Abstract
Very little is known about the developmental stages of Aspergillus fumigatus during invasive aspergillosis. We performed real-time reverse transcription-PCR analysis on lung samples from mice with invasive pulmonary aspergillosis to determine the expression of A. fumigatus genes that are expressed at specific stages of development. In established infection, A. fumigatus exhibited mRNA expression of genes specific to developmentally competent hyphae, such as stuA. In contrast, mRNA of genes expressed by conidia and precompetent hyphae was not detected. Many genes required for mycotoxin synthesis, including aspHS, gliP, mitF, and metAP, are known to be expressed by developmentally competent hyphae in vitro. Interestingly, each of these genes was expressed at significantly higher levels during invasive infection than in vitro. The expression of gliP mRNA in vitro was found to be highly dependent on culture conditions. Furthermore, gliP expression was found to be dependent on the transcription factor StuA both in vitro and in vivo. Therefore, developmentally competent hyphae predominate during established invasive infection, and many mycotoxin genes are expressed at high levels in vivo. These results highlight the importance of the evaluation of putative virulence factors expressed by competent hyphae and analysis of gene expression levels during invasive infection rather than in vitro alone.
Aspergillus fumigatus is a ubiquitous saprophytic fungus that produces abundant airborne spores (conidia). Invasive infection with this organism can develop in patients who are immunocompromised from a variety of causes including chemotherapy, bone marrow or solid-organ transplantation, tumor necrosis factor inhibitor therapy, or underlying hematologic malignancy. In such patients, invasive aspergillosis is the most common filamentous fungal infection and is associated with a mortality rate of 30% to 90% (7, 10, 14, 17).
The typical portal of entry for A. fumigatus is the respiratory tract. Airborne conidia are inhaled and, because of their small size (2 to 3 μm), are deposited in the alveoli. In the absence of a robust immune response, these conidia germinate and invade the lung tissue before disseminating to other deep organs. Hyphae are the only form of the organism observed during invasive infection (12, 17). Surprisingly, little is known about the developmental cycle of A. fumigatus in vivo. In vitro, A. fumigatus conidia germinate to produce hyphae, which are initially unable to produce asexual reproductive structures in response to stimuli. After a fixed time period after germination, these hyphae become able to produce asexual reproduction structures (1). This shift from a state in which hyphae cannot undergo asexual reproduction to one in which they can is termed the acquisition of developmental competence. The time to acquisition of developmental competence is affected by temperature but is independent of nutrient status (25). Although mutagenesis studies in Aspergillus nidulans have identified mutants with a delayed onset of developmental competence, the specific genes controlling this developmental event have not yet been identified (3). While precompetent and competent hyphae are indistinguishable morphologically, the transition to developmental competence coincides with a coordinated alteration in the expression of over 400 genes in vitro (21). While some of the genes expressed by competent hyphae govern the production of conidiophores, many putative virulence factors are upregulated in response to the onset of developmental competence, including genes encoding mycotoxins, proteases, and allergens. In vitro, the transcription factor StuA plays a central role in governing the expression of these competence-associated genes. However, the deletion of stuA does not impair the acquisition of developmental competence (21). It was previously unknown whether hyphae become developmentally competent in vivo or if the stuA developmental pathway is active during invasive infection.
The determination of developmental competence is performed in vitro by exposing hyphae to conidiation signals and measuring the delay to the formation of conidiophores. Classically, this involves subculturing hyphal mats from liquid culture to an agar surface and visually observing the production of conidiophores (3). Given the few hyphae present during pulmonary infection, this method is not feasible for the determination of the timing of developmental competence in vivo. However, we recently performed whole-genome transcriptional analysis of A. fumigatus during the acquisition of developmental competence and have identified a subset of genes whose expression is specific to pre- or postcompetence (21). The expression of these genes can therefore provide a useful surrogate measure for determining the onset of developmental competence in vivo.
