Abstract
mntABC from Neisseria gonorrhoeae encodes an ABC permease which includes a periplasmic divalent cation binding receptor protein of the cluster IX family, encoded by mntC. Analysis of an mntC mutant showed that growth of N. gonorrhoeae could be stimulated by addition of either manganese(II) or zinc(II) ions, suggesting that the MntABC system could transport both ions. In contrast, growth of the mntAB mutant in liquid culture was possible only when the medium was supplemented with an antioxidant such as mannitol, consistent with the view that ion transport via MntABC is essential for protection of N. gonorrhoeae against oxidative stress. Using recombinant MntC, we determined that MntC binds Zn2+ and Mn2+ with almost equal affinity (dissociation constant of ∼0.1 μM). Competition assays with the metallochromic zinc indicator 4-(2-pyridylazo)resorcinol showed that MntC binds Mn2+ and Zn2+ at the same binding site. Analysis of the N. gonorrhoeae genome showed that MntC is the only Mn/Zn metal binding receptor protein cluster IX in this bacterium, in contrast to the situation in many other bacteria which have systems with dedicated Mn and Zn binding proteins as part of distinctive ABC cassette permeases. Both the mntC and mntAB mutants had reduced intracellular survival in a human cervical epithelial cell model and showed reduced ability to form a biofilm. These data suggest that the MntABC transporter is of importance for survival of Neisseria gonorrhoeae in the human host.
Neisseria gonorrhoeae is a mucosal pathogen often associated with the genitourinary tract, and it is the etiological agent of the sexually transmitted disease gonorrhea (31). Gonorrhea is often characterized by a localized inflammatory response involving inflamed urogenital tissues and activated polymorphonuclear leukocytes (PMNs) (26). This innate immune response includes production of superoxide radical (O2−), hydrogen peroxide (H2O2), and reactive nitrogen species. The accumulation of reactive oxygen species around the site of gonococcal infection can exert a cytotoxic effect, which results from oxidant interaction with proteins, lipids, and nucleic acids (18, 32). Despite the constant environmental stress elicited by O2− and H2O2, gonococci are routinely isolated from PMN-laden purulent exudates. Furthermore, studies suggest that gonococci not only survive within PMNs but may also replicate within these cells (27, 29, 36). The ability of gonococci to survive in environments high in reactive oxygen species suggests that this bacterium has an efficient antioxidant defense system.
Previous studies show that the accumulation of manganese (Mn), via the Mn transporter, MntABC, is important for protection of N. gonorrhoeae from O2− and H2O2 killing via a mechanism that is independent of superoxide dismutase (34) and catalase (30). MntABC is an ATP binding cassette (ABC) permease, and it contains a periplasmic metal binding receptor protein (MBR), MntC (34). Protein phylogenetic analysis indicates that MntC is a member of the cluster IX family of solute binding proteins that interact with metal ions (9). Generally, cluster IX MBRs demonstrate specificity for Zn2+, Mn2+, and (occasionally) Fe2+ (7). The high-resolution crystal structures of several cluster IX MBRs have been determined. These include ZnuA, from Synechocystis sp. strain 6803 (3); PsaA, from Streptococcus pneumoniae (20) and TroA, from Treponema pallidium (21). PsaA consists of two (β/α)4 domains linked by a single α-helical “backbone” (20). A single Zn2+ ion was found to be coordinated between the two domains. The presence of a single linking helix was a significant departure from previously characterized solute binding proteins associated with ABC permeases. Solute binding proteins involved in binding large substrates such as SO42− invariably have a flexible hinge region linking the two domains which allows binding and release of the substrate and may signal transport. A single helical linker in the MBRs limits the ability of the domains to move, which may stabilize the protein and allow metal binding. Since the structure of PsaA was solved, others, including T. pallidum TroA (21), Synechocystis ZnuA (3) and MntC (28), and Escherichia coli ZnuA (22), have all been found to have two domains linked by a long helix. In all structures the metal ion (Zn2+ or Mn2+) is bound at the interface between the domains, and it is not clear how the structure influences metal specificity. An interesting feature of some of these proteins is the presence of an unstructured, flexible histidine-rich sequence that is located near the metal binding site. Studies with ZnuA (Synechocystis) have shown that this loop has a very weak affinity for Zn2+ but does not affect the main high-affinity site (37). It has been suggested that this loop may be involved in the sensing of zinc concentrations rather than directing Zn2+ to the main site. The histidine-rich region is absent in zinc transporters such as TroA and varies in length. Thus, its exact role remains unclear.
Although purified PsaA contains Zn, all of the available evidence indicates that in S. pneumoniae the PsaABC permease transports Mn2+ into the cell and is required for protection against oxidative stress (23). In S. pneumoniae a distinct ABC transporter, AdcCBA, is required for Zn2+ transport as well as optimal growth of S. pneumoniae (10). In T. pallidum, the TroABCD permease was reported to transport both Zn2+ and Mn2+ with similar affinities, suggesting multiple metal specificities of TroA.
Our in vitro experiments have suggested that MntABC is important in defense against oxidative stress in the gonococcus (34), and this defense was dependent upon the presence of Mn2+ ions. To provide a better understanding of the influence of metal ions on oxidative stress defense mechanisms in the gonococcus, we have purified recombinant MntC (rMntC), investigated the metal binding properties of this protein, and characterized further the phenotypic properties of mntC and mntAB mutants in relation to the ability of gonococci to invade human cervical epithelial cells and form a biofilm.
MATERIALS AND METHODS
Bacterial media and growth.
N. gonorrhoeae 1291 strains were cultured on solid brain heart infusion (BHI) agar (Becton Dickinson) with IsoVitaleX (Becton Dickinson) and levithal (1) and incubated at 37°C in a 5% elevated carbon dioxide chamber overnight. Fresh colonies were subsequently transferred to BHI broth (Oxoid) with shaking at 180 rpm at 37°C overnight. For growth studies, the gonococcus colonies were selected from agar, grown in BHI broth until mid-log phase, and then pelleted and resuspended in same medium to a desired starting optical density at 600 nm (OD600) of 0.001. Various concentrations of Zn(II) and Mn(II) sulfate and mannitol were added for gonococcus phenotype rescue experiments. The construction of mntC and mntAB mutants has been described previously (34, 38). Both mutations were constructed by insertion of a kanamycin resistance cassette inserted in an orientation to allow transcriptional readthrough into the distal genes. In this way polar effects of the insertional mutagenesis are avoided. The cell growth was monitored at regular intervals by measuring the OD600. E. coli cells were grown aerobically in Luria-Bertani (LB) medium with appropriate antibiotic and shaking (180 rpm) at 37°C.
