Abstract
Acinetobacter baumannii is a bacterial pathogen of increasing medical importance. Little is known about its mechanisms of pathogenesis, and safe reliable agents with predictable activity against A. baumannii are presently nonexistent. The availability of relevant animal infection models will facilitate the study of Acinetobacter biology. In this report we tested the hypothesis that the rat pneumonia and soft-tissue infection models that our laboratory had previously used for studies of extraintestinal pathogenic Escherichia coli were clinically relevant for A. baumannii. Advantages of these models over previously described models were that the animals were not rendered neutropenic and they did not receive porcine mucin with bacterial challenge. Using the A. baumannii model pathogen 307-0294 as the challenge pathogen, the pneumonia model demonstrated all of the features of infection that are critical for a clinically relevant model: namely, bacterial growth/clearance, an ensuing host inflammatory response, acute lung injury, and, following progressive bacterial proliferation, death due to respiratory failure. We were also able to demonstrate growth of 307-0294 in the soft-tissue infection model. Next we tested the hypothesis that the soft-tissue infection model could be used to discriminate between the inherent differences in virulence of various A. baumannii clinical isolates. The ability of A. baumannii to grow and/or be cleared in this model was dependent on the challenge strain. We also hypothesized that complement is an important host factor in protecting against A. baumannii infection in vivo. In support of this hypothesis was the observation that the serum sensitivity of various A. baumannii clinical isolates in vitro roughly paralleled their growth/clearance in the soft-tissue infection model in vivo. Lastly we hypothesized that the soft-tissue infection model would serve as an efficient screening mechanism for identifying gene essentiality for drug discovery. Random mutants of 307-0294 were initially screened for lack of growth in human ascites in vitro. Selected mutants were subsequently used for challenge in the soft-tissue infection model to determine if the disrupted gene was essential for growth in vivo. Using this approach, we have been able to successfully identify a number of genes essential for the growth of 307-0294 in vivo. In summary, these models are clinically relevant and can be used to study the innate virulence of various Acinetobacter clinical isolates and to assess potential virulence factors, vaccine candidates, and drug targets in vivo and can be used for pharmacokinetic and chemotherapeutic investigations.
Acinetobacter species are highly prevalent in the environment and have been cultured from moist skin in healthy humans, but increased colonization of skin and respiratory and gastrointestinal tracts occurs in individuals in long-term care and hospital facilities. The overwhelming majority of infections described until recently have been acquired mainly in hospitals, to a lesser degree in long-term care facilities, and only rarely from the community. Acinetobacter baumannii accounts for 1 to 3% of hospital-acquired infections and 2 to 10% of infections in intensive care units (14, 33, 49). Both sporadic and epidemic infections occur, usually after the first week of hospitalization (3, 13, 14, 19, 51). Importantly, the incidence of Acinetobacter infection is increasing worldwide (13, 16, 33). The respiratory tract, particularly in ventilated patients (6.9% of hospital-acquired pneumonias in 2003 based on National Nosocomial Infection Surveillance System data); the urinary tract; intravenous devices; surgical sites; and decubitus or diabetic ulcers are favored sites of infection. Mortality rates associated with Acinetobacter infection range from 19 to 54% (16). Interestingly, A. baumannii has been reported to uncommonly cause severe community-acquired pneumonia, usually in abnormal hosts (e.g., alcoholics), with the preponderance of cases reported from warm and humid geographic locales (1, 2, 6). Further, the importance of Acinetobacter infections in war-related injuries is now established. A. baumannii was the most common gram-negative bacillus recovered from traumatic injuries to the lower extremities during the Vietnam War (48). Most recently a new series of infections due to A. baumannii has been reported in U.S. service members injured in Iraq, Kuwait, and Afghanistan (5, 10, 11, 18, 44). The majority of patients sustained traumatic injuries, and infectious syndromes included soft-tissue infection, osteomyelitis, pneumonia, and bacteremia (30). Lastly, Acinetobacter emerged as an important pathogen in survivors of the Asian tsunami in 2004 (15, 24). In summary, the changing epidemiology and incidence of infections due to Acinetobacter establish it as a pathogen of increasing medical importance.
In many centers the incidence of infections due to highly antibiotic-resistant strains is making treatment challenging (17, 29, 32, 45, 47, 50). Particularly problematic are panresistant strains. In a 1999 New York City outbreak, 12% of A. baumannii isolates were resistant to all standard antimicrobials (23). These rates are higher outside the United States. Safe reliable agents with predictable activity against A. baumannii are presently nonexistent.
The need for an increased understanding of Acinetobacter pathogenesis, identification of virulence factors, and the identification and testing of vaccine candidates and new antimicrobial targets is more pressing than ever (28, 46). In order to accomplish these goals it is critical to identify/develop relevant animal models of infection. To date, Acinetobacter has been used in a variety of animal models, including murine (21, 22, 25, 26, 31, 36) and guinea pig (4) pneumonia models, a rat thigh infection model (27), and a rabbit endocarditis model (35). However, the clinical relevance and suitability of these models vary depending on the hypothesis being tested. In this report we tested the hypothesis that the rat pneumonia and soft-tissue infection models that our laboratory had previously used for studies of extraintestinal pathogenic Escherichia coli were clinically relevant for studying A. baumannii infection. Next we tested the hypothesis that the soft-tissue infection model could be used to discriminate between the inherent differences in virulence of various A. baumannii clinical isolates. We also hypothesized that complement is an important host factor in protecting against A. baumannii infection in vivo. Lastly we hypothesized that the soft-tissue infection model would serve as an efficient screening mechanism for establishing gene essentiality for drug discovery. The results suggest that these models are well suited and clinically relevant for testing and answering a variety of questions related to infections due to A. baumannii.
MATERIALS AND METHODS
Bacterial strains and media.
A. baumannii strain 307-0294 (blood isolate, sequence type 15 [ST15], clonal group 1 based on the work of Ecker et al. [12]) was isolated from a patient hospitalized at Erie County Medical Center, Buffalo, NY. A. baumannii strains 853 (OIFC031) (blood isolate, ST11, clonal group II), 855 (OIFC075) (axillary isolate, ST14, clonal group III), 900 (OIFC111) (perineal isolate, ST19, clonal group undetermined), and 979 (OIFC327) (environmental isolate, ST24, clonal group undetermined) were isolated from infected military personnel serving in Iraq and Afghanistan (kindly provided by David Craft and Paul Scott of the Walter Reed Army Medical Center). All strains were grown in Luria-Bertani (LB) medium unless stated otherwise and were maintained at −80°C in 50% LB broth and 50% glycerol. Ascites plates consisted of 80% human ascites and 20% water. Two hundred milliliters of water and 15 g of Bacto agar were autoclaved and cooled to 45°C, 800 ml of ascites and kanamycin (40-μg/ml final concentration) was added, and plates were poured. For quantitative growth curves in ascites, 100% human ascites was utilized.
Rat pneumonia model.