Here, we describe a method to analyze gene expression in vivo in the mouse model of invasive aspergillosis. Using this technique, we demonstrated that A. fumigatus hyphae express competence-associated but not precompetence-associated genes during invasive infection. We confirmed the mRNA expression of the developmental regulator stuA and of stuA-dependent genes in vivo (21). Finally, we analyzed the in vivo expression of a selection of genes essential for the biosynthesis of A. fumigatus mycotoxins and identified significant differences between their expression in vitro and their expression in vivo.
MATERIALS AND METHODS
Strains and media.
A. fumigatus strain Af293, a clinical isolate, was a generous gift from P. T. Magee, University of Minnesota. The ΔstuA mutant and the complemented strain were described previously (21). Strains were grown on Sabouraud dextrose agar plates for 10 days at 37°C, and conidia were collected by flooding the plates with sterile phosphate-buffered saline containing 0.2% (vol/vol) Tween 80 (Sigma-Aldrich, St. Louis, MO). The conidia were concentrated by centrifugation and counted using a hemacytometer. For in vitro studies, 100-ml YPD (1% yeast extract, 2% peptone, 2% glucose) cultures were inoculated with 1 × 106 conidia/ml and grown in a shaking incubator for the indicated times at 37°C. Under these growth conditions, developmental competence occurs reproducibly between 10 and 12 h (21).
Animal experiments.
Male BALB/c mice (National Cancer Institute, Bethesda, MD), 18 to 22 g, were used for these experiments. All experiments involving mice were approved by the institutional animal care and use committee according to the National Institutes of Health guidelines for animal housing and care. Mice were immunosuppressed at day −2 and day +3 (relative to infection) by intraperitoneal injection with cyclophosphamide (Western Medical Supply, Arcadia, CA) at 250 mg/kg and 200 mg/kg, respectively as well as with 250 mg/kg cortisone acetate (Sigma-Aldrich) subcutaneously on both days. Two methods of infection were used. For the study of later time points, mice were infected in an inhalational chamber by aerosolized conidia (1.2 × 1010 conidia), which resulted in an average inoculum of 2.4 × 103 conidia per mouse as described previously (22). For analysis of gene expression by the ΔstuA mutant and for testing gene expression at early time points, an intranasal model of infection was used because the ΔstuA mutant produced insufficient conidia for inoculation in the aerosol chamber (21). Therefore, for these experiments, mice were anesthetized with isofluorane (Western Medical Supply) and then infected by the intranasal instillation of a 25-μl volume containing 5 × 105 conidia of the various strains. All mice received ceftazidime (Western Medical Supply) at 5 mg per day subcutaneously to protect against bacterial infection. For all in vivo experiments, animals were sacrificed for gene expression studies only during the period where 90% of infected animals remained alive in order to avoid healthy survivor bias.
Isolation of total RNA.
RNA was isolated from in vitro cultures as described previously (19). Briefly, A. fumigatus mycelia were harvested by filtration through Whatman filter paper, washed with sterile water, and then ground under liquid nitrogen using a mortar and pestle. Total RNA was isolated from ground mycelia using the RNeasy Plant Mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. To extract total RNA from in vivo samples, lungs from infected mice were removed and immediately frozen in liquid nitrogen. Frozen lungs were then ground under liquid nitrogen, and the powder was resuspended in 600 μl TES buffer (1 mM Tris-HCl, 1 mM EDTA, 0.5% sodium dodecyl sulfate). Total RNA was then isolated using the hot phenol method. Briefly, after adding 600 μl acidic phenol, the solution was incubated for 30 min at 65°C and then cooled on ice for 5 min. The suspension was centrifuged at 16,000 × g for 3 min in a microcentrifuge, and the aqueous phase was transferred into a new tube. After phenol-chloroform extraction, the RNA was precipitated with LiCl, suspended in RNase-free treated water, ethanol precipitated, and finally resuspended in 60 μl RNase-free water.
Real-time RT-PCR.
