Abstract
Neisseria meningitidis LpxL1 lipopolysaccharide (LPS) bearing penta-acylated lipid A is considered a promising adjuvant candidate for inclusion in future N. meningitidis vaccines, as it elicits a markedly reduced endotoxic response in human macrophages relative to that in wild-type (hexa-acylated) LPS, while it is an equally effective adjuvant in mice. As dendritic cells (DC) and Toll-like receptors (TLR) are regarded as central mediators in the initiation of an immune response, here we evaluated the ability of LpxL1 LPS to mature and to activate human DC and examined its TLR4-/MD-2-activating properties. Unexpectedly, purified LpxL1 LPS displayed minimal human DC-stimulating properties compared to wild-type LPS. Although whole bacteria induced DC maturation and activation irrespective of their type of LPS, the LpxL1 mutant failed to activate the human recombinant TLR4/MD-2 complex expressed in HeLa cells. Similarly, purified LpxL1 LPS was unable to activate human TLR4/MD-2 and it even acted as an antagonist of wild-type LPS. Both wild-type and LpxL1 LPSs activated the murine TLR4/MD-2 complex, consistent with their abilities to induce maturation and activation of murine DC. Assays with cells transfected with different combinations of human and murine TLR4 and MD-2 indicated that TLR4 was a more-major determinant of the LPS response than MD-2. The species-specific activation of the TLR4/MD-2 complex by LpxL1 LPS may have an impact on the use of LpxL1 LPS as an adjuvant and the use of murine immunization models in human meningococcal vaccine development.
Neisseria meningitidis group B is the most common cause of invasive meningococcal disease in industrialized countries. Extensive efforts to generate a universally effective vaccine against this pathogen have not yet been successful (26). Evaluation in humans of outer membrane vesicle (OMV) vaccines depleted of the majority of lipopolysaccharides (LPS) to minimize the risk of vaccine reactogenicity (7, 29, 40) revealed that their immunogenicity is low, as reflected in the requirement of four doses to induce an adequate immune response (3, 4, 25). The major current challenge is to boost immunogenicity of the OMV vaccines by inclusion of the appropriate adjuvant(s).
N. meningitidis LPS is composed of a hydrophobic lipid A portion that anchors it to the bacterial outer membrane and a hydrophilic oligosaccharide core, which is exposed on the bacterial surface (16, 19). The majority of the biological activity of LPS is determined by its lipid A part (8). The phosphorylation status as well as the composition and length of the fatty acyl chains in lipid A influences its endotoxic properties (35). N. meningitidis lipid A mutants expressing a fatty acyl pattern different from that expressed by wild-type lipid A induce lower levels of proinflammatory cytokines in human macrophages (32, 39). Similar immunogenic properties exist for these lipid A mutants when used in a mouse immunization model (32-34, 39). These N. meningitidis lipid A mutants with improved pharmacological properties are regarded as interesting adjuvant candidates for inclusion in future meningococcal OMV vaccines.
Microbially derived adjuvants such as LPS exert their function by activation of immature dendritic cells (imDC) via Toll-like receptors (TLR). TLR consist of leucine-rich repeat regions involved in the binding of conserved microbial patterns and a signaling domain homologous to the signaling component of the type I interleukin 1 receptor (IL-1R) (18, 23). TLR engagement by microbially derived products on dendritic cells (DC) activates a variety of signaling pathways resulting in the activation of NF-κB and transcription of immunomodulatory genes, leading to the production of cytokines, like IL-10 and IL-12-p70, and the expression of costimulatory molecules, including CD40, CD80, and CD86 (18, 22). This process, also known as DC maturation, is required for the ability of DC to activate and instruct naive T cells (2). Thus, the activation of DC via TLR sets the stage for the development of an adequate immune response involving both innate and adaptive arms of the immune system.
Among the mammalian TLR known to date, TLR4 has been identified to form the mammalian LPS receptor complex together with the secreted glycoprotein MD-2 that directly interacts with the extracellular domain of TLR4 (20, 28). Upon recognition of LPS by the serum protein LPS-binding protein, LPS oligomeric micelles are converted to monomers for delivery to CD14, which in turn transfers the LPS to the TLR4/MD-2 complex (10). The lipid A portion of LPS plays a crucial role in this interaction, and the structural variation in lipid A is being discriminated by TLR4/MD-2 as endotoxic or antiendotoxic. Moreover, species-specific differences in recognition of lipid A by TLR4/MD-2 have been reported (1, 11, 17, 21).