The rat pneumonia and soft-tissue infection model animal studies were reviewed and approved by the University at Buffalo and Veterans Administration Institutional Animal Care Committee. An established rat (Long-Evans) model for studying pulmonary damage was used as reported previously (39, 41). In brief, Long-Evans rats (250 to 300 g) were anesthetized with 3.5% halothane in 100% oxygen until unconscious and then maintained at 3.5% halothane. The trachea was exposed surgically, and a 4-in. piece of 1-0 silk was slipped under the trachea to facilitate instillation of the inoculum. The animals were suspended in a supine position on a 60°-incline board. Pulmonary instillation of bacteria prepared in 1× phosphate-buffered saline (PBS) (pH 7.4) was introduced intratracheally (1.2 ml/kg of body weight) via a 1-ml syringe and 26-gauge needle, and the incision was closed with surgical staples. Animals were sacrificed at 3, 6, 24, 48, 72, and 168 h postinoculation for assessments of bacterial growth/clearance, the pulmonary inflammatory response, and lung injury. At harvest, halothane anesthesia was performed, a fraction of inspired oxygen (FiO2) of 98% was administered, and a midline incision was made through the peritoneum and thoracic cavity. An arterial blood gas sample was obtained from the descending aorta in a 1-ml heparinized syringe. The pulmonary vasculature was flushed of residual blood by injecting the right ventricle with 20 ml of 1× Hanks’ balanced salt solution plus 7.5% NaHCO3 (pH 7.2) using a 22-gauge needle. Next, bronchoalveolar lavage (BAL) was performed with 15 ml of normal saline (37°C) that was administered into the lungs by gravity via a tracheal cannula. The recovered BAL fluid (BALF) was kept on ice. A 500-μl aliquot of recovered BALF was removed for subsequent measurement of bacterial CFU. The remaining BALF was centrifuged at 1,500 × g to pellet the cellular fraction. The supernatant was removed and frozen at −80°C for cytokine/chemokine assessments (see subsequent section on pulmonary inflammatory response). The cellular fraction was resuspended in 3 ml of ice-cold 1× PBS, carefully layered over 2 ml of cold-filtered 2% bovine serum albumin in 1× PBS, centrifuged at 150 × g, and resuspended in 4 ml of ice-cold 1× PBS. Lastly, the lungs were removed and kept at 4°C.
(i) Assessment of bacterial growth/clearance.
Intact, excised post-BAL lungs were weighed and suspended in normal saline to a total weight of 10 g (assumed to equate to 10 ml). The addition of saline served as a vehicle for homogenization and to generate a constant volume for each so that titers could be easily calculated. Lungs were then homogenized on ice (three bursts of 3-s duration each) using a Polytron PT-2000 homogenizer (Brinkman Instruments, Westbury, NY). Total Acinetobacter titers were determined by enumerating combined bacteria in BALF plus post-BAL lung tissue as described previously (41).
(ii) Assessment of the pulmonary host response. (a) Measurement of rat TNF-α, IL-1β, and CINC-1 levels in BALF.
Sandwich enzyme-linked immunosorbent assays utilizing commercial antibodies and standards (R&D Systems Inc., Minneapolis, MN) were employed in measuring levels of rat tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and cytokine-induced neutrophil chemoattractant 1 (CINC-1) as described previously (40).
(b) Measurement of BALF cell counts.
A 50-μl aliquot was diluted 1:200 in Isoton II solution (Beckman Coulter), and the leukocyte concentration was determined using a Multisizer 3 Coulter Counter (Beckman Coulter). A cytoslide was prepared by diluting cells to a final concentration of 5 × 104 leukocytes, using a Cytospin 3 cytocentrifuge (Shandon, Pittsburgh, PA), staining with Diff-Quik reagents (Baxter, Miami, FL), and examination by light microscopy (Nikon Microscope ECLIPSE 80i; Nikon Instruments Inc., Melville, NY).
(iii) Assessment of lung injury.
Arterial blood oxygenation was measured (ABL5; Radiometer America, Westlake, OH) and reported as the arterial partial pressure of oxygen divided by the fraction of inspired oxygen (PaO2/FiO2 ratio). Albumin concentrations in cell-free BAL were measured by enzyme-linked immunosorbent assay using a polyclonal rabbit anti-mouse albumin antibody (a gift from Daniel Remick, University of Michigan, Ann Arbor, MI) and horseradish peroxidase-labeled goat anti-rabbit immunoglobulin (Pharmingen, San Diego, CA) (9). Rat albumin (Sigma, St. Louis, MO) was used as a standard.
Rat soft-tissue infection model.
Long-Evans rats (200 to 250 g) were anesthetized as described for the pneumonia model. A subcutaneous space was created on the back of each rat by injecting 50 ml of air subcutaneously and then injecting a mixture of sterile vegetable oil (975 μl) and croton oil (25 μl) in the space created. After 7 days this resulted in an encapsulated “pouch” that was filled with an exudative fluid (pouch fluid), mimicking a subcutaneous abscess. This model has been well characterized (7, 8). It is a dynamic model, as evidenced by the fact that neutrophils will migrate into the pouch in response to appropriate stimuli. Bacteria can be introduced into the pouch, and pouch fluid can be easily removed over time, enabling the study of both microbial and host responses. On day 8, approximately 5 × 106 CFU of the A. baumannii strain being assessed was injected into the pouches of anesthetized animals, resulting in estimated starting pouch concentrations of 1 × 105 to 2 × 105 CFU/ml. Within 1 minute after the bacteria were injected into the pouch, 0.5 ml of pouch fluid was removed to measure the actual starting bacterial titer. Fluid aliquots (0.5 ml) were subsequently obtained from anesthetized animals 3, 6, 24, and 48 h after the initial bacterial challenge, and bacterial titers were determined by enumerating bacterial CFU in pouch fluid by serial 10-fold dilutions in 1× PBS.
Serum bactericidal assay.
Complement-mediated bactericidal assays were performed by measuring the change in bacterial titer over time in the presence of 90% active or inactive (heated at 56°C for 30 min) human serum. An input bacterial titer of approximately 1 × 105 CFU was utilized, and titers were measured at 0, 1, 2, and 3 h as described previously (38, 43).
Transposon mutagenesis and screen for lack of growth on ascites plates.
Electrocompetent cells were generated by growing AB307-0294 in Mueller-Hinton (MH) broth to an A600 of around 0.4. Fifteen milliliters of cells was washed once with 1 ml of ice-cold sterile milli-Q-filtered H2O (Millipore, Billerica, MA), followed by two washes with 10% ice-cold, sterile glycerol. After the last wash, the cells were resuspended in 75 μl and either used immediately or stored at −80°C prior to use. EZ-Tn5<kan-2>Tnp Transposome (60 ng in 3 μl) (Epicentre Biotechnologies; catalog no. TSM99K2; Madison, WI) was electroporated into 75 μl electrocompetent AB307 (Bio-Rad Gene Pulser; 25 mF/2.5 kV/200 Ω) using an 0.2-cm-gap, ice-cold EPchamber (Bio-Rad Laboratories, Hercules, CA). Immediately after electroporation cells were resuspended in S.O.C. medium (Invitrogen, Carlsbad, CA) and grown at 37°C for 1 h. Aliquots were then plated on MH plates supplemented with kanamycin (40 μg/ml), and isolated colonies were purified on the same medium. These 307 mutants (presumably 307-0294::Tn5<kan-2>) were subsequently gridded onto ascites-kanamycin plates. 307-0294 mutants that were confirmed to have minimal or no growth on the ascites-kanamycin plates were numbered consecutively and stored at −80°C.
DNA sequencing and analysis.
The location of the transposon insertion in mutant derivatives of 307-0294 was determined by chromosomal sequencing. Chromosomal DNA was prepared from the 307 mutants of interest by using a Qiagen DNeasy tissue kit (Qiagen, Valencia, CA) following the protocol for gram-negative bacteria. UV spectroscopy was used to determine concentrations. DNA was used immediately or frozen at −20°C. Cycle sequencing was performed off the EZ-Tn5<kan-2> Transposon (Epicentre Biotechnologies) using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), following the protocol for sequencing genomic DNA. The KAN-2 FP-1 forward primer included in the transposon kit was used. Cycle sequencing was performed on 20-μl reaction mixtures with the following protocol: 96°C for 5 min, 96°C for 30 s, 50°C for 10 s, 60°C for 4 min (40 cycles)/4°C, and ramping at 1°C/s. Next DNA was precipitated by adding 30 ml of 80% isopropanol to each reaction mixture. Reaction mixtures were allowed to stand in the dark for 15 min and then centrifuged for 15 min at 2,097 × g at 4°C in a 5415 R refrigerated centrifuge (Eppendorf). Supernatant was removed, and 70% ethanol was added to each reaction mixture. Reaction mixtures were again centrifuged for 15 min at 2,097 × g and 4°C. Supernatants were removed, and reaction mixtures were air dried for 10 min in the dark. Afterwards 20 ml HiDi formamide (Applied Biosystems) was added to each reaction mixture. Each was vortexed for 15 s. Sanger sequencing was performed on a 3130xl Genetic Analyzer DNA sequencer (Applied Biosystems) according to the manufacturer's protocol. Sequence comparisons were performed via BLAST analysis of the nonredundant GenBank sequences.