Contaminating genomic DNA was removed from RNA samples by treatment with 1 μl of Turbo-DNase (Ambion, Austin, TX) for 30 min at room temperature. DNase was then removed using an RNA Clean-Up kit (Zymo Research, Orange, CA). First-strand cDNA synthesis was performed using the Retroscript first-strand synthesis kit (Ambion). Gene-specific primers for expression analysis were designed with the assistance of online primer design software (Genscript, Piscataway, NJ). These primers are listed in Table 1. Whenever possible, the primers spanned an intron. For each primer pair, the amplification efficiency was determined by serial dilution experiments, and the resulting efficiency coefficient was used for the quantification of the products (20). Gene expression was analyzed with 500 nM primers by using the QuantiTect Sybr green PCR kit (Qiagen) and an ABI Prism 7000 thermocycler. Cycle conditions were 10 min at 90°C and 40 cycles of 15 s at 95°C and 1 min at 60°C. Single PCR products were confirmed with the heat dissociation protocol at the end of the PCR cycles. Except where noted, the relative quantification of the mRNA levels of the target genes was determined using the ΔCT method (18). Briefly, the amount of target was normalized to the endogenous reference gene TEF1: ΔCT = CT (target gene) − CT (TEF1), where CT represents the cycle number required to reach a defined threshold target abundance. The relative mRNA levels were calculated as xΔCT (where x is primer efficiency) (20). All reactions were performed in duplicate, and the mixture included a negative no-reverse-transcription (RT) control in which reverse transcriptase was omitted.
TABLE 1.
Genes tested in this study and the primers used in real-time RT-PCR assays
| Gene | Oligonucleotide sequence (5′-3′)a | Amplicon size (bp) | Gene accession no. or identifier (source or reference) |
|---|---|---|---|
| tef1 | CCATGTGTGTCGAGTCCTTC (F) | 84 | Afu1g06390 |
| GAACGTACAGCAACAGTCTGG (R) | |||
| sspA | GACCGTCACTCTGACCTCAA (F) | 71 | Afu8g03930 (this work) |
| CTGTGGGAAGGGTAGTGCTT (R) | |||
| stuA | GAGGACGAAGGGAGTCTCTG (F) | 83 | Afu2g07900 (21) |
| ACCGTTGATCATGTGGTTGT (R) | |||
| hsp70 | TGTCATCACCGTACCAGCTT (F) | 93 | Afu8g03930 (this work) |
| TGATGATGCGGAGAACATTT (R) | |||
| ura7 | CATCTTCGGCTCACAAGAGA (F) | 113 | Afu2g03930 (this work) |
| CCAAAGTGAACATCCGATTG (R) | |||
| gliP | TCCAACAGTCAGAGGCATTC (F) | 103 | AY838877 (13) |
| CTTGAGGGATAATCGGTGGT (R) | |||
| aspF1 | TACCCGCACTGGTTCACTAA (F) | 94 | M83781 (2) |
| GACGGTCACAGTCGGCTT (R) | |||
| 18S rRNA | GGCCCTTAAATAGCCCGGT (F) | 62 | AB008401 (6) |
| TGAGCCGATAGTCCCCCTAA (R) | |||
| mitF | AGCCGTGTCTGTTCTAGCTG (F) | 72 | X58278 (16) |
| AGCTGTTGGTTGATGCATGT (R) | |||
| dmaW | GAATGGTTCTTACCGGGTTG (F) | 118 | XM_749235 (8) |
| GTGTTGAATGGGTCTTGTGC (R) | |||
| metAP | GTTGTCCACGCTACGGAGTA (F) | 132 | Afu8g00410 (this work) |
| CGGTGATTCTCAGCTTCTCA (R) | |||
| aspHS | AGTCCACTGGGACTGTCCAT (F) | 108 | D16501 (11) |
| GCACCACCATACTTGTTCCA (R) |
F, forward; R, reverse.
RESULTS
Hyphae express stuA mRNA and competence-associated genes in vivo.