Chemicals.
All buffers were prepared using MilliQ deionized water. MES (morpholineethanesulfonic acid), Tris and HEPES buffer salts, and 4-(2-pyridylazo)resorcinol (PAR) were obtained from Sigma. All chromatography materials were obtained from GE Healthcare.
Cloning of the mntC gene.
The mntC coding sequence was PCR amplified from N. gonorrhoeae 1291 using forward primer 5′-AAA GAG CTC GCA CCC CTT CCG GT-3′ and reverse primer 5′-ACC CTG CAG TTA TTG CTT CAT CGC-3′, creating the SacI and PstI restriction sites. The purified PCR product was double digested with SacI and PstI and subsequently cloned into the SacI and PstI sites of expression vector pBad/His (Invitrogen). The plasmid was transformed into competent E. coli Top10 cells. Transformants were selected on LB agar containing 100 μg/ml ampicillin.
Expression and purification of rMntC in E. coli.
E. coli Top10 cells carrying the recombinant pBad-mntC plasmid were grown overnight in LB and ampillicin at 37°C. The overnight culture was diluted 1:100 into fresh LB medium and incubated at 37°C with shaking at 180 rpm until an OD600 of 0.4 was obtained. His-tagged MntC expression was induced by adding 0.2% (wt/vol) l-arabinose, and the cells were allowed to grow overnight. Cells were harvested by centrifugation, and the cell pellets were resuspended in 20 ml lysis buffer (50 mM NaH2PO4 [pH 8.0], 300 mM NaCl, and 10 mM imidazole). The cells were lysed by passage through a French press (1.4 × 108 lb/in2) and centrifuged twice to remove any unlysed cells and cell debris. The resultant supernatant was loaded into a preequilibrated nickel-chelated Sepharose fast-flow gel column (GE Healthcare). Purification of His-tagged MntC was conducted using methods described in the ProBond purification kit (Invitrogen). The purified proteins were analyzed by polyacrylamide gel electrophoresis. Electrophoretic analysis of protein samples was conducted using either 10% (wt/vol) or 12% (wt/vol) sodium dodecyl sulfate-polyacrylamide gels. Purified MntC was then dialyzed against storage buffer (50 mM MES, pH 6.0). The protein concentration was determined using the calculated molar extinction coefficient at 280 nm of 23,380 M−1 cm−1 (12). Protein samples were concentrated using Centricon Ultra 15 centrifugal filters with YM-10 MW membranes (10,000-molecular-weight cutoff) (Millipore) in accordance with manufacturer's instructions.
Cleavage and purification of MntC.
The hexahistidine tag from His-MntC was cleaved using an enterokinase, EKMAX (Invitrogen). Cleaved MntC was purified using a PD-10 column (GE Healthcare). The eluate was collected in 0.2-ml fractions and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with Coomassie blue staining. Fractions containing protein were pooled and stored at −80°C. Western blot analysis was used to determine the presence or absence of the hexahistidine tags in the expressed recombinant protein. Proteins were transferred to a nitrocellulose membrane (Protran; Schleicher & Schuell) after polyacrylamide gel electrophoresis, using a Hoeffer minigel system. The membrane was then treated according to the manufacturer's instructions for monoclonal antipolyhistidine clone His-1 (Sigma, St. Louis, MO).
Production of apoprotein.
Metal-free protein was formed by denaturation and refolding the native MntC protein. The native protein was denatured by dialysis using a Slide-A-Lyzer (Pierce) with denaturing buffer [6 M guanidine-HCl, 2× N,N,N′,N′-tetrakis-(2-pyridylmethyl)ethylenediamine, 2 mM Na2EDTA, dithiothreitol [DTT]) overnight at 25°C. The denatured protein was then refolded through a series of dialyses performed at 4°C with pH 7.5 solutions: (i) buffer A (6 M urea, 2 mM Na2EDTA, and 1 mM DTT) for 4 hours, (ii) buffer B (2 M urea, 2 mM Na2EDTA, and 1 mM DTT) for 4 hours, and (iii) buffer C (2 mM Na2EDTA and 0.75 ml of 1 M DTT solution) overnight.
Determination of metal content using ICP-MS.
To determine the metal content of N. gonorrhoeae, an acid digestion was performed prior to inductively coupled plasma-mass spectrometry (ICP-MS) sample analysis. The culture was grown overnight in BHI broth, pelleted, and resuspended in 1 ml BHI broth, and then 0.5 ml of the culture was transferred to aluminum foil and left to dry overnight in 68°C oven. The dry weight of the culture was noted, and 1 ml of concentrated nitric acid was added to 0.5 ml of the remaining liquid culture. The sample was then allowed to stand at room temperature for 20 min or until the effervescence ceased. Subsequently, the sample was placed in a boiling water bath for 9 h. The sample was then diluted to a final solution of 2% nitric acid, and samples were analyzed by EnTox (The University of Queensland, Brisbane, Australia). The results were corrected using appropriate buffers as references.
Determination of relative metal affinity of MntC by monitoring of fluorescence quenching.
Fluorescence studies were carried out using a Perkin-Elmer model LS55 spectrofluorimeter. Apo-MntC was prepared as described above. Titration with Mn(II) and Zn(II) sulfate salts was carried out at room temperature in 50 mM MES, pH 6.0. Aliquots of metal salts solutions were added from a gas-tight Hamilton syringe to protein solutions. Following each addition, the sample solution was mixed well, and the fluorescence emission spectrum was collected. The sample was excited at 280 nm, and the emission was scanned between 320 nm and 400 nm. The dilution effect after each addition of metal salt solution was taken into account. Spectra were fitted using Graphpad Prism software (33) using different binding models.