Statistical analyses.
Data are presented as means ± standard errors of the means. P values of 0.05/n (n = number of comparisons) are considered statistically significant based on the Bonferroni correction for multiple comparisons, and P values of >0.05/n but <0.05 are considered as representing a trend. In vivo and in vitro data were shown to be normally distributed by the Kolmogorov-Smirnov normality test (P > 0.10, alpha = 0.05) (Prism 4 for MacIntosh; GraphPad Software Inc.) and were analyzed using two-tailed unpaired t or log rank tests.
RESULTS
The rat pneumonia model is clinically relevant for assessing Acinetobacter infection.
Acinetobacter is capable of causing both community-acquired and nosocomial pneumonia. Therefore, we tested the hypothesis that the immunocompetent rat pneumonia model that our laboratory had previously used for studies of extraintestinal pathogenic E. coli was clinically relevant for A. baumannii (39-42). The pertinent features of gram-negative bacterial pneumonia were evaluated: namely, bacterial growth (or clearance), the ensuing host inflammatory response, acute lung injury, and death due to respiratory failure following progressive bacterial proliferation.
Bacterial growth/clearance.
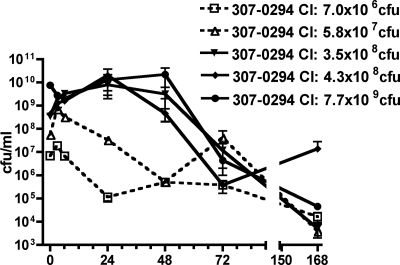
Rats underwent intratracheal challenge with various titers (7.0 × 106 to 7.7 × 109 CFU) of A. baumannii strain 307-0294 (blood isolate, ST15, clonal group 1) to determine whether growth or clearance occurred in this in vivo pneumonia model over 7 days (Fig. 1). Overall, growth/clearance roughly correlated with the magnitude of the challenge inoculum. For the highest challenge titer (7.7 × 109 CFU) net growth occurred over the first 48 h, and for the next two highest challenge titers (3.5 × 108 and 4.3 × 108 CFU) net growth occurred over the first 24 h. However, by 72 h net clearance occurred for all challenge titers. By day 7, although clearance occurred for all challenge titers, it was not complete. Taken together, these data support the contention that A. baumannii is able to survive and grow in the rat pneumonia model and that this is dependent on the challenge inoculum.
FIG. 1.
Growth/clearance of different titers of A. baumannii strain 307-0294 in the rat pneumonia model. Rats were challenged with 7.0 × 106, 5.8 × 107, 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU of 307-0294 (blood isolate, ST15, clonal group 1) by intratracheal instillation, and total lung bacterial titers were determined at 0, 3, 6, 24, 48, 72, and 168 h. Data are means ± standard errors of the means for n = 3 for each challenge titer and time point.
Survival.
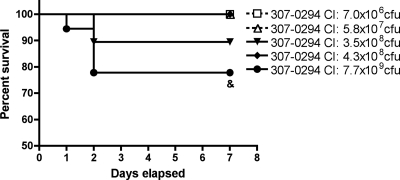
Rats underwent intratracheal challenge with various titers (7.0 × 106 to 7.7 × 109 CFU) of 307-0294 to determine if mortality occurred in this in vivo pneumonia model over 7 days (Fig. 2). No deaths occurred after challenge with 7.0 × 106 (n = 18), 5.8 × 107 (n = 19), and 4.3 × 108 (n = 18) CFU. Two and four deaths occurred 24 to 48 h after challenge with 3.5 × 108 (n = 19) and 7.7 × 109 (n = 18) CFU, respectively. There was a trend for increased survival in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 7.7 × 109 CFU (P = 0.03). These data support the contention that A. baumannii is able to cause lethal pulmonary infection in the rat pneumonia model and that this is dependent on the challenge inoculum. Further, growth/clearance (see “Bacterial growth/clearance” above), oxygenation, and BALF albumin data (see “Acute lung injury” below) suggest that death, at least in part, is due to progressive bacterial proliferation and associated respiratory failure.
FIG. 2.
Survival of rats in the pneumonia model post-challenge with different titers of A. baumannii strain 307-0294. Rats were challenged with 7.0 × 106 (n = 18), 5.8 × 107 (n = 19), 3.5 × 108 (n = 19), 4.3 × 108 (n = 18), and 7.7 × 109 (n = 18) CFU of 307-0294 by intratracheal instillation, and survival was recorded. There was a trend for increased survival in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 7.7 × 109 CFU (&, P = 0.03).
Acute lung injury.
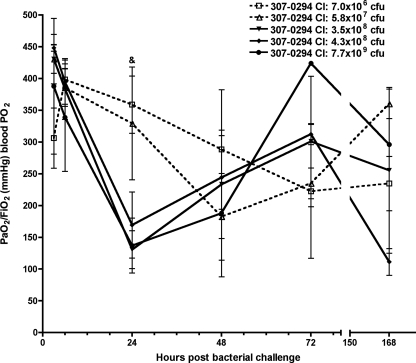
To determine the effect of A. baumannii on acute lung injury, arterial oxygenation (PaO2/FiO2 ratio) and BALF albumin were measured at 3, 6, 24, 48, 72, and 168 h following challenge of rats with various titers of 307-0294. After challenge with 307-0294, the decreases in oxygenation levels, which reflect acute lung injury, were similar and most pronounced with the three highest challenge inocula (3.5 × 108 to 7.7 × 109 CFU). A lesser effect on oxygenation was observed with the two lowest challenge inocula (7.0 × 106 and 5.8 × 107 CFU) (Fig. 3). The greatest decrease in oxygenation occurred at 24 h for the three highest 307-0294 challenge inocula (3.5 × 108 to 7.7 × 109 CFU), at 48 h for the next highest challenge inoculum (5.8 × 107 CFU), and at 72 h for the lowest challenge inoculum (7.0 × 106 CFU). Levels recovered thereafter but not back to baseline. There was a trend for increased oxygenation at 24 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 and 7.7 × 109 CFU (P = 0.02 and 0.04, respectively). These data demonstrated that when A. baumannii was able to grow (e.g., 307-0294 challenge inocula of 3.5 × 108 to 7.7 × 109 CFU), the decrease in oxygenation was greatest and occurred more quickly than when A. baumannii was cleared (e.g., 307-0294 challenge inocula of 7.0 × 106 and 5.8 × 107 CFU).
FIG. 3.
Oxygenation concentration from rats at 3, 6, 24, 48, 72, and 168 h post-challenge with different titers of A. baumannii strain 307-0294. Rats were given 7.0 × 106, 5.8 × 107, 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU of 307-0294 by intratracheal instillation, and aortic blood was obtained at 3, 6, 24, 48, 72, and 168 h for determination of PaO2/FiO2 (mm Hg). There was a trend for increased oxygenation at 24 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 and 7.7 × 109 CFU (&, P = 0.02 and 0.04, respectively). Data are means ± standard errors of the means for n = 3 for each challenge titer and time point.