The central role of DC and TLR in the establishment of innate and adaptive immune responses enables the use of DC as an ex vivo model system for the evaluation of the immunogenic properties of microbially derived adjuvants such as LPS. Indeed, we previously demonstrated that LPS derived from N. meningitidis strain H44/76 and its LpxL1 derivative that carries a penta-acylated lipid A instead of a hexa-acylated lipid A induces maturation and activation of bone marrow-derived mouse DC in vitro and exhibits potent immune stimulating properties in mice in vivo (34). To get more insight into the adjuvant potential of H44/76 and LpxL1 LPS for human vaccines, here we evaluated the biological activities of both purified and membrane-bound LpxL1 LPS with respect to the maturation and activation of human monocyte-derived DC and the recognition by the TLR4/MD-2 complex.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The characteristics of N. meningitidis strain H44/76 and its LpxL1 derivative carrying penta-acylated LPS have been described previously (12, 39). Both strains were grown on GC agar (Difco, Basingstoke, United Kingdom) supplemented with Vitox (Oxoid Ltd.) in an atmosphere of 5% CO2 in air at 37°C. For use in experiments, bacteria grown for 18 h were fixed in 0.5% paraformaldehyde (PFA) in RPMI 1640 medium without phenol red (Gibco-BRL) for 20 min. To remove the PFA, the bacteria were repeatedly pelleted by centrifugation and washed with RPMI 1640 medium without phenol red. Fluorescein isothiocyanate (FITC)-labeled bacteria were prepared by incubating (20 min, 37°C) PFA-fixed bacteria in 0.5 mg/ml of FITC (Sigma, Poole, United Kingdom) in RPMI 1640 medium without phenol red. Free FITC was removed by repeated cycles of pelleting of the labeled bacteria and by resuspending them in RPMI 1640 medium without phenol red. Both H44/76 and LpxL1 bacteria were equally intensely labeled with FITC as analyzed by fluorescence-activated cell sorting (FACS). PFA-fixed (FITC-labeled) bacteria were resuspended in RPMI 1640 medium without phenol red to an optical density at 600 nm of 109 bacteria/ml and stored at −20°C until further use.
Isolation of LPS.
N. meningitidis H44/76 and LpxL1 LPS were isolated by the hot-phenol extraction method described by Westphal and Jann (41). LPS stock solutions of 1 mg/ml were prepared based on LPS quantification in a 3-deoxy-d-manno-2-octulosonic acid assay (13) with 3-deoxy-d-manno-2-octulosonic acid-NH3 as a standard.
Isolation DC and cell culture.
Human imDC were generated from human peripheral blood mononuclear cells (PBMC) as described previously (5, 27) with minor modifications. Briefly, PBMC were isolated from heparinized blood from healthy volunteers by using density gradient centrifugation over a Ficoll gradient (Amersham Biosciences). Recovered PBMC fractions were rinsed three times in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Bodinco B.V.). Next, monocytes were prepared from PBMC by centrifugation (1,750 × g, 45 min, 20°C) over a three-layer Percoll gradient (60%, 47.5%, and 34% Percoll in RPMI 1640-10% FCS; GE Healthcare Bio-Sciences AB). Monocytes were harvested from the upper interface, rinsed three times with RPMI 1640-10% FCS medium, and incubated in six-well plates (4 ml per well, 0.5 × 106 cells/ml) in RPMI 1640-10% FCS, supplemented with 2.4 mM l-glutamine (Sigma-Aldrich), 100 U/ml penicillin-streptomycin (Gibco), 100 ng/ml of human recombinant granulocyte-macrophage colony-stimulating factor (Peprotech), and 50 ng/ml of human recombinant IL-4 (Strathmann-Biotec AG). After 6 days of culture, imDC were harvested. imDC were CD14− CD83− CD86low HLA-DRlow and expressed CD40 and CD11c, as assessed by flow cytometry on a FACSCalibur using Cell Quest software (Becton Dickinson).
The HeLa 57A cell line stably transfected with an NF-κB luciferase reporter construct (24) was generously provided by R. T. Hay (Institute of Biomolecular Sciences, University of St. Andrews, St. Andrews, Scotland, United Kingdom). Cells were routinely cultured in 25-cm2 tissue culture flasks (Corning) in Dulbecco modified Eagle medium (DMEM)-10% FCS at 37°C in a 5% CO2 atmosphere.
Stimulation of DC.
imDC resuspended at a concentration of 0.5 × 106 cells/ml in 4 ml of RPMI 1640-10% FCS were coincubated with either PFA-fixed N. meningitidis H44/76 or the LpxL1 mutant at a multiplicity of infection (MOI) of 50 or 200, respectively, or purified LPS at concentrations between 0.001 and 1,000 ng/ml. Unstimulated imDC served as a control in all experiments. DC were harvested after 24 h and directly stained for expression of cell surface markers. Culture supernatants were harvested and stored at −80°C.
Flow cytometric analysis of cell surface markers.
Surface expressions of maturation markers and costimulatory molecules on imDC and stimulated DC were assessed by flow cytometry (FACSCalibur). Cells were harvested, rinsed with RPMI 1640-10% FCS, and resuspended in filter-sterilized phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (FACS buffer). Next, cells were incubated (30 min, 4°C) with the following antibodies: FITC-conjugated anti-human CD11c (mIgG1) and CD83 (mIgG1), phycoerythrin-conjugated anti-human CD86 (mIgG1) and CD40 (mIgG1), antigen-presenting-cell-conjugated anti-human CD14 (mIgG1) and HLA-DR (mIgG2b), and appropriate fluorochrome-labeled isotype controls (CD11c, CD40, and CD14 from eBioscience; CD83, CD86, and HLA-DR from BD Pharmingen).
Cytokine measurements.
Human IL-10 and IL-12p70 concentrations in the supernatants of imDC and stimulated DC were determined using OptEIA enzyme-linked immunosorbent assay (ELISA) kits (BD Biosciences Pharmingen) or CytoSets ELISA kits (Biosource Europe S.A.) according to the manufacturer's instructions. Statistical significance was confirmed by a paired ratio t test. A two-tailed P value of <0.05 was taken to be significant.
Bacterial adhesion and internalization assay.