PAR competition assays.
PAR was employed in a competition assay with MntC. PAR (200 μM) was mixed with ZnSO4 (50 μM) in 50 mM HEPES (pH 7.4), and the optical spectrum was recorded. Small aliquots of MntC were added, and the spectrum was recorded after each addition. Spectra were corrected for dilution effects and by subtraction of the “apo-spectrum.” For the Mn2+ competition assay, MntC (50 μM) was loaded with one equivalent of Zn2+ (50 μM) in the presence of PAR (200 μM) in 50 mM HEPES, pH 7.4. Mn sulfate salt was subsequently titrated into the solution. Following each titration of Mn2+, the spectrum was recorded and corrected as described above.
EPR spectroscopy.
Electron paramagnetic resonance (EPR) spectra were acquired on a Bruker Elexsys E580 instrument operating in continuous-wave mode at X-band frequency (∼9.4 GHz). Calibration of the magnetic field was achieved with an ER036TM teslameter. Room temperature spectra were collected using a Super High-Q cavity incorporating a Bruker AquaX flow cell. The flow cell was connected in-line to a peristaltic pump. To monitor Mn(II) binding, apo-MntC was titrated with Mn(II) sulfate salts (12.5 μM MntC and 2.5 to 50 μM Mn2+), and the solution mixed by pumping through the flow cell for 5 min. The pump was stopped prior to acquiring EPR data. All spectra were zeroed and baseline corrected prior to analysis.
Assay for survival of N. gonorrhoeae in primary human cervical epithelial cells.
Primary human cervical epithelial (pex) cells were procured and maintained as described previously (11), and cell monolayers were grown to confluence in 35-mm tissue culture dishes (Falcon). Wild-type and mutant N. gonorrhoeae cells that were allowed to grow overnight (37°C, 5% CO2) on GC-IsoVitaleX agar plates were harvested using a sterile swab and resuspended in sterile saline. Culture density was determined spectrophotometrically, where an OD600 of 1 was equivalent to 109 bacteria ml−1 of cell culture. Bacterial cells were then used to challenge 105 pex cells at a multiplicity of infection of 100 (i.e., 107 bacteria). To confirm the actual number of bacteria used to challenge pex cells, the inoculum used for each N. gonorrhoeae strain (1291, 1291 mntAB, or 1291 mntC) was quantitated by plating the bacterial suspension and enumerating CFU. To determine the ability of N. gonorrhoeae wild-type and mutant strains to associate with, invade, and survive within pex cells, the infection was then allowed to progress at 37°C in a 5% CO2 atmosphere. For association assays, the infection medium was removed at 90 min postinfection and the cells rinsed with phosphate-buffered saline (PBS). For invasion assays, pex cells were incubated for a further 30 min with medium containing 100 μg of gentamicin (Gibco) per ml to kill extracellular bacteria. Survival assays were performed in a similar manner with the exception that following gentamicin treatment, the infected cell monolayers were again rinsed with PBS. Fresh antibiotic-free medium was then added to each infected cell monolayer before 1 h or 3 h of incubation. Following each assay, pex cells were lysed with 0.5% saponin to release invasive bacteria, and serial dilutions were plated to determine CFU. Association, invasion, and survival assays in which the same wild-type or mutant bacterial solution was used to infect pex cell monolayers were performed in parallel on three separate occasions. The percent association, invasion, or survival was determined as a function of the original inoculum and the number of colonies formed with subsequent plating of the cellular lysate. P values were determined using a Kruskal-Wallis nonparametric analysis of variance.
Assay for biofilm formation.
For examination of biofilm formation via confocal microscopy, the N. gonorrhoeae 1291 strain and the mntAB and mntC mutants were transformed with a plasmid encoding green fluorescent protein (GFP) (pLES98 containing GFP was a gift from Virginia Clark, University of Rochester, NY). Strains were propagated from frozen stock cultures on GC agar with IsoVitaleX (Becton Dickinson) and incubated at 37°C with 5% CO2. Overnight plate cultures were used to create cell suspensions for inoculation of biofilm flow chambers. N. gonorrhoeae was grown in continuous-flow chambers in 1:10 GC broth (19) diluted in PBS with 1% IsoVitaleX, 100 mM sodium nitrite, 5 mM mannitol, and 5 mg/ml chloramphenicol to maintain pGFP. Approximately 2 × 108 CFU/ml of each strain was suspended in biofilm medium, and 1 ml of each suspension was used to inoculate two flow cell chamber wells (37 by 5 by 5 mm). Flow chambers were incubated under static conditions at 37°C for 1 h postinoculation to facilitate bacterial attachment before flow was initiated at 180 ml/min. After 48 h, the biofilm effluent was cultured to confirm purity, and biofilm formation was assessed via confocal microscopy.
Z-series photomicrographs of flow chamber biofilms were taken with the Nikon PCM-2000 confocal microscope scanning system (Nikon, Melville, NY) using a modified stage for flow cell microscopy. GFP was excited at 450 to 490 nm for biofilm imaging. Three-dimensional images of the biofilms were created from each z series using Volocity High Performance 3D Imaging software (Improvision Inc., Lexington, MA). The images were adjusted to incorporate the pixel sizes for the x, y, and z axes of each image stack. Quantitative analysis of each z series was performed using COMSTAT (16) (http://www.cbm.biocentrum.dtu.dk/English/Services/Resources/COMSTAT.aspx). An information file was created for each z series to adjust for the pixel sizes of the x, y, and z axes and the number of images in each z series. COMSTAT was then used to threshold the images to reduce background. The biomass and average and maximum thicknesses in each z series were calculated by COMSTAT from the threshold images.
RESULTS
Comparison of gonococcal MntC with known Zn2+ and Mn2+ binding proteins of the cluster IX family.