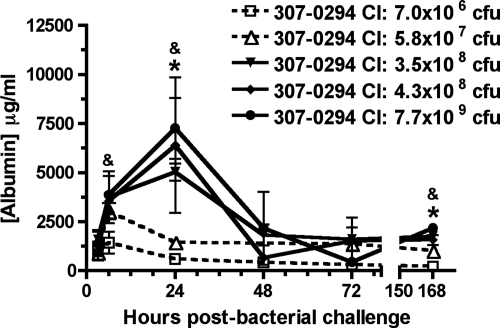
Leakage of albumin from the vasculature into the alveolar spaces is another measure of acute lung injury. After challenge with 307-0294, the increases in BALF albumin levels were similar and most pronounced with the three highest challenge inocula (3.5 × 108 to 7.7 × 109 CFU) and a lesser effect on BALF albumin was observed with the two lowest challenge inocula (7.0 × 106 and 5.8 × 107 CFU) (Fig. 4). The greatest increase in BALF albumin occurred at 24 h for the three highest 307-0294 challenge inocula (3.5 × 108 to 7.7 × 109 CFU) and at 6 h for the two lowest challenge inocula (5.8 × 107 and 7.0 × 106 CFU); levels decreased thereafter. There was a significant decrease or trend toward a significant decrease in the concentration of BALF albumin at various times in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU (Fig. 4). These data demonstrated that the increase in BALF albumin was greatest when A. baumannii was able to grow (e.g., 307-0294 challenge inocula of 3.5 × 108 to 7.7 × 109 CFU) compared to when A. baumannii was cleared (e.g., 307-0294 challenge inocula of 7.0 × 106 and 5.8 × 107 CFU).
FIG. 4.
BALF albumin concentration from rats at 3, 6, 24, 48, 72, and 168 h post-challenge with different titers of A. baumannii strain 307-0294. Rats were given 7.0 × 106, 5.8 × 107, 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU of 307-0294 by intratracheal instillation, and cell-free BALF was obtained at 3, 6, 24, 48, 72, and 168 h for determination of albumin. There was a significant decrease in the concentration of BALF albumin at 24 h in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 3.5 × 108 CFU (*, P = 0.0008 and 0.002, respectively) and at 168 h in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 3.5 × 108 CFU (*, P = 0.006 and 0.008, respectively). There was a trend for a decrease in the concentration of BALF albumin at 6 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 4.3 × 108 CFU (&, P = 0.03), at 24 h in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 7.7 × 109 CFU (&, P = 0.01 and 0.02, respectively), and at 168 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 4.3 × 108 CFU (&, P = 0.01). Data are means ± standard errors of the means for n = 3 for each challenge titer and time point.
Host inflammatory response.
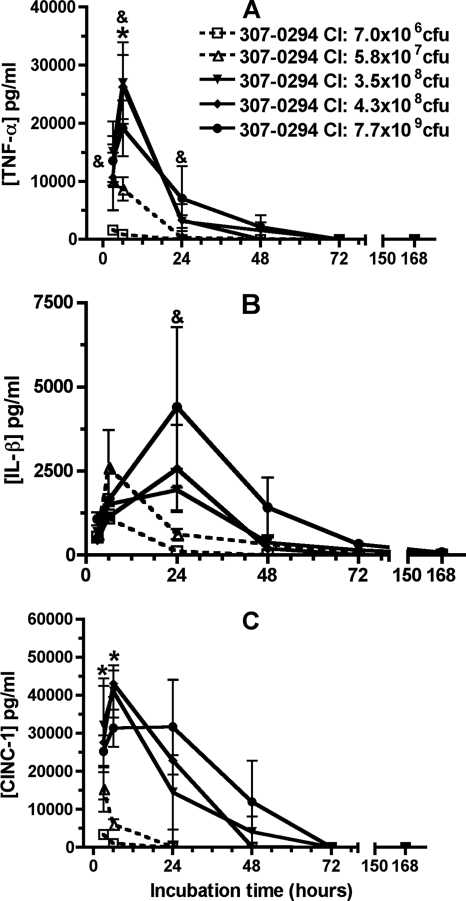
To determine the effect of A. baumannii on pulmonary levels of TNF-α, IL-1β, and CINC-1 in vivo, these mediators were assessed in cell-free BALF supernatants at 3, 6, 24, 48, 72, and 168 h following challenge of rats with various titers of 307-0294. After challenge with 307-0294, levels of TNF-α, IL-1β, and CINC-1 were barely measurable after a challenge inoculum of 7.0 × 106 CFU, increased after a challenge inoculum of 5.7 × 107 CFU, and were of similar orders of magnitude after challenge inocula of 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU (Fig. 5A to C). After challenge with 307-0294, TNF-α and CINC-1 levels peaked at 6 h and essentially were at the limit of detection by 72 h (Fig. 5A and C), whereas IL-1β levels peaked at 24 h and were at the limit of detection by 72 h (Fig. 5B). There was a significant decrease or trend toward a significant decrease in TNF-α, IL-1-β, and CINC-1 levels at various times in rats challenged with 7.0 × 106 and 5.8 × 107 CFU compared to rats challenged with 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU (Fig. 5). These data demonstrated that after challenge with 307-0294 levels of TNF-α, IL-1β, and CINC-1 increased with increasing challenge inocula from 7.0 × 106 to 3.5 × 108 CFU and reached plateau levels thereafter. Further, levels of these early proinflammatory cytokines decreased after 6 h for TNF-α and CINC-1 and after 24 h for IL-1β. These data support the contention that the magnitude of the challenge inoculum is the dominant factor affecting levels of TNF-α, IL-1β, and CINC-1.
FIG. 5.
TNF-α, IL-1β, and CINC-1 cell-free BALF concentrations from rats at 3, 6, 24, 48, 72, and 168 h post-challenge with different titers of A. baumannii strain 307-0294. (A) TNF-α. (B) IL-1β. (C) CINC-1. Rats were given 7.0 × 106, 5.8 × 107, 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU of 307-0294 (blood isolate, ST15, clonal group 1) by intratracheal instillation, and BALF was obtained for determination of cytokine/chemokine levels at 3, 6, 24, 48, 72, and 168 h. (A) There was a significant decrease in TNF-α levels at 6 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 4.3 × 108 CFU (*, P = 0.01) and a trend for decreased TNF-α levels at 3 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 7.7 × 109 CFU (&, P = 0.049), at 6 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 and 7.7 × 109 CFU (&, P = 0.02), at 6 h in rats challenged with 5.8 × 107 CFU compared to rats challenged with 4.3 × 108 CFU (&, P = 0.04), and at 24 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 CFU (&, P = 0.04). (B) There was a trend for decreased IL-1β levels at 24 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 CFU (&, P = 0.04). (C) There was a significant decrease in CINC-1 levels at 3 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 7.7 × 109 CFU (*, P = 0.007) and at 6 h in rats challenged with 7.0 × 106 compared to rats challenged with 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU (*, P = 0.004, 0.0003, and 0.004, respectively) and in rats challenged with 5.8 × 107 CFU compared to rats challenged with 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU (*, P = 0.007, 0.0006, and 0.008, respectively). Data are means ± standard errors of the means for n = 3 for each challenge titer and time point.
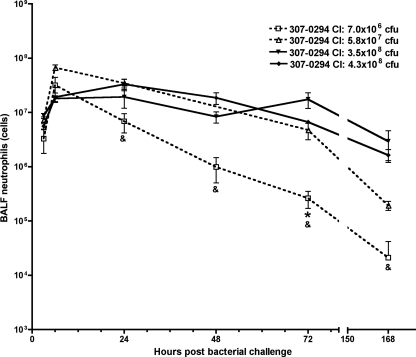
Pulmonary neutrophils present in BALF were also assessed after challenge with various titers of 307-0294 (except for the 7.7 × 109 CFU challenge inoculum). Neutrophil counts peaked at 6 h and decreased thereafter (Fig. 6). The total numbers of neutrophils were similar after challenge with all titers of 307-0294, except after challenge with the lowest titer (7.0 × 106 CFU) at 24, 48, 72, and 168 h, where there was a significant decrease or a trend toward a significant decrease in the number of neutrophils compared to that for rats challenged with 5.7 × 107, 3.5 × 108, and 4.3 × 108 CFU (Fig. 6). The timing of maximal neutrophil counts corresponded to the timing of maximal TNF-α and CINC-1 levels, but unlike cytokines, neutrophil counts decreased more slowly and persisted for 168 h. Further, the maximal neutrophil response was seen even with the lowest 307-0294 challenge titers.