Binding and internalization of bacteria by DC were determined with a combination of flow cytometry and confocal microscopy as described previously (37). For detection by flow cytometry, DC were incubated with FITC-labeled bacteria for 0.5 to 6 h, fixed in FACSFix (BD Biosciences), washed, and analyzed on a FACSCalibur. DC with associated bacteria were easily identified by fluorescence within the DC gated population. For confocal microscopy, DC stimulated with FITC-labeled N. meningitidis were allowed to adhere for 10 min to an adhesion slide (Bio-Rad Laboratories Ltd.). To stop internalization, DC were fixed with 4% PFA in PBS for 10 min, and DC were visualized by staining with 5 μg/ml of anti-major histocompatibility complex class II monoclonal antibody (Dako). After washing, the specimen was incubated (1 h) with 5 μg/ml of Texas Red-conjugated goat antimouse antibody (Molecular Probes). Slides were washed and mounted in Citifluor medium (Citifluor Ltd.). Confocal images were obtained using a Leica SP2 confocal laser scanning microscope system (Leica) fitted with appropriate filter sets. To identify intracellular bacteria, 15 to 20 optical sections (0.2 to 0.5 μm) spanning the entire DC were projected and superimposed with Leica confocal imaging software.
Construction of expression plasmids.
The gene encoding murine TLR4 (mTLR4) was amplified by PCR using Pfu polymerase (Promega) from mTLR4 cDNA derived from C3H/HeJ murine macrophages that had previously been stably transfected with the intact mTLR4 gene (19). mCD14 was amplified by PCR from the same cell line. Both PCR products were cloned into pTracer-CMV2 (Invitrogen), yielding pTracer-mTLR4 and pTracer-mCD14, respectively (38). hCD14 (GenBank accession number NM_000591) was PCR amplified from chromosomal DNA of HEK293 cells and cloned into pTracer-CMV2 (Invitrogen), yielding pTracer-hCD14. Expression plasmids pUNO-hTLR4-A, pUNO-hMD-2, and pUNO-mMD-2 were purchased from InvivoGen.
Transient transfection.
HeLa 57A cells were grown in 24-well tissue culture plates in DMEM-10% FCS until 70% confluence was reached (∼24 h). Then, cells were transiently transfected in DMEM-10% FCS by using FuGene 6 (Roche Diagnostics) at a lipid-to-DNA ratio of 3 to 1 (15) Plasmids carrying the desired inserts were added at a concentration of 125 ng/plasmid. Variable amounts of empty vector were included to equalize the total amounts of transfected plasmid DNA (500 ng) added to the cells. In all transfections, the pTK-LacZ vector was used for normalization of transfection efficiency. After 24 h of incubation (37°C) of the cells in the presence of the DNA/FuGene 6 mixture, the medium was replaced with fresh medium containing DMEM-10% FCS and stimulated with the different ligands.
Luciferase assay.
TLR signaling was assessed using the NF-κB-luciferase reporter system, as described previously (14) Cells were stimulated with either PFA-fixed bacteria or purified LPS for 5 h, rinsed three times with 0.5 ml of Dulbecco's PBS (pH 7.4), and immediately lysed in 0.2 ml of reporter lysis buffer (Promega). Firefly luciferase activity was measured with the luciferase assay system (Promega) by using a luminometer (TD-20/20; Turner Designs). For normalization of the efficiency of transfection, luciferase values were adjusted to β-galactosidase values as determined with the β-galactosidase assay (Promega). Results were expressed in relative luciferase units (RLU) and represent the mean ± standard error of the mean (SEM) values of three independent experiments in duplicate.
RESULTS
Interaction of N. meningitidis LpxL1 LPS with human DC.
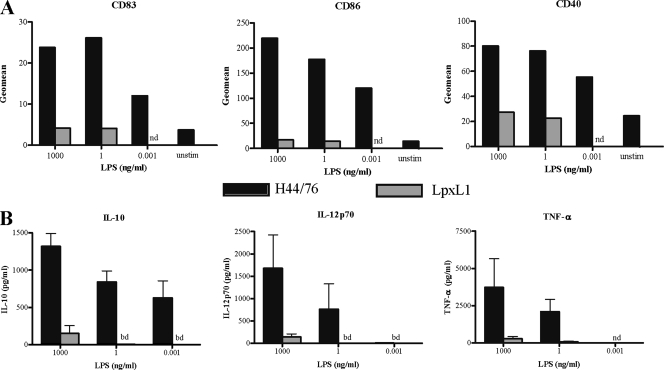
To assess the ability of N. meningitidis LpxL1 LPS to mature and activate human DC, monocyte-derived imDC were incubated with various concentrations of purified LPS of wild-type strain H44/76 and the LpxL1 mutant. Expression of costimulatory markers CD83, CD86, and CD40 and induction of IL-10, IL-12p70, and tumor necrosis factor alpha (TNF-α) were determined at 18 h of stimulation. H44/76 LPS stimulated the expression of CD83, CD86, and CD40, even at the lowest concentration of LPS tested (0.001 ng/ml) (Fig. 1A). Similarly, IL-10 production was observed for DC stimulated with H44/76 LPS at a concentration of 0.001 ng/ml and IL-12p70 and TNF-α at 1 ng/ml (Fig. 1B). In contrast, purified LpxL1 LPS did not induce expression of CD83, CD86, or CD40 even at the highest concentration (1,000 ng/ml) (Fig. 1A) and yielded only minimal production of cytokines (Fig. 1B). Thus, purified LpxL1 LPS lack the ability to mature and activate human DC.
