Abstract
Glutathione (GSH) and its derivative phytochelatin are important binding factors in transition-metal homeostasis in many eukaryotes. Here, we demonstrate that GSH is also involved in chromate, Zn(II), Cd(II), and Cu(II) homeostasis and resistance in Escherichia coli. While the loss of the ability to synthesize GSH influenced metal tolerance in wild-type cells only slightly, GSH was important for residual metal resistance in cells without metal efflux systems. In mutant cells without the P-type ATPase ZntA, the additional deletion of the GSH biosynthesis system led to a strong decrease in resistance to Cd(II) and Zn(II). Likewise, in mutant cells without the P-type ATPase CopA, the removal of GSH led to a strong decrease of Cu(II) resistance. The precursor of GSH, γ-glutamylcysteine (γEC), was not able to compensate for a lack of GSH. On the contrary, γEC-containing cells were less copper and cadmium tolerant than cells that contained neither γEC nor GSH. Thus, GSH may play an important role in trace-element metabolism not only in higher organisms but also in bacteria.
Under aerobic growth conditions, either glutathione (GSH; l-γ-glutamyl-l-cysteine-glycine) or the small 12-kDa protein thioredoxin (TrxB) is essential to maintain a reduced environment in the cytosol of Escherichia coli cells (5, 27, 60, 65). Since E. coli thioredoxin reductase can transfer electrons from NADH to glutaredoxin 4 (Grx4, GrxD, or YdhD) and Grx4 can reduce Grx1 (GrxA) and Grx3 (GrxC) (14), E. coli is able to catalyze the reduction of disulfides without GSH. Thus, GSH by itself is not essential for the survival of this bacterium (17).
The cellular GSH is kept almost completely reduced (2, 30): the reduced GSH-oxidized GSH (GSSG) couple has a standard redox potential at pH 7.0 of −240 mV (66). Using a potential of about −260 mV in vivo (29) and the Nernst equation results in the calculation of a GSH concentration of about 5 mM and a GSSG concentration of about 5 μM. Therefore, any change in the GSH concentration is likely to influence the cellular metabolism by changing the redox potential of the cytoplasm and maybe also that of the periplasm (59). A decrease of the GSH concentration by half would increase the cytoplasmic redox potential by 18 mV.
GSH is also involved in the osmoadaptation of E. coli (39). As a response to highly osmotic conditions, a mutant strain unable to synthesize trehalose as an osmoprotectant accumulates GSH to a concentration about 10-fold that under normal conditions. The first and quickest response of E. coli to changing osmotic conditions is to change the cytoplasmic potassium concentration (39), and indeed, GSH is needed for the regulation of this pool (13), probably through interaction with the GSH-gated potassium efflux system KefC-YabF (43). Moreover, GSH is involved in the detoxification of methylglyoxal (13), although there are GSH-independent pathways of methylglyoxal degradation (44), and resistance to chlorine compounds (7, 67). In bacteria other than E. coli, GSH is essential for thiamine synthesis (19) and is also involved in defense against oxidative (31) and acidic (64) stress. Bacteria unable to synthesize GSH are sometimes able to import GSH from the growth medium, as shown previously for Haemophilus influenzae and for lactic acid bacteria (34, 71, 77), or to use other thiol compounds like mycothiol or γ-glutamylcysteine (γEC) instead (12, 45, 47). Some gram-positive bacteria use cysteine as the main cellular thiol against oxidative stress (26).
These data illustrate the importance of GSH and related compounds to the cellular biochemistry. In contrast, not much is known about the interplay of heavy metals and GSH in bacteria, except that GSH influences resistance to arsenite and mercury in E. coli (32) and cadmium tolerance in Rhizobium leguminosarum bv. viciae (15, 35). On the other hand, GSH is heavily involved in transition-metal homeostasis in eukaryotes. In Saccharomyces cerevisiae, GSH is essential for full cadmium resistance (18). In most plants and fungi, GSH is the major substrate for the synthesis of the heavy-metal binding polypeptide phytochelatin (8, 40). The sequestration of heavy metal cations by phytochelatin and the consecutive transport of the bis-glutathionato complexes into vacuoles seem to be the main route of heavy-metal detoxification in these organisms. Phytochelatins can be produced successfully in transgenic bacteria, leading to enhanced metal accumulation in the bacterial cytoplasm (3, 11, 28, 69, 72) without the compartmentation ability of a vacuole.
