Abstract
The importance of toll-like receptor 4 (TLR4) in immunity to rickettsiae remains elusive. To investigate the role of TLR4 in protection against rickettsioses, we utilized C3H/HeJ mice, which are naturally defective in TLR4 signaling, and compared the responses of C3H/HeN and C3H/HeJ mice following intravenous inoculation with Rickettsia conorii. Mice genetically defective in TLR4 signaling developed overwhelming, fatal rickettsial infections when given an inoculum that was nonfatal for TLR4-competent mice. In addition, mice lacking the ability to signal through TLR4 had significantly greater rickettsial burdens in vivo. Moreover, we observed greater concentrations of the cytokines interleukin 6 (IL-6), tumor necrosis factor alpha, IL-12p40, IL-12p70, and IL-17 in the sera of mice with intact TLR4 function as well as significantly greater quantities of activated CD4+ and CD8+ T lymphocytes. Additionally, we also observed that Th17 cells were present only in TLR4-competent mice, suggesting an important role for TLR4 ligation in the activation of this subset. In agreement with these data, we also observed significantly greater percentages of immunosuppressive regulatory T cells in the spleen during infection in TLR4-defective mice. Together, these data demonstrate that, while rickettsiae do not contain endotoxic lipopolysaccharide, they nevertheless initiate TLR4-specific immune responses, and these responses are important in protection.
The importance of toll-like receptors (TLR) in initiating and polarizing immune responses toward pathogens has been extensively chronicled (8, 17, 35). TLR4 ligation is important in activating dendritic cells (DC) toward the initiation of Th1-type or Th17 responses (40). Conversely, lack of TLR4 stimulation during antigen (Ag) presentation leads to disproportionate expansion of regulatory T lymphocytes (Treg) and subsequent suppression of immunity (26, 27).
Many important immune effector mechanisms against rickettsiae have been elucidated using a Rickettsia conorii-infected C3H/HeN mouse model (39). Owing to the obligately intracellular lifestyle of rickettsiae, cytotoxic T lymphocytes (CTL) and endothelial production of NO subsequent to gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) stimulation are critical in immunity (9, 10). Evidence suggests that NK cell activity and early NK cell-derived IFN-γ production are also important (4). Nevertheless, the early triggers leading to protective immunity, particularly the significance of TLR ligation, have not been determined. Previous studies have demonstrated that a spotted fever group rickettsia, namely, Rickettsia africae, and a phylogenetically related organism, a Wolbachia sp., are capable of inducing cellular activation through TLR4 and TLR2, for which the natural ligands are lipopolysaccharide (LPS) and peptidoglycan, respectively (5, 6).
Our laboratory has previously demonstrated that ligation of TLR may be important in initiating immunity to rickettsioses. Transfer of DC stimulated with Escherichia coli LPS induced partial protection against an ordinarily lethal rickettsial infection, suggesting that TLR4 ligation is important in protection (16). These data led us to examine the importance of TLR4 signaling in initiating a protective immune response in vivo.
To this end, we compared our well-established C3H/HeN-R. conorii model with the genetically related mouse strain C3H/HeJ. C3H/HeJ mice possess a missense mutation in the TLR4 gene, which leads to a single amino acid change in the cytoplasmic portion of TLR4, impeding signal transduction and leading to a phenotype similar to that of TLR4 knockout mice (15, 29, 30). Therefore, C3H/HeJ mice are defective in TLR4 signaling and defective in responding to LPS [TLR4(LPS-d)].
TLR4(LPS-d) mice are predisposed to developing overwhelming, fatal rickettsial infections when given an inoculum that is nonfatal to TLR4-competent C3H/HeN mice, and they develop significantly greater rickettsial burdens and decreased proinflammatory cytokine levels throughout infection. Infected TLR4(LPS-d) mice had a significantly greater percentage of Treg cells in the spleen and significantly fewer activated CD4+ and CD8+ T lymphocytes.
MATERIALS AND METHODS
Rickettsiae.
R. conorii strain Malish 7, a human isolate from South Africa, was obtained from the American Type Culture Collection (Manassas, VA) (ATCC VR 613) and cloned by plaque purification in our laboratory. The rickettsial stock for animal infections was prepared as an infected 10% yolk sac suspension and stored at −80°C. Rickettsiae for in vitro Ag stimulation were purified by density gradient centrifugation. Briefly, monolayers of Vero cells were infected with R. conorii from the 10% yolk sac stock (105 PFU per 150-cm2 flask). After 7 to 10 days of incubation at 34°C, Vero cells were harvested and lysed by ultrasonication. Rickettsiae were purified from cell debris by discontinuous density gradient centrifugation in Renografin (13). Viable rickettsiae were then collected in sucrose-phosphate-glutamic acid (SPG) buffer (0.218 M sucrose, 3.8 mM KH2PO4, 7.2 mM K2HPO4, 4.9 mM monosodium glutamic acid [pH 7.0]), stored at −80°C, and quantified by a plaque assay prior to use.