To determine the developmental status of hyphae during infection, we compared the in vitro and in vivo expression levels of stuA and three other genes that exhibited different regulation during the onset of developmental competence by microarray analyses in vitro (21). To ensure the detection of subpopulations of both types of hyphae, we examined two genes (stuA and sspA) that were upregulated in competent hyphae and two genes (hsp70 and ura7) that were upregulated in precompetent hyphae in vitro (21). Details of these genes are presented in Table 1.
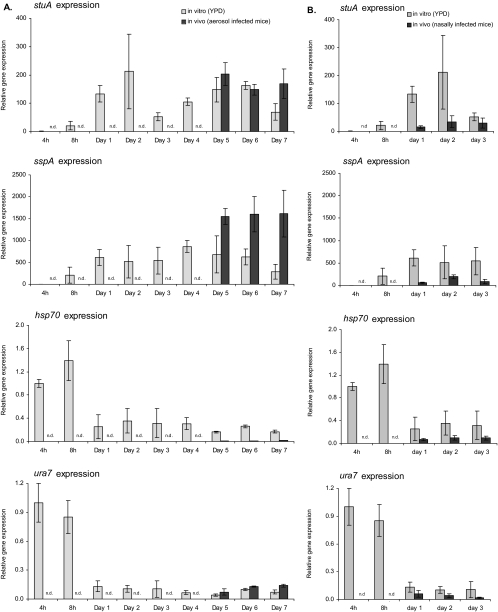
As predicted by our previous transcriptional profiling studies (21), developmentally competent hyphae grown in liquid culture displayed increased levels of expression of stuA and sspA after 24 h of culture compared to those after 4 and 8 h (Fig. 1). Conversely, the precompetence genes hsp70 and ura7 were strongly expressed after 4 and 8 h, but were markedly downregulated by 24 h, during the acquisition of developmental competence under these growth conditions (Fig. 1). When real-time RT-PCR was performed using samples from infected mouse lungs, the expression patterns of all four genes were similar to that of developmentally competent hyphae in vitro for all the time points examined between days 1 and 7 postinfection. Even using the higher-dose intranasal model of infection, we were unable to reliably detect mRNA from A. fumigatus from time points earlier than 24 h and thus cannot exclude a role for precompetent hyphae early in the establishment of disease. Collectively, these data suggest that after invasive aspergillosis is established, developmentally competent hyphae predominate, and there are few to no precompetent hyphae or conidia present.
FIG. 1.
Comparative in vitro and in vivo expression levels of stuA, sspA, hsp70, and ura7 determined by real-time RT-PCR. RNA in vitro was derived from cultures that were grown for 4 h, 8 h, and 1 to 7 days at 37°C in YPD medium. For in vivo data, mice were infected with wild-type strain Af293 in an inhalational chamber (A) or intranasally (B). At the indicated time points, infected lungs were removed, and total RNA was then used for real-time RT-PCR analysis (n = 3 per time point). The expression levels of all genes were normalized to the expression level of the endogenous control gene TEF1. The expression value at 4 h for each gene was used as the baseline. Arrows indicate the onset of developmental competence in vitro. n.d. indicates that fungal RNA was not detected at this time point.
Expression of sspA is StuA dependent in vitro and in vivo.
