaves the signal peptide from glyceride-modified prolipoproteins, while they are translocated across the cytoplasmic membrane (37). It was shown recently that overexpression of R. typhi LspA in E. coli confers resistance to globomycin, a specific inhibitor of SPase II (18), and genetically complements the growth defect of a SPase II-deficient E. coli mutant (38). Although the percentage of amino acid identity between R. typhi LspA and E. chaffeensis LspA is only 36%, five domains and catalytic residues that are considered to be essential for the enzymatic activity of bacterial SPase II (37) are conserved in the LspA proteins of E. chaffeensis and other members of the family Anaplasmataceae (Fig. 1), suggesting that E. chaffeensis LspA might also have SPase II activity.
FIG. 1.
Alignment of protein sequences of LspA from Ehrlichia chaffeensis Arkansas (E_chaf) (GenBank accession no. YP_507845), Anaplasma phagocytophilum HZ (A_phago) (YP_505700), Neorickettsia sennetsu Miyayama (N_sennetsu) (GenBank accession no. YP_506779), Wolbachia pipientis wMel (wMel) (GenBank accession no. NP_966516), Rickettsia typhi Wilmington (R_typhi) (GenBank accession no. YP_067353), and Escherichia coli K-12 (E_coli) (GenBank accession no. NP_414568). Sequences were aligned using the MegAlign program by the Clustal W method in the Lasergene software package. The conserved domains (boxes A through E) are marked on top, and the sequences are shown in bold. The catalytic residues are underlined and marked by asterisks.
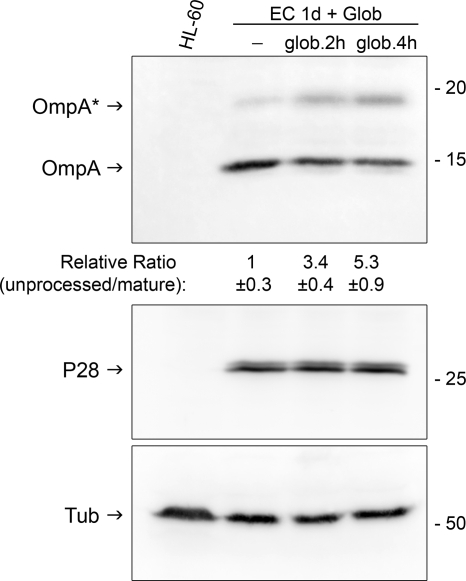
To confirm E. chaffeensis SPase II activity, we examined the effect of globomycin on the processing of E. chaffeensis lipoprotein OmpA, one of the highly expressed low-molecular-mass lipoproteins based on our proteomic analysis (Table 1). Western blotting results showed that most OmpA protein from untreated (data not shown) or DMSO-treated E. chaffeensis-infected HL-60 cells was detected at around 15 kDa, which approximately corresponds to the mature form without the signal peptide (Fig. 2). However, treatment of E. chaffeensis-infected HL-60 cells at 1 day p.i. with 25 μg/ml globomycin for 2 or 4 h partially blocked the cleavage of the OmpA lipoprotein signal peptide, resulting in significantly increased ratios of the band density of 20-kDa uncleaved OmpA to that of 15-kDa cleaved OmpA (Fig. 2). Furthermore, densitometric analysis of three independent experiments showed that the maturation of OmpA was significantly inhibited by globomycin treatment in a time-dependent manner (P < 0.01). The observed molecular mass of unprocessed OmpA by Western blot analysis matched the predicted molecular mass (19.95 kDa); however, there was a slight discrepancy between the observed (∼15 kDa) and the predicted (17.7 kDa) molecular mass of the mature OmpA after the cleavage of the predicted signal peptide (MKHKLVFIKFMLLCLILSS). This could be due to an anomalous electrophoretic mobility of membrane proteins (43), or the cleaved signal peptide might be longer than predicted. The amounts of E. chaffeensis P28 outer membrane protein and host cell α-tubulin, used as protein-loading controls for bacteria and host cells, did not change during the 4-h globomycin treatment (Fig. 2).
FIG. 2.