Mouse infections.
Male C3H/HeN (H-2k) (Harlan Sprague Dawley, Indianapolis, IN) or C3H/HeJ (H-2k) (Jackson Laboratories, Bar Harbor, ME) mice between the ages of 6 and 12 weeks were housed under animal biosafety level-3 specific-pathogen-free conditions according to a protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch (Galveston). In preliminary experiments, C3H/HeJ and C3H/HeN mice were challenged intravenously with serial dilutions of R. conorii to determine the relative susceptibilities of the mouse strains. The dose of 8 × 103 PFU, approximately 1 50% lethal dose (LD50) in C3H/HeN mice, was determined to cause uniform lethality in C3H/HeJ mice and was used for subsequent experiments. Mice were mock infected by diluting SPG buffer as for animal inoculations; however, R. conorii was not present in the inoculum. Mock-infected mice were unaffected by the inoculum and were phenotypically similar to mice that were not injected. After infection, mice were monitored twice daily for survival. Serial sacrifice studies were conducted to collect sera by cardiocentesis, determine rickettsemia levels, detect pathological lesions in target organs, and determine the relative and absolute numbers of immune effector cells in peripheral lymph nodes and the spleen. For experiments determining the recall response after survival of rickettsial infection, mice were inoculated with 2 × 102 PFU of R. conorii. Both strains of mice survived infection with this dose.
Brain microvascular endothelial cell isolation.
Isolation of mouse brain endothelial cells (MBEC) was adapted from previously reported protocols (28, 34). Briefly, whole brains from male C3H/HeN or C3H/HeJ mice were removed aseptically and briefly soaked with 70% ethanol to render leptomeningeal vessels nonviable. The fresh brains were stored in ice-cold Dulbecco's modified Eagle medium (DMEM)-F-12 containing 2% fetal calf serum (FCS) prior to homogenization. Brains were homogenized using a glass Dounce homogenizer and centrifuged. The resulting pellet was resuspended in 15% dextran (molecular mass, 70 kDa) and centrifuged at 11,400 × g for 10 min at 4°C to remove the myelin-containing cells. The pellet was washed once more in DMEM-F-12 with 2% fetal bovine serum (FBS) and incubated at 37°C for 1.5 h in 1 mg/ml collagenase/dispase containing 10 U/ml DNase I and 0.147 μg/ml Nα-tosyl-l-lysine chloromethyl ketone (TLCK) with constant agitation. Following the digestion step, the crude microvessels were washed in medium and plated on rat tail collagen-coated plates in growth medium containing DMEM-F-12, 10% FCS, 10% normal horse serum, 100 μg/ml endothelial cell growth supplement (Biomedical Technologies, Stoughton, MA), 100 μg/ml heparin, and 3 μg/ml puromycin. After 3 days of incubation, the puromycin was removed from the culture medium, and the cultures then consisted of pure brain endothelial cells. The cells were maintained at 37°C under 5% CO2.
Determination of Ag-specific IFN-γ production.
DC-T-cell cocultures were performed to determine the ability of DCs to present rickettsial Ags to naïve and immune CD4+ and CD8+ T cells. For comparison, T cells were cocultured with macrophages; 106 DC or splenic macrophages were inoculated with heat-killed R. conorii (multiplicity of infection equivalent to 5) and cocultivated with 5 × 106 T-cells in 1 ml in 24-well plates (ratio of antigen-presenting cells to T cells, 1:5). After 48 h of culture, the supernatant was collected as described above for assessment of cytokine production.
Flow cytometry.
Phenotypic analysis of immune effector cells was accomplished by staining cell suspensions in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 1% FBS and 0.1% NaN3) with fluorochrome-conjugated monoclonal antibodies (MAbs). Prior to being stained with fluorochrome-conjugated MAbs, Fc receptors were blocked with anti-CD16/32 (Fc Block, 2.4G2). The intensity of fluorescence was measured on a FACScan or FACScalibur flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using CellQuest (BD Biosciences) or FlowJo (TreeStar, San Carlos, CA) software. For analysis of intracellular cytokines, cell surface molecules were stained essentially as described above. Thereafter, cells were washed twice with FACS buffer and were fixed and permeabilized with 100 μl Cytofix/Cytoperm (BD Biosciences) for 20 min on ice. Cells were then washed two times in Perm/Wash (BD Biosciences) and resuspended in 50 μl Perm/Wash. Applicable cytokine-specific fluorochrome-conjugated MAbs were then added, and cells were incubated for 20 min. Subsequently, cells were washed with FACS buffer and analyzed as described above. Antibodies used included anti-CD3e (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD25 (7D4), anti-CD69 (H1.2F3), anti-IL-17 (TC11-18H10), anti-IFN-γ (XMG1.2), anti-FoxP3 (150D) (BioLegend, San Diego, CA), and appropriate isotype controls. Antibodies were obtained from BD Biosciences (San Diego, CA) unless otherwise noted.
