Abstract
Highly purified protein antigens are usually poor immunogens; in practice, adjuvants are needed to obtain satisfactory immune responses. Plasmodium yoelii 19-kDa merozoite surface protein 1 (MSP119) is a weak antigen, but mice vaccinated with this antigen in strong adjuvants can survive an otherwise lethal parasite challenge. Fusion proteins comprising this antigen fused to the oligomerization domain of the murine complement inhibitor C4-binding protein (C4bp) and a series of homologues have been produced. These C4bp domains acted as adjuvants for the fused antigen; the MSP119-murine C4bp fusion protein induced protective immunity in BALB/c mice. Because this fusion protein also induced antibodies against circulating murine C4bp, distantly related C4bp oligomerization domains fused to the same antigen were tested. These homologous domains did not induce antibodies against murine C4bp and, surprisingly, induced higher antibody titers against the antigen than the murine C4bp domain induced. These results demonstrate a new adjuvantlike effect of C4bp oligomerization domains.
Adjuvants have been called immunologists' “dirty little secret” (11) and are used because most purified antigens are weakly immunogenic. Classical antigens, such as the tetanus and diphtheria toxins, can generate protective immune responses in humans when they are formulated in the weak adjuvant alum, but there are several antigens which need much stronger adjuvants to protect experimental animals and these adjuvants have not been approved for use in humans. For example, the C-terminal region of merozoite surface protein 1 (referred to as 19-kDa merozoite surface protein 1 [MSP119]), a conserved antigen found on the surface of Plasmodium merozoites, can elicit protection against parasite challenges in both mice and monkeys (6, 10, 16, 17), but such protection requires formulations unacceptable for human use. In one challenge study with Aotus monkeys, the protective formulation had to include Freund's adjuvant; with six other adjuvants suitable for human use no protection was obtained (13).
Using proteins as adjuvants might overcome the problem posed by the limited number of adjuvants available for vaccinating humans, and such methods have been known for some time. In one approach, it has been shown that antigens coupled to monoclonal antibodies can induce high antibody titers (1, 4). Alternatively, fusing the model antigen hen egg white lysozyme to three tandem copies of the complement protein C3d rendered it 10,000-fold more immunogenic than hen egg white lysozyme alone (7). However, neither approach seems to be promising for producing vaccines. The first approach is limited by the significant costs of first producing the monoclonal antibody and then coupling it to the antigen, while the second approach is handicapped by the genetic instability of tandem C3d genes (2).
In this paper, we describe a novel and more practical protein adjuvant, a small domain derived from the complement inhibitor C4-binding protein (C4bp). C4bp is an abundant plasma protein first discovered in mice (9). Its natural function is to inhibit the classical and lectin pathways of complement activation (for a review, see reference 3). Three isoforms of C4bp, composed of different numbers of alpha and beta chains, are found in human plasma, but there is only one isoform of murine C4bp, a homo-heptamer of alpha chains, because the beta chain gene is a pseudogene (20). The last exon of C4bp alpha chain genes encodes the only domain in the protein which does not belong to the complement control protein family. This non-complement control protein domain contains 57 amino acid residues in humans and 54 amino acid residues in mice and is both necessary (12) and sufficient (5, 15, 19, 21) for the oligomerization of C4bp or other polypeptides fused to it. Related oligomerization sequences have also been found in other proteins (18). It was speculated many years ago that fusing proteins to C4bp domains would confer novel biological properties, including prolonged plasma half-lives and increased immunogenicity (M. P. Pasek, G. Winkler, and T. R. Liu, 8 August 1991, Patent Cooperation Treaty application WO91/11461). It has since been demonstrated that some peptides and proteins fused to the human oligomerization domain do have increased half-lives in mice (5, 8, 21), but increased immunogenicity of a protein fused to C4bp domains has not been reported. Nor, apparently, has such a protein been tested.
Recombinant fusion proteins consisting of the MSP119 protein from the rodent malaria parasite Plasmodium yoelii fused to a series of C4bp oligomerization domains from different species were purified and used to immunize mice. Immunization with the MSP119-murine C4bp fusion protein (referred to below as IMX108) could protect mice against an otherwise lethal parasite challenge, but it also induced antibodies which recognized the host C4bp (9). To eliminate the potential risks associated with such autoimmune antibodies, distantly related homologues of the C4bp domain were tested, and some of these domains proved to be superior in mice to the murine C4bp domain. Such domains may prove to be useful as adjuvants for vaccinating a wide range of species, including humans.
MATERIALS AND METHODS
Protein expression and purification. (i) IMX108, IMX180, IMX262, IMX313, IMX 314, and IMX317.