FIG. 1.
Maturation and activation of DC stimulated with purified LPS derived from N. meningitidis H44/76 and the LpxL1 mutant. imDC were incubated with the indicated LPS preparations for 20 h and analyzed for expression of maturation markers CD83, CD86, and CD40 by FACS (A) and production of IL-10, IL-12p70, and TNF-α by ELISA (B). Representative results (A) and the mean ± SEM values (B) of three separate experiments with three different donors are shown. unstim, unstimulated; nd, not determined; bd, below detection level.
Interaction of N. meningitidis LpxL1 bacteria with human DC.
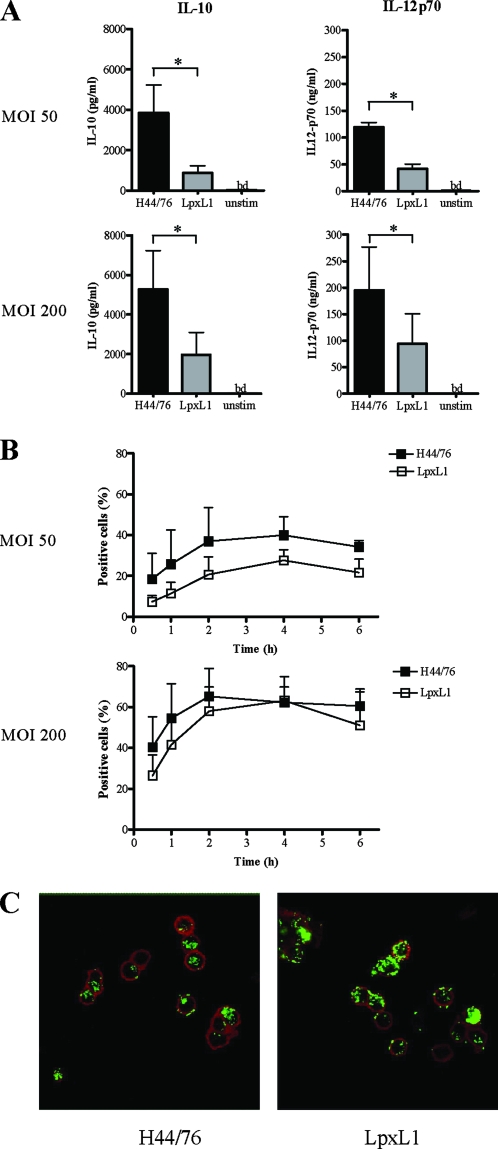
As N. meningitidis requires LPS for bacterial internalization and this event aids optimal cytokine production (37), we determined the effect of intact bacteria expressing LpxL1 LPS on DC maturation and activation. The addition of the LpxL1 strain to DC stimulated the expression of costimulatory markers to an extent similar to the expression stimulated by the parental H44/76 strain (data not shown). Moreover, DC stimulated with LpxL1 bacteria produced IL-10 and IL-12p70 at both concentrations tested (Fig. 2A). However, this cytokine production was significantly lower than the production of IL-10 and IL-12p70 by DC stimulated with the H44/76 parent strain.
FIG. 2.
Interaction of the N. meningitidis wild type and the LpxL1 mutant with DC. (A) ImDC were incubated with the PFA-fixed wild type, H44/76, or the LpxL1 mutant at 1:50 and 1:200 ratios for 20 h and analyzed for the production of IL-10 and IL-12p70 by ELISA. unstim; unstimulated; bd, below detection level; *, P < 0.05. (B and C) imDC were incubated with the PFA-fixed, FITC-labeled wild type, H44/76, or the LpxL1 mutant at 1:50 and 1:200 ratios. At the indicated time points, DC were PFA fixed, washed, and analyzed by FACS (B) and by confocal microscopy (C) (2 h; 1:200 ratio is shown). The mean ± SEM values (A and B) and representative results (C) of three separate experiments with three different donors are shown.
To assess whether the observed differences were caused by an altered interaction of the LpxL1 bacteria with DC, the levels of association and internalization of FITC-labeled bacteria were measured. At an MOI of 50, LpxL1 bacteria showed a trend toward a level of association with DC that was slightly lower than that displayed by H44/76 (Fig. 2B). However, at an MOI of 200, no differences were observed in the associations (Fig. 2B) and internalizations (Fig. 2C) of the LpxL1 strain and of the wild type. These data suggest that the reduced cytokine production observed for the LpxL1 mutant at an MOI of 200 is not caused by a reduced interaction of the mutant with DC.
The LpxL1 LPS fails to stimulate the hTLR4/hMD-2 complex.