Determination of cytokine production.
The levels of interleukin 2 (IL-2), IL-4, IL-6, IL-10, IL-12p40, IL-12p70, IL-17, RANTES, TNF-α, and IFN-γ in the supernatants of DC activation assays and the sera of animals after R. conorii infection were quantified using the BioPlex chemiluminescent bead assay from Bio-Rad Systems. IL-23 was quantitated using a commercial enzyme-linked immunosorbent assay (eBioscience), and data were collected using a Versa Max microplate reader and SoftMAX pro software (Molecular Devices, Sunnyvale, CA).
Real-time PCR quantitation of rickettsemia levels.
Rickettsiae in tissues were measured as described previously (16). Briefly, 1-mm3 pieces (approximately 2 mg) of brain, lung, and spleen were harvested on days 1, 3, and 5 postinfection and were stored at −20°C until processing. Tissue pieces were homogenized, and DNA was purified using the DNeasy tissue kit (Qiagen, Valencia, CA). Plasmids containing rickettsial gltA and murine β-actin PCR products were constructed using the TOPO 2.1 and TOPO 4 cloning kits, respectively. Rickettsial gltA was amplified using the forward primer CS-5 (5′-GAGAGAAAATTATATCCAAATGTTGAT) and the reverse primer CS-6 (5′-AGGGTCTTCGTGCATTTCTT). Murine β-actin DNA was amplified using the forward primer 5′-AGAGGGAAATCGTGCGTGAC and the reverse primer 5′-CAATAGTGATGACCTGGCCGT. Real-time PCR was performed using Sybr green Supermix, 1 μl DNA, and primers (0.2 μM) on an iCycler real-time PCR apparatus (Bio-Rad, Hercules, CA). Standard curves were generated using plasmids containing cloned PCR products. All PCRs were performed using the following protocol: 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 50°C for 15 s, and 60°C for 15 s. Data are expressed as the average number of rickettsial gltA copies per 10,000 copies of β-actin.
Histopathology and immunohistology.
Necropsies were performed on mice sacrificed on days 0, 1, 3, and 5 postinfection. Brain, lung, and spleen were immersed in zinc fixative (BD Biosciences) for immunohistology or histopathology, embedded in paraffin, and sectioned at a thickness of 5 μm. Sections were processed and stained with hematoxylin and eosin for histopathologic evaluation. For immunohistology, a rabbit anti-R. conorii polyclonal antibody was used to detect rickettsial Ag (37, 39) using Vectastain ABC reagents (Vector Laboratories, Burlingame, CA) and either Vector Red or 3,3′-diaminobenzidine.
Statistics.
Data are expressed as means ± standard errors of the means (SEM) or standard deviations (SD), and the significant differences between two series of results were determined using Student's unpaired t test with Sigma Stat software. P values of <0.05 were considered significant.
RESULTS
TLR4 dysfunction leads to increased susceptibility to severe R. conorii infection.
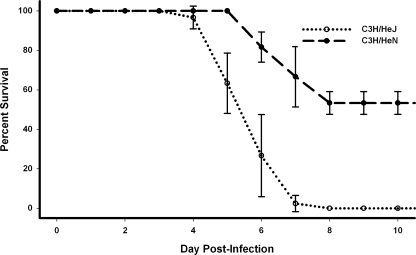
In order to determine the significance of TLR4 signaling in immunity to spotted-fever rickettsiae, we used our well-established murine model of spotted-fever rickettsioses in the C3H/HeN mouse and compared it to the genetically related mouse with defective TLR4 function, C3H/HeJ [TLR4(LPS-d)] mice. TLR4(LPS-d) mice given an R. conorii inoculum that is near the LD50 in C3H/HeN mice were all lethally infected (Fig. 1). Both TLR4(LPS-d) and C3H/HeN mice survived mock infection (data not shown). Additionally, TLR4(LPS-d) mice succumbed to infection earlier than C3H/HeN mice. It is noteworthy also that lethally infected TLR4(LPS-d) mice appeared ill (characterized by hunched posture, lethargy, and ruffled hair) later than C3H/HeN mice.
FIG. 1.
Deficiency in TLR4 leads to greater susceptibility to fatal R. conorii infection in mice. C3H/HeJ [TLR4(LPS-d)] and C3H/HeN mice were infected intravenously with 8 × 103 PFU R. conorii and monitored for survival (n = 10 mice per group). Each curve demonstrates the mean survival per day postinfection for three independent experiments. Error bars, SD. Mice that survived to day 10 postinfection remained alive and were immune to reinfection. Mock-infected mice were phenotypically similar to uninfected controls and survived challenge (data not shown).
These data led us to investigate the susceptibility of TLR4(LPS-d) mice more fully. While the typical LD50 of R. conorii in C3H/HeN mice is between 8 × 103 and 1 × 104 PFU, infecting TLR4(LPS-d) mice with doses as low as 7.5 × 102 PFU of R. conorii led to >60% mortality, while C3H/HeN mice all survived infection at this dose (Table 1).