FIG. 6.
Neutrophil numbers in BALF from rats at 3, 6, 24, 48, 72, and 168 h post-challenge with different titers of A. baumannii strain 307-0294. Rats were given 7.0 × 106, 5.8 × 107, 3.5 × 108, 4.3 × 108, and 7.7 × 109 CFU of 307-0294 by intratracheal instillation; BALF was collected at 3, 6, 24, 48, 72, and 168 h; and cells were harvested from BALF and then enumerated by Coulter counting. Neutrophil numbers are based on leukocyte differentials obtained from stained cytoslides (Materials and Methods). Cell counts were not done from animals challenged with 7.7 × 109 CFU. There was a significant decrease in the number of neutrophils at 72 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 4.3 × 108 CFU (*, P = 0.0003) and a trend for a decrease in the number of neutrophils at 24 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 5.7 × 107 and 4.3 × 108 CFU (&, P = 0.019 and 0.041, respectively), at 48 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 and 4.3 × 108 CFU (&, P = 0.025 and 0.019, respectively), at 72 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 3.5 × 108 CFU (&, P = 0.037), and at 168 h in rats challenged with 7.0 × 106 CFU compared to rats challenged with 5.7 × 107 and 4.3 × 108 CFU (&, P = 0.022 and 0.022, respectively). Data are means ± standard errors of the means for n = 3 for each challenge titer and time point.
Taken together, these data demonstrate that bacterial growth, acute lung injury, and an appropriate host inflammatory response occur in the rat pneumonia model after challenge with Acinetobacter. This supports our hypothesis that the immunocompetent rat pneumonia model is clinically relevant for assessing A. baumannii infection.
The rat soft-tissue infection model is clinically relevant for assessing Acinetobacter infection.
Surgical sites, ulcers, and patients who have sustained traumatic (e.g., battlefield) injuries have been at risk for soft-tissue infection due to Acinetobacter (30, 48). Therefore, we tested our hypothesis that the immunocompetent rat soft-tissue infection model is clinically relevant for assessing A. baumannii infection. In brief, a subcutaneous fluid-filled space is created that can be inoculated with the bacterial strain being assessed, samples can be withdrawn, and bacterial titers can be measured over time (for at least 7 days). The growth of 307-0294 was assessed over 48 h (Fig. 7). Clearance was observed over the first 6 h, followed by growth and achievement of plateau density by 24 h. The ability of 307-0294 to survive the host's defenses and proliferate within this soft-tissue environment is consistent with its ability to cause soft-tissue infection in humans. These data support our hypothesis that this model is clinically relevant and can be used to study Acinetobacter in this setting.
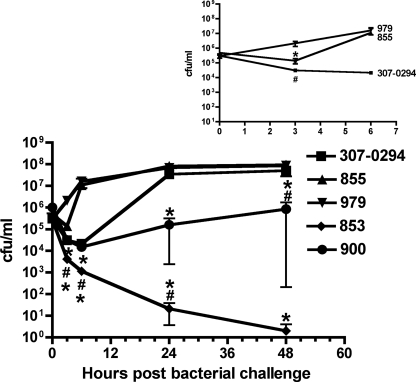
FIG. 7.
Growth/clearance of A. baumannii strains 307-0294, 853, 855, 900, and 979 in the rat soft-tissue infection model. Rats were prepared and challenged with the bacterial strains being assessed as described in Materials and Methods. Bacterial titers were determined at 0, 3, 6, 24, and 48 h. There was a significant decrease or trend toward a significant decrease in survival of 853 and 900 at various times compared to 307-0294, 855, and 979. Data are means ± standard errors of the means for n = 4 to 9. *, 853 compared to 307-0294, P < 0.001 and 0.0005 at 3 and 6 h, respectively; *, 853 compared to 855, P = 0.0004 at 48 h; *, 853 compared to 979, P = 0.0005 and 0.003 at 24 and 48 h, respectively. #, 853 compared to 307-0294, P = 0.045 at 24 h; #, 853 compared to 855, P = 0.03 at 24 h; #, 853 compared to 979, P = 0.02 and 0.03 at 3 and 6 h, respectively; *, 900 compared to 307-0294, P = 0.007 at 24 h; *, 900 compared to 855, P = 0.004 and <0.0001 at 24 and 48 h, respectively; *, 900 compared to 979, P = 0.002, 0.005, <0.0001, and <0.0001 at 3, 6, 24, and 48 h, respectively; #, 900 compared to 307-0294, P = 0.019 at 48 h. (Inset) Enlargement of data from 307-0294, 855, and 979 from 0 to 6 h. There was both a trend and a significant difference in growth of 307-0294 and 855 compared to 979. *, 855 compared to 979, P = 0.005; #, 307-0294 compared to 979, P = 0.02.
The rat soft-tissue infection model can be used to assess the relative virulence of various A. baumannii clinical isolates.
Next we tested the hypothesis that the soft-tissue infection model could be used to discriminate between the inherent differences in virulence of various A. baumannii clinical isolates. The growth of 853 (OIFC031) (blood isolate, ST11, clonal group II), 855 (axillary isolate, ST14, clonal group III), 900 (perineal isolate, ST19, clonal group undetermined), and 979 (OIFC327) (environmental isolate, ST24, clonal group undetermined) was compared to that of 307-0294 in this model. Three patterns were observed: growth (979), clearance (853), or a variable degree of clearance over 3 to 6 h followed by different rates of growth (855 > 307-0294 > 900) (Fig. 7). There was a significant decrease or trend toward a significant decrease in survival of 853 and 900 at various times compared to 307-0294, 855, and 979. This model was quite sensitive in differentiating the abilities of different A. baumannii strains to survive in vivo. Interestingly, although the number of strains assessed was small, one might predict that survival of the blood isolates 307-0294 and 853 would be the greatest, but this was not the case. These data support our hypothesis that this model can be used to discriminate between the inherent differences in virulence of various A. baumannii clinical isolates.
Serum sensitivity of Acinetobacter isolates in vitro correlates with their growth in the soft-tissue infection model.
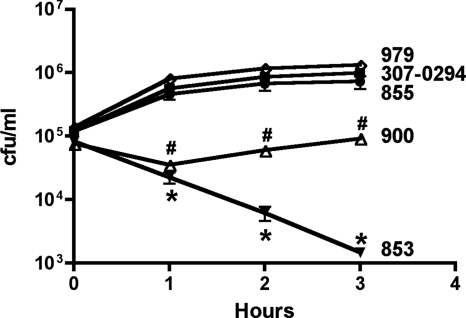
Complement and neutrophils are innate host defense factors known to be active in the soft-tissue infection model (7, 8). Since various strains of Acinetobacter were variably cleared in this model, we hypothesized that complement is an important host factor in protecting against A. baumannii infection in vivo. Therefore, the serum sensitivity of A. baumannii strains 307-0294, 853, 855, 900, and 979 was assessed over 3 h in vitro (Fig. 8). All strains grew similarly in 90% serum that was heated at 56°C for 30 min to inactivate complement-mediated killing and LB medium (data not shown). When these strains were exposed to 90% active serum, the growth of 307-0294, 855, and 900 was similar. In contrast, 853 underwent an approximate 2-log killing and 900 underwent an approximate 0.5-log killing followed by recovery to the starting titer at 3 h; these differences were significant compared to 307-0294, 855, and 979. These data roughly correlate with growth/clearance in the soft-tissue infection model (Fig. 7). However, the soft-tissue infection model was more discriminatory, with 307-0294, 855, and 979 demonstrating differing growth/clearance capabilities, whereas in the serum sensitivity assay, the survival capabilities of 307-0294, 855, and 979 were similar. This is not surprising since multiple host defense factors (e.g., professional phagocytes and antimicrobial peptides) in addition to complement are present in the soft-tissue infection model. Nonetheless, these data support our hypothesis that complement is one host defense factor that contributes to the clearance of A. baumannii in vivo.