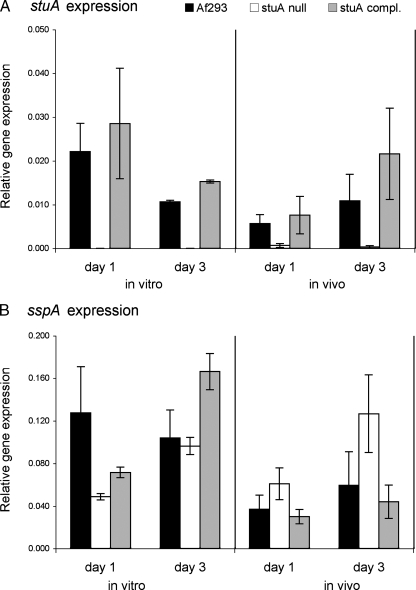
The results of our previous transcriptional profiling experiments indicated that the expression of sspA is governed by StuA in vitro (21). Therefore, we determined if sspA mRNA expression was dependent on StuA in vivo using a ΔstuA mutant. We first verified that sspA expression required StuA in vitro. When the ΔstuA mutant was grown in liquid YPD medium, sspA expression was reduced after 1 day but not 3 days compared to that for the wild-type strain (Fig. 2). Complementation of the ΔstuA mutant with an intact copy of stuA restored sspA mRNA expression to wild-type levels. Next, we determined whether sspA expression was dependent on stuA in the lungs of mice with invasive aspergillosis. Surprisingly, we observed that in vivo, stuA deletion was associated with increased levels of expression of sspA after both 24 and 72 h of infection (Fig. 2B). Therefore, a factor other that StuA must also govern sspA expression during pulmonary infection.
FIG. 2.
mRNA expression of sspA is differentially dependent on StuA in vitro and in vivo. (A) In vitro and in vivo expression of stuA mRNA as determined by real-time RT-PCR. (B) Relative mRNA expression of sspA determined by real-time RT-PCR in vitro and in vivo. In vitro cultures of each strain were grown for 24 h at 37°C in YPD. Mice were infected intranasally with the wild-type strain Af293, the ΔstuA mutant, and the stuA-complemented strain (n = 3 mice per time point), and lungs were removed for expression analysis at 24 and 72 h after infection. The expression levels of all genes were normalized to the expression level of the endogenous control gene TEF1.
Expression of mycotoxins differs in vitro and in vivo.
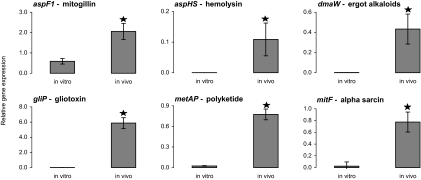
A. fumigatus secretes a number of toxic molecules in vitro. However, it is unknown whether the genes encoding most of these molecules are expressed in vivo. We therefore compared the in vitro and in vivo expression levels of genes involved in the biosynthesis of six putative mycotoxins (aspF1, dmaW, metAP, gliP, aspHS, and mitF) (Table 2). The expression levels of the first three of these genes are predicted to be StuA dependent and those of the latter three genes are predicted to be StuA independent based on our previous microarray studies (21). All of the mycotoxin synthetic genes were significantly upregulated during experimental aerosol infection with strain Af293 compared with their level of expression during growth for the same period of time in liquid YPD medium (Fig. 3).
TABLE 2.
Genes encoding or necessary for synthesis of toxins that were analyzed in this study
| Gene | Toxin | Cellular activity |
|---|---|---|
| gliP | Gliotoxin | Cytotoxic; NF-κB inhibitor |
| aspF1 | Mitogillin | RNase; inhibition of protein synthesis |
| mitF | Alpha-sarcin | RNase; inhibition of protein synthesis |
| aspHS | Asp-hemolysin | Red blood cell lysis |
| metAP1 | Unknown polyketide | Unknown |
| dmaW | Fumitremorgin? | Neurotoxic |
FIG. 3.
Mycotoxin expression is induced in vivo. Comparative in vitro and in vivo expression levels of gliP, aspHS, aspF1, metAP1, dmaW, and mitF were determined by real-time RT-PCR. RNA in vitro was derived from cultures that were grown for 5 to 8 days at 37°C in YPD medium. For in vivo data, mice were infected with wild-type strain Af293 in an inhalational chamber. On days 5 to 8 after infection, the infected lungs were removed, and total RNA was then used for real-time RT-PCR analysis (n = 3 mice per time point, with 12 mice total). Results from all four time points in vivo were very similar and were combined. Average expression values for each gene were normalized to values for TEF1 expression. ★ indicates statistically different results from in vitro expression by log-rank test (P < 0.05).