Inhibitory effect of globomycin on E. chaffeensis OmpA lipoprotein processing. HL-60 cells were infected with host cell-free E. chaffeensis (EC). At 1 day (1d) p.i., cells were treated with the solvent control, DMSO, or 25 μg/ml globomycin (Glob) and were cultured in the presence of these reagents for 2 or 4 h. Cells were lysed in RIPA buffer, and Western blotting was performed using antibodies against E. chaffeensis OmpA, E. chaffeensis P28, or human α-tubulin. The arrows with OmpA or OmpA* designate mature or unprocessed precursor protein, respectively. The relative migration of molecular mass standards (in kilodaltons) is shown on the right. The values under each lane with anti-OmpA antiserum show the relative ratios ± standard deviations of unprocessed to mature OmpA band densities, with the ratio of the DMSO control group arbitrarily set at 1. Results are representative of three independent experiments. ANOVA showed that these three groups were significantly different from each other (P < 0.01; n = 3).
Globomycin causes the accumulation of the modified lipoprotein precursors in E. coli inner membrane and prevents proper lipoprotein localization (14-16), resulting in the inhibition of bacterial growth (14). The sensitivity of E. chaffeensis lipoprotein processing to globomycin suggests that E. chaffeensis infection might be also sensitive to globomycin. While bactericidal concentrations of globomycin have not been reported, the minimal globomycin concentrations that inhibit the growth of wild-type E. coli and Salmonella enterica serovar Typhimurium in vitro are 20 and >80 μg/ml, respectively, and the bactericidal concentrations are expected to be greater than these values (24). E. chaffeensis was found to be highly sensitive to globomycin. Treatment with 10 μg/ml globomycin for 1 day at day 1 p.i. greatly reduced E. chaffeensis infection in HL-60 cells, showing inclusions with sparsely populated bacteria and accumulation of amorphous materials (Fig. 3A). The total number of organisms was significantly decreased, to around 10% of that with the solvent control (this percentage was ∼30% with 1.0 μg/ml globomycin [Fig. 3B]). No toxicity was observed in HL-60 cells with 10-μg/ml globomycin treatment for 1 day, based on cell morphology (data not shown) and semiquantitive RT-PCR analysis of human GAPDH mRNA levels in HL-60 cells (Fig. 3C). These results are consistent with inhibitory effects of globomycin on lipoprotein processing by E. chaffeensis.
FIG. 3.
Inhibitory effect of globomycin on E. chaffeensis infection in HL-60 cells. HL-60 cells were infected with host cell-free E. chaffeensis (EC) in 6-well plates. At 1 day (d) p.i., cells were treated with the DMSO solvent control or with 1 or 10 μg/ml globomycin and cultured in the presence of these reagents for 1 day. (A) Representative Diff-Quik-stained images of E. chaffeensis-infected cells at 1 day p.i. treated with 10 μg/ml globomycin or DMSO for 1 day. Bar, 10 μm. (B) Numbers of E. chaffeensis organisms counted in 100 cells in triplicate. *, significantly different from the DMSO solvent control by Student's t test (P < 0.01; n = 3). (C) Semiquantitive RT-PCR analysis of host gapdh mRNA amount. The same amount of total RNA, prepared from the infected cells incubated with or without globomycin, was reverse transcribed (RT+) using an oligo(dT) primer and subsequently PCR amplified using primers specific to the human gapdh gene under linear amplification conditions (16 cycles). RT−, negative control without reverse transcriptase; CTL, DMSO solvent control; Globo: globomycin (in μg/ml). The relative sizes of molecular mass standards are shown (in base pairs) on the left.
Lipoprotein-encoding mammalian expression vectors induce humoral and cell-mediated immune responses in dogs.