The IMX108, IMX180, IMX262, IMX313, IMX314, and IMX317 proteins differ only in C4bp oligomerization domain; each coding sequence starts with DNA encoding amino acids 1619 to 1753 of P. yoelii MSP119 (14) fused to an oligomerization domain (Tables 1 and 2) through a BamHI site (encoding glycine and serine residues) in a T7 promoter vector. IMX108 contains the murine C4bp domain, while IMX180 contains the human domain. The remaining domains were derived directly or indirectly from the chicken genome: IMX314 contains the oligomerization domain from the complement regulatory secretory protein (CRES) described by Oshiumi et al. (18); IMX262 contains a previously undescribed C4bp domain designated chicken2 here; IMX317 is identical to IMX262 except that the last 7 amino acids have been removed from the C terminus; and IMX313 differs from IMX317 in that four consecutive amino acids from IMX314 replace the equivalent amino acids from IMX262 in order to reduce the similarity to human (and other mammalian) C4bp domains. Fusion proteins were expressed in Escherichia coli strain C41(DE3) after induction by isopropyl-β-d-thiogalactopyranoside (IPTG) and purified. Cells were harvested and lysed, and proteins were purified by a three-step procedure. First, the fusion proteins were bound to a HiTrap SP FastFlow column (GE Healthcare) and eluted in a 0 to 1 M NaCl gradient. Then the proteins were denatured at 4°C with 8 M (final concentration) urea and applied to a Superdex S200 column equilibrated with 8 M urea. Dialysis against 20 mM Tris-HCl (pH 7.5)-150 mM NaCl overnight at 4°C was followed by chromatography on a Superdex S200 column in phosphate-buffered saline (PBS).
TABLE 1.
Recombinant proteins used in this studya
| Protein | Antigen | Oligomerization domain | Elution vol (ml) |
|---|---|---|---|
| IMX183 | MSP119 | None | |
| IMX269 | MSP119 | None | |
| IMX108 | MSP119 | Murine C4bp | 163 |
| IMX180 | MSP119 | Human C4bp | 164 |
| IMX272 | MSP119 | Archaeal Sm | 152 |
| IMX262 | MSP119 | Chicken2 C4bp | 165 |
| IMX314 | MSP119 | Chicken1 C4bp | |
| IMX317 | MSP119 | Chicken2 C4bpΔ7 | 161 |
| IMX313 | MSP119 | Hybrid C4bpΔ7 | 162 |
| IMX63 | DsbA | None | |
| IMX253 | DsbA | Murine C4bp | |
| IMX259 | IF tag | Chicken2 C4bp | |
| IMX117 | IF tag | Human C4bp | |
| IMX273 | IF tag | Archaeal Sm | |
| IMX315 | None | Chicken2 C4bp |
Recombinant proteins used in this study were all prepared from E. coli. The IF tag functions like a histidine tag and facilitates purification; however, the murine C4bp domain was not well expressed using this tag, and it was replaced by the DsbA protein.
TABLE 2.
Increasing the dose of IMX108 increases the antibody responsea
| Antigen | Dose (μg) | Dose (nmol) | IFA titer | ELISA titer |
|---|---|---|---|---|
| IMX108 | 5 | 0.24 | 1/10,240 | 1/102,400 |
| 10 | 0.48 | 1/10,240 | 1/102,400 | |
| 20 | 0.95 | 1/10,240 | 1/102,400 | |
| 40 | 1.9 | 1/20,480 | 1/204,800 | |
| 80 | 3.8 | 1/20,480 | 1/204,800 | |
| 140 | 6.7 | 1/40,960 | 1/409,600 | |
| 200 | 9.5 | 1/40,960 | 1/409,600 | |
| IMX183 | 23 | 1.9 | 1/80 | <100 |
Groups of three BALB/c mice were immunized subcutaneously three times at 4-week intervals with different amounts of the IMX108 protein (MSP119-murine C4bp) without additional adjuvant. Control mice received IMX183 (MSP119 alone). Antibody titers were determined by IFA or by ELISA using pooled serum samples collected from each group 3 weeks after the third immunization. The IFA provided a measure of the amount of antibody binding to native MSP1 on the parasite, whereas the ELISA provided a measure of the amount of antibody binding to the recombinant MSP119.
(ii) IMX269.
Fusion protein glutathione S-transferase (GST)-P. yoelii MSP119 was purified by affinity chromatography (GE Healthcare); the GST tag was separated from the antigen by cleavage with thrombin, and the MSP119 (IMX269) was purified using Superdex S75 equilibrated in PBS. This form of MSP119 proved to be much simpler to prepare than IMX183.
(iii) IMX183.
To prepare IMX183, the unfused antigen was expressed and purified like IMX108 with the following differences: strain BL21(DE3) was used instead of C41(DE3), and gel filtration was performed with a Superdex S75 column rather than an S200 column.
(iv) IMX272.
The gene encoding the archaebacterial protein Sm (22) was amplified from Pyrococccus abyssi genomic DNA using primers 5′-GGGGGATCCGCCGAGACACCTCTTGATG and 5′-CCCCTCGAGTCACTCTTCAGTCGGTG. The underlined C residue in the former primer creates an arginine-to-threonine mutation at residue 3 which prevents dimerization of the heptmeric rings (22). The PCR product was digested with BamHI and XhoI and cloned in place of the human C4bp oligomerization domain in plasmid pIMX180. The IMX272 protein was purified using the procedures used for the C4bp oligomerization domain fusion proteins.
(v) Murine C4bp.