TABLE 1.
TLR4(LPS-d) mice exhibit enhanced susceptibility to R. conorii infection throughout infective dose rangesa
| R. conorii dose (PFU) | No. of mice that died/no. infected (% mortality)
|
|
|---|---|---|
| C3H/HeN | C3H/HeJ (TLR4(LPS-d)) | |
| 2.4 × 104 | 6/6 (100) | 6/6 (100) |
| 1.2 × 104 | 5/6 (83) | 6/6 (100) |
| 6 × 103 | 3/6 (50) | 6/6 (100) |
| 3 × 103 | 1/6 (17) | 6/6 (100) |
| 1.5 × 103 | 0/6 (0) | 4/6 (67) |
| 7.5 × 102 | 0/6 (0) | 4/6 (67) |
| 3.7 × 102 | 0/6 (0) | 3/6 (50) |
C3H/HeN and TLR4(LPS-d) mice were infected intravenously with twofold dilutions of R. conorii and were monitored for survival. The established LD50 for this stock is approximately 8 × 103 PFU in C3H/HeN mice.
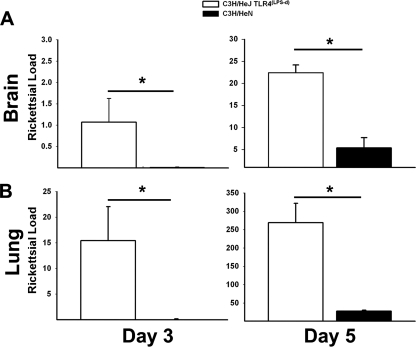
Rickettsial loads in the organs are significantly greater in mice deficient in TLR4 function.
To evaluate whether the enhanced susceptibility observed in TLR4(LPS-d) mice correlated with rickettsial burden differences in vivo, we determined the relative rickettsial loads throughout infection in the brain and lung. TLR4(LPS-d) mice had significantly greater numbers of rickettsiae in the brain and lungs on days 3 and 5 postinfection (Fig. 2A and B). Rickettsial gltA was undectectable in mock-infected controls (data not shown). It is noteworthy that rickettsial loads were nearly undetectable in C3H/HeN mice, relative to those in TLR4(LPS-d) mice, suggesting significant control of rickettsial growth in TLR4-competent mice. Moreover, it should be noted that in both strains of mice, rickettsial loads in the lungs were approximately 1 log unit greater than those observed in the brain, suggesting that following tail vein injection, many rickettsiae infect the endothelium of the alveolar capillaries, delaying spread to the brain until later in infection. The differences in rickettsial loads in the brain and lung were also observed by immunohistochemistry. Rickettsial Ag was observed, by staining, at greater intensity and in greater quantity for mice lacking TLR4 function (data not shown).
FIG. 2.
Increased susceptibility of TLR4(LPS-d) mice to R. conorii is associated with increased rickettsial burdens. Rickettsial loads in the brains (A) and lungs (B) of mice on days 3 and 5 postinfection were quantitated by quantitative real-time PCR. Data are expressed as rickettsial gltA copies per 10,000 murine β-actin copies and represent means for 3 mice per treatment group per day ± SD. *, P < 0.05. Similar statistically significant differences were observed in the second experiment.
TLR4(LPS-d) mice have fewer splenic CD8 T lymphocytes after rickettsial infection in association with increased Treg cells in lymph nodes.
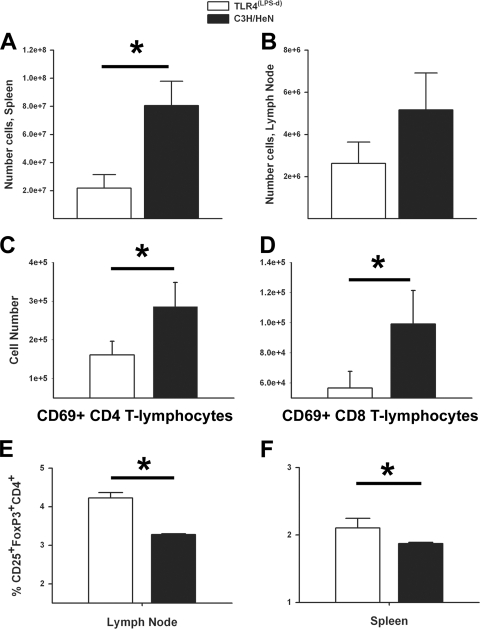
In light of the observation that TLR4 ligation appears to significantly limit rickettsial proliferation in vivo, we focused on evaluating the differences in cellular immunity after R. conorii infection. Necropsies performed on day 5 postinfection revealed marked differences in the sizes of the spleen between TLR4(LPS-d) mice and mice with competent TLR4 responses (data not shown). Consistent with a grossly enlarged spleen, mice with functional TLR4 responses had significantly greater splenic lymphocyte contents on day 5 postinfection, although significant differences in lymphocyte quantities were not present in the peripheral lymph nodes (Fig. 3A and B).