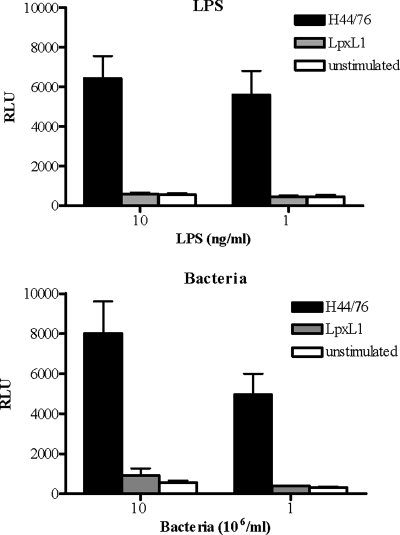
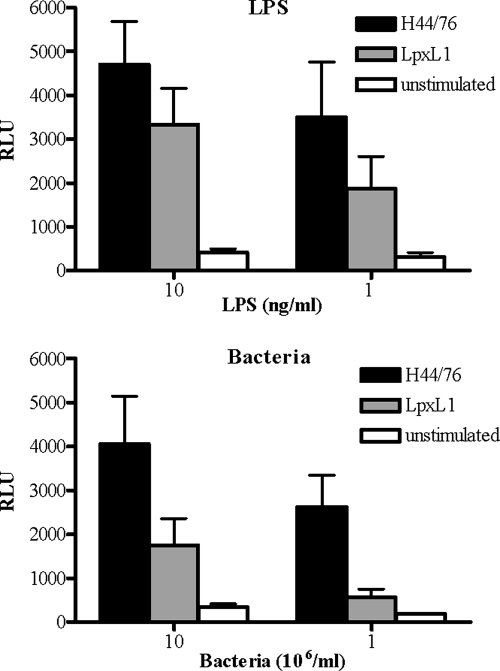
In search of the basis of the reduced bioactivity of the LpxL1 strain and the apparent inability of purified LpxL1 LPS to stimulate DC, we measured the activities of the bacteria and LPS by using HeLa 57A cells transfected with the human TLR4 (hTLR4), hMD-2, and hCD14. The activation of this receptor complex was measured using the NF-κB-luciferase reporter system present in the HeLa 57A cells. In this system, H44/76 bacteria as well as its LPS stimulated hTLR4/hMD-2specific NF-κB activation (Fig. 3). In contrast, LpxL1 bacteria or LpxL1 LPS did not induce luciferase activity, irrespective of whether low or high concentrations were tested (Fig. 3). Similar patterns of TLR4 activation were observed for live H44/76 and LpxL1 bacteria (data not shown).
FIG. 3.
Differential activation of hTLR4/hMD-2 by H44/76 and LpxL1. HeLa 57A cells transfected with hTLR4, hMD-2, and hCD14 were stimulated with purified H44/76 LPS or LpxL1 LPS (A) or PFA-fixed N. meningitidis H44/76 or the LpxL1 mutant (B) at the indicated concentrations. After 5 h, NF-κB luciferase activities, indicated in RLU, were measured. The values are the means ± SEMs of at least three experiments.
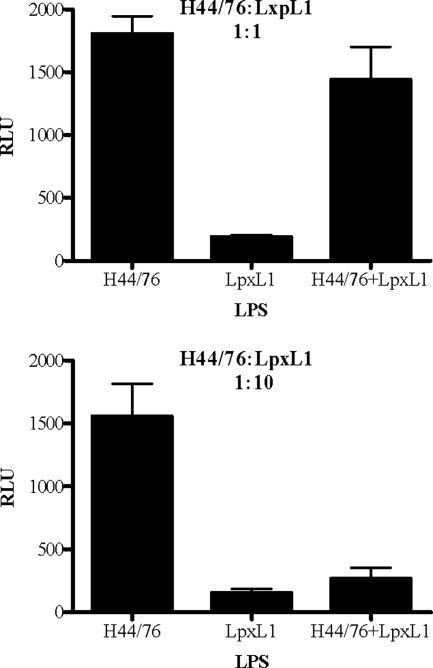
To dissect whether the lack of response of LpxL1 was caused by the inability to bind to the hTLR4/hMD-2 complex or by a more downstream effect on cell signaling, we tested whether LpxL1 LPS displayed possible properties antagonistic toward the stimulatory effect of wild-type LPS. HeLa 57A cells transfected with hTLR4/hMD-2/hCD14 were incubated with mixtures of H44/76 and LpxL1 LPS at a 1:1 or 1:10 ratio. These assays demonstrated that the stimulatory effect of H44/76 LPS was partially antagonized when equal amounts of LpxL1 LPS were present and even completely inhibited when 10-times-more LpxL1 LPS than H44/76 LPS was used (Fig. 4). Together, these results indicate that the LpxL1 LPS still interacts with the hTLR4/hMD-2 complex but fails to activate NF-κB.
FIG. 4.
Inhibition of LPS-induced activation of TLR4/MD-2 by LpxL1 LPS. HeLa 57A cells transfected with hTLR4, hMD-2, and hCD14 were stimulated with purified H44/76 LPS, LpxL1 LPS, or mixtures of these LPS preparations at ratios of 1:1 and 1:10 (1 = 10 ng/ml). After 5 h of incubation, NF-κB luciferase activities were measured. Values are expressed in RLU and represent the means ± SEMs for the results of at least three experiments.
LpxL1 LPS activates mTLR4/mMD-2.
LpxL1 LPS has been reported to be an activator of mouse DC that is equally as potent as H44/76 LPS (34). This suggests that LpxL1 LPS is able to activate mTLR4/mMD-2 and, thus, that LpxL1 may display species-specific interactions with the mTLR4/mMD-2 complex. We addressed this topic by assessing the potencies of LpxL1 and H44/76 LPS in activating HeLa 57A cells transfected with mTLR4, mMD-2, and mCD14. Both LpxL1 LPS and H44/76 LPS activated mTLR4-/mMD-2-/mCD14-transfected HeLa 57A cells, although LpxL1 LPS appeared to be less potent (Fig. 5). As expected, LpxL1 LPS displayed no antagonistic effect on the stimulatory effect of wild-type LPS on mTLR4/mMD-2 (data not shown). A comparison of the effects of the LPS mutant and those of the parent strain yielded similar results (Fig. 5). These data support the notion that the LpxL1 LPS is able to activate mTLR4/mMD-2 but not hTLR4/hMD-2 (Fig. 3).