Native murine C4bp was purified as described previously (9), with some modifications. Pevikon block electrophoresis was replaced by ion-exchange chromatography with SP-Sepharose. A HiTrap heparin column was added after gel filtration. Contaminants were removed by incubation with Ni-nitrilotriacetic acid resin, to which they bound, and protein L agarose was used to remove immunoglobulin M (IgM).
(vi) IMX63, IMX117, IMX 253, IMX259, IMX273, and IMX315.
The IMX63, IMX117, IMX 253, IMX259, IMX273, and IMX315 proteins were all expressed as described above for the C4bp fusion proteins.
(vii) IMX63 (DsbA).
The supernatant obtained after bacterial lysis at pH 6.5 was loaded on a DEAE F.F. column, to which it bound only weakly, and the protein eluted in an essentially pure form when the column was washed with the loading buffer.
(viii) IMX253 (DsbA-murine C4bp).
After lysis, the supernatant was loaded on a DEAE F.F. column in 20 mM Tris (pH 7.5) and eluted with an NaCl gradient. Pooled fractions containing IMX253 were dialyzed overnight against Tris buffer (pH 7.0) and then loaded on a HiTrap Q F.F. column at pH 7.0. After elution with a salt gradient, pooled positive fractions were purified with a Superdex S200 column.
(ix) IMX273, IMX315, IMX117, and IMX259.
The supernatant obtained after centrifugation of the lysed bacteria was heated at 75°C for 20 min and then recentrifuged. The second supernatant was incubated for 1 h at 4°C with Ni-nitrilotriacetic acid resin, which was then washed first with 50 mM NaPO4-250 mM NaCl (pH 7.5) and then with the same buffer supplemented with 20 mM imidazole. The IMX273 protein was eluted using a buffer containing 200 mM imidazole (pH 8.0) and was then further purified by gel filtration on a Superdex S75 column. IMX315 was purified in exactly the same way. The IMX117 and IMX259 proteins were purified like the IMX273 and IMX315 proteins except that the heating step was omitted.
Endotoxin levels in all proteins used for immunization were measured by the Limulus amoebocyte lysate method (QCL kit; Lonza), and all samples containing >300 endotoxin units/mg were treated with Detoxi-Gel (Pierce) until the levels were below this limit. The endotoxin content of each IMX108 protein batch used for the challenge experiments was less than 10 endotoxin units/mg without treatment with Detoxi-Gel.
All recombinant proteins were examined by electrospray mass spectrometry (Applied Biosystems SCIEX API165; M. Becchi, IBCP, Lyon, France); the molecular masses were within 2 Da of the molecular masses calculated from the primary structure. One protein, IMX180, was characterized by dynamic light scattering with a Malvern Zetasizer Nano. At a concentration of 1 mg/ml in PBS at 20°C, a single protein species with a diameter of 12.19 nm was found.
Immunization protocols.
For all immunizations except those in the dose-response and challenge experiments, groups of three BALB/c mice were immunized subcutaneously twice with a 4-week interval between the immunizations with 2 nmol of the recombinant protein either with or without Freund's adjuvant (complete Freund's adjuvant for the first immunization and incomplete Freund's adjuvant for the immunization). Antibody titers were determined by an enzyme-linked immunosorbent assay (ELISA) using serum samples collected 2 weeks after the second immunization. Three different lots of IMX108 and IMX262 and two different lots of IMX313 were tested. For the mixture of IMX269 and IMX315, 2 nmol of each protein was used.
Dose-response experiment and parasite challenge.
Groups of three BALB/c mice were immunized three times subcutaneously with 4-week intervals between the immunizations with 5, 10, 20, 40, 80, 140, or 200 μg of the IMX108 protein. A control group was immunized with 23 μg (1.9 nmol) of IMX183 (MSP119 alone), which was the molar equivalent of 40 μg of IMX108. Antibody titers were determined by an indirect immunofluorescence assay (IFA) and an ELISA for pooled serum samples collected 21 days after the last injection.
In the challenge study, groups of six BALB/c mice were immunized as described above with either 40 μg of IMX108 or 23 μg of the IMX183 protein without additional adjuvants. Unimmunized mice served as controls. Two weeks after the last injection, samples were taken to determine antibody levels, and then 4 days later each mouse received 5,000 P. yoelii YM-infected red blood cells (RBCs) intravenously. From day 3 to day 21 after the infection, Giemsa-stained blood smears were examined and the parasitemia was determined (17). Mice were killed if the parasitemia exceeded 75%.
Immunochemical methods.