Why do bacteria detoxify heavy metals mainly by efflux (49, 50) even though they should be able to sequester metals to the phytochelatin educt GSH? In GSH, two cysteine residues and four carboxyl groups should be able to form octahedral bis-glutathionato complexes that are very stable (76). To address this question, we deleted the GSH synthesis pathway in E. coli and also genes for various efflux systems and investigated if GSH might be a crucial transition-metal binding factor of the bacterial cell.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli was grown in Luria-Bertani (LB) medium or in Tris-buffered mineral salts medium (41) containing 2 ml of glycerol and 3 g of Casamino Acids per liter (TMM). Solid medium contained 20 g of agar/liter. Antibiotics (chloramphenicol at 25 μg/ml, kanamycin at 25 μg/ml, and ampicillin at 125 μg/ml) and metals were added where appropriate.
Dose response growth experiments.
Overnight cultures of E. coli strains were diluted 1:100 in fresh LB medium or 1:1,000 in fresh TMM. After 2 h, they were diluted 1:10,000 in fresh LB medium with increasing metal cation concentrations and cultivated for 16 h with shaking at 37°C. The turbidity at 600 nm was measured using a SmartSpec3000 photometer (Bio-Rad, Munich, Germany).
Growth experiments.
Overnight cultures of E. coli strains were diluted 1:100 in fresh LB medium. After 2 h, they were diluted 1:100 in fresh LB medium with or without metal and cultivated with shaking at 37°C. The turbidity over 18 to 22 h was monitored by a Klett photometer.
Gene deletions and other genetic techniques.
Genes were deleted by the insertion of resistance cassettes using the λ Red recombinase system (9). Initial deletions in E. coli strain BW25113, in which the target genes were exchanged for a chloramphenicol resistance cassette (cat), were transferred by general transduction with phage P1 into E. coli strain W3110 or its derivatives. The mutant strains harboring copA::Km or zntA::Km were constructed by insertion of kanamycin resistance genes through homologous recombination (62, 63). Multiple deletion mutants were constructed by the FLP recombination target-dependent elimination of the respective resistance cassette with the assistance of flipase from plasmid pCP20 (9) and subsequent general phage P1 transduction. Otherwise, standard molecular genetic techniques were used (68). PCR was performed with Taq or Taq/Pwo DNA polymerase (Roche, Mannheim, Germany). All primer sequences can be obtained upon request.
GSH content determination.
Overnight cultures were diluted 1:100 in fresh LB medium or TMM with or without metals and cultivated with shaking at 37°C until the optical density (at 600 nm) reached 2.25 ± 0.25 (the late exponential phase of growth). Volumes of cells corresponding to 2.5 to 5 mg (dry weight) were harvested by centrifugation (15 min at 4,500 × g and 4°C), and the cells were suspended in 1 ml of 0.1 N HCl and disrupted by sonication for 2 min on ice with a UniEquip UW60 at 60 W and a 70% time interval. Cell debris was removed by centrifugation (15 min at 15,300 × g and 4°C). The supernatant was used to determine the protein concentration by the bicinchoninic acid assay with an assay kit from Sigma GmbH (Osterode) incorporating bovine serum albumin as the standard. The supernatant was also used to measure the GSH content by high-performance liquid chromatography (HPLC) analysis.
HPLC analyses of thiols were performed using monobromobimane derivatization as described previously (46). A volume of 0.12 ml of the cell-free supernatant was added to a mixture of 0.18 ml of 0.2 M CHES buffer [2-(cyclohexyl-amino-ethanesulfonate), pH 9.3] and 30 μl of 5 mM dithiothreitol. After incubation on ice for 1 h, 10 μl of monobromobimane (30 mM in methanol [MeOH]) was added for thiol derivatization and incubation was continued for 15 min in the dark at room temperature. The reaction was stopped with 5% acetic acid (vol/vol). HPLC analyses were carried out on a Lichrospher 60 RP Select B column (4 by 250 mm; particle size, 5 μm; Merck, Darmstadt, Germany) using a Merck-Hitachi LaChrom system equipped with a D-7000 interface, an L-7100 pump, an L-7200 autosampler, and a D-7480 fluorescence detector (excitation wavelength, 420 nm; emission wavelength, 520 nm). Mobile phase A consisted of a solution of 2% MeOH (vol/vol) in H2O with 2.5 ml of glacial acetic acid liter−1, adjusted to pH 4.3 with 10 N NaOH. Mobile phase B was composed of a solution of 90% MeOH (vol/vol) in H2O with 2.5 ml of glacial acetic acid liter−1, adjusted pH 3.9 with 0.1 N NaOH. HPLC running conditions are available upon request.