FIG. 8.
Effect of 90% normal human serum on the viability of the A. baumannii strains 307-0294, 853, 855, 900, and 979 in vitro. Assays were performed as described in Materials and Methods. All strains were also assessed in the presence of 90% heat-inactivated (56°C for 30 min) normal human serum, and their growth rates were similar; therefore, these data are not shown. The survival of 853 and 900 was significantly decreased compared to that of 307-0294, 855, and 979. Data are means ± standard errors of the means for n = 4 to 6. *, 853 compared to 307-0294, 855, and 979. P is <0.0001 for all comparisons between 853 and 307-0294 and 979. P is <0.0001, 0.0006, and 0.002 for 853 compared to 855 for 1, 2, and 3 h, respectively. #, 900 compared to 307-0294, 855, and 979. P is < 0.0001, 0.0006, and <0.0001 for 900 compared to 307-0294; P = 0.002, 0.008, and 0.009 for 900 compared to 855; P = 0.0004, <0.0001, and < 0.0001 for 900 compared to 979 for 1, 2, and 3 h, respectively.
The rat soft-tissue infection model can be used to efficiently assess gene essentiality in vivo.
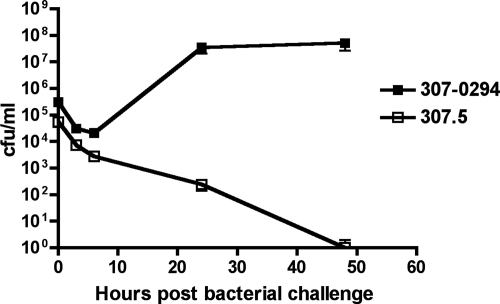
Because safe reliable agents with predictable activity against A. baumannii are presently nonexistent, there is a need to identify new antimicrobial targets in Acinetobacter. Although multiple approaches to accomplish this goal exist, a starting point for one strategy is to identify Acinetobacter genes essential for growth. The rat soft-tissue infection model is an efficient model for assessing in vivo growth/survival because multiple samples can be obtained from a single animal over time. Therefore, we hypothesized that the soft-tissue infection model would serve as an effective screening tool for establishing gene essentiality for drug discovery. We randomly mutagenized 307-0294 using the transposon EZ-Tn5<kan-2>. Mutants isolated on MH plates containing kanamycin were subsequently gridded onto ascites plates supplemented with kanamycin. Chromosomal sequencing (using primers from the ends of EZ-Tn5<kan-2>) was performed on mutants that displayed no or minimal growth on ascites plates, enabling the localization of the transposon insertion. One of these mutants (307.5) contains a transposon insertion in the gene that codes for phosphoribosylaminoimidazole-succinocarboxamide synthase. Quantitative growth curves performed in LB medium demonstrated that there was no difference in growth between 307-0294 and 307.5 (data not shown). To test our hypothesis that the soft-tissue infection model could be used to establish gene essentiality in vivo, we assessed the growth of AB307.5 in this model (Fig. 9). 307.5, a mutant derivative of 307-0294, was cleared, thereby supporting our hypothesis.
FIG. 9.
Growth/clearance of A. baumannii strain 307-0294 and its mutant derivative 307.5 in the rat soft-tissue infection model. Rats were prepared and challenged with the bacterial strains being assessed as described in Materials and Methods. Bacterial titers were determined at 0, 3, 6, 24, and 48 h.
DISCUSSION
Acinetobacter is a pathogen of increasing medical importance (13, 14, 19, 33). Little is known about its mechanisms of pathogenesis, vaccine candidates have not been identified, and an increasing proportion of strains are highly resistant to antimicrobials (29, 32). The availability of clinically relevant animal infection models will facilitate studies on the innate virulence of various Acinetobacter clinical isolates, potential virulence factors, vaccine candidates, and drug targets in vivo and can be used for pharmacokinetic and chemotherapeutic investigations. In this report we tested the hypotheses that the rat pneumonia and soft-tissue infection models that our laboratory had previously used for studies of extraintestinal pathogenic E. coli were clinically relevant for assessing A. baumannii (37, 41, 42). The lung is an important site of Acinetobacter infection in humans, and after challenge with A. baumannii, the pneumonia model demonstrated all of the features of infection that are critical for a clinically relevant model: namely, bacterial growth/clearance, an ensuing host inflammatory response, acute lung injury, and death due to respiratory failure following progressive bacterial proliferation (Fig. 1 to 6). Soft tissue has been increasingly recognized as an important site of Acinetobacter infection in battlefield injuries, surgical sites, and ulcers (5, 11, 18). We were also able to demonstrate growth of 307-0294 in the soft-tissue infection model, as occurs in soft-tissue infection, thereby supporting our hypothesis. We also hypothesized that the soft-tissue infection model could be used to discriminate between the inherent levels of virulence possessed by various Acinetobacter strains. In support of this, various patterns of growth were observed for the Acinetobacter strains tested (Fig. 7). Although it is beyond the scope of this report, these biologic data could be used in conjunction with molecular epidemiologic data as a means of predicting the virulence of Acinetobacter isolates. Given the variable growth of Acinetobacter strains in the soft-tissue infection model and the fact that complement is an active host defense factor in this site, we hypothesized that complement is an important host factor in protecting against A. baumannii infection in vivo. Growth/clearance of the A. baumannii strains in the soft-tissue infection model roughly correlated with growth/clearance in in vitro serum sensitivity studies (Fig. 8), thereby supporting this hypothesis. Lastly, we supported our hypothesis that the soft-tissue infection model could be used to establish the essentiality of Acinetobacter genes in vivo, an important characteristic for potential drug targets (Fig. 9). Although we did not utilize these models to assess vaccine candidates or virulence factors, it is self-evident that they can be used for this purpose as well.
The models described in this report have certain advantages over the previously described Acinetobacter animal infection models (4, 21, 22, 25-27, 31, 36). First, it is critical to be able to have bacterial growth over a significant period of time in a pneumonia model. In our rat model, with the highest challenge inocula we were able to demonstrate growth for 24 to 48 h post-bacterial challenge (Fig. 1). Further, at the highest challenge inocula some rats succumbed to infection (Fig. 2). Given that at these challenge inocula significant acute lung injury also occurred (Fig. 3 to 4), it is reasonable to infer that these animals died from respiratory insufficiency (as opposed to “cytokine storm”). Since we did not perform blood cultures in these animals, we cannot exclude the possibility of concomitant bacteremia, which could also contribute to mortality in this setting. However, even if bacteremia did occur in these animals, bacteremia also occurs in severe pneumonia in humans and as a result does not make this model less relevant. Taken together, these data support this model as being particularly relevant for studying all aspects of Acinetobacter pneumonia: namely, bacterial growth/clearance, acute lung injury, and the host response. In contrast, bacteria are often cleared in murine pneumonia models. Therefore, in the majority of Acinetobacter murine pneumonia models reported on to date, the animals received porcine mucin with the bacterial challenge (4, 25, 26, 34, 36) or were rendered neutropenic (20, 21), permutations that are not present in most patients who develop Acinetobacter pneumonia. In one report that used a murine model, Acinetobacter grew over the first 4 h but underwent significant clearance by 24 h (22). A major advantage of the soft-tissue infection model is that multiple sampling can be performed over time in each animal, making it time and cost efficient for initial assessment of strains in vivo. In contrast, in the pneumonia models and thigh infection model (27) animals need to be euthanized for measurement of bacterial growth/clearance, injury, and the host response. Further, in the thigh infection model animals were rendered neutropenic (27). Although the lungs and soft tissue are clearly different host environments that contain different growth and host defense factors, clinical isolates and mutant derivatives of 307-0294 that were cleared in the soft-tissue infection model were also cleared when assessed in the pneumonia model (data not shown). Although we did not measure the host response in our A. baumannii studies, this can be done in the soft-tissue infection model as previously reported (7, 8). Therefore, the soft-tissue infection model is more cost-effective and high throughput for answering certain biologic questions. It would appear that this model could be used for an initial, efficient means to assess the growth/clearance of Acinetobacter in vivo.