FIG. 3.
TLR4 ligation leads to significantly greater numbers of activated T lymphocytes in vivo after R. conorii infection. Splenic (A) and lymph node (B) single-cell suspensions were enumerated on day 5 postinfection in order to determine the total cellular numbers. Percentages of early-activated (CD69+) CD4+ (C) and CD8+ (D) T lymphocytes in the spleen were assessed by flow cytometry, and total numbers were determined by multiplying the percentages obtained by the numbers of cells recovered. The percentages of regulatory T lymphocytes (CD4+ CD25+ FoxP3+) in peripheral lymph nodes (E) and spleen (F) on day 3 postinfection were assessed by flow cytometry. Data are means for three mice per time point. Error bars, SD. *, P < 0.05.
In addition to determining absolute lymphocyte populations, we also determined the percentages and absolute numbers of immune effector cells in the spleen. TLR4(LPS-d) mice had significantly fewer activated (CD69+) CD4+ and CD8+ T lymphocytes in the spleen on day 5 of infection (Fig. 3C and D). Despite these dramatic differences in absolute cell quantities, it should be noted that the percentages of the lymphocyte subsets did not differ significantly (data not shown); therefore, although TLR4 leads to significant augmentation of the cellular immune response, it does not alter the overall proportions of immune cells in response to rickettsial infection.
Our observation of possible immunosuppression in TLR4(LPS-d) mice, indicated by decreased immune cell proliferation and significantly fewer early-activated T lymphocytes, also spurred investigation into the role of Treg cells in these susceptible mice. TLR4(LPS-d) mice had a significantly greater percentage of Treg cells (FoxP3+ CD25+ CD4+ T lymphocytes) in peripheral lymph nodes than C3H/HeN mice (Fig. 3E) on day 3 postinfection. TLR4(LPS-d) mice also had greater numbers of Treg cells in the spleen (Fig. 3F).
Mice with competent TLR4 responses produce significantly greater quantities of proinflammatory cytokines in vivo.
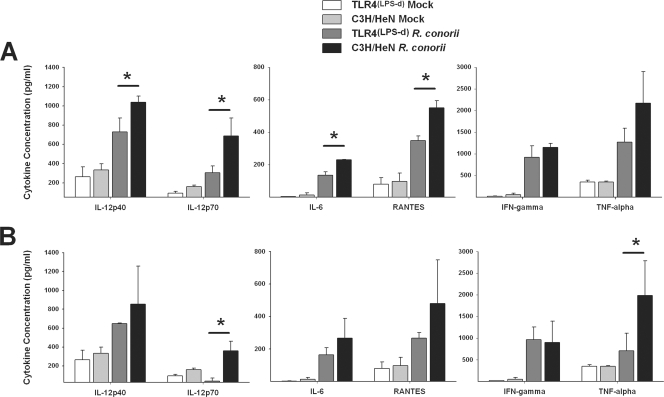
Our evaluation of the immune effector cell response, specifically the expansion of immune effector subtypes, revealed that mice that possessed functional TLR4 responses had significantly greater numbers of immune effector cells (activated CD4 and CD8 T lymphocytes), while TLR4(LPS-d) mice had a greater percentage of Treg cells in lymph nodes and spleen. We, therefore, examined the cytokine concentrations in the sera on days 3 and 5 after infection with R. conorii in order to determine the effect that TLR4 ligation had on systemic cytokine production. Mice possessing functional TLR4 receptors had significantly higher levels of the prototypical Th1-inducing cytokines IL-12p40 and IL-12p70 than TLR4(LPS-d) mice 3 days postinfection (Fig. 4A). TLR4-competent mice also had significantly higher levels of the leukocyte chemotactic factor RANTES (CCL5) and IL-6, although levels of two cytokines implicated in antirickettsial activity, TNF-α and IFN-γ, were not significantly higher than in TLR4(LPS-d) mice.
FIG. 4.
TLR4 dysfunction significantly decreases serum proinflammatory cytokine production in vivo. Sera from TLR4(LPS-d) and C3H/HeN mice, either infected with R. conorii or mock infected, were obtained on days 3 (A) and 5 (B) after infection by cardiac puncture. Quantitation of serum cytokines was determined by a BioPlex assay. Data are means for 3 mice per time point; error bars indicate SD. *, P < 0.05.
On day 5 postinfection, similar cytokine profiles prevailed; however, levels of IL-12p40 and RANTES were no longer statistically different. However, IL-12p70 levels still remained significantly higher in TLR4-competent mice than in TLR4(LPS-d) mice, and TNF-α levels were also significantly elevated in TLR4-competent mice (Fig. 4B). Of note, IL-12p70 was nearly undetectable in TLR4(LPS-d) mice, suggesting a significant failure in polarization toward a Th1 response.