In vivo expression of gliP (gliotoxin) is dependent on stuA.
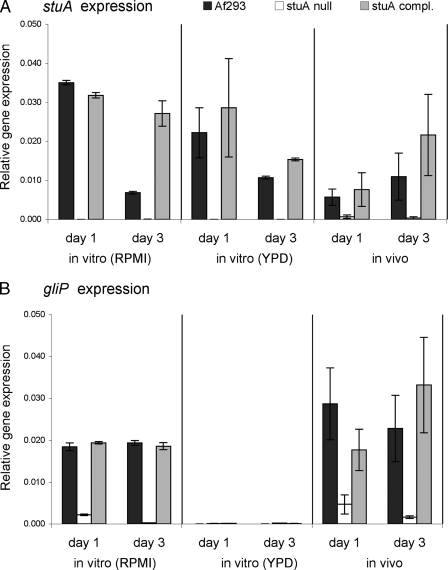
The high level of expression of gliP by competent hyphae in vivo suggests that gliotoxin production may be linked to developmental competence. These data, however, contrast with data from our previous microarray study in which the gliotoxin biosynthetic cluster was not found to be developmentally regulated. An important point is that this study was performed using organisms grown in liquid YPD medium, in which gliP expression is very low (21). We therefore hypothesized that gliP expression may be developmentally regulated only under the appropriate permissive growth conditions. To test this hypothesis, we examined the expression of gliP in wild-type strain Af293, the ΔstuA mutant, and the stuA-complemented strain both in vivo and in different liquid culture media. For in vitro experiments, we tested gliP expression at 24 h and at 72 h in both YPD and RPMI 1640 media, the latter of which was previously shown to induce high levels of gliotoxin production and is commonly used to mimic in vivo growth (4, 15).
During in vitro growth in YPD medium, very low levels of gliP mRNA expression were detectable in all strains. In contrast, growth in RPMI medium induced high levels of both stuA and gliP mRNA in strain Af293 but not in the ΔstuA mutant, while complementation of the ΔstuA mutant with an intact copy of stuA restored both stuA expression and gliP expression (Fig. 4). Similarly, during intranasal infection, the expression levels of stuA and gliP were markedly reduced in samples from mice infected with the ΔstuA mutant (Fig. 4). Complementation of the ΔstuA mutant with an intact copy of stuA also restored both stuA and gliP expression in vivo. Collectively, these results suggest that gliP expression and, by extension, gliotoxin production are likely dependent on StuA during murine infection.
FIG. 4.
Comparative in vitro and in vivo expression levels of gliP determined by real-time RT-PCR. Expression values are normalized to TEF1 expression values. (A) RNA in vitro was derived from cultures of wild-type strain Af293, the ΔstuA mutant, or the stuA-complemented strain, which were grown for 24 h and 72 h at 37°C in YPD or RPMI medium. Note that data for stuA expression in YPD and in vivo from Fig. 2 are used for comparative purposes. (B) Mice were infected by intranasal instillation of wild-type strain Af293, the ΔstuA mutant, or the stuA-complemented strain. Twenty-four hours and 72 h after infection, infected lungs were removed for total RNA extraction, and the mRNA levels of gliP were determined by real-time RT-PCR. All experiments were repeated in triplicate on at least two separate occasions.