The IFA, the ELISA, and Western blotting were performed essentially as described previously (16, 17). Thin blood smears were fixed with acetone and incubated with test sera serially diluted twofold in PBS. Specific antibodies were detected with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma) and, for isotype determination, with fluorescein isothiocyanate-labeled goat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotechnology). Titers were expressed as the reciprocal of the last positive dilution with clear fluorescence as determined by UV microscopy. For the ELISA, antigens diluted to a concentration of 1 μg ml−1 in 0.1 M sodium carbonate/bicarbonate (pH 9.6) were used to coat the wells of MaxiSorb plates (Nunc-Immulon, Denmark). Twofold serial dilutions of the test sera were added to the wells, and following washing, bound antibodies were detected with donkey anti-mouse IgG (Jackson ImmunoResearch Labs) conjugated to horseradish peroxidase. Absorbance at 490 nm was determined after o-phenylenediamine (Sigma) and H2O2 were added and the reaction was stopped with 1 M sulfuric acid. The antibody titer was defined as the reciprocal dilution at which the absorbance was 3 standard deviations above that of the control. For Western blotting, 50 ng of DsbA, DsbA-murine C4bp, or native murine C4bp was electrophoresed and blotted onto a polyvinylidene difluoride membrane. After blocking with 5% milk, the membrane was incubated with pooled sera from mice immunized with either IMX108 or IMX180; antibody binding was detected using donkey anti-mouse IgG (Jackson ImmunoResearch Labs) conjugated to horseradish peroxidase and the ECL method according to the manufacturer's instructions (GE Healthcare).
RESULTS
C4bp oligomerization domain fusion proteins can be expressed in E. coli in a soluble, heptameric form.
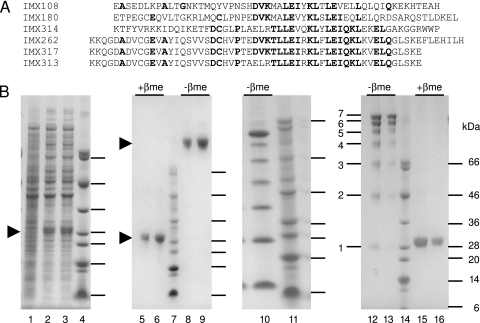
We found that fusion proteins containing murine, human, or chicken C4bp oligomerization domains (Fig. 1A) can be produced in a soluble, oligomeric form in E. coli (Fig. 1B). A series of fusion proteins were prepared, each containing the same antigen, P. yoelii MSP119, fused to a different C4bp oligomerization domain (Table 1). After three chromatography steps, the fusion proteins were at least 95% pure (Fig. 1B). Analysis on polyacrylamide gels in the absence of a reducing agent (Fig. 1B) indicated that the fusion proteins had spontaneously assembled into a heptameric complex of identical subunits. All C4bp oligomerization domain fusions with the MSP119 antigen eluted in almost identical volumes (161 to 165 ml) (Table 1) when size exclusion chromatography was used, which corresponded to a molecular mass of 150 kDa. The IMX180 protein was examined by dynamic light scattering, and the results showed that there was only a single protein species. When mass spectrometry analysis was used, both monomeric and heptameric forms were observed, with molecular masses of approximately 21 and 150 kDa, respectively.
FIG. 1.
C4bp oligomerization domains and expression and purification of fusion proteins. (A) Alignment of C4bp oligomerization domains which were fused to the P. yoelii MSP119 antigen. IMX108 is the murine C4bp domain; IMX180 is the human C4bp domain; IMX314 is from chicken CRES (20); IMX262 is a previously undescribed chicken C4bp homolog; IMX317 is a truncated version of IMX262 with improved solubility; and IMX313 is a hybrid of IMX314 and IMX317 (four residues exchanged). Bold type indicates residues that are present in four or more sequences. (B) Fusion protein expression and purification. Coomassie blue-stained 4 to 12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels contained lysates of E. coli uninduced (lane 1) and induced (lanes 2 and 3) cells expressing IMX180 and purified IMX180 protein electrophoresed under reducing conditions with β-mercaptoethanol (+βme) (lanes 5 and 6) and under nonreducing conditions (−βme) (lanes 8 and 9). Three micrograms of protein was loaded in lanes 5 and 8, and 6 μg of protein was loaded in lanes 6 and 9. Lane 10 contained the IMX259 protein, which is identical to the IMX262 protein except that a polyhistidine tag is present instead of the antigen. This protein was purified as a heptamer and then promptly electrophoresed under nonreducing conditions in order to demonstrate the presence of intermediates which had not yet formed all the disulfide bonds. All intermediates from monomers to heptamers were clearly seen. Lanes 12, 13, 15, and 16 contained purified IMX262 protein in the presence or absence of β-mercaptoethanol. In the absence of β-mercaptoethanol, the incompletely oxidized cysteines produced a ladder of monomeric to heptameric forms (bands labeled 1 to 7 on the left). Lanes 13 and 16 contained 2.5 μg of protein, while lanes 12 and 15 contained 5 μg. Lanes 4, 7, 11 and 14 contained molecular mass markers.
Fusing the MSP119 antigen to C4bp oligomerization domains substantially increases its immunogenicity.