Initially, we searched the N. gonorrhoeae FA1090 genome for open reading frames that encoded an MBR of the cluster IX family (Gonococcal Genome Sequencing Project, University of Oklahoma GenBank accession number AE004969). In contrast to the situation in many other bacterial pathogens, mntC (NGO0168) was the only gene encoding a cluster IX MBR that could be identified in the gonococcal genome. Gonococcal MntC exhibited 23% sequence identity to MntC from Synechocystis sp. strain PC6803 and PsaA from Streptococcus pneumoniae, which are MBR proteins of known Mn permease systems, and it showed 22% and 24% sequence identity, respectively, to S. pneumoniae AdaC and Synechocystis sp. strain PC6803 ZnuA, which are MBRs from Zn permease systems. However, MntC showed the highest sequence identity (27%) to T. pallidum TroA, an MBR that is known to bind Zn or Mn with similar affinity (14). TroA coordinates a single Zn(II) ion in a pentavalent complex using three histidine ligands and a bidentate aspartate side chain, while ZnuA binds Zn(II) in a distorted tetrahedral coordinated geometry with a water molecule providing the fourth coordinating ligand (15). Alignment of the primary structures of gonococcal MntC with those of TroA and ZnuA (see Fig. S1 in the supplemental material) showed that these histidine residues are present in MntC. The conserved aspartate is also present in gonococcal MntC. In ZnuA this aspartate residue points away from the metal binding site, but it is a fourth ligand in TroA and Mn binding MBRs. It has also been suggested that a key difference between TroA and ZnuA may lie in the amino acid sequence between the conserved aspartate ligand and the conserved glutamate (E290 in ZnuA) that is part of the secondary shell of interacting residues. Alignment of sequences indicated that gonococcal MntC had highest sequence similarity in this region to Mn binding MBRs such as Synechocystis MntC. Based on the above analysis, these data suggested that the gonococcal MntC should bind Mn2+ since it exhibits homology to Mn2+ binding MBRs. However, a feature of some zinc binding MBRs such as ZnuA is the presence of a histidine-rich loop that is located near the metal ion binding cleft. This histidine-rich loop is present in gonococcal MntC, suggesting that gonococcal MntC might also bind Zn2+.
Zn2+ or Mn2+ can restore optimal growth to an mntC mutant, but growth of an mntAB mutant requires the presence of an antioxidant.
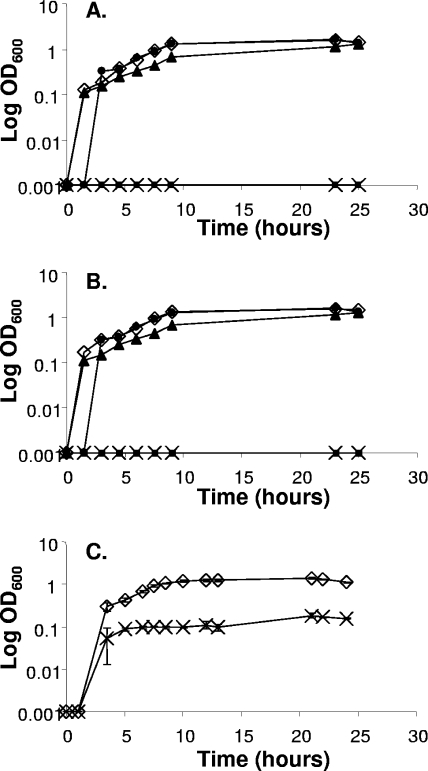
The presence of only one MBR of the cluster IX family in N. gonorrhoeae raised the question of whether the MntABC permease could transport both Zn and Mn. In an earlier publication we observed that Mn ions could protect N. gonorrhoeae against killing via oxidative stress and that this protection was dependent upon MntC (34). During measurements of growth of gonococci in liquid culture, we observed that the mntC mutant was unable to grow in BHI medium from a small inoculum unless it was supplemented with either Zn2+ (Fig. 1A) or Mn2+ (Fig. 1B). Optimal growth was achieved with a minimum of 3 μM Zn2+ or 5 μM Mn2+. It was previously observed that the mntAB mutant exhibited a more severe growth defect than the mntC mutant; the mntAB mutant will form only small colonies on solid medium and will not grow in BHI liquid medium (34). We now extend this work by showing that the mntAB mutant, in liquid medium, cannot be rescued by addition of Zn2+ or Mn2+ (Fig. 1A and B). However, we observed that addition of the antioxidant mannitol could restore growth of this mutant (Fig. 1C). This observation supports our previous conclusion that mutation of the mntABC system leads to oxidative stress in N. gonorrhoeae.
FIG. 1.
Growth of wild-type N. gonorrhoeae 1291 and the mntAB and mntC mutants in liquid culture on BHI medium supplemented with zinc sulfate (A), manganese sulfate (B), or mannitol (C). (A) ⋄, N. gonorrhoeae wild-type 1291; ×, mntAB mutant with 100 μM Zn2+; ▪, mntC mutant; ▴, mntC mutant with 3 μM Zn2+; •, mntC mutant with 100 μM Zn2+. (B) ⋄, wild-type N. gonorrhoeae 1291; ×, mntAB mutant with 100 μM Mn2+; ▪, mntC mutant; ▴, mntC mutant with 5 μM Mn2+; •, mntC mutant with 100 μM Mn2+. (C) ⋄, N. gonorrhoeae 1291 with 5 mM mannitol; ×, mntAB mutant with 5 mM mannitol. The data are the means ± standard errors from three independent experiments.
MntC binds Zn2+ and Mn2+ with similar affinities.
The growth assays indicated that gonococcal MntABC permease is able to transport both Zn and Mn ions, suggesting that MntC should be able to bind both of these ions. This is observed for TroA, which has very similar affinities for Zn2+ and Mn2+ (15). To investigate metal binding to MntC, we overexpressed and purified the protein from E. coli. In order to ensure that any bound transition metal ions were removed from rMntC, the protein was denatured, dialyzed against metal-free buffers, and then refolded. The secondary structure of the refolded rMntC was similar to that of native rMntC, as indicated by circular dichroism spectroscopy (data not shown). Metal analysis using ICP-MS revealed that refolded rMntC did not contain any transition metal ions; this allowed us to investigate metal binding to rMntC.