RESULTS
Cellular GSH in E. coli.
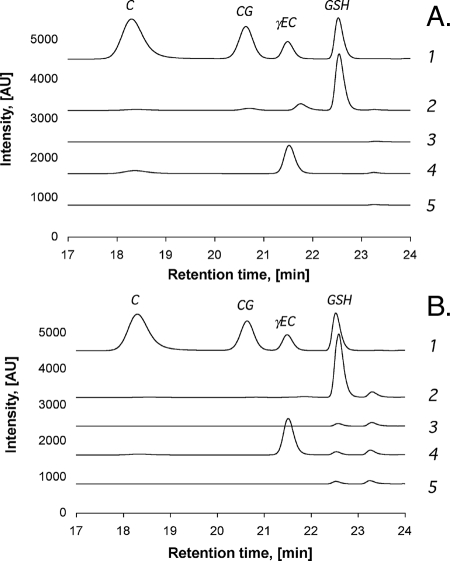
The gshA gene (encoding the γ-Glu-Cys synthetase), the gshB gene (encoding the GSH synthetase), or both genes were deleted from the chromosome of the E. coli wild-type strain W3110. The deletion of these genes resulted in a decrease of the cellular GSH concentrations in cells grown in TMM and in LB medium-grown cells (Fig. 1). The crude extracts from wild-type cells contained a mean ± a standard deviation of 13.7 ± 1.6 mg (TMM-grown cells) or 18.5 ± 2.4 mg (LB medium-grown cells) of GSH/g of total protein, and those from the ΔgshB mutant strain contained 27.4 ± 4.9 mg (TMM-grown cells) or 34.1 ± 6.4 mg (LB medium-grown cells) of γEC/g of total protein. Thus, the ΔgshB mutant strain contained a level of γ-Glu-Cys (γEC) higher than the level of GSH in the wild-type strain, which can be explained by the lack of GSH feedback inhibition of the γEC synthetase GshA in the ΔgshB mutant strain (1).
FIG. 1.
Thiol compounds in the crude extracts from E. coli wild-type and Δgsh mutant strains. HPLC profiles of the crude extracts prepared from E. coli wild-type cells (lanes 2), the ΔgshA mutant strain (lanes 3), the ΔghsB mutant strain (lanes 4), and the ΔgshA ΔghsB double mutant strain (lanes 5), each cultivated in TMM (A) or in LB medium (B), are shown. Lanes 1 represent a standard (8 μg/ml) containing peaks corresponding to cysteine (C), cysteine-glycine (CG), γEC, and GSH. All chromatograms were horizontally shifted for better visibility. The minor peak in panel A, lane 2, does not represent γEC but a nonreproducible artifact. AU, arbitrary units.
When cultivated in LB medium, all three types of mutant cells exhibited small GSH-derivative signals in HPLC analyses, indicating cytoplasmic GSH concentrations to be an order of magnitude smaller than that in wild-type cells (Fig. 1B). Since LB medium contains yeast extract and, therefore, GSH (mean value for various LB medium batches, 26 ± 6 μM) (data not shown), this residual GSH in the mutant cells may be the result of the uptake of external GSH by the GsiABCD uptake system (73). Even though LB medium-grown mutant cells were not entirely GSH free, E. coli strains with multiple deletions in gsh genes plus genes for metal efflux systems were cultivated in LB medium because that minor residual amount of the tripeptide (<5% of the wild-type level) (Fig. 1B) should not be able to affect toxic metal concentrations.
The deletion of gshA or of both gsh genes did not influence the growth rate of E. coli in LB medium, but the deletion of gshB alone decreased the growth rate by half (data not shown). Growth rates, expressed as the increase in turbidity at 600 nm, were 1.52 ± 0.34 h−1 (wild type), 1.34 ± 0.18 h−1 (ΔgshA mutant), 0.77 ± 0.17 h−1 (ΔgshB mutant), and 1.43 ± 0.36 h−1 (double mutant). Thus, γEC, present in the ΔgshB mutant cells, was not able to compensate for the missing GSH since a decreased growth rate was observed, even without additional heavy-metal stress.
GSH and copper.