Thirteen of 15 lipoprotein-coding DNA sequences were successfully cloned into the mammalian expression vector VR1020 and confirmed by sequencing the entire length of each insert. GM-CSF is a hematopoietic cytokine that has been used in several immunization and DNA vaccine studies to enhance immunity by increasing the efficacy of antigen processing (44, 46, 47). As such, we cloned canine GM-CSF cDNA into the mammalian expression vector VR1055 and coadministered it with lipoprotein DNAs to enhance the efficacy of immunization. DNA constructs of three lipoproteins (ECH_0482, ECH_0558, and ECH_0929) were randomly selected to determine in situ protein expression and immunogenicity in dogs. All three lipoprotein DNA constructs induced specific antibody development in dogs as detected by Western blot analysis using the respective recombinant proteins, indicating that DNA constructs were expressed as proteins in dogs (Fig. 4A). Preimmune sera did not contain, and control group dogs did not develop, these specific antibodies. Since a positive DTH response is an indicator of cell-mediated immunity, dogs were tested for DTH reactions to the respective lipoproteins. All six dogs developed DTH reactions to the positive control PHA, showing their normal immune status. The DNA construct induced, at cutaneous sites of deposition, significantly larger red zones against the respective lipoprotein stimulants than the VR1020 vector plus the GM-CSF control (Fig. 4B). Thus, relative to the VR1020 vector control, all six dogs were considered DTH-positive to the E. chaffeensis lipoprotein DNA constructs. These results support the notion that the presence of antibody and DTH reactions to lipoproteins can be used to detect lipoprotein expression by E. chaffeensis in infected dogs.
FIG. 4.
Immune responses in dogs after lipoprotein DNA immunization. (A) Humoral immune responses as determined by Western blot analysis. Plasma samples were collected 1 week after the third immunization with ECH_0482 (dogs 3 and 4), ECH_0558 (dogs 5 and 6), or ECH_0929 (dogs 7 and 8) construct DNA and were used as primary antisera (diluted 1:100). CTL, control lysate of E. coli transformed with the blank vector (pET29a+). ECH_0482, ECH_0558, and ECH_0929, respectively, represent His tag-purified recombinant ECH_0482 and lysates of E. coli expressing ECH_0558 and ECH_0929. The approximate molecular mass of each band (in kilodaltons) is shown to the right of each panel. (B) DTH reactions. Shown are the diameters of red zones in lipoprotein-immunized dogs 72 h after intradermal inoculation with lipoprotein constructs. PHA is the positive control. *, significantly different from the result with the vector control (VR1020) by Student's t test (n = 6; P < 0.05).
E. chaffeensis-infected dogs develop antibodies specific to lipoproteins and DTH reactions against lipoproteins encoded in mammalian expression constructs.
To determine whether E. chaffeensis expresses lipoproteins in infected dogs, we inoculated two dogs with E. chaffeensis-infected DH82 cells and one dog with DH82 host cells as a control. Serum from dog A, infected with E. chaffeensis Arkansas strain on day 31, recognized recombinant ECH_0482, ECH_0558, ECH_0929, and ECH_0498 expressed in E. coli by Western blot analysis (Fig. 5). In order to confirm the specificity of the immune reaction and the absence of antigenic cross-reactivity among E. chaffeensis lipoproteins, the serum from dog A was absorbed with the lysate from ECH_0482-, ECH_0558-, or ECH_0929-transformed E. coli. Western blotting results showed that absorption with one of the recombinant lipoproteins reduced the reactivity (band density) to this lipoprotein only, not to the other three lipoproteins (Fig. 5). These data indicate that these four lipoproteins were expressed by E. chaffeensis and that they induced humoral immune responses in the infected dog.
FIG. 5.
Four lipoproteins were expressed and induced humoral immune responses in E. chaffeensis-infected dogs. Either the serum from E. chaffeensis-infected dog A (Dog α-EC) was preabsorbed with a control E. coli BL21 lysate transformed with a blank pET29a(+) vector only (-BL lys.), or the E. coli-preabsorbed serum was further absorbed with an E. coli lysate transformed with recombinant protein ECH_0929, ECH_0482, or ECH_0558. The preabsorbed sera were incubated with an E. coli BL21 lysate transformed with the blank pET29a(+) vector only (lanes CTL) or with ECH_0482 (lanes 482), ECH_0498 (lanes 498), ECH_0929 (lanes 929), or ECH_0558 (lanes 558). The relative migration of molecular mass standards is shown on the left, and the molecular mass of each recombinant lipoprotein is shown in parentheses on the right. The value under each lane is the relative ratio of the band density of Dog α-EC serum absorbed with the indicated recombinant protein to that of Dog α-EC serum absorbed with the control lysate (-BL lys.). Asterisks indicate reduced band density relative to that with Dog α-EC (-BL lys.). Results are representative of three independent experiments.