Although it was not the main goal of this report, interesting aspects of A. baumannii biology were illuminated during this study. The soft-tissue infection model was more discriminating for growth differences between strains than for in vitro serum sensitivity testing (Fig. 7 and 8). This is not surprising since in the soft-tissue infection model host factors critical in protecting against extracellular bacterial pathogens like Acinetobacter are present including complement, antimicrobial peptides, and professional phagocytes. Second, A. baumannii strains 307-0294 and 853 were both blood isolates but displayed diametrically opposed growth/clearance patterns in vivo. These data highlight the fact that the innate virulence of clinical isolates may not necessarily be predicted by site of isolation.
In summary, we have supported our hypotheses on the utility of rat pneumonia and soft-tissue infection models for studying Acinetobacter biology. Both of the models described in this report are clinically relevant and possess certain advantages over previously described models. These models can be used to study the innate virulence of various Acinetobacter clinical isolates and to assess potential virulence factors, vaccine candidates, and drug targets in vivo and can be used for pharmacokinetic and chemotherapeutic investigations.
Acknowledgments
We gratefully acknowledge the financial support of a VA Merit Review from the Department of Veterans Affairs (T.A.R.) and a US Army Medical Research Acquisition Activity under contract W81XWH-05-1-0627 (T.A.R. and A.C.).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Anstey, N. M., B. J. Currie, M. Hassell, D. Palmer, B. Dwyer, and H. Seifert. 2002. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J. Clin. Microbiol. 40685-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey, N. M., B. J. Currie, and K. M. Withnall. 1992. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis. 1483-91. [DOI] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernabeu-Wittel, M., C. Pichardo, A. Garcia-Curiel, M. E. Pachon-Ibanez, J. Ibanez-Martinez, M. E. Jimenez-Mejias, and J. Pachon. 2005. Pharmacokinetic/pharmacodynamic assessment of the in-vivo efficacy of imipenem alone or in combination with amikacin for the treatment of experimental multiresistant Acinetobacter baumannii pneumonia. Clin. Microbiol. Infect. 11319-325. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2004. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004. MMWR Morb. Mortal. Wkly. Rep. 531063-1066. [PubMed] [Google Scholar]
- 6.Chen, M. Z., P. R. Hsueh, L. N. Lee, C. J. Yu, P. C. Yang, and K. T. Luh. 2001. Severe community-acquired pneumonia due to Acinetobacter baumannii. Chest 1201072-1077. [DOI] [PubMed] [Google Scholar]
- 7.Dalhoff, A., G. Frank, and G. Luckhaus. 1982. The granuloma pouch: an in vivo model for pharmacokinetic and chemotherapeutic investigations. I. Biochemical and histological characterization. Infection 10354-360. [DOI] [PubMed] [Google Scholar]
- 8.Dalhoff, A., G. Frank, and G. Luckhaus. 1983. The granuloma pouch: an in vivo model for pharmacokinetic and chemotherapeutic investigations. II. Microbiological characterization. Infection 1141-46. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, B., P. Knight, J. Helinski, N. Nader, T. Shanley, and K. Johnson. 1999. The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology 91486-499. [DOI] [PubMed] [Google Scholar]
- 10.Davis, K. A., K. A. Moran, C. K. McAllister, and P. J. Gray. 2005. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 111218-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Veterans Affairs. 2004. Update of the Colleagues’ Letter sent April 23, 2004 concerning Acinetobacter baumannii. Department of Veterans Affairs, Veterans Health Administration, Washington, DC.
- 12.Ecker, J. A., C. Massire, T. A. Hall, R. Ranken, T. T. Pennella, C. A. Ivy, L. B. Blyn, S. A. Hofstadler, T. P. Endy, P. T. Scott, L. Lindler, T. Hamilton, C. Gaddy, K. Snow, M. Pe, J. Fishbain, D. Craft, G. Deye, S. Riddell, E. Milstrey, B. Petruccelli, S. Brisse, V. Harpin, A. Schink, D. J. Ecker, R. Sampath, and M. W. Eshoo. 2006. Identification of Acinetobacter species and genotyping of Acinetobacter baumannii by multilocus PCR and mass spectrometry. J. Clin. Microbiol. 442921-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas, M. E., and E. A. Karveli. 2007. The changing global epidemiology of Acinetobacter baumannii infections: a development with major public health implications. Clin. Microbiol. Infect. 13117-119. [DOI] [PubMed] [Google Scholar]
- 14.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42692-699. [DOI] [PubMed] [Google Scholar]
- 15.Garzoni, C., S. Emonet, L. Legout, R. Benedict, P. Hoffmeyer, L. Bernard, and J. Garbino. 2005. Atypical infections in tsunami survivors. Emerg. Infect. Dis. 111591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41848-854. [DOI] [PubMed] [Google Scholar]
- 17.Jain, R., and L. H. Danziger. 2004. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann. Pharmacother 381449-1459. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, E. N., T. C. Burns, R. A. Hayda, D. R. Hospenthal, and C. K. Murray. 2007. Infectious complications of open type III tibial fractures among combat casualties. Clin. Infect. Dis. 45409-415. [DOI] [PubMed] [Google Scholar]
- 19.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11868-873. [DOI] [PubMed] [Google Scholar]
- 20.Joly-Guillou, M. L., M. Wolff, R. Farinotti, A. Bryskier, and C. Carbon. 2000. In vivo activity of levofloxacin alone or in combination with imipenem or amikacin in a mouse model of Acinetobacter baumannii pneumonia. J. Antimicrob. Chemother. 46827-830. [DOI] [PubMed] [Google Scholar]
- 21.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp, S., C. W. Wieland, S. Florquin, R. Pantophlet, L. Dijkshoorn, N. Tshimbalanga, S. Akira, and T. van der Poll. 2006. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173122-129. [DOI] [PubMed] [Google Scholar]
- 23.Landman, D., J. M. Quale, D. Mayorga, A. Adedeji, K. Vangala, J. Ravishankar, C. Flores, and S. Brooks. 2002. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, N.Y.: the preantibiotic era has returned. Arch. Intern. Med. 1621515-1520. [DOI] [PubMed] [Google Scholar]
- 24.Maegele, M., S. Gregor, E. Steinhausen, B. Bouillon, M. M. Heiss, W. Perbix, F. Wappler, D. Rixen, J. Geisen, B. Berger-Schreck, and R. Schwarz. 2005. The long-distance tertiary air transfer and care of tsunami victims: injury pattern and microbiological and psychological aspects. Crit. Care Med. 331136-1140. [DOI] [PubMed] [Google Scholar]
- 25.Montero, A., J. Ariza, X. Corbella, A. Domenech, C. Cabellos, J. Ayats, F. Tubau, C. Borraz, and F. Gudiol. 2004. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J. Antimicrob. Chemother. 541085-1091. [DOI] [PubMed] [Google Scholar]