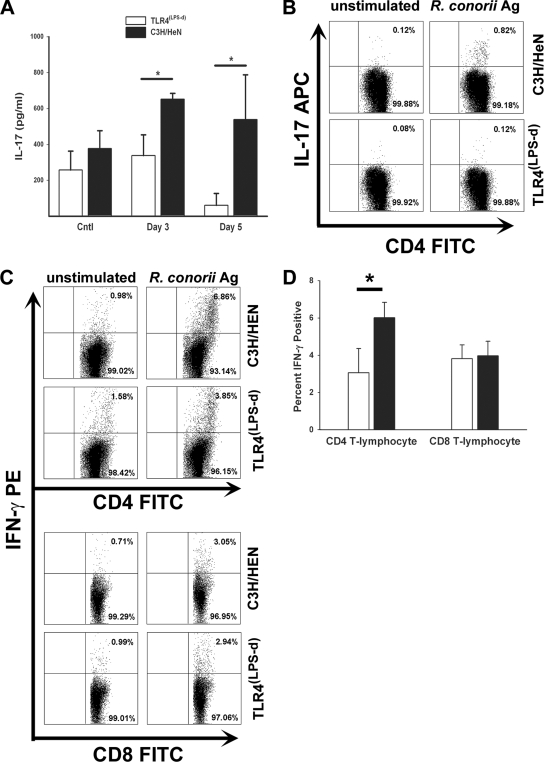
Mice with functional TLR4 responses generated Ag-specific Th17 cells and had significantly greater levels of IFN-γ-producing CD8+ and CD4+ T lymphocytes during acute rickettsial infection.
Our data suggested that despite the perception of diminished proinflammatory cytokine responses and blunted recall responses, memory responses were still generated despite a lack of TLR4 signaling capability. We therefore sought to further characterize the differences in the immune responses during acute infection. Previous research had suggested that in other bacterial infections, TLR4 ligation was necessary for the generation of IL-17-producing T lymphocytes. To determine if rickettsial infection induced IL-17 production in response to TLR4 stimulation, we collected serum by cardiac puncture on days 3 and 5 postinfection and then isolated splenocytes on day 5, exposed them to rickettsial Ag for 24 h in vitro, and determined the levels of IL-17 in serum and the quantities of IFN-γ-secreting CD4+ and CD8+ T lymphocytes. Mice with competent TLR4 responses had significantly higher IL-17 levels in the serum on both days 3 and 5 postinfection than mice deficient in TLR4 signaling (Fig. 5A). Moreover, we demonstrated that TLR4-competent mice had significantly more Th17 cells (IL-17-producing CD4+ T lymphocytes) on day 5 postinfection (Fig. 5B). In addition to significantly higher levels of serum IL-17 and Th17 cells, TLR4-competent mice also had a greater number of Ag-specific Th1 cells (IFN-γ-producing CD4+ T lymphocytes) on day 5 postinfection; however, we did not observe a significant difference in the numbers of IFN-γ-producing CD8+ T lymphocytes (Fig. 5C and D).
FIG. 5.
TLR4-competent mice had significantly higher levels of Th17 and Th1 responses during rickettsial infection. (A) IL-17 concentrations in serum were assayed prior to infection and on days 3 and 5 postinfection. (B to D) Splenocytes were collected on day 5 postinfection and were stimulated in vitro for 24 h. Thereafter, cells were subjected to flow cytometry to determine the phenotype of cells producing IL-17 (B) and IFN-γ (C and D). Data are means for three animals per treatment group per time point ± SD (error bars). *, P < 0.05.
DISCUSSION
In the current study, we have demonstrated the importance of TLR4 signaling in rickettsial infection. Specifically, we have determined that mice with competent TLR4 responses were more resistant to rickettsial infection; moreover, this resistance was associated with an increase in the number of known antirickettsial effector cells, specifically Th1-polarized CD4+ and CD8+ T lymphocytes. Substantial information exists on the role of TLR stimulation, particularly TLR4 stimulation, in augmenting adaptive immunity (3, 7, 20, 38). TLR4 stimulation in DC by gram-negative pathogens causes polarization toward Th1 cellular differentiation, in part due to IL-12 production (23, 31). Additionally, lack of TLR4 stimulation during Ag presentation leads to Treg expansion and the suppression of proinflammatory immune responses. (26, 27). This study corroborated previous findings on the importance of TLR4 stimulation in the initiation of Th1- and Th17-driven proinflammatory responses. Additionally, we have demonstrated, for the first time, that TLR4 stimulation is important in the expansion of Th1 T lymphocytes in rickettsial infection.