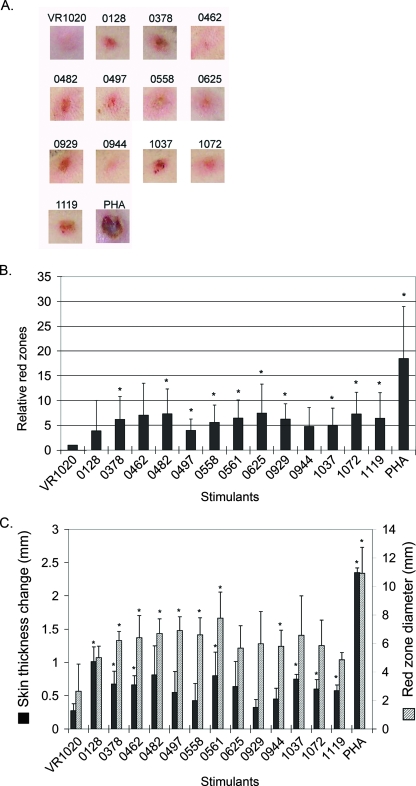
To determine DTH reactions in infected dogs, infected dog A was intradermally inoculated with 13 lipoprotein DNA constructs, each at a different place, on day 46 p.i. Red zones were formed at the injection site 3 days after DNA administration, and representative photographs of the spots are shown in Fig. 6A. Red zones for several lipoprotein constructs were significantly darker than that for the vector control (VR1020 plus GM-CSF) by densitometric analysis (Fig. 6B). Similarly, dog B, infected with the E. chaffeensis St. Vincent strain, was also intradermally inoculated with 13 lipoprotein DNA constructs, each at a different place, after the last E. chaffeensis inoculation on day 53. Because this dog had dark skin, DTH reactions were measured based on skin thickness and red-zone diameter. Increased skin thickness was detected for several lipoprotein constructs (Fig. 6C). Significantly larger red zones were also detected for several lipoprotein constructs (Fig. 6C). Both dogs A and B developed strong DTH reactions to the positive control, PHA, showing their normal immune status. In uninfected dog C, except for the PHA positive control and one spot for ECH_0482, no red zones were formed after the DTH assay (data not shown). The change in skin thickness was insignificant in dog C compared with those in dogs A and B for all lipoproteins constructs (data not shown). Thus, relative to the uninfected dog and the VR1020 vector control, the infected dogs were considered to be DTH positive to several E. chaffeensis lipoprotein DNA constructs. This result is consistent with the Western blotting result, indicating that multiple E. chaffeensis lipoproteins are expressed in infected dogs.
FIG. 6.
Lipoproteins encoded in mammalian expression constructs induced DTH reactions in E. chaffeensis-infected dogs. (A) Representative red zones formed at injection sites 72 h after the administration of stimulants to E. chaffeensis Arkansas-infected dog A. VR1020 is the vector-alone control. The numbers are the genomic locus numbers for lipoproteins, and PHA is the positive control. (B) Densitometric analysis of red zone intensity 72 h after the administration of stimulants in E. chaffeensis Arkansas-infected dog A. *, significantly different from the vector-alone control (n = 5; P < 0.05). (C) DTH reactions to lipoprotein constructs in E. chaffeensis St. Vincent-infected dog B. The skin thickness changes and red zone diameters (in millimeters), indicating DTH reactions that developed at the sites of injection, were measured 72 h after administration. The genomic locus numbers for lipoproteins are shown along the x axis. *, significantly different from the vector-alone control by Student's t test (n = 4; P < 0.05).
DISCUSSION
The present study demonstrates that multiple lipoproteins and three lipoprotein-processing enzymes (LspA, Lgt, and CutE) were expressed by E. chaffeensis in cell culture. Combined with the rare detection of signal peptides in E. chaffeensis lipoproteins and the sensitivity of E. chaffeensis OmpA signal peptide processing to globomycin treatment, these results suggest that a globomycin-sensitive lipoprotein biogenesis pathway is present in E. chaffeensis. The inhibition of E. chaffeensis infection by globomycin treatment in vitro suggests that lipoproteins are required for E. chaffeensis infection of host cells. Unfortunately, the limited availability of globomycin precludes in vivo experiments, and globomycin analogs of higher antimicrobial activity, once developed, are not currently available (21, 22). Based on the present data, investigation of SPase II inhibitors deserves more attention, especially since doxycycline is the only choice of antibiotic for HME (54).