- 26.Pachon-Ibanez, M. E., F. Fernandez-Cuenca, F. Docobo-Perez, J. Pachon, and A. Pascual. 2006. Prevention of rifampicin resistance in Acinetobacter baumannii in an experimental pneumonia murine model, using rifampicin associated with imipenem or sulbactam. J. Antimicrob. Chemother. 58689-692. [DOI] [PubMed] [Google Scholar]
- 27.Pantopoulou, A., E. J. Giamarellos-Bourboulis, M. Raftogannis, T. Tsaganos, I. Dontas, P. Koutoukas, F. Baziaka, H. Giamarellou, and D. Perrea. 2007. Colistin offers prolonged survival in experimental infection by multidrug-resistant Acinetobacter baumannii: the significance of co-administration of rifampicin. Int. J. Antimicrob. Agents 2951-55. [DOI] [PubMed] [Google Scholar]
- 28.Paterson, D. L., and Y. Doi. 2007. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin. Infect. Dis. 451179-1181. [DOI] [PubMed] [Google Scholar]
- 29.Perez, F., A. M. Hujer, K. M. Hujer, B. K. Decker, P. N. Rather, and R. A. Bonomo. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 513471-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen, K., M. S. Riddle, J. R. Danko, D. L. Blazes, R. Hayden, S. A. Tasker, and J. R. Dunne. 2007. Trauma-related infections in battlefield casualties from Iraq. Ann. Surg. 245803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renckens, R., J. J. Roelofs, S. Knapp, A. F. de Vos, S. Florquin, and T. van der Poll. 2006. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J. Infect. Dis. 193187-195. [DOI] [PubMed] [Google Scholar]
- 32.Rice, L. B. 2006. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis. 43S100-S105. [DOI] [PubMed] [Google Scholar]
- 33.Richet, H., and P. E. Fournier. 2006. Nosocomial infections caused by Acinetobacter baumannii: a major threat worldwide. Infect. Control Hosp. Epidemiol. 27645-646. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Hernandez, M. J., L. Cuberos, C. Pichardo, F. J. Caballero, I. Moreno, M. E. Jimenez-Mejias, A. Garcia-Curiel, and J. Pachon. 2001. Sulbactam efficacy in experimental models caused by susceptible and intermediate Acinetobacter baumannii strains. J. Antimicrob. Chemother. 47479-482. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Hernandez, M. J., M. E. Jimenez-Mejias, C. Pichardo, L. Cuberos, A. Garcia-Curiel, and J. Pachon. 2004. Colistin efficacy in an experimental model of Acinetobacter baumannii endocarditis. Clin. Microbiol. Infect. 10581-584. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Hernandez, M. J., J. Pachon, C. Pichardo, L. Cuberos, J. Ibanez-Martinez, A. Garcia-Curiel, F. J. Caballero, I. Moreno, and M. E. Jimenez-Mejias. 2000. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J. Antimicrob. Chemother. 45493-501. [DOI] [PubMed] [Google Scholar]
- 37.Russo, T., Y. Liang, and A. Cross. 1994. The presence of K54 capsular polysaccharide increases the pathogenicity of Escherichia coli in vivo. J. Infect. Dis. 169112-118. [DOI] [PubMed] [Google Scholar]
- 38.Russo, T., G. Sharma, C. Brown, and A. Campagnari. 1995. The loss of the O4 antigen moiety from the lipopolysaccharide of an extraintestinal isolate of Escherichia coli has only minor effects on serum sensitivity and virulence in vivo. Infect. Immun. 631263-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo, T. A., L. A. Bartholomew, B. A. Davidson, J. D. Helinski, U. B. Carlino, P. R. Knight III, M. F. Beers, E. N. Atochina, R. H. Notter, and B. A. Holm. 2002. Total extracellular surfactant is increased but abnormal in a rat model of gram-negative bacterial pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 283L655-L663. [DOI] [PubMed] [Google Scholar]
- 40.Russo, T. A., B. A. Davidson, J. M. Beanan, R. Olson, B. A. Holm, R. H. Notter, and P. R. Knight III. 2007. Capsule and O-antigen from an extraintestinal isolate of Escherichia coli modulate cytokine levels in rat macrophages in vitro and in a rat model of pneumonia. Exp. Lung Res. 33337-356. [DOI] [PubMed] [Google Scholar]
- 41.Russo, T. A., B. A. Davidson, U. B. Carlino-MacDonald, J. D. Helinski, R. L. Priore, and P. R. Knight III. 2003. The effects of Escherichia coli capsule, O-antigen, host neutrophils, and complement in a rat model of Gram-negative pneumonia. FEMS Microbiol. Lett. 226355-361. [DOI] [PubMed] [Google Scholar]
- 42.Russo, T. A., B. A. Davidson, S. A. Genagon, N. M. Warholic, U. Macdonald, P. D. Pawlicki, J. M. Beanan, R. Olson, B. A. Holm, and P. R. Knight III. 2005. E. coli virulence factor hemolysin induces neutrophil apoptosis and necrosis/lysis in vitro and necrosis/lysis and lung injury in a rat pneumonia model. Am. J. Physiol. Lung Cell. Mol. Physiol. 289L207-L216. [DOI] [PubMed] [Google Scholar]
- 43.Russo, T. A., M. C. Moffitt, C. H. Hammer, and M. M. Frank. 1993. TnphoA-mediated disruption of K54 capsular polysaccharide genes in Escherichia coli confers serum sensitivity. Infect. Immun. 613578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott, P., G. Deye, A. Srinivasan, C. Murray, K. Moran, E. Hulten, J. Fishbain, D. Craft, S. Riddell, L. Lindler, J. Mancuso, E. Milstrey, C. T. Bautista, J. Patel, A. Ewell, T. Hamilton, C. Gaddy, M. Tenney, G. Christopher, K. Petersen, T. Endy, and B. Petruccelli. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin. Infect. Dis. 441577-1584. [DOI] [PubMed] [Google Scholar]
- 45.Sunenshine, R. H., M. O. Wright, L. L. Maragakis, A. D. Harris, X. Song, J. Hebden, S. E. Cosgrove, A. Anderson, J. Carnell, D. B. Jernigan, D. G. Kleinbaum, T. M. Perl, H. C. Standiford, and A. Srinivasan. 2007. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 1397-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talbot, G. H., J. Bradley, J. E. Edwards, Jr., D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42657-668. [DOI] [PubMed] [Google Scholar]
- 47.Tognim, M. C., S. S. Andrade, S. Silbert, A. C. Gales, R. N. Jones, and H. S. Sader. 2004. Resistance trends of Acinetobacter spp. in Latin America and characterization of international dissemination of multi-drug resistant strains: five-year report of the SENTRY Antimicrobial Surveillance Program. Int. J. Infect. Dis. 8284-291. [DOI] [PubMed] [Google Scholar]
- 48.Tong, M. J. 1972. Septic complications of war wounds. JAMA 2191044-1047. [PubMed] [Google Scholar]
- 49.van Dessel, H., T. E. Kamp-Hopmans, A. C. Fluit, S. Brisse, A. M. de Smet, L. Dijkshoorn, A. Troelstra, J. Verhoef, and E. M. Mascini. 2002. Outbreak of a susceptible strain of Acinetobacter species 13 (sensu Tjernberg and Ursing) in an adult neurosurgical intensive care unit. J. Hosp. Infect. 5189-95. [DOI] [PubMed] [Google Scholar]
- 50.Van Looveren, M., and H. Goossens. 2004. Antimicrobial resistance of Acinetobacter spp. in Europe. Clin. Microbiol. Infect. 10684-704. [DOI] [PubMed] [Google Scholar]
- 51.Villegas, M. V., and A. I. Hartstein. 2003. Acinetobacter outbreaks, 1977-2000. Infect. Control Hosp. Epidemiol. 24284-295. [DOI] [PubMed] [Google Scholar]