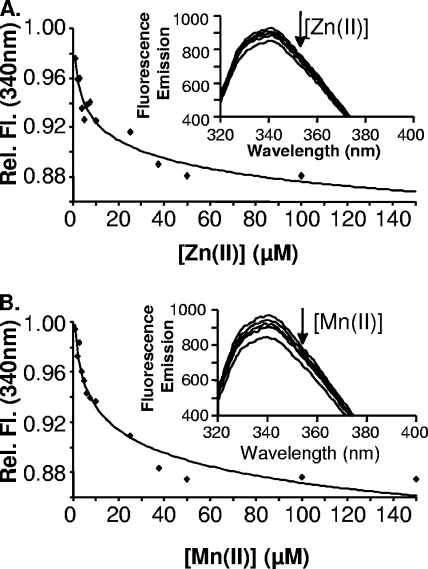
MntC contains a single tryptophan residue (Trp 138) located near the putative metal binding site between the (β/α)4 domains. This allowed us to use changes in tryptophan fluorescence emission at 340 nm to monitor metal ion binding to rMntC. Addition of Zn2+ or Mn2+ to rMntC caused a decrease in fluorescence at 340 nm (Fig. 2A and B), which suggests a metal ion-induced alteration to the tryptophan environment within the protein. The change in fluorescence quenching was measured as a function of metal ion concentration, and this yielded curves that fitted best to a 1:1 binding model for the binding of Zn2+ or Mn2+ to rMntC (Fig. 2A and B, insets). The dissociation constants for the interaction of Zn2+ and Mn2+ with rMntC were calculated to be 104 ± 5 nM and 100 ± 8 nM, respectively. These values are of a similar magnitude to those described for binding of Zn2+ and Mn2+ to TroA (8). Circular dichroism spectra of native rMntC and metal-saturated rMntC from fluorescence quenching were identical and show that metal binding does not induce changes to major secondary structural elements (data not shown). This is supported by the fluorescence data that show no change to the maximum tryptophan wavelength as Zn2+ or Mn2+ is titrated in. Indeed, the 340-nm maximum absorption indicates that the tryptophan is initially in a solvent-shielded environment and remains so after metal addition. To determine whether the observed tryptophan quenching was due to a specific interaction between Zn2+ and Mn2+ with MntC, we titrated Fe(II). Titration of MntC with Fe2+ showed no quenching of tryptophan fluorescence, suggesting a lack of binding (data not shown). This result suggests that rMntC is specific for Zn2+ and Mn2+, similar to previous results for the homologue TroA (15).
FIG. 2.
MntC titration with Zn2+ and Mn2+. (A) Relative change in fluorescence emission at 340 nm during Zn2+ titration. Inset, decrease in fluorescence emission spectrum as increasing concentrations of Zn2+ were added to 25 μM MntC. (B) Relative change in fluorescence emission at 340 nm during Mn2+ titration. Inset, decrease in fluorescence emission spectrum as increasing concentrations of Mn2+ were added to 25 μM MntC. The arrow denotes the decrease in the relative fluorescence emission at 340 nm upon Zn2+ (A) or Mn2+ (B) titration into MntC.
The binding of Mn(II) to MntC was confirmed using EPR spectroscopy. Mn(II) is a paramagnetic metal ion for which S = 5/2 and I = 5/2. Hexaquo-Mn(II) has a distinctive six-line EPR signal at room temperature. When Mn(II) binds to a protein, solvent ligands are displaced and the room temperature X-band signal disappears. The loss of signal is due to broadening via the zero field splitting arising from disturbances to the ligand field of the bound metal. Thus, Mn(II) resonances observed in room temperature solution EPR spectra in the presence of MntC arise only from unbound Mn(II) ions free in solution. Upon addition of MntC to the Mn(II) solution, there was a decrease in the amplitude of the EPR signal, consistent with the binding of Mn(II) to MntC (see Fig. S2 in the supplemental material).
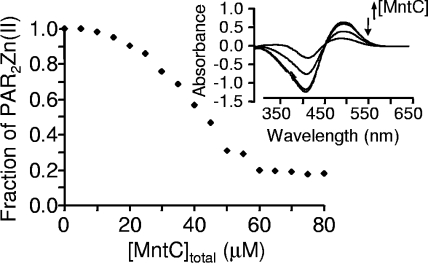
In order to qualitatively verify that Zn2+ was bound by MntC, a competition assay with the metallochromic Zn2+ indicator PAR was performed. Under solution conditions (pH 7.4), PAR is calculated to form 1:1 and 2:1 complexes with Zn2+ with stepwise affinity constants of 9.7 × 106 and 6.1 × 105 M−1, respectively (17). Thus, an excess of PAR was used in the experiments described here to ensure that [Zn(PAR)2] was the only Zn(II) complex in solution prior the addition of protein. The addition of Zn2+ to PAR results in an intense absorption band at 500 nm. Upon the addition of MntC, a decrease in the absorption band at 500 nm was observed (Fig. 3). This indicates that Zn2+ was displaced from [Zn(PAR)2] to presumably form an MntC-Zn complex and is consistent with our fluorescence measurements (Fig. 2A) that indicated that MntC has a higher affinity for Zn2+ than PAR. This observation is consistent with the Zn dissociation constant value for MntC obtained from the fluorescence spectra data (nanomolar to micromolar).
FIG. 3.
Measurement of Zn2+ binding to MntC via a PAR competition assay. A binding isotherm was generated from 200 μM PAR and 50 μM Zn2+ titration with increasing concentration of MntC (0 to 80 μM). Inset, representative spectra obtained during MntC titration. The downward arrow denotes the decrease in absorbance at 500 nm, indicating the removal of Zn2+ from the PAR2Zn complex by adding (upward arrow) various concentration of MntC to form MntC-Zn2+.
Zn and Mn ions compete for the same site in MntC.
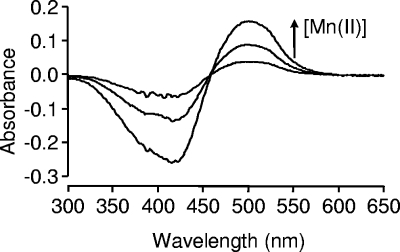
The observation that MntC would displace Zn(II) from [Zn(PAR)2] allowed us to use a PAR-based competitive assay to determine whether MntC has a single binding site for both Mn2+ and Zn2+. The displacement assay was performed using a 1:1 MntC-Zn complex in the presence of excess PAR. Increasing concentrations of Mn2+ were titrated into the MntC-Zn and PAR reaction mixture. The titration of Mn(II) resulted in an absorbance increase at 500 nm, indicative of [Zn(PAR)2] formation (Fig. 4). These data show that Mn(II) can displace Zn(II) from the MntC-Zn complex to form an MntC-Mn complex. This result suggests that Zn2+ and Mn2+ utilize the same ligands (binding site) to coordinate MntC. Furthermore, these data indicate that Mn2+ has a higher affinity for the site than does Zn2+.