While small numbers of E. chaffeensis bacteria present in the peripheral blood monocytes of infected dogs precluded direct determination of lipoprotein expression in the blood of infected dogs, at least four lipoproteins were expressed in dogs based on the development of lipoprotein-specific antibodies. Bacterial lipoproteins are known to induce proinflammatory cytokines and to be involved in the pathogenesis of various bacterial infections (4, 42, 45). All members of the family Anaplasmataceae are LPS deficient, but genes encoding orthologs of most of the 15 E. chaffeensis lipoproteins as well as the 3 lipoprotein-processing enzymes are found in members of three other genera in the family Anaplasmataceae (see Table S4 in the supplemental material) (10, 29). Lipoproteins, thus, need to be taken into consideration in understanding pathogenesis and immune responses to these obligately intracellular bacteria. Along these lines, the present proteomics data will facilitate future studies on the roles of individual lipoproteins in E. chaffeensis pathogenesis.
In addition to their roles in pathogenesis, bacterial lipoproteins can activate microbicidal activities of monocytes and macrophages through Toll-like receptor 2 (TLR2) (6, 27, 34, 49). Since E. chaffeensis infection leads to the downregulation of TLR2 in the host cells in vitro (30), it is expected that E. chaffeensis lipoproteins cannot induce effective antimicrobicidal activity in already infected monocytes and macrophages. However, it is possible that E. chaffeensis lipoproteins can activate uninfected monocytes and macrophages to make them refractory to infection in vitro and in vivo. Furthermore, E. chaffeensis lipoproteins may be able to activate TLR2-expressing neutrophils, NK cells, dendritic cells, or other types of cells, which are not usually infected with E. chaffeensis, to modulate the overall host immune response. It was reported recently that in mouse Th1 cells, stimulation by TLR2, but not by other TLRs, directly induces gamma interferon production, cell proliferation, and cell survival without the stimulation of T-cell receptors (17). Future detailed analysis of cell types activated by E. chaffeensis lipoproteins in animals experimentally infected with E. chaffeensis may shed light on this critical innate immune response in HME.
The present study showed that E. chaffeensis lipoproteins could induce DTH reactions in dogs, which are elicited by CD4+ and CD8+ T cells of the Th1 subset through macrophage activation and inflammation induction (23). In fact, lipoproteins are the first components of E. chaffeensis shown to induce DTH reactions. Although DTH reactions in HME patients have not been determined, both gamma interferon and antibodies are effective at reducing the Ehrlichia burden and clinical signs in a mouse model of HME (5, 19, 25, 26). Thus, some lipoproteins may be considered as potential candidates for a vaccine against HME. Future studies on T-cell specificity and reactivity to each E. chaffeensis lipoprotein would help refine the mammalian expression constructs prepared in the present study.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01 AI30100 and R01 AI47885. Proteomics analysis was performed in the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
We appreciate Vical, Incorporated, for kindly providing VR1020 and VR1055, and Shunichi Miyakoshi at Sankyo Pharmaceutical Co. for kindly providing globomycin.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 19 May 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adkins, J. N., H. M. Mottaz, A. D. Norbeck, J. K. Gustin, J. Rue, T. R. Clauss, S. O. Purvine, K. D. Rodland, F. Heffron, and R. D. Smith. 2006. Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol. Cell. Proteomics 51450-1461. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, B. E., J. E. Dawson, D. C. Jones, and K. H. Wilson. 1991. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 292838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, B. E., J. W. Sumner, J. E. Dawson, T. Tzianabos, C. R. Greene, J. G. Olson, D. B. Fishbein, M. Olsen-Rasmussen, B. P. Holloway, E. H. George, et al. 1992. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 30775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bas, S., L. Neff, M. Vuillet, U. Spenato, T. Seya, M. Matsumoto, and C. Gabay. 2008. The proinflammatory cytokine response to Chlamydia trachomatis elementary bodies in human macrophages is partly mediated by a lipoprotein, the macrophage infectivity potentiator, through TLR2/TLR1/TLR6 and CD14. J. Immunol. 1801158-1168. [DOI] [PubMed] [Google Scholar]