FIG. 5.
Activation of mTLR4/mMD-2 by H44/76 and the LpxL1 mutant. HeLa 57A cells transfected with mTLR4, mMD-2, and mCD14 were stimulated with purified H44/76 LPS or LpxL1 LPS (A) or PFA-fixed N. meningitidis H44/76 or the LpxL1 mutant (B) at the indicated concentrations. After 5 h, NF-κB luciferase activities were measured. Values are expressed in RLU and represent the means ± SEMs for the results of at least three experiments.
Species-specific recognition of LpxL1 LPS mediated by TLR4.
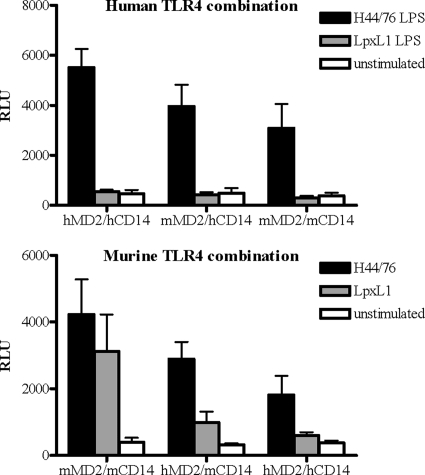
The basis of the apparent species-specific recognition of LpxL1 LPS was further assessed by transfecting HeLa 57A cells with hTLR4 in combination with human or murine MD-2 and CD14 or with mTLR4 in combination with human or murine MD-2 and CD14. Activation of the cells carrying mTLR4/hMD-2 or hTLR4/mMD-2 by H44/76 LPS was lower than that of the cells carrying TLR4 and MD-2 from the same species (Fig. 6). Activation of HeLa 57A cells in response to LpxL1 LPS was observed for cells transfected with mMD-2 or hMD-2 in combination with mTLR4 but not with hTLR4 (Fig. 6). Although the activation of cells carrying mTLR4 by LpxL1 LPS is significantly higher with mMD-2 than with hMD-2, the inability of LpxL1 LPS to activate hTLR4 in the presence of mMD-2 favors an essential function for TLR4 rather than for MD-2 in the observed species-specific recognition.
FIG. 6.
Species-specific recognition of N. meningitidis LpxL1 LPS. HeLa 57A cells transfected with hTLR4 (A) or mTLR4 (B) in combination with human or murine MD-2 and CD14 were stimulated with 10 ng/ml of H44/76 LPS or LpxL1 LPS. After 5 h of incubation, NF-κB luciferase activities were measured. Values are expressed in RLU and are the means ± SEMs for the results of at least three experiments.
DISCUSSION
LPS is a potent inducer of DC maturation through activation of the TLR4/MD-2 complex. For N. meningitidis, we previously demonstrated that both hexa-acylated wild-type LPS and penta-acylated LpxL1 LPS induce maturation of bone marrow-derived mouse DC (34) and cause potent immune stimulating properties in mice (33, 39). This together with the low potency of an LpxL1 mutant in inducing TNF-α in human macrophages led to the notion that LpxL1 LPS may be a suitable adjuvant candidate for inclusion in human OMV vaccines (34, 39). In the present study, we provide evidence that purified LpxL1 LPS barely induce maturation and production of cytokines by human DC, in contrast to wild-type LPS. The impaired response of LpxL1 LPS could be attributed to the failure to activate the human but not the murine TLR4/MD-2 complex. This species-specific activation of the TLR4/MD-2 complex by LpxL1 LPS and the even inhibitory effect of LpxL1 LPS on hTLR4/hMD-2 may explain the reduced toxicity of this LPS variant but raises questions about the adjuvant potential of purified LpxL1 LPS for use in human vaccines. Importantly, LpxL1 LPS retained some capacity to stimulate human DC, and it is likely that this adjuvant activity is occurring via a TLR4-independent mechanism (9).
Despite the lack of activation of hTLR4/hMD-2 by purified LpxL1 LPS and the LpxL1 mutant in the transfected HeLa cells, the mutant strain was still able to mature and activate human DC. This effect is in line with the finding that the LpxL1 strain induces TNF-α in human macrophages (34, 39). The activity of the mutant strain is likely caused by the presence of other bacterial constituents, such as lipopeptides, CpG DNA, and bacterial porins, which can activate other TLR expressed in DC but not in HeLa cells. Synergy between different TLR ligands might also contribute to the different response seen in the DC system. The biological activity of non-LPS components is evidenced by the PBMC and DC stimulating capacity of a completely LPS-deficient N. meningitidis mutant (5, 31) as well as the ability of purified meningococcal porin PorB to mature DC via activation of TLR2 (30). Both the LPS-deficient strain and the LpxL1 mutant display reduced levels of IL-10 and IL-12p70 compared to those of the wild-type N. meningitidis strain but similar expression levels of DC maturation markers (37; this work). In the case of the LPS-deficient mutant, the reduced cytokine production has been suggested to result from impaired bacterial internalization by DC (37). As LpxL1 and wild-type bacteria display equal levels of DC association and internalization (Fig. 2B and C), this cannot account for the reduced DC cytokine response of the LpxL1 mutant. Therefore, it would appear that the ability of lipid A to activate TLR4/MD-2 may be more critical for DC cytokine production than bacterial internalization.