These results are particularly significant due to the fact that rickettsiae do not possess classical endotoxic LPS yet are still capable of initiating a TLR4-mediated response (2). Previously, it was believed that rickettsial LPS possessed little immunologic significance due to the fact that its endotoxic activity required substantially higher doses than typical LPS of gram-negative bacteria (33). The chemical composition of rickettsial LPS is somewhat different from that of enterobacterial LPS (2). Moreover, the death of IFN-γ-primed RAW 264.7 cells upon exposure to Rickettsia prowazekii occurs independently of the effects of LPS from E. coli 0111:B4 (36). Additionally, treatment of SCID mice with antibodies to rickettsial LPS does not provide protection against fatal rickettsial infection (11). However, we now demonstrate that TLR4, presumably signaling due to ligation with rickettsial LPS, is an important mediator of immunity to rickettsiae.
We observed significant differences in the adaptive immune responses. TLR4 stimulation is important in polarizing adaptive immune responses toward a Th1-type immune response associated with the production of IL-12p40 and IL-23 (1, 12, 14, 32). In previous studies, we demonstrated that DC stimulated by rickettsiae produce Th1-type cytokines similar to those produced by LPS-stimulated DC in vitro, particularly IL-23 and IL-12p40 (16). IL-23 promotes the expansion of a distinct population of Th17 cells, which play a critical role in autoimmunity yet are also implicated in protective immune responses to bacterial pathogens (14, 18, 19). In the current study, we observed a reduced Th1-type response in TLR4(LPS-d) mice during R. conorii infection; in particular, TLR4(LPS-d) mice produced significantly less IL-12p40, IL-12p70, and IL-23. The production of these cytokines in rickettsia-infected TLR4-competent mice was also associated with the expansion of activated (CD69+) CD4+ and CD8+ T lymphocytes in the spleen.
We also observed that TLR4(LPS-d) mice had a significantly greater percentage of Treg cells in their peripheral lymph nodes, which could potentially suppress proinflammatory responses through the production of IL-10 or transforming growth factor β (25) and limit the initiation of adaptive immunity by decreasing the number of effector cells during Ag stimulation (22, 24). Under some conditions, mice that lack Treg cells eventually succumb to massive immune-mediated damage (21). Thus, deleterious expansion of Treg cells during infection may limit protective immunity. In this study, we have demonstrated that TLR4(LPS-d) mice given an ordinarily sublethal dose of R. conorii had a defect in the generation of Ag-specific T lymphocytes. Both CD4+ and CD8+ T lymphocytes from TLR4(LPS-d) mice produced significantly less Ag-specific IFN-γ than mice with competent TLR4 responses, suggesting that TLR4 signaling leads to a greater number of Ag-specific memory T lymphocytes. However, we observed that TLR4(LPS-d) mice that survived rickettsial infection were resistant to reinfection with a normally lethal dose, suggesting that a protective anamnestic response can occur in the absence of TLR4 signaling (data not shown).
In conclusion, the present study has established that TLR4 signaling plays an important role in both innate and adaptive protective immunity against R. conorii infection in vivo, inducing NK cell proliferation and cytotoxic activity, limiting rickettsial growth in vivo, and polarizing the response toward a Th1 antirickettsial phenotype.
Acknowledgments
This work was supported by a grant (A121242) from the National Institute of Allergy and Infectious Diseases.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. van Dyke, and B. Pulendran. 2003. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 1714984-4989. [DOI] [PubMed] [Google Scholar]
- 2.Amano, K., M. Fujita, and T. Suto. 1993. Chemical properties of lipopolysaccharides from spotted fever group rickettsiae and their common antigenicity with lipopolysaccharides from Proteus species. Infect. Immun. 614350-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banus, H. A., R. J. Vandebriel, H. de Ruiter, J. A. Dormans, N. J. Nagelkerke, F. R. Mooi, B. Hoebee, H. J. van Kranen, and T. G. Kimman. 2006. Host genetics of Bordetella pertussis infection in mice: significance of Toll-like receptor 4 in genetic susceptibility and pathobiology. Infect. Immun. 742596-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billings, A. N., H. M. Feng, J. P. Olano, and D. H. Walker. 2001. Rickettsial infection in murine models activates an early anti-rickettsial effect mediated by NK cells and associated with production of gamma interferon. Am. J. Trop. Med. Hyg. 6552-56. [DOI] [PubMed] [Google Scholar]
- 5.Brattig, N. W., C. Bazzocchi, C. J. Kirschning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173437-445. [DOI] [PubMed] [Google Scholar]
- 6.Damas, J. K., M. Jensenius, T. Ueland, K. Otterdal, A. Yndestad, S. S. Froland, J. M. Rolain, B. Myrvang, D. Raoult, and P. Aukrust. 2006. Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J. Immunol. 1772699-2706. [DOI] [PubMed] [Google Scholar]
- 7.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 1681704-1709. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, A. D., S. P. Manickasingham, R. Sporri, S. S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 1693652-3660. [DOI] [PubMed] [Google Scholar]
