Abstract
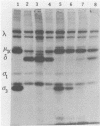
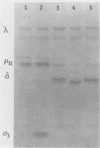
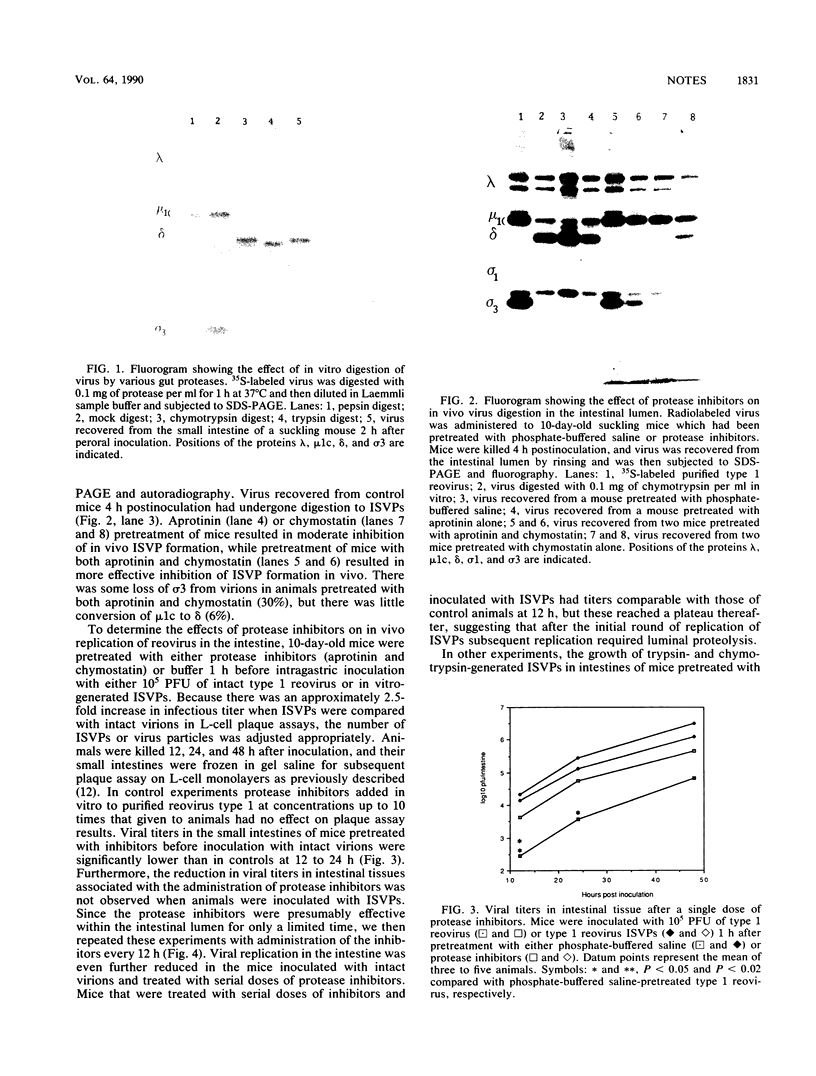
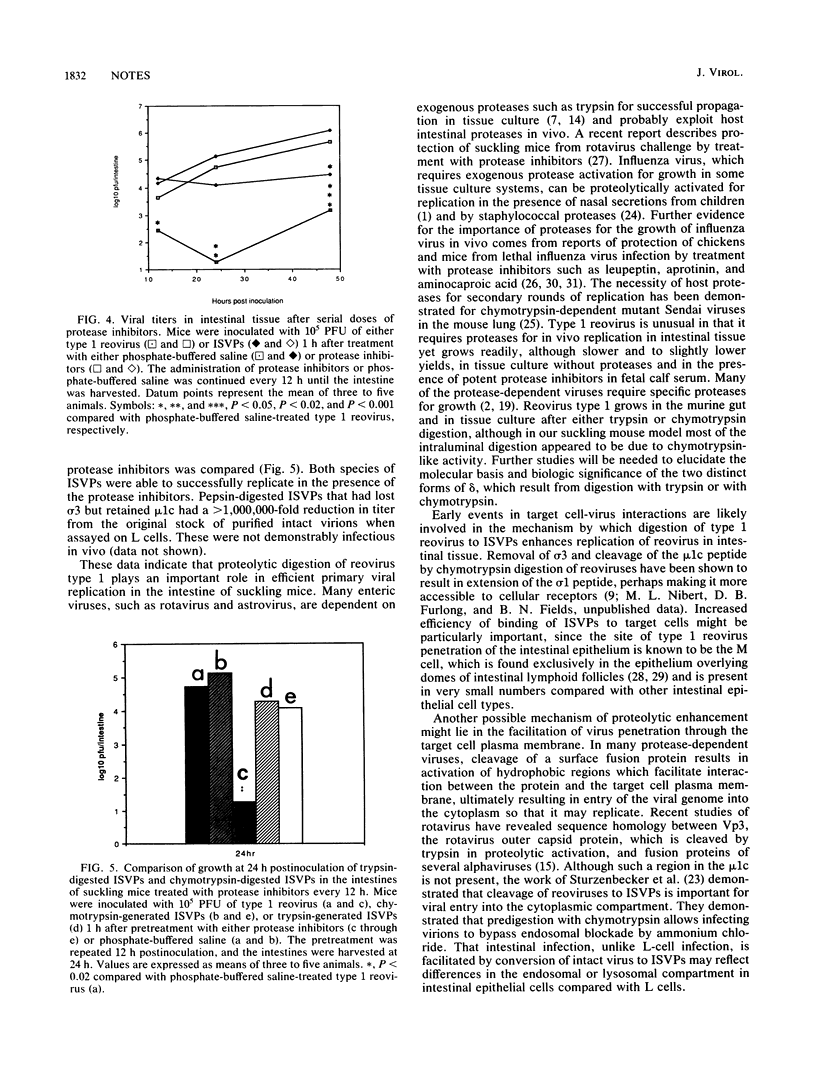
Oral inoculation of suckling mice with reovirus serotype 1 (strain Lang) results in the conversion of intact virions to intermediate subviral particles (ISVPs) in the intestinal lumen. Digestion of virus in vitro with chymotrypsin or trypsin reveals two distinct forms of ISVPs, while the predominant species of ISVPs found in the small intestinal lumen appears to be identical to the chymotrypsin product. The in vivo conversion of virions to ISVPs was blocked by pretreatment of mice with protease inhibitors, resulting in inefficient replication of reovirus in intestinal tissue. The early inhibition of viral replication in suckling mice pretreated with protease inhibitors was not observed when suckling mice were inoculated with ISVPs generated by in vitro digestion with either chymotrypsin or trypsin. However, replication was decreased during secondary rounds of replication in mice receiving repeated doses of protease inhibitors, suggesting that luminal proteolytic digestion is important in rendering progeny virions infectious in the gut.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbey-Morel C. L., Oeltmann T. N., Edwards K. M., Wright P. F. Role of respiratory tract proteases in infectivity of influenza A virus. J Infect Dis. 1987 Apr;155(4):667–672. doi: 10.1093/infdis/155.4.667. [DOI] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Clark M. L. Effect of enzymes on rotavirus infectivity. J Clin Microbiol. 1979 Jul;10(1):111–113. doi: 10.1128/jcm.10.1.111-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Trier J. S., Dambrauskas R., Wolf J. L. Reovirus type I infection of small intestinal epithelium in suckling mice and its effect on M cells. Lab Invest. 1988 Feb;58(2):226–235. [PubMed] [Google Scholar]
- Bodkin D. K., Nibert M. L., Fields B. N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989 Nov;63(11):4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Copps T. P., Sargent M. D., Long D. G., Chapman J. D. New intermediate subviral particles in the in vitro uncoating of reovirus virions by chymotrypsin. J Virol. 1973 Apr;11(4):552–564. doi: 10.1128/jvi.11.4.552-564.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Morash B. D., Sargent M. D., Copps T. P., Lievaart P. A., Szekely J. G. Two modes of entry of reovirus particles into L cells. J Gen Virol. 1979 Oct;45(1):161–170. doi: 10.1099/0022-1317-45-1-161. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Activation and characterization of the reovirus transcriptase: genetic analysis. J Virol. 