DISCUSSION
The developmental transcription factor StuA is required for the normal production of conidia. However, the mRNA expression of stuA coincides with the onset of developmental competence and is maintained at high levels in competent hyphae even in the absence of conidiation (21). Our finding that stuA is expressed in vivo therefore suggests that hyphae are developmentally competent during pulmonary infection and that they at least have the potential to produce conidia, even though it is still doubtful that conidia are produced within the host. The sspA, hsp70, and ura7 genes are also highly differentially regulated during the onset of developmental competence. The expression pattern of all three genes during the infection is consistent with that of developmentally competent hyphae grown in vitro. These data support the hypothesis that hyphae are competent during established invasive pulmonary infection. These results have important implications given that the gene expression profile of competent hyphae is very different from that of precompetent hyphae (21). For example, when investigating the interactions of A. fumigatus with host cells in vitro, it may be relevant to test competent hyphae in addition to the more commonly used germlings. One caveat to our study is that we were unable to reliably detect mRNA from A. fumigatus at very early time points. Thus, it remains likely that precompetent hyphae may play a role in initiating pulmonary infection.
Transcriptional profiling of A. fumigatus genes in vivo is a valuable tool for investigating the pathogenesis of invasive disease. As demonstrated by the results of this study, direct extrapolation of in vitro transcriptional analyses must be performed with caution. While expression levels of some genes (such as stuA and the precompetence-associated genes) were similar in vitro and in vivo, important discrepancies in the expression levels of several mycotoxins were observed. Although most mycotoxin genes were expressed at higher levels in mice than in vitro, the most striking difference between in vivo and in vitro gene expression was found with gliP. We detected only extremely low gliP mRNA levels during the growth of the wild-type strain in YPD medium, while high-level expression was easily detected from mouse lungs infected with the same strain using both intranasal and inhalational models of invasive aspergillosis. Indeed, growth in RPMI 1640 medium was required to induce levels of gliP mRNA expression comparable to those seen during pulmonary infection. The importance of this observation is exemplified by our finding that gliP expression, and likely gliotoxin production, is dependent on StuA in vivo. In our previous microarray studies (21), this StuA dependence was not observed because the organisms were grown in YPD medium, which resulted in very low levels of gliP expression in the wild-type strain as well as in the ΔstuA mutant. Thus, although StuA is necessary but not sufficient for gliP expression, other signal transduction pathways must also govern the expression of this gene. Interestingly, similar differences between in vivo and in vitro toxin expression levels were also found when the global regulator laeA was studied. The disruption of laeA resulted in impaired gliotoxin production in vitro; however, gliotoxin was still detected in mice infected with the ΔlaeA strain (5).
Collectively, these studies of genes that exhibit StuA dependence in vivo suggest that the stuA pathway is active during invasive infection and governs the expression of key candidate virulence genes such as gliP. Gliotoxin production has proven to be an important virulence factor in the nonneutropenic murine model of invasive aspergillosis through the induction of leukocyte apoptosis (23, 24). In previous studies of the role of stuA in virulence, the deletion of stuA was not associated with a reduction in virulence despite the impaired gliotoxin production (21). These studies, however, were performed in neutropenic mice, in which the absence of gliotoxin has no effect on virulence (5, 9, 23, 24). These findings may also reflect the precocious germination of the ΔstuA null mutant or the contribution of other StuA-dependent genes to overall virulence.
In addition, we found that the levels of expression of a number of other StuA-independent genes that encode toxin biosynthetic genes are upregulated during infection. Although the role of most of these metabolites in virulence remains undefined, their expression during infection suggests that they may represent interesting candidates for further detailed study by gene disruption and other methods.
Acknowledgments
This work was supported in part by grants M01RR00425, R21AI064511, and R01AI073829 as well as contract no. N01-AI-30041 from the National Institutes of Health. D.C.S. was supported in part by a clinician-scientist award and an operating grant from the Canadian Institutes of Health.
Editor: A. Casadevall
Footnotes
Published ahead of print on 19 May 2008.