FIG. 4.
Displacement of Zn2+ from MntC by Mn2+, showing spectra of Mn2+ (0 to 100 μM) titrated into MntC-Zn2+ (50 μM) and excess PAR (200 μM). The arrow indicates the increase in absorbance at 500 nm, indicating the generation of a PAR2Zn complex.
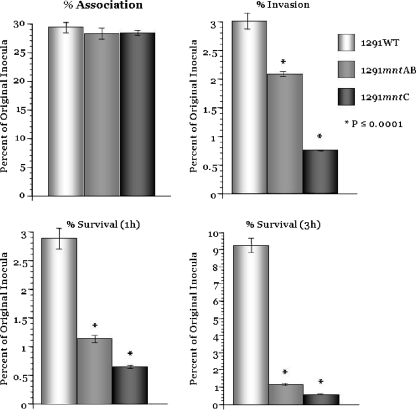
mntC and mntAB mutants have a reduced ability to invade primary ectocervical cells.
N. gonorrhoeae adherence to and invasion of cervical epithelial cells is a complex process that is mediated by several factors (11, 24). The ability of wild-type and mutant gonococci to associate with, invade, and survive within primary cervical (pex) cells was determined as described previously (38). Figure 5 shows that both the mntAB and mntC mutants showed a similar level of association to pex cells as did wild-type cells; however, there was a significant reduction in the level of invasion, and this was particularly noticeable for the mntC mutant. Extending the study of the invasion produced some interesting results. After 1 h there was no difference in CFU for wild-type cells, while CFU for the mntAB mutant were reduced by 50%. The CFU count of the mntC mutant was the lowest of all three strains tested after 1 h of invasion, and this value essentially did not change after 3 h of invasion. Similarly, the mntAB mutant CFU count did not increase after 3 h, but, in contrast, the wild-type cells had shown clear evidence of growth, as indicated by recovery of CFU. Taken together, these data show that the MntABC permease is critically important for intracellular survival of N. gonorrhoeae in cervical epithelial cells.
FIG. 5.
Association/invasion/survival assays performed using wild-type (WT) N. gonorrhoeae 1291, 1291 mntAB, and 1291 mntC with 90 min of infection without (association) or with (invasion) 30 min of gentamicin treatment (100 μg/ml) and then a 1-h or 3-h second incubation (survival) in antibiotic-free medium. Gonococcal association, invasion, and survival within pex cells was determined in parallel in a single assay; however, the data obtained have been plotted as individual panels. Data shown are the means (variances) obtained from three separate assays performed in triplicate.
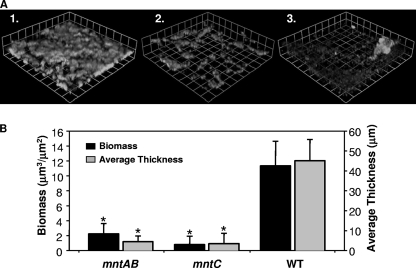
mntC and mntAB mutants are deficient in forming biofilms.
Studies performed in continuous-flow chambers have recently shown that N. gonorrhoeae strain 1291 can form a biofilm on glass coverslips as well as on primary cervical cells without loss of viability of the epithelial cells (13). The abilities of the N. gonorrhoeae mntAB and mntC mutant strains to form biofilm compared to that of the wild-type strain were evaluated after 2 days of growth under continuous-flow conditions (Fig. 6). COMSTAT (16) was used to quantitatively assess the biomass and the average and maximum thicknesses of confocal z-series photomicrographs taken for each flow chamber. COMSTAT analysis showed that both the mntAB and mntC mutants are deficient in biofilm formation compared to the wild type (Fig. 6B). Specifically, the mntAB mutant had 19.8% of the biomass of the wild type, while the mntC mutant had 7.0% of the biomass of the wild type. Both mutants also form biofilms with lower average thicknesses than the wild type. The mntAB and mntC mutants possessed 10.0% and 11.7% the average thickness of the wild type, respectively. Differences in biomass and average thickness were determined to be statistically significant using a Student t test (P < 0.000001). Three-dimensional images of these biofilms were created in Volocity. These images show that overall both mutants form thinner and more diffuse biofilms with large gaps between biofilm clusters compared to wild type.
FIG. 6.
Biofilm formation by wild-type N. gonorrhoeae 1291 and the mntAB and mntC mutants. (A) Biofilm mass over 2 days of growth for (1) wild-type N. gonorrhoeae 1291, (2) the mntAB mutant, and (3) the mntC mutant. These images are stacked z series taken at a magnification of ×200. (B) COMSTAT analysis of the biomass and average thickness of biofilm stacks. The error bars represent ±1 standard deviation of the mean. Asterisks denote statistically significant differences in biomass and average thickness compared to the wild type. These experiments were repeated on three different occasions, and a representative result is shown. There is a statistically significant difference in the mean biomasses of the mntAB and mntC mutants relative to the wild-type (P values of 3.1 × 10−7 and 7.1 × 10−8, respectively, as determined using Student's t test). There is also a statistically significant difference in the average thicknesses of the biofilm of the mntAB and mntC mutants relative to the wild type (P values of 1.3 × 10−8 and 1.5 × 10−9, respectively).