In preliminary experiments, we tested the antibody responses induced by different doses of IMX108 protein in the absence of any adjuvant. Groups of mice were immunized with various amounts of IMX108 (from 5 to 200 μg protein) on three occasions, and the antibody titers in pooled serum samples were determined by an IFA using parasite-infected erythrocytes and by an ELISA using recombinant MSP119 as the antigen (Table 2). The IFA detects antibodies that bind to native MSP1 on the parasite, and the ELISA measures antibodies that bind to recombinant protein, but the two assays cannot be compared directly. The mice immunized using 5-, 10-, or 20-μg doses of IMX108 had IFA titers of 1/10,240, and the mice immunized with 40 or 80 μg had titers of 1/20,480, while the mice which received 140 or 200 μg per injection had titers of 1/40,960. The titers determined by ELISA were 10-fold higher. A mouse immunized with 23 μg of MSP119 alone (IMX183), which contained the same molar amount of antigen as 40 μg of IMX108, had a serum IFA titer of 1/80. The isotypes of the antibodies induced by immunization with 40-μg doses of IMX108 were also determined by IFA. The results were similar to those obtained after immunization of mice with a GST-MSP119 fusion protein in the presence of adjuvant (16); the predominant antibody was IgG1 (titer, 1/10,240) and there was a much smaller amount of IgG2a (titer, 1/160), which was indicative of a predominantly Th2 response.
Animals immunized with IMX108 without any adjuvant other than the C4bp domain are protected against a parasite challenge.
It has been shown repeatedly that a high antibody titer against P. yoelii MSP119 can protect mice against a challenge with parasite-infected RBCs (6, 10, 16, 17). Given the high serum antibody titers obtained by immunization of mice with the IMX108 protein in the absence of any additional adjuvant, it was of interest to determine whether the mice would be protected against a parasite challenge. Two groups of mice were immunized on three occasions with either IMX183 (the P. yoelii MSP119 antigen alone) or IMX108; a third group was not immunized. All three groups were challenged with parasite-infected RBCs, and the parasitemia levels, expressed as the percentage of infected RBCs, were monitored for 21 days (Fig. 2). In the unimmunized mice and the mice that received MSP119 alone, parasites were detected in blood smears 4 days after the challenge, and the percentage of infected RBCs rose rapidly, so that all animals had either died or had to be sacrificed by day 7. In contrast, four of the six mice in the group that received IMX108 had no detectable parasitemia, a fifth mouse had a transient relatively low-grade parasitemia, and only one mouse was unable to control parasite growth and was killed on day 19. The antibody titers of serum samples taken prior to the parasite challenge were examined. In unimmunized mice and in the mice that received MSP119 alone, no antibodies were detected by IFA at the lowest serum dilution examined (1/320). In the group immunized with IMX108, five mice had titers of 1/20,480; the sixth mouse had a lower titer (1/10,240) and succumbed to the challenge infection. Essentially identical results (five of six mice protected) were obtained when the parasite challenge experiment was repeated (data not shown).
FIG. 2.
Challenge with P. yoelii-infected erythrocytes following immunization. Mice were immunized with the IMX108 fusion protein (red lines) (six mice) or with MSP119 alone (blue lines) (six mice) or were not immunized (black lines) (two mice). Following a challenge infection with 5,000 infected erythrocytes, the parasitemia percentage was determined daily.
IMX108 induces antibodies recognizing the C4bp domain and circulating C4bp.
Given the high titers of antibodies induced by IMX108 against MSP119, it was important to see whether antibodies were also induced against the murine C4bp oligomerization domain in the fusion protein and against circulating native C4bp in the host. It has been suggested that C4bp domains could be useful for therapeutic purposes because they should be nonimmunogenic in the species of origin (15, 19). As shown in Table 3, antibodies that bound the recombinant murine C4bp domain were detected in the sera of mice that had been immunized with IMX108, but not in the sera of mice that had been immunized with IMX180, which contained the human C4bp domain. IMX180 induced antibodies that bound the recombinant human C4bp domain.
TABLE 3.
Antibody titers against P. yoelii MSP119 induced by different oligomerization domainsa
| Immunogen(s) | Oligomerization domain | Freund's adjuvant | Titer with coated antigen
|
||||
|---|---|---|---|---|---|---|---|
| P. yoelii Msp119b | Murine C4bp | Human C4bp | Chicken2 C4bp | Archaeal Sm | |||
| IMX269 | None | − | <100 (0) | ||||
| IMX269 + IMX315 | Chicken2 C4bp | − | <100 (0) | ||||
| IMX269 | None | + | 3,200 (7,390) | ||||
| IMX272 | Archeal Sm | − | 12,800 (6,350) | <100 | <100 | 102,400 | |
| IMX180 | Human C4bp | − | 12,800 (13,418) | <100 | 6,400 | <100 | |
| IMX180 | Human C4bp | + | 51,200 (51,731) | ||||
| IMX108 | Murine C4bp | − | 25,600 (9,776) | ||||
| IMX108 | Murine C4bp | − | 25,600 (9,776) | 3,200 | <100 | ||
| IMX108 | Murine C4bp | − | 51,200 (28,637) | 3,200 | <100 | <100 | <100 |
| IMX108 | Murine C4bp | + | 102,400 (90,000) | 3,200 | <100 | <100 | <100 |
| IMX314 | Chicken1 C4bp | − | 51,200 (29,560) | ||||
| IMX262 | Chicken2 C4bp | − | 102,400 (0) | <100 | 12,800 | ||
| IMX262 | Chicken2 C4bp | − | 102,400 (48,036) | ||||
| IMX262 | Chicken2 C4bp | − | 102,400 (78,209) | ||||
| IMX317 | Chicken2 C4bpΔ7 | − | 102,400 (103,467) | ||||
| IMX313 | Hybrid C4bpΔ7 | − | 204,800 (0) | ||||
| IMX313 | Hybrid C4bpΔ7 | − | 204,800 (0) | ||||
Groups of three BALB/c mice were immunized as described in Materials and Methods. Antibody titers were determined by ELISA in serum samples collected 2 weeks after the second immunization (week 6). Three different lots of IMX108 and IMX262 and two different lots of IMX313 were tested, and the results for each test are shown on a separate line. If there is no entry, no test was done.