- 5.Bitsaktsis, C., J. Huntington, and G. Winslow. 2004. Production of IFN-γ by CD4 T cells is essential for resolving Ehrlichia infection. J. Immunol. 1726894-6901. [DOI] [PubMed] [Google Scholar]
- 6.Cabral, E. S., H. Gelderblom, R. L. Hornung, P. J. Munson, R. Martin, and A. R. Marques. 2006. Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J. Infect. Dis. 193849-859. [DOI] [PubMed] [Google Scholar]
- 7.Dawson, J. E., B. E. Anderson, D. B. Fishbein, J. L. Sanchez, C. S. Goldsmith, K. H. Wilson, and C. W. Duntley. 1991. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 292741-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson, J. E., K. L. Biggie, C. K. Warner, K. Cookson, S. Jenkins, J. F. Levine, and J. G. Olson. 1996. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am. J. Vet. Res. 571175-1179. [PubMed] [Google Scholar]
- 9.Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2005. Epidemiology of human ehrlichiosis and anaplasmosis in the United States, 2001-2002. Am. J. Trop. Med. Hyg. 73400-409. [PubMed] [Google Scholar]
- 10.Dunning Hotopp, J. C., M. Lin, R. Madupu, J. Crabtree, S. V. Angiuoli, J. Eisen, R. Seshadri, Q. Ren, M. Wu, T. R. Utterback, S. Smith, M. Lewis, H. Khouri, C. Zhang, H. Niu, Q. Lin, N. Ohashi, N. Zhi, W. Nelson, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, J. Sundaram, S. C. Daugherty, T. Davidsen, A. S. Durkin, M. Gwinn, D. H. Haft, J. D. Selengut, S. A. Sullivan, N. Zafar, L. Zhou, F. Benahmed, H. Forberger, R. Halpin, S. Mulligan, J. Robinson, O. White, Y. Rikihisa, and H. Tettelin. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge, Y., and Y. Rikihisa. 2007. Surface-exposed proteins of Ehrlichia chaffeensis. Infect. Immun. 753833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, S. D., and H. C. Wu. 1991. Identification and subcellular localization of apolipoprotein N-acyltransferase in Escherichia coli. FEMS Microbiol. Lett. 6237-41. [DOI] [PubMed] [Google Scholar]
- 13.Han, J., and R. J. Ulevitch. 2005. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 61198-1205. [DOI] [PubMed] [Google Scholar]
- 14.Hussain, M., S. Ichihara, and S. Mizushima. 1980. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J. Biol. Chem. 2553707-3712. [PubMed] [Google Scholar]
- 15.Ichihara, S., M. Hussain, and S. Mizushima. 1981. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J. Biol. Chem. 2563125-3129. [PubMed] [Google Scholar]
- 16.Ichihara, S., M. Hussain, and S. Mizushima. 1982. Mechanism of export of outer membrane lipoproteins through the cytoplasmic membrane in Escherichia coli. Binding of lipoprotein precursors to the peptidoglycan layer. J. Biol. Chem. 257495-500. [PubMed] [Google Scholar]
- 17.Imanishi, T., H. Hara, S. Suzuki, N. Suzuki, S. Akira, and T. Saito. 2007. TLR2 directly triggers Th1 effector functions. J. Immunol. 1786715-6719. [DOI] [PubMed] [Google Scholar]
- 18.Inukai, M., M. Takeuchi, K. Shimizu, and M. Arai. 1978. Mechanism of action of globomycin. J. Antibiot. (Tokyo) 311203-1205. [DOI] [PubMed] [Google Scholar]
- 19.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-α by CD8+ type 1 cells and down-regulation of IFN-γ production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 1721786-1800. [DOI] [PubMed] [Google Scholar]
- 20.Kelso, A., and N. M. Gough. 1989. Differential inhibition by cyclosporin A reveals two pathways for activation of lymphokine synthesis in T cells. Growth Factors 1165-177. [DOI] [PubMed] [Google Scholar]