Our data demonstrate significant levels of IL-12 in culture supernatants from DC stimulated with purified meningococcal LPS. Dixon et al. previously demonstrated that purified meningococcal LPS does not induce detectable levels of intracellular IL-12 in DC (5). These seemingly conflicting results are most likely because of the different methods used to measure cytokine production. Levels of intracellular IL-12 are measured in gated cells with the phenotypic characteristics of DC. In contrast, ELISAs of culture supernatants will detect not only IL-12 secreted from DC but also IL-12 made by other cells, including monocytes and macrophages that exist in the cultures after 7 days of incubation with granulocyte-macrophage colony-stimulating factor and IL-4.
Previous studies addressing the molecular basis of the endotoxin potency of neisserial penta-acylated LPS have focused on the interaction with the hTLR4/hMD-2 complex (36, 42). This work, which did not include comparisons with mTLR4/mMD-2 activation, revealed that penta-acylated LPS derived from an lpxL1 (msbB) mutant of N. meningitidis strain NMB or from Neisseria gonorrhoeae still activated hTLR4/hMD-2 albeit with reduced potency, in contrast to the results presented here. A possible explanation for this different result may be the differences in the degrees of substitution of the lipid A 1 and 4′ positions with phosphate and/or phosphoethanolamine groups. Indeed, upon mass spectrometry analysis, we found that the LpxL1 LPS consists of different species, with forms carrying only two phosphate groups and forms carrying two phosphates plus one phosphoethanolamine being the most common (our unpublished results).
The molecular basis of the apparent species-specific activation of the TLR4/MD-2 complex by LpxL1 LPS was investigated by assessing LpxL1 activation in cells expressing different combinations of human and/or mouse TLR4 and MD-2. This approach revealed that the inability of penta-acylated LpxL1 LPS to activate hTLR4/hMD-2 is predominantly caused by the characteristics of hTLR4. The finding that the replacement of hTLR4 by mTLR4 but not the exchange of hMD-2 with mMD-2 conferred LPS responsiveness resembles observations made with penta-acylated Rhodobacter sphaeroides and Pseudomonas aeruginosa LPS (11, 17). Our findings are also in line with the work of Teghanemt et al. (36) which indicates that the reduced potency of penta-acylated N. meningitidis NMB LPS does not result from differences in the efficiency of formation of the LPS/MD-2 complex but rather from the interaction of this complex with hTLR4. In contrast to these observations, Zimmer et al. (42) concluded that a reduced affinity of MD-2 for penta-acylated meningococcal LPS is likely to be responsible for the reduced potency of this LPS. However, these MD-2 affinity studies were performed in the absence of CD14 and, as such, do not resemble the physiological conditions in which LPS is presented as a monomeric complex with CD14, which is known to markedly enhance MD-2 activity (10).
The immunogenic properties of potential vaccines against N. meningitidis and also against many other organisms are often evaluated in mouse models before being tested in human individuals. Clearly, the species-specific differences in recognition of penta-acylated LpxL1 LPS by TLR4/MD-2 imply that the adjuvant properties as determined in mice (6, 33, 39) may not reflect the response in humans. On the other hand, the low endotoxic properties of LpxL1 LPS found on human cells together with its antagonistic function demonstrated here may decrease the risk of inducing severe vaccine reactogenicity when an LpxL1 LPS-expressing strain is applied in humans and, thus, may leave space to evaluate its properties in clinical trials.
Acknowledgments
This work was supported by grants from ZonMw (906-02-058 and 9120-6150), the Meningitis Research Foundation (project 08/02), the Edward Jenner Institute for Vaccine Research, which is sponsored by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, and the United Kingdom Department of Health.