1982 Jan;41(1):110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972 Sep;49(3):700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman R. S., Wolf J. L., Finberg R., Trier J. S., Fields B. N. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983 Jan 30;124(2):403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lee T. W., Kurtz J. B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J Gen Virol. 1981 Dec;57(Pt 2):421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. H., Kornstein M. J., Anderson A. O. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985 Feb;53(2):391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. D., Long D. G., Borsa J. Functional analysis of the interactions between reovirus particles and various proteases in vitro. Virology. 1977 May 1;78(1):354–358. doi: 10.1016/0042-6822(77)90110-6. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Storz J., Rott R., Kaluza G. Enhancement of plaque formation and cell fusion of an enteropathogenic coronavirus by trypsin treatment. Infect Immun. 1981 Mar;31(3):1214–1222. doi: 10.1128/iai.31.3.1214-1222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Ciborowski P., Reinacher M., Pulverer G., Klenk H. D., Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology. 1987 Apr;157(2):421–430. doi: 10.1016/0042-6822(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983 Feb;39(2):879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Klenk H. D., Rott R. Inhibitory effect of a protease inhibitor, leupeptin, on the development of influenza pneumonia, mediated by concomitant bacteria. J Gen Virol. 1987 Jul;68(Pt 7):2039–2041. doi: 10.1099/0022-1317-68-7-2039. [DOI] [PubMed] [Google Scholar]
- Vonderfecht S. L., Miskuff R. L., Wee S. B., Sato S., Tidwell R. R., Geratz J. D., Yolken R. H. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J Clin Invest. 1988 Dec;82(6):2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Kauffman R. S., Finberg R., Dambrauskas R., Fields B. N., Trier J. S. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983 Aug;85(2):291–300. [PubMed] [Google Scholar]
- Wolf J. L., Rubin D. H., Finberg R., Kauffman R. S., Sharpe A. H., Trier J. S., Fields B. N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981 Apr 24;212(4493):471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P., Ovcharenko A. V., Bukrinskaya A. G. Protective effect of protease inhibitors in influenza virus infected animals. Arch Virol. 1982;73(3-4):263–272. doi: 10.1007/BF01318080. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P., Ovcharenko A. V., Bukrinskaya A. G. Suppression of influenza virus replication in infected mice by protease inhibitors. J Gen Virol. 1984 Jan;65(Pt 1):191–196. doi: 10.1099/0022-1317-65-1-191. [DOI] [PubMed] [Google Scholar]