The values in parentheses are standard deviations.
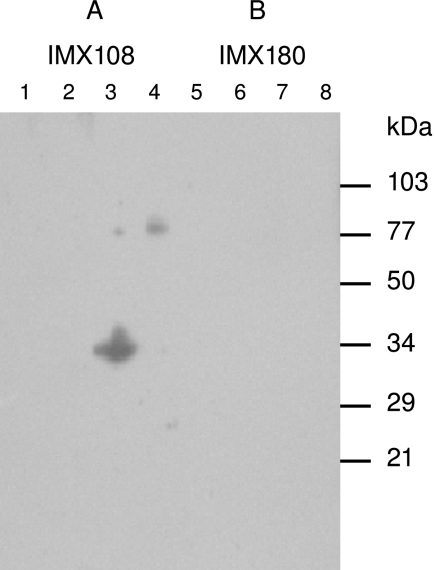
To test whether antibodies were induced by IMX108 that could recognize the native protein, murine C4bp was purified from plasma (9) and probed using Western blotting. Figure 3 shows that antibodies recognizing circulating C4bp were present in the pooled sera of mice immunized with the IMX108 protein.
FIG. 3.
Antibodies induced by immunization with IMX108 react with murine C4bp. A single Western blot was cut in two and probed with antisera raised by immunization with either IMX108 (A) or IMX180 (B). Lanes 1 and 5 contained molecular mass standards, lanes 2 and 6 contained DsbA protein (50 ng), lanes 3 and 7 contained DsbA-murine C4bp (50 ng), and lanes 4 and 8 contained murine C4bp purified from plasma (50 ng).
Distant homologues of the murine C4bp domain are more effective in increasing immunogenicity.
Given that antibodies recognizing native murine C4bp are induced when mice are immunized with IMX108, we tested homologues of the C4bp domain whose sequences were only weakly related to see if they could retain the “adjuvantlike” effect without inducing antibodies against murine C4bp. The first version tested was IMX180, in which the human C4bp domain replaced the murine C4bp domain. While it was encouraging that immunization with IMX180 did not induce antibodies that cross-reacted with native murine C4bp (Fig. 3) or with a recombinant version of the murine domain (Fig. 3 and Table 3), it was clear that the human domain was less effective as an adjuvant in mice than the murine domain in eliciting antibodies (Table 3). It is noteworthy that Freund's adjuvant increased the immunogenicity of both the IMX108 and IMX180 proteins.
An archaeal oligomerization domain, which is not homologous to C4bp, was tested to determine the contribution that oligomerization per se might make to the increased immune response. This domain, used in the IMX272 protein, is the Sm core protein from P. abyssi (22). It contains 74 amino acids and also spontaneously forms heptamers in E. coli. It is clear from the ELISA results that oligomerization of the antigen by this unrelated domain significantly improved antibody responses to the antigen; in fact, the titers obtained were greater than those obtained by administering the MSP119 antigen (IMX269) in Freund's adjuvant (Table 3). It is also noteworthy that the archaeal domain itself was very immunogenic. This domain may augment immunogenicity by contributing strong T-cell help, rather than just simply through oligomerization of the antigen.
Two very distant C4bp relatives, both encoded by genes found in the chicken genome (Fig. 1A), were also tested. The first domain, used in the IMX314 protein, was found in the C4bp homologue described by Oshiumi and colleagues, who called this protein CRES (18). The second domain, in the IMX262 protein, has not been described previously. Both homologues were cloned in place of the murine C4bp domain fused to the MSP119 antigen, and the immunogenicity of each of the purified fusion proteins was measured. Given the disappointing immunogenicity of IMX180, it was particularly surprising that both chicken homologues greatly boosted the antigen's immunogenicity; Table 3 shows that the ELISA titers obtained by immunization with the IMX262 and IMX314 proteins were higher than even those obtained when IMX108 was used.
Two modifications were made in order to reduce both the size of the domain and its similarity to the human C4bp domain. The first modification, in the IMX317 protein, truncated the second chicken domain by 7 amino acids. This truncation has been shown to improve substantially the solubility of some antigen-C4bp fusion proteins (J.-B. Marchand and F. Hill, unpublished data). The truncation had no effect on immunogenicity. The second modification replaced four consecutive residues (EDVK) in the newly described chicken domain with four residues (AELR) from the original chicken domain. This reduced the similarity of the hybrid domain both to the human C4bp domain, in which the sequence EDVK is also present, and to the mouse domain because three of the four residues are also identical (HDVK). Surprisingly, combining these two modifications in the IMX313 protein created the most effective domain, both in terms of the immunogenicity of the antigen and in terms of ensuring that all mice responded equally in two different immunizations with independently prepared batches of the protein.