We thank Garth Dixon for his intellectual contribution to this area of work.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Akashi, S., Y. Nagai, H. Ogata, M. Oikawa, K. Fukase, S. Kusumoto, K. Kawasaki, M. Nishijima, S. Hayashi, M. Kimoto, and K. Miyake. 2001. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int. Immunol. 131595-1599. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 3.Cartwright, K., R. Morris, H. Rumke, A. Fox, R. Borrow, N. Begg, P. Richmond, and J. Poolman. 1999. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 172612-2619. [DOI] [PubMed] [Google Scholar]
- 4.de Kleijn, E. D., R. de Groot, J. Labadie, A. B. Lafeber, G. van den Dobbelsteen, L. van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer membrane vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 181456-1466. [DOI] [PubMed] [Google Scholar]
- 5.Dixon, G. L., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 694351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisseha, M., P. Chen, B. Brandt, T. Kijek, E. Moran, and W. Zollinger. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 734070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, and U. Rye. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1467-79. [PubMed] [Google Scholar]
- 8.Galanos, C., O. Luderitz, E. T. Rietschel, O. Westphal, H. Brade, L. Brade, M. Freudenberg, U. Schade, M. Imoto, H. Yoshimura, et al. 1985. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 1481-5. [DOI] [PubMed] [Google Scholar]
- 9.Gavin, A. L., K. Hoebe, B. Duong, T. Ota, C. Martin, B. Beutler, and D. Nemazee. 2006. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science 3141936-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gioannini, T. L., A. Teghanemt, D. Zhang, N. P. Coussens, W. Dockstader, S. Ramaswamy, and J. P. Weiss. 2004. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA 1014186-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3354-359. [DOI] [PubMed] [Google Scholar]
- 12.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karkhanis, Y. D., J. Y. Zeltner, J. J. Jackson, and D. J. Carlo. 1978. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal. Biochem. 85595-601. [DOI] [PubMed] [Google Scholar]
- 14.Keestra, A. M., M. R. de Zoete, R. A. van Aubel, and J. P. van Putten. 2007. The central leucine-rich repeat region of chicken TLR16 dictates unique ligand specificity and species-specific interaction with TLR2. J. Immunol. 1787110-7119. [DOI] [PubMed] [Google Scholar]
- 15.Keestra, A. M., M. R. de Zoete, R. A. van Aubel, and J. P. van Putten. 2008. Functional characterization of chicken TLR5 reveals species-specific recognition of flagellin. Mol. Immunol. 451298-1307. [DOI] [PubMed] [Google Scholar]
- 16.Kulshin, V. A., U. Zahringer, B. Lindner, C. E. Frasch, C. M. Tsai, B. A. Dmitriev, and E. T. Rietschel. 1992. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 1741793-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388394-397. [DOI] [PubMed] [Google Scholar]
- 19.Pavliak, V., J. R. Brisson, F. Michon, D. Uhrin, and H. J. Jennings. 1993. Structure of the sialylated L3 lipopolysaccharide of Neisseria meningitidis. J. Biol. Chem. 26814146-14152. [PubMed] [Google Scholar]
- 20.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 21.Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. USA 972163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rescigno, M., M. Martino, C. L. Sutherland, M. R. Gold, and P. Ricciardi-Castagnoli. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1882175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez, M. S., J. Thompson, R. T. Hay, and C. Dargemont. 1999. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J. Biol. Chem. 2749108-9115. [DOI] [PubMed] [Google Scholar]
- 25.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 634642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenstein, N. E., M. Fischer, and J. W. Tappero. 2001. Meningococcal vaccines. Infect. Dis. Clin. N. Am. 15155-169. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1791109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1891777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14195-207. [PubMed] [Google Scholar]
- 30.Singleton, T. E., P. Massari, and L. M. Wetzler. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 1743545-3550. [DOI] [PubMed] [Google Scholar]
- 31.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70283-288. [PubMed] [Google Scholar]
- 32.Steeghs, L., M. Berns, J. ten Hove, A. de Jong, P. Roholl, L. van Alphen, J. Tommassen, and P. van der Ley. 2002. Expression of foreign LpxA acyltransferases in Neisseria meningitidis results in modified lipid A with reduced toxicity and retained adjuvant activity. Cell. Microbiol. 4599-611. [DOI] [PubMed] [Google Scholar]
- 33.Steeghs, L., B. Kuipers, H. J. Hamstra, G. Kersten, L. van Alphen, and P. van der Ley. 1999. Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect. Immun. 674988-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steeghs, L., J. Tommassen, J. H. Leusen, J. G. van de Winkel, and P. van der Ley. 2004. Teasing apart structural determinants of ‘toxicity’ and ‘adjuvanticity’: implications for meningococcal vaccine development. J. Endotoxin Res. 10113-119. [DOI] [PubMed] [Google Scholar]
- 35.Takada, H., and S. Kotani. 1989. Structural requirements of lipid A for endotoxicity and other biological activities. Crit. Rev. Microbiol. 16477-523. [DOI] [PubMed] [Google Scholar]
- 36.Teghanemt, A., D. Zhang, E. N. Levis, J. P. Weiss, and T. L. Gioannini. 2005. Molecular basis of reduced potency of underacylated endotoxins. J. Immunol. 1754669-4676. [DOI] [PubMed] [Google Scholar]
- 37.Uronen-Hansson, H., L. Steeghs, J. Allen, G. L. Dixon, M. Osman, P. van der Ley, S. Y. Wong, R. Callard, and N. Klein. 2004. Human dendritic cell activation by Neisseria meningitidis: phagocytosis depends on expression of lipooligosaccharide (LOS) by the bacteria and is required for optimal cytokine production. Cell. Microbiol. 6625-637. [DOI] [PubMed] [Google Scholar]
- 38.van Aubel, R. A., A. M. Keestra, D. J. Krooshoop, W. van Eden, and J. P. van Putten. 2007. Ligand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cells. Mol. Immunol. 443702-3714. [DOI] [PubMed] [Google Scholar]
- 39.van der Ley, P., L. Steeghs, H. J. Hamstra, G. J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity and adjuvant activity. Infect. Immun. 695981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Ley, P., J. van der Biezen, and J. T. Poolman. 1995. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine 13401-407. [DOI] [PubMed] [Google Scholar]
- 41.Westphal, O., and J. K. Jann. 1965. Bacterial liopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 583-91. [Google Scholar]
- 42.Zimmer, S. M., S. M. Zughaier, Y. L. Tzeng, and D. S. Stephens. 2007. Human MD-2 discrimination of meningococcal lipid A structures and activation of TLR4. Glycobiology 17847-856. [DOI] [PubMed] [Google Scholar]