- 21.Kiho, T., M. Nakayama, K. Yasuda, S. Miyakoshi, M. Inukai, and H. Kogen. 2004. Structure-activity relationships of globomycin analogues as antibiotics. Bioorg. Med. Chem. 12337-361. [DOI] [PubMed] [Google Scholar]
- 22.Kiho, T., M. Nakayama, K. Yasuda, S. Miyakoshi, M. Inukai, and H. Kogen. 2003. Synthesis and antimicrobial activity of novel globomycin analogues. Bioorg. Med. Chem. Lett. 132315-2318. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, K., K. Kaneda, and T. Kasama. 2001. Immunopathogenesis of delayed-type hypersensitivity. Microsc. Res. Tech. 53241-245. [DOI] [PubMed] [Google Scholar]
- 24.Lai, J. S., W. M. Philbrick, S. Hayashi, M. Inukai, M. Arai, Y. Hirota, and H. C. Wu. 1981. Globomycin sensitivity of Escherichia coli and Salmonella typhimurium: effects of mutations affecting structures of murein lipoprotein. J. Bacteriol. 145657-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, E. H., and Y. Rikihisa. 1997. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IκB-α and activation of NF-κB. Infect. Immun. 652890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 1661855-1862. [DOI] [PubMed] [Google Scholar]
- 27.Liang, M. D., A. Bagchi, H. S. Warren, M. M. Tehan, J. A. Trigilio, L. K. Beasley-Topliffe, B. L. Tesini, J. C. Lazzaroni, M. J. Fenton, and J. Hellman. 2005. Bacterial peptidoglycan-associated lipoprotein: a naturally occurring toll-like receptor 2 agonist that is shed into serum and has synergy with lipopolysaccharide. J. Infect. Dis. 191939-948. [DOI] [PubMed] [Google Scholar]
- 28.Lin, M., A. den Dulk-Ras, P. J. Hooykaas, and Y. Rikihisa. 2007. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell. Microbiol. 92644-2657. [DOI] [PubMed] [Google Scholar]
- 29.Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 715324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, M., and Y. Rikihisa. 2004. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-κB, ERK 1/2 and p38 MAPK in host monocytes. Cell. Microbiol. 6175-186. [DOI] [PubMed] [Google Scholar]
- 31.Lin, M., M. X. Zhu, and Y. Rikihisa. 2002. Rapid activation of protein tyrosine kinase and phospholipase C-γ2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect. Immun. 70889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda, K., N. Markowitz, R. C. Hawley, M. Ristic, D. Cox, and J. E. McDade. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316853-856. [DOI] [PubMed] [Google Scholar]
- 33.Mott, J., R. E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 671368-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller, S. D., M. R. Muller, M. Huber, U. Esche Uv, C. J. Kirschning, H. Wagner, W. G. Bessler, and K. Mittenbuhler. 2004. Triacyl-lipopentapeptide adjuvants: TLR2-dependent activation of macrophages and modulation of receptor-mediated cell activation by altering acyl-moieties. Int. Immunopharmacol. 41287-1300. [DOI] [PubMed] [Google Scholar]
- 35.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 1637-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paddock, C. D., J. W. Sumner, G. M. Shore, D. C. Bartley, R. C. Elie, J. G. McQuade, C. R. Martin, C. S. Goldsmith, and J. E. Childs. 1997. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J. Clin. Microbiol. 352496-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paetzel, M., A. Karla, N. C. Strynadka, and R. E. Dalbey. 2002. Signal peptidases. Chem. Rev. 1024549-4580. [DOI] [PubMed] [Google Scholar]
- 38.Rahman, M. S., S. M. Ceraul, S. M. Dreher-Lesnick, M. S. Beier, and A. F. Azad. 2007. The lspA gene, encoding the type II signal peptidase of Rickettsia typhi: transcriptional and functional analysis. J. Bacteriol. 189336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rikihisa, Y. 1999. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1367-376. [DOI] [PubMed] [Google Scholar]
- 40.Rikihisa, Y. 1997. Ehrlichiae, emerging human pathogens, p. 332-345. In Y. Yanagihara and T. Masuzawa (ed.), Proceedings of the 2nd International Symposium on Lyme Disease. University of Shizuoka, Shizuoka, Japan.