DISCUSSION
ABC permeases which contain an MBR of the cluster IX family have emerged as key transporters that are required for virulence in several bacterial pathogens. In a number of bacteria, for example, S. pneumoniae, there are two distinct permeases which appear to have biologically distinct roles as transporters of Zn or Mn ions (9). The presence of a single MBR of the cluster IX family in N. gonorrhoeae raised the question of its metal binding specificity in relation to its role in growth and survival of this bacterium. We have previously shown that MntC is required for accumulation of Mn2+ in gonococci to levels that are sufficient to confer protection against killing via oxidative stress (34). The present data showed that in the absence of MntC, gonococci required addition of either Zn2+ or Mn2+ to liquid medium in order to facilitate growth. In an earlier report, Chen and Morse (5) identified an ABC permease in N. gonorrhoeae strain F62 which was essential for uptake of zinc. This permease, described as ZnuABC, is homologous to the MntABC that we have described in the present study. We have made a similar observation in the case of a psaA mutant of S. pneumoniae, where we observed that Mn2+ supplementation of liquid media was required to promote growth (23). These results are consistent with the expected biological role of MBRs, which is to confer a high affinity on the permease system. In the absence of the MBR, the permease can still function, but it will have a lower affinity for metal. The loss of mntAB produced a more severe phenotype with respect to growth of gonococci on plates and in liquid culture, and it was not possible to facilitate growth of the mntAB mutant with Zn or Mn supplementation. These data suggest that the MntABC system is the dominant Mn and Zn transporter in gonococci. The mntAB mutant could be rescued by addition of the antioxidant mannitol or tiron, suggesting that the effect of Mn2+ and Zn2+ transport by the MntABC system is to protect N. gonorrhoeae against oxidative stress. We have previously focused on Mn-dependent protection of N. gonorrhoeae against oxidative stress and have shown that MntC is required for this phenomenon. Mn and Zn are both known to possess antioxidant properties, but their modes of action are quite different. Mn is able to cycle between the Mn2+ and Mn3+ states and can quench both superoxide ions and hydrogen peroxide. In many bacteria Mn is a component of superoxide dismutase (SodA), although in N. gonorrhoeae the sole type of superoxide dismutase appears to be the Fe-Sod (SodB). However, Mn can provide protection against oxidative stress to a sodB mutant of N. gonorrhoeae, consistent with the view that chemical quenching of superoxide by Mn has a potentially important role as an antioxidant in this bacterium.
Zn lacks redox activity, and it has ligand binding properties different from those of Mn. Zn2+ is known to bind cysteine thiolates as well as N ligands (imidazole). As such, it is able to stabilize dithiols in a reduced state and prevent disulfide bridge formation (4). It is established that release of Zn2+ from a ribosomal pool is an important part of the oxidative stress response in Bacillus subtilis (25). Zn2+ is bound via at least one ribosomal protein, L31, but during oxidative stress expression of an L31 protein paralogue which lacks the cysteine residues capable of binding Zn leads to displacement of the Zn-L31 from the ribosome and an increase in cytoplasmic Zn. N. gonorrhoeae possesses both forms of L31, and expression of the Zn-free L31 has been shown to be increased in a PerR mutant of this bacterium (38). Although we have named this Fur paralogue in N. gonorrhoeae PerR, it seems more likely that this transcriptional repressor is responding to Zn2+. This transcription factor also represses mntC expression, suggesting that it is Zn that controls expression of MntABC. A similar situation has been observed in T. pallidum, where the repressor of troABCD expression responds to Zn2+ levels but not to Mn2+ within a physiological range (8).
In S. pneumoniae it is established that PsaA is a virulence factor that is required for disease (9). The importance of MntC in the pathogenesis of the gonococcus, in vivo, is more difficult to assess. Nevertheless, our observation that MntC is required for invasion and survival within primary cervical epithelial cells as well as biofilm formation is strong evidence that Mn and/or Zn transport via the MntABC permease is important in gonococcal pathogenesis. Our results show that MntC from N. gonorrhoeae is able to bind Mn2+ or Zn2+ with almost equal affinity. This situation has also been reported for TroA from T. pallidum, where a single ABC cassette system containing a cluster IX MBR was able to transport both Mn2+ and Zn2+ (8). Unlike T. pallidum, gonococci do not possess a dedicated Zn(II) transporter. It is possible that transport of both Zn2+ and Mn2+ via the MntABC transporter is relevant to growth and survival of gonococci. However, the bioavailability of manganese and zinc in different settings is hard to assess. Lactic acid bacteria are present as commensals in the female genitourinary tract, and they may act to concentrate Mn2+ (2). It is possible that this may lead to increased bioavailability of Mn2+ in that environment. Although zinc is more abundant, in human serum the total Zn concentration is approximately 20 μM and the concentration of Mn is near 100 nM (6, 35); it is also the case that Zn2+ is usually bound tightly to proteins, while Mn2+ exchanges ligands rapidly. The presence of the histidine-rich loop in gonococcal MntC may be an additional feature that allows Zn2+ to be acquired.
Supplementary Material
Acknowledgments
This work was supported by program grant 284214 from the National Health and Medical Research Council of Australia to M.P.J. and A.G.M. J.L.E. received funding from the Research Institute at Nationwide Children's Hospital. This work was also supported in part by grant AI045728 from the National Institutes of Health to M.A.A.
We acknowledge the Cooperative Human Tissue Network (Columbus, Ohio) for providing cervical tissue specimens.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 21 April 2008.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alexander, H. E. 1965. The Haemophilus group, p. 724-741. In R. J. Dubos and J. G. Hirsch (ed.), Bacterial and mycotic infection in man. Pitman Medical Publishing, London, United Kingdom.