REFERENCES
- 1.Adams, T. H., W. A. Hide, L. N. Yager, and B. N. Lee. 1992. Isolation of a gene required for programmed initiation of development by Aspergillus nidulans. Mol. Cell. Biol. 123827-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arruda, L. K., T. A. Platts-Mills, J. W. Fox, and M. D. Chapman. 1990. Aspergillus fumigatus allergen I, a major IgE-binding protein, is a member of the mitogillin family of cytotoxins. J. Exp. Med. 1721529-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelrod, D. E., M. Gealt, and M. Pastushok. 1973. Gene control of developmental competence in Aspergillus nidulans. Dev. Biol. 349-15. [DOI] [PubMed] [Google Scholar]
- 4.Belkacemi, L., R. C. Barton, V. Hopwood, and E. G. Evans. 1999. Determination of optimum growth conditions for gliotoxin production by Aspergillus fumigatus and development of a novel method for gliotoxin detection. Med. Mycol. 37227-233. [PubMed] [Google Scholar]
- 5.Bok, J. W., D. Chung, S. A. Balajee, K. A. Marr, D. Andes, K. F. Nielsen, J. C. Frisvad, K. A. Kirby, and N. P. Keller. 2006. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 746761-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 453474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brakhage, A. A., and K. Langfelder. 2002. Menacing mold: the molecular biology of Aspergillus fumigatus. Annu. Rev. Microbiol. 56433-455. [DOI] [PubMed] [Google Scholar]
- 8.Coyle, C. M., and D. G. Panaccione. 2005. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 713112-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramer, R. A., Jr., M. P. Gamcsik, R. M. Brooking, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, C. J. Balibar, J. R. Graybill, J. R. Perfect, S. N. Abraham, and W. J. Steinbach. 2006. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23608-615. [DOI] [PubMed] [Google Scholar]
- 11.Ebina, K., H. Sakagami, K. Yokota, and H. Kondo. 1994. Cloning and nucleotide sequence of cDNA encoding Asp-hemolysin from Aspergillus fumigatus. Biochim. Biophys. Acta 1219148-150. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, R. S. 1993. Pulmonary aspergillosis: pathologic and pathogenetic features. Pathol. Annu. 28231-277. [PubMed] [Google Scholar]
- 13.Gardiner, D. M., A. J. Cozijnsen, L. M. Wilson, M. S. Pedras, and B. J. Howlett. 2004. The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans. Mol. Microbiol. 531307-1318. [DOI] [PubMed] [Google Scholar]
- 14.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347408-415. [DOI] [PubMed] [Google Scholar]
- 15.Kosalec, I., S. Pepeljnjak, and M. Jandrlic. 2005. Influence of media and temperature on gliotoxin production in Aspergillus fumigatus strains. Arh. Hig. Rada Toksikol. 56269-273. [PubMed] [Google Scholar]
- 16.Lamy, B., and J. Davies. 1991. Isolation and nucleotide sequence of the Aspergillus restrictus gene coding for the ribonucleolytic toxin restrictocin and its expression in Aspergillus nidulans: the leader sequence protects producing strains from suicide. Nucleic Acids Res. 191001-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 19.Monroy, F., and D. C. Sheppard. 2005. Taf1: a class II transposon of Aspergillus fumigatus. Fungal Genet. Biol. 42638-645. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard, D. C., T. Doedt, L. Y. Chiang, H. S. Kim, D. Chen, W. C. Nierman, and S. G. Filler. 2005. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol. Biol. Cell 165866-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 481908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spikes, S., R. Xu, C. K. Nguyen, G. Chamilos, D. P. Kontoyiannis, R. H. Jacobson, D. E. Ejzykowicz, L. Y. Chiang, S. G. Filler, and G. S. May. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197479-486. [DOI] [PubMed] [Google Scholar]
- 24.Sugui, J. A., J. Pardo, Y. C. Chang, K. A. Zarember, G. Nardone, E. M. Galvez, A. Mullbacher, J. I. Gallin, M. M. Simon, and K. J. Kwon-Chung. 2007. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 61562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, J., and B. L. Miller. 1997. Aspergillus asexual reproduction and sexual reproduction are differentially affected by transcriptional and translational mechanisms regulating stunted gene expression. Mol. Cell. Biol. 176191-6201. [DOI] [PMC free article] [PubMed] [Google Scholar]