In the presence of 3 mM Cu(II), the three Δgsh mutant strains and the wild type exhibited growth rates that were not significantly different (data not shown). Growth rates were 0.95 ± 0.08 h−1 (wild type), 1.23 ± 0.26 h−1 (ΔgshA mutant), 1.22 ± 0.26 h−1 (ΔgshB mutant), and 1.37 ± 0.58 h−1 (double mutant). However, the lag phase for the ΔgshB cells was markedly extended compared to those of the other strains (data not shown). This finding indicates that Cu(II) delayed the growth of the γEC-containing cells. As demonstrated by dose response curves for cells grown in LB medium, the deletion of gshA or of gshA and gshB did not influence copper resistance but ΔgshB mutant cells exhibited decreased copper resistance (Fig. 2A). Thus, GSH was not required for full copper resistance in this genetic background and copper toxicity was increased slightly in ΔgshB mutants. This increase was reversed in the double mutant, suggesting that the presence of γEC contributes to the increased copper toxicity. Similar effects were obtained in TMM but to a lesser extent (data not shown).
FIG. 2.
Effects of deletions of gsh genes on copper resistance. Dose response curves (over 16 h at 37°C in LB medium) were recorded for the E. coli wild type (black circles) and the corresponding ΔgshB (black squares), ΔgshA (white circles), and ΔgshA ΔgshB (white squares) mutants (A); the ΔcopA (black triangles), ΔcopA ΔgshA (white triangles), and ΔcopA ΔgshB (gray triangles) mutant strains (B); and the copA::Km ΔcusA ΔcueO (black inverted triangles), ΔcopA ΔcusCFBA ΔcueO ΔgshA (white inverted triangles), and copA::Km ΔcusA ΔcueO ΔgshB (gray inverted triangles) mutant strains (C). Note that the scales of the x axes in panels B and C are identical but different from that in panel A. Mean values with standard deviations (error bars) from at least three repeats are shown.
Cytoplasmic copper is detoxified mainly by the Cu(I)-exporting CPx-type ATPase CopA (62). As expected, the deletion of copA led to decreased copper resistance (Fig. 2B). In a ΔcopA mutant background, the further deletion of gshA or of gshB led to a decrease in copper resistance (Fig. 2B), and again, the ΔcopA ΔgshB mutant strain was less copper resistant than the ΔcopA ΔgshA mutant strain. Thus, GSH was required for copper resistance in E. coli when the cells were unable to detoxify cytoplasmic copper by efflux, and increased γEC was not able to compensate for missing GSH. Interestingly, low copper concentrations slightly stimulated the growth of the ΔcopA ΔgshA strain but not that of the ΔcopA ΔgshB cells. This result may indicate a beneficial effect of GSH, but not of γEC, on cellular copper allocation (Fig. 2B).
As reported previously (24), the inactivation of the periplasmic detoxification systems CusCFBA and CueO decreased the copper resistance of E. coli such that the 50% lethal dose for the ΔcopA mutant was 1.25 mM and that for the triple mutant strain was 0.83 mM (Fig. 2C). However, the additional deletion of gshA or of gshB in the triple mutant strain did not diminish copper resistance much further (Fig. 2C). In this mutant background, the deletion of gshA and that of gshB had similar effects on growth in the presence of copper.
GSH and zinc.
In the wild-type background, the deletion of the gsh genes had no effect on zinc resistance, either in dose response experiments performed with LB medium (Fig. 3A) or TMM (data not shown) or in time-dependent growth experiments [performed with 850 μM Zn(II) in LB medium] (data not shown). Thus, in these types of cells, the presence of GSH did not correlate with full zinc resistance and the presence of γEC instead of GSH did not correlate with increased zinc sensitivity.
FIG. 3.
Effects of deletions of gsh genes on zinc resistance. Dose response curves (over 16 h at 37°C in LB medium) were recorded for E. coli wild-type (WT) and mutant cells. For easier comparison, the dose response of the ΔzntA mutant cells (black triangles) is shown in all panels. (A) Results for wild-type (black circles), ΔgshB (black squares), ΔgshA (white circles), and ΔgshA ΔgshB (white squares) cells. (B) Results for ΔzntA cells (black triangles) and ΔgshA (gray circles), ΔgshB (white triangles), and ΔgshA ΔgshB (gray squares) mutants thereof. (C) Results for ΔzitB cells (black inverted triangles) and ΔgshA (gray circles), ΔgshB (white inverted triangles), and ΔgshA::Cm ΔgshB (gray squares) mutants thereof. (D) Results for ΔzntA ΔzitB cells (black diamonds) and ΔgshA (gray circles), ΔgshB (white diamonds), and ΔgshA::Cm ΔgshB::Km (gray squares) mutants thereof. The x-axis scales in panels A and C are identical, as are those in panels B and D. Mean values with standard deviations (error bars) from at least three repeats are shown.
The deletion of the gene for the CPx-type ATPase ZntA led to strongly decreased zinc resistance, as expected (70). The deletion of gshB, gshA, or both (Fig. 3B) in the ΔzntA background led to a further decrease of zinc resistance in all three cases. The ΔzntA ΔgshA mutant was slightly more zinc resistant than the other two strains. It is not clear why the absence of GSH in this mutant had an effect different from that of the absence of GSH in the ΔzntA ΔgshA ΔgshB mutant; the integrity of the constructs was verified continuously. Nevertheless, the differences were small, and as in the case of copper, GSH seemed to be important for zinc resistance in the absence of the cytoplasm-detoxifying CPx-type ATPase.
Another protein able to detoxify the cytoplasm of E. coli is the cation diffusion facilitator ZitB (20, 33). The deletion of zitB (in the presence of ZntA) led to no decrease in zinc resistance, and the further deletion of gsh genes in the ΔzitB mutant led to only small decreases in zinc resistance (Fig. 3C). Finally, the ΔzntA ΔzitB double mutant displayed decreased zinc resistance compared to that of the ΔzntA mutant, and the deletion of gsh genes decreased zinc resistance even more (Fig. 3D), especially in the ΔgshB mutant containing only γEC. Thus, the absence of GSH correlated with decreased zinc resistance when the efflux pump ZntA was missing, and γEC seemed not to be able to compensate for missing GSH.
GSH and chromate resistance.
GSH was also required for full chromate resistance (Fig. 4). The ΔgshA and ΔgshA ΔgshB mutant strains showed some decrease in chromate resistance compared to that of the wild type, while the ΔgshB mutant was the most sensitive strain of those tested. There was no effect of the gsh mutations on resistance to Ni(II) or Co(II) (data not shown).
FIG. 4.
Influence of GSH on chromate resistance. Dose response curves for the E. coli wild type (black circles) and the corresponding ΔgshA (white circles), ΔgshB (black squares), and ΔgshA ΔgshB (white squares) mutant strains over 16 h at 37°C in LB medium containing increasing concentrations of potassium chromate were recorded. Mean values with standard deviations (error bars) from triple determinations are shown.
GSH and cadmium.
In the presence of Cd(II), all mutant cells were less metal resistant than wild-type cells, in TMM and in LB medium (Fig. 5). While the ΔgshB mutant cells showed the smallest degree of cadmium resistance in either medium, the ΔgshA mutant cells and the ΔgshA ΔgshB double mutant cells displayed intermediate levels of cadmium resistance (Fig. 5). Thus, in contrast to that in the cases of zinc and copper resistance, the absence of GSH could not be fully complemented by efflux systems. The presence of γEC in the ΔgshB mutant correlated with decreased, not increased, cadmium resistance.
FIG. 5.
Influence of GSH on cadmium resistance. Dose response curves for the E. coli wild type (black circles) and the corresponding ΔgshA (white circles), ΔgshB (black squares), and ΔgshA ΔgshB (white squares) mutant strains over 16 h at 37°C in LB medium (A) or TMM (B) containing increasing concentrations of cadmium(II) chloride were recorded. Mean values with standard deviations (error bars) from at least three repeats are shown.
In the ΔzntA and ΔzntA ΔzitB mutant strains, the deletion of gsh genes led to strongly decreased cadmium resistance (Fig. 6; the cadmium concentration is expressed on a logarithmic scale). The difference between ΔgshA mutant derivatives (containing GSH) and ΔgshB mutant derivatives (containing γEC) with the wild-type background was greater than that between the corresponding mutant derivatives with ΔzntA and ΔzntA ΔzitB mutant backgrounds (Fig. 5 and 6). Reminiscent of the findings for zinc and copper, this result confirmed GSH to be an important tool in metal homeostasis in the absence of the efflux system and γEC to be unable to compensate for the missing GSH.
FIG. 6.