- 2.Archibald, F. 1986. Manganese: its acquisition by and function in the lactic acid bacteria. Crit. Rev. Microbiol. 1363-109. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S., B. Wei, M. Bhattacharyya-Pakrasi, H. B. Pakrasi, and T. J. Smith. 2003. Structural determinants of metal specificity in the zinc transport protein ZnuA from Synechocystis 6803. J. Mol. Biol. 3331061-1069. [DOI] [PubMed] [Google Scholar]
- 4.Berg, J. M., and Y. G. Shi. 1996. The galvanization of biology: a growing appreciation for the roles of zinc. Science 2711081-1085. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. Y., and S. A. Morse. 2001. Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 20267-71. [DOI] [PubMed] [Google Scholar]
- 6.Christianson, D. W. 1997. Structural chemistry and biology of manganese metalloenzymes. Prog. Biophys. Mol. Biol. 67217-252. [DOI] [PubMed] [Google Scholar]
- 7.Claverys, J. P. 2001. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 152231-243. [DOI] [PubMed] [Google Scholar]
- 8.Desrosiers, D. C., Y. C. Sun, A. A. Zaidi, C. H. Eggers, D. L. Cox, and J. D. Radolf. 2007. The general transition metal (Tro) and Zn2+ (Znu) transporters in Treponema pallidum: analysis of metal specificities and expression profiles. Mol. Microbiol. 65137-152. [DOI] [PubMed] [Google Scholar]
- 9.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25727-739. [DOI] [PubMed] [Google Scholar]
- 10.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with putative lipoprotein homologues to a family of streptococcal adhesins. Res. Microbiol. 148119-131. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, J. L., E. J. Brown, S. Uk-Nham, J. G. Cannon, M. S. Blake, and M. A. Apicella. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell. Microbiol. 4571-584. [DOI] [PubMed] [Google Scholar]
- 12.Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. Walker (ed.), The proteomics protocols handbook. Humana Press, Totowa, NJ.
- 13.Greiner, L. L., J. L. Edwards, J. Shao, C. Rabinak, D. Entz, and A. A. Apicella. 2005. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 731964-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardham, J. M., L. V. Stamm, S. F. Porcella, J. G. Frye, N. Y. Barnes, J. K. Howell, S. L. Mueller, J. D. Radolf, G. M. Weinstock, and S. J. Norris. 1997. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene 19747-64. [DOI] [PubMed] [Google Scholar]
- 15.Hazlett, K. R. O., F. Rusnak, D. G. Kehres, S. W. Bearden, C. J. La Vake, M. E. La Vake, M. E. Maguire, R. D. Perry, and J. D. Radolf. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J. Biol. Chem. 27820687-20694. [DOI] [PubMed] [Google Scholar]
- 16.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. Ersbøll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 1462395-2407. [DOI] [PubMed] [Google Scholar]
- 17.Hunt, J. B., S. H. Neece, and A. Ginsburg. 1985. The use of 4-(2-pyridylazo)resorcinol in studies of zinc release from Escherichia coli aspartate transcarbamoylase. Anal. Biochem. 146150-157. [DOI] [PubMed] [Google Scholar]
- 18.Imlay, J. A. 2002. How oxygen damages microbes: Oxygen tolerance and obligate anaerobiosis, Adv. Microbial Physiol. 46111-153. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg, D. S., W. L. Peacock, C. I. Pirkle, W. E. Deacon, and L. Brown. 1963. Neisseria gonorrhoeae. 1. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 61553-1561. [DOI] [PubMed] [Google Scholar]
- 21.Lee, Y. H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA is a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6628-633. [DOI] [PubMed] [Google Scholar]
- 22.Li, H., and G. Jogl. 2007. Crystal structure of the zinc-binding transport protein ZnuA from Escherichia coli reveals an unexpected variation in metal coordination. J. Mol. Biol. 3681358-1366. [DOI] [PubMed] [Google Scholar]
- 23.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53889-901. [DOI] [PubMed] [Google Scholar]
- 24.Merz, A. J., and M. So. 2000. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16423-457. [DOI] [PubMed] [Google Scholar]
- 25.Nanamiya, H., G. Akanuma, Y. Natori, R. Murayama, S. Kosono, T. Kudo, K. Kobayashi, N. Ogasawara, S. M. Park, K. Ochi, and F. Kawamura. 2004. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol. Microbiol. 52273-283. [DOI] [PubMed] [Google Scholar]
- 26.Naumann, M., T. Rudel, and T. F. Meyer. 1999. Host cell interactions and signalling with Neisseria gonorrhoeae. Curr. Opin. Microbiol. 262-70. [DOI] [PubMed] [Google Scholar]
- 27.Parsons, N. J., C. W. Penn, D. R. Veale, and H. Smith. 1979. More than one antigen contributes to the immunogenicity of Neisseria gonorrhoeae in the guinea-pig chamber model. J. Gen. Microbiol. 11397-104. [DOI] [PubMed] [Google Scholar]
- 28.Rukhman, V., R. Anati, M. Melamed-Frank, and N. Adir. 2005. The MntC crystal structure suggests that import of Mn2+ in cyanobacteria is redox controlled. J. Mol. Biol. 348961-969. [DOI] [PubMed] [Google Scholar]
- 29.Seib, K. L., M. P. Simons, H.-J. Wu, A. G. McEwan, W. M. Nauseef, M. A. Apicella, and M. P. Jennings. 2005. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 735269-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seib, K. L., H. J. Tseng, A. G. McEwan, M. A. Apicella, and M. P. Jennings. 2004. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J. Infect. Dis. 190136-147. [DOI] [PubMed] [Google Scholar]
- 31.Seib, K. L., H. J. Wu, S. P. Kidd, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2188-194. [DOI] [PubMed] [Google Scholar]
- 33.Swillens, S. 1995. Interpretation of binding curves obtained with high receptor concentrations—practical aid for computer-analysis. Mol. Pharmacol. 471197-1203. [PubMed] [Google Scholar]
- 34.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 401175-1186. [DOI] [PubMed] [Google Scholar]
- 35.Vallee, B. L., and K. H. Falchuk. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 7379-118. [DOI] [PubMed] [Google Scholar]
- 36.Veale, D. R., M. Goldner, C. W. Penn, J. Ward, and H. Smith. 1979. Intracellular survival and growth of gonococci in human phagocytes. J. Gen. Microbiol. 113383-393. [DOI] [PubMed] [Google Scholar]
- 37.Wei, B. X., A. M. Randich, M. Bhattacharyya-Pakrasi, H. B. Pakrasi, and T. J. Smith. 2007. Possible regulatory role for the histidine-rich loop in the zinc transport protein, ZnuA. Biochemistry 468734-8743. [DOI] [PubMed] [Google Scholar]
- 38.Wu, H. J., K. L. Seib, Y. N. Srikhanta, S. P. Kidd, J. L. Edwards, T. L. Maguire, S. M. Grimmond, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2006. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol. Microbiol. 60401-416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.