To examine whether antibodies induced by a chicken-derived oligomerization domain could recognize the murine domain, ELISAs were performed using recombinant murine C4bp and its homologues as coated antigens with sera produced by immunizing mice with the IMX262 protein (containing the second chicken domain). The results (Table 3) show that, as expected, antibodies were induced against the second chicken domain present in the protein with which the mice were immunized, but there were no antibodies which recognized the murine C4bp domain.
Antigen mixed with, but not fused to, the C4bp oligomerization domain remains poorly immunogenic.
To investigate whether the C4bp oligomerization domains could act like a classical adjuvant, both the MSP119 antigen (IMX269) and the hybrid chicken oligomerization domain (IMX315) were prepared as separate proteins and mixed (2 nmol of each protein) before immunization. Antibody titers against the antigen were determined by an ELISA, and they proved to be the same as those obtained after injection of the antigen alone (<100). This shows that the C4bp domain is not strictly comparable to classical adjuvants but resembles other protein adjuvants, such as C3d or monoclonal antibodies, where fusion or coupling of the antigen to the protein adjuvant is also required (1, 4, 7).
DISCUSSION
The results of this study show that the murine C4bp oligomerization domain acts as an adjuvant when it is fused to P. yoelii MSP119 and substantially increases the antigen's immunogenicity. A clear and direct relationship was observed between the dose of recombinant fusion protein used and the antibody response obtained. The serum antibody titers observed are comparable to those obtained using MSP119 with strong adjuvants, such as Freund's adjuvant (17) (Table 3) and SBAS2 (16). Immunization with the MSP119-murine C4bp fusion protein could also protect mice against an otherwise lethal parasite challenge.
Immunization with the MSP119-murine C4bp fusion protein also induced antibodies which recognized the host C4bp (9). It is possible that some of the antibodies binding to the murine C4bp domain recognized an epitope exposed by the absence of glycosylation in the recombinant protein, but it is clear nevertheless that tolerance to circulating C4bp can be broken by immunization with the IMX108 protein. To obviate this problem, further fusion proteins were made using MSP119 and oligomerization domains from a variety of sources, including domains that were modified to reduce both their size and their similarity to mouse or human C4bp. These fusion proteins proved to be superior to those containing the murine C4bp domain. It is striking that it was only in immunizations using the second chicken domain or the truncated hybrid domain that no variation between different animals was found. The truncated hybrid C4bp domain exhibits only 20% identity to either the murine domain (11 residues in 54 identical residues) or the human domain (10 residues in 51 identical residues). It remains to be seen whether the antibodies induced to the novel C4bp oligomerization domains can neutralize their adjuvantlike effect. Repeated immunization of the same animals using different antigens fused to the same oligomerization domain is needed to determine this.
The results reported here clearly show that fusion of both known and novel C4bp oligomerization domains to the weak antigen MSP119 enhances the immunogenicity of the parasite protein. Using the murine C4bp domain, strong antibody responses capable of protecting against a parasite challenge were induced. The mode of action of the C4bp domains remains to be determined, and while oligomerization per se may be an important element, the significant differences between the different C4bp domains (all of which form heptamers) show that there are other factors that contribute to the effects seen, which may include the fortuitous presence of T-cell epitopes.
Many interesting questions are raised by the results presented here, apart from questions concerning the mechanism(s) involved. For example, it remains to be seen whether T-cell responses to the MSP119 antigen are also increased by fusing this antigen to C4bp domains and whether the same increase in immunogenicity occurs with other antigens and in other species. The first question was clearly answered affirmatively in a companion study (S. J. Draper, A. C. Moore, A. L. Goodman, C. A. Long, A. A. Holder, S. C. Gilbert, F. Hill, and A. V. S. Hill, submitted for publication), while the answers to the other questions will determine whether the C4bp oligomerization domains described here will be useful in malaria vaccine development and in vaccination strategies for other diseases in a wide range of species.
Acknowledgments
We thank Michael Steward and Richard Smith, then at Adprotech, for providing the MSP119 expression plasmid; Florence Constantinesco of the Institut de Génétique et Microbiologie, Université Paris XI, for providing genomic DNA from P. abyssi; Laurence Garnier for constructing expression plasmids for IMX108, IMX180, and IMX183; Michel Julien and Emmanuel Risse for contributing to the purification of early batches of these three proteins; and Jérôme Pansanel for his help with the figures.
F.H. is a shareholder in Imaxio SA; the other authors have no conflicting financial interests.