Cadmium resistance of E. coli mutants with deletions in genes involved in cadmium detoxification. Dose response curves (over 16 h at 37°C in LB medium) were recorded for E. coli ΔzntA (black circles), ΔzntA ΔgshA (white circles), ΔzntA ΔgshB (black triangles), and ΔzntA ΔgshA ΔgshB (white triangles) cells (A) and for E. coli ΔzntA ΔzitB (black squares), ΔzntA ΔzitB ΔgshA (white squares), ΔzntA ΔzitB ΔgshB (black inverted triangles), and ΔzntA ΔzitB ΔgshA ΔgshB (white inverted triangles) cells (B). Mean values with standard deviations (error bars) from at least three repeats are shown. The x axis is on a logarithmic scale.
DISCUSSION
Transition-metal ion homeostasis in general.
How can cellular heavy-metal homeostasis be described in terms of an interplay of transport and binding reactions? Transition-metal cations are taken up, bind to cytoplasmic and periplasmic components, and are exported by efflux systems. Upon first consideration, the cytoplasmic metal concentration seems to be in a flow equilibrium that is the result of uptake and efflux processes, but efflux systems may not be able to reach some of the metal cations in the cytoplasm because they are bound too tightly. Thus, superimposed on the kinetic flow equilibrium of uptake and efflux processes, there is a process of partitioning between cytoplasmic metal binding sites, including regulatory sites, and the substrate binding sites of the efflux proteins. Much is known about the uptake and efflux systems that compose the transport flow backbone of the cellular metal homeostasis, but binding and partitioning reactions have hardly been characterized.
Zinc.
In the transport flow backbone of transition metals in E. coli, Zn2+ is imported into the E. coli cells by fast and unspecific uptake systems like ZupT (21). Additionally, conditions of zinc starvation induce the ZnuABC uptake system (58). Inside the cells, the essential trace element zinc binds to a multitude of proteins, especially to the RNA polymerase (56). Excess zinc in the cytoplasm induces the expression of the CPx-type efflux ATPase ZntA (70) and of the cation diffusion facilitator family protein ZitB (20, 33), which export transition metals. Additionally, E. coli contains another cation diffusion facilitator protein, FieF (YiiP), that binds Zn(II) and transports Zn(II) in vitro (37) but may be an Fe(II) efflux system in vivo (22). The expression of the ZnuABC uptake system is under the control of the Zur protein (58), a paralog of the main iron homeostasis regulator, Fur. The expression of the main zinc efflux pump, ZntA, is under the control of the MerR-type regulator ZntR (4, 57). The sigmoidal activity functions of Zur and ZntR overlap, keeping the free cytoplasmic Zn2+ concentration in a narrow window between 2 × 10−16 M (the half-maximum-induction point for Zur regulation) and 10−15 M (the half-maximum-induction point for ZntR regulation) (57). In a previous study, the expression of the zitB gene was induced by zinc in a reporter gene assay (20), but the regulator has not yet been identified. So, partitioning events involved in cytoplasmic zinc homeostasis should occur between the unaccounted-for cytoplasmic binding sites of Zn2+ and the substrate binding sites of ZntA, ZitB, or even FieF.
Copper.
Copper homeostasis in E. coli is a more complicated process than zinc homeostasis. A great deal of copper homeostasis occurs in the periplasm. The resistance-nodulation-cell division-driven (RND) efflux system CusCBA seems to transport periplasmic copper from the periplasm to the outside of the cell (16, 24). Additionally, copper is bound by the small periplasmic protein CusF (16, 36), and Cu(I) is oxidized by the multicopper oxidase CueO into the less toxic form Cu(II) (23). It is unclear how periplasmic copper enters the cytoplasm and when and where it is reduced to Cu(I). It is exported, however, by the CPx-type ATPase CopA (62), which together with CueO is under the regulatory control of CueR (6).
Cobalt.
Any influence of GSH on cobalt resistance was not observed here (data not shown), although such influence in Salmonella enterica has been shown previously (74). However, the gshA mutant of S. enterica was a transposon mutant (74), indicating the possibility of polar effects and rearrangements, especially since the arrangement of genes on the S. enterica chromosome is slightly different from that on the E. coli chromosome (http://biocyc.org). In E. coli, cobalt toxicity is based mainly on the competition of Co(II) with Fe(II) during the synthesis of iron-sulfur clusters (61), which fits with the higher affinity of Co(II) than of Fe(II) for sulfur- and oxygen-containing ligands in complex compounds (48).
Chromate.
Like in other bacteria (52, 53), chromate is probably imported into E. coli cells by sulfate uptake systems, but E. coli does not contain a CHR-type efflux system for chromate (51). In the cytoplasm, chromate