- 41.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 322107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander, P., M. Rezwan, B. Walker, S. K. Rampini, R. M. Kroppenstedt, S. Ehlers, C. Keller, J. R. Keeble, M. Hagemeier, M. J. Colston, B. Springer, and E. C. Bottger. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 521543-1552. [DOI] [PubMed] [Google Scholar]
- 43.Schägger, H. 2003. SDS electrophoresis techniques, p. 85-103. In C. Hunte, G. von Jagow, and H. Schägger (ed.), Membrane protein purification and crystallization. Academic Press, San Diego, CA.
- 44.Sedegah, M., W. Weiss, J. B. Sacci, Jr., Y. Charoenvit, R. Hedstrom, K. Gowda, V. F. Majam, J. Tine, S. Kumar, P. Hobart, and S. L. Hoffman. 2000. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J. Immunol. 1645905-5912. [DOI] [PubMed] [Google Scholar]
- 45.Sha, J., S. L. Agar, W. B. Baze, J. P. Olano, A. A. Fadl, T. E. Erova, S. Wang, S. M. Foltz, G. Suarez, V. L. Motin, S. Chauhan, G. R. Klimpel, J. W. Peterson, and A. K. Chopra. 2008. Braun lipoprotein (Lpp) contributes to virulence of yersiniae: potential role of Lpp in inducing bubonic and pneumonic plague. Infect. Immun. 761390-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin, J. I., J. J. Kim, K. E. Ugen, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1998. Enhancement of protective humoral (Th2) and cell-mediated (Th1) immune responses against herpes simplex virus-2 through co-delivery of granulocyte-macrophage colony-stimulating factor expression cassettes. Eur. J. Immunol. 283530-3540. [DOI] [PubMed] [Google Scholar]
- 47.Somasundaram, C., H. Takamatsu, C. Andreoni, J. C. Audonnet, L. Fischer, F. Lefevre, and B. Charley. 1999. Enhanced protective response and immuno-adjuvant effects of porcine GM-CSF on DNA vaccination of pigs against Aujeszky's disease virus. Vet. Immunol. Immunopathol. 70277-287. [DOI] [PubMed] [Google Scholar]
- 48.Tajima, T., and Y. Rikihisa. 2005. Cytokine responses in dogs infected with Ehrlichia canis Oklahoma strain. Ann. N. Y. Acad. Sci. 1063429-432. [DOI] [PubMed] [Google Scholar]
- 49.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 2911544-1547. [DOI] [PubMed] [Google Scholar]
- 50.Unver, A., H. Huang, and Y. Rikihisa. 2006. Cytokine gene expression by peripheral blood leukocytes in dogs experimentally infected with a new virulent strain of Ehrlichia canis. Ann. N. Y. Acad. Sci. 1078482-486. [DOI] [PubMed] [Google Scholar]
- 51.Unver, A., Y. Rikihisa, R. W. Stich, N. Ohashi, and S. Felek. 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 704701-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wellman, M. L., S. Krakowka, R. M. Jacobs, and G. J. Kociba. 1988. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In Vitro Cell. Dev. Biol. 24223-229. [DOI] [PubMed] [Google Scholar]
- 53.Winslow, G. M., E. Yager, K. Shilo, D. N. Collins, and F. K. Chu. 1998. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffeensis. Infect. Immun. 663892-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wormser, G. P., A. Filozov, S. R. Telford III, S. Utpat, R. S. Kamer, D. Liveris, G. Wang, L. Zentmaier, I. Schwartz, and M. E. Aguero-Rosenfeld. 2006. Dissociation between inhibition and killing by levofloxacin in human granulocytic anaplasmosis. Vector Borne Zoonotic Dis. 6388-394. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, X. F., J. Z. Zhang, S. W. Long, R. P. Ruble, and X. J. Yu. 2003. Experimental Ehrlichia chaffeensis infection in beagles. J. Med. Microbiol. 521021-1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.