All animal studies were approved by the authors' institutional review boards.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Barber, B. H. 1997. The immunotargeting approach to adjuvant-independent subunit vaccine design. Semin. Immunol. 9293-301. [DOI] [PubMed] [Google Scholar]
- 2.Barrault, D. V., M. Steward, V. F. Cox, R. A. Smith, and A. M. Knight. 2005. Efficient production of complement (C3d)3 fusion proteins using the baculovirus expression vector system. J. Immunol. Methods 304158-173. [DOI] [PubMed] [Google Scholar]
- 3.Blom, A. M., B. O. Villoutreix, and B. Dahlback. 2004. Complement inhibitor C4b-binding protein—friend or foe in the innate immune system? Mol. Immunol. 401333-1346. [DOI] [PubMed] [Google Scholar]
- 4.Carayanniotis, G., and B. H. Barber. 1987. Adjuvant-free IgG responses induced with antigen coupled to antibodies against class II MHC. Nature 32759-61. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen, D., P. Devaux, B. Reveil, A. Evlashev, B. Horvat, J. Lamy, C. Rabourdin-Combe, J. H. Cohen, and D. Gerlier. 2000. Octamerization enables soluble CD46 receptor to neutralize measles virus in vitro and in vivo. J. Virol. 744672-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 612462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey, P. W., M. E. Allison, S. Akkaraju, C. C. Goodnow, and D. T. Fearon. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271348-350. [DOI] [PubMed] [Google Scholar]
- 8.Dervillez, X., A. Huther, J. Schuhmacher, C. Griesinger, J. H. Cohen, D. von Laer, and U. Dietrich. 2006. Stable expression of soluble therapeutic peptides in eukaryotic cells by multimerisation: application to the HIV-1 fusion inhibitory peptide C46. Chem. Med. Chem. 1330-339. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira, A., M. Takahashi, and V. Nussenzweig. 1977. Purification and characterization of mouse serum protein with specific binding affinity for C4 (Ss protein). J. Exp. Med. 1461001-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 1593400-3411. [PubMed] [Google Scholar]
- 11.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp. Quant. Biol. 541-13. [DOI] [PubMed] [Google Scholar]
- 12.Kask, L., A. Hillarp, B. Ramesh, B. Dahlback, and A. M. Blom. 2002. Structural requirements for the intracellular subunit polymerization of the complement inhibitor C4b-binding protein. Biochemistry 419349-9357. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 682215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis, A. P. 1989. Cloning and analysis of the gene encoding the 230-kilodaltion merozoite surface antigen of Plasmodium yoelii. Mol. Biochem. Parasitol. 36271-282. [DOI] [PubMed] [Google Scholar]
- 15.Libyh, M. T., D. Goossens, S. Oudin, N. Gupta, X. Dervillez, G. Juszczak, P. Cornillet, F. Bougy, B. Reveil, F. Philbert, T. Tabary, D. Klatzmann, P. Rouger, and J. H. Cohen. 1997. A recombinant human scFv anti-Rh(D) antibody with multiple valences using a C-terminal fragment of C4-binding protein. Blood 903978-3983. [PubMed] [Google Scholar]
- 16.Ling, I. T., S. A. Ogun, P. Momin, R. L. Richards, N. Garcon, J. Cohen, W. R. Ballou, and A. A. Holder. 1997. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine 151562-1567. [DOI] [PubMed] [Google Scholar]
- 17.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 1663-67. [DOI] [PubMed] [Google Scholar]
- 18.Oshiumi, H., K. Shida, R. Goitsuka, Y. Kimura, J. Katoh, S. Ohba, Y. Tamaki, T. Hattori, N. Yamada, N. Inoue, M. Matsumoto, S. Mizuno, and T. Seya. 2005. Regulator of complement activation (RCA) locus in chicken: identification of the chicken RCA gene cluster and functional RCA proteins. J. Immunol. 1751724-1734. [DOI] [PubMed] [Google Scholar]
- 19.Oudin, S., M. T. Libyh, D. Goossens, X. Dervillez, F. Philbert, B. Reveil, F. Bougy, T. Tabary, P. Rouger, D. Klatzmann, and J. H. Cohen. 2000. A soluble recombinant multimeric anti-Rh(D) single-chain Fv/CR1 molecule restores the immune complex binding ability of CR1-deficient erythrocytes. J. Immunol. 1641505-1513. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez de Cordoba, S., M. Perez-Blas, R. Ramos-Ruiz, P. Sanchez-Corral, F. Pardo-Manuel de Villena, and J. Rey-Campos. 1994. The gene coding for the beta-chain of C4b-binding protein (C4BPB) has become a pseudogene in the mouse. Genomics 21501-509. [DOI] [PubMed] [Google Scholar]
- 21.Shinya, E., X. Dervillez, F. Edwards-Levy, V. Duret, E. Brisson, L. Ylisastigui, M. C. Levy, J. H. Cohen, and D. Klatzmann. 1999. In vivo delivery of therapeutic proteins by genetically-modified cells: comparison of organoids and human serum albumin alginate-coated beads. Biomed. Pharmacother. 53471-483. [DOI] [PubMed] [Google Scholar]
- 22.Thore, S., C. Mayer, C. Sauter, S. Weeks, and D. Suck. 2003. Crystal structures of the Pyrococcus abyssi Sm core and its complex with RNA. Common features of RNA binding in archaea and eukarya. J. Biol. Chem. 2781239-1247. [DOI] [PubMed] [Google Scholar]