- 9.Feng, H., V. L. Popov, G. Yuoh, and D. H. Walker. 1997. Role of T lymphocyte subsets in immunity to spotted fever group rickettsiae. J. Immunol. 1585314-5320. [PubMed] [Google Scholar]
- 10.Feng, H. M., V. L. Popov, and D. H. Walker. 1994. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect. Immun. 621952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, H. M., T. Whitworth, J. P. Olano, V. L. Popov, and D. H. Walker. 2004. Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 722222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flohe, S. B., J. Bruggemann, S. Lendemans, M. Nikulina, G. Meierhoff, S. Flohe, and H. Kolb. 2003. Human heat shock protein 60 induces maturation of dendritic cells versus a Th1-promoting phenotype. J. Immunol. 1702340-2348. [DOI] [PubMed] [Google Scholar]
- 13.Hanson, B. A., C. L. Wisseman, Jr., A. Waddell, and D. J. Silverman. 1981. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect. Immun. 34596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins, S. C., A. G. Jarnicki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 1777980-7989. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1623749-3752. [PubMed] [Google Scholar]
- 16.Jordan, J. M., M. E. Woods, H. M. Feng, L. Soong, and D. H. Walker. 2007. Rickettsiae-stimulated dendritic cells mediate protection against lethal rickettsial challenge in an animal model of spotted fever rickettsiosis. J. Infect. Dis. 196629-638. [DOI] [PubMed] [Google Scholar]
- 17.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2278-83. [DOI] [PubMed] [Google Scholar]
- 18.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8369-377. [DOI] [PubMed] [Google Scholar]
- 19.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70 is available. J. Immunol. 175788-795. [DOI] [PubMed] [Google Scholar]
- 20.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15396-401. [DOI] [PubMed] [Google Scholar]
- 21.Liston, A., A. G. Farr, Z. Chen, C. Benoist, D. Mathis, N. R. Manley, and A. Y. Rudensky. 2007. Lack of Foxp3 function and expression in the thymic epithelium. J. Exp. Med. 204475-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mempel, T. R., M. J. Pittet, K. Khazaie, W. Weninger, R. Weissleder, H. von Boehmer, and U. H. von Andrian. 2006. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25129-141. [DOI] [PubMed] [Google Scholar]
- 23.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor, G. M., O. M. Hart, and C. M. Gardiner. 2006. Putting the natural killer cell in its place. Immunology 1171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oida, T., L. Xu, H. L. Weiner, A. Kitani, and W. Strober. 2006. TGF-β-mediated suppression by CD4+ CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 1772331-2339. [DOI] [PubMed] [Google Scholar]
- 26.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 2991033-1036. [DOI] [PubMed] [Google Scholar]
- 27.Pasare, C., and R. Medzhitov. 2004. Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21733-741. [DOI] [PubMed] [Google Scholar]
- 28.Perriere, N., P. Demeuse, E. Garcia, A. Regina, M. Debray, J. P. Andreux, P. Couvreur, J. M. Scherrmann, J. Temsamani, P. O. Couraud, M. A. Deli, and F. Roux. 2005. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J. Neurochem. 93279-289. [DOI] [PubMed] [Google Scholar]
- 29.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 2822085-2088. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 27637692-37699. [DOI] [PubMed] [Google Scholar]
- 32.Rolland, A., E. Jouvin-Marche, C. Viret, M. Faure, H. Perron, and P. N. Marche. 2006. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 1767636-7644. [DOI] [PubMed] [Google Scholar]
- 33.Schramek, S., R. Brezina, and R. Kazar. 1977. Some biological properties of an endotoxic lipopolysaccharide from the typhus group rickettsiae. Acta Virol. 21439-441. [PubMed] [Google Scholar]
- 34.Song, L., and J. S. Pachter. 2003. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell. Dev. Biol. Anim. 39313-320. [DOI] [PubMed] [Google Scholar]
- 35.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 36.Turco, J., and H. H. Winkler. 1994. Relationship of tumor necrosis factor alpha, the nitric oxide synthase pathway, and lipopolysaccharide to the killing of gamma interferon-treated macrophage-like RAW 264.7 cells by Rickettsia prowazekii. Infect. Immun. 622568-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valbuena, G., J. M. Jordan, and D. H. Walker. 2004. T cells mediate cross-protective immunity between spotted fever group rickettsiae and typhus group rickettsiae. J. Infect. Dis. 1901221-1227. [DOI] [PubMed] [Google Scholar]
- 38.Viriyakosol, S., M. A. Matthias, M. A. Swancutt, T. N. Kirkland, and J. M. Vinetz. 2006. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar Icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect. Immun. 74887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, D. H., V. L. Popov, J. Wen, and H. M. Feng. 1994. Rickettsia conorii infection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab. Investig. 70358-368. [PubMed] [Google Scholar]
- 40.Wynn, T. A. 2005. TH-17: a giant step from TH1 and TH2. Nat. Immunol. 61069-1070. [DOI] [PubMed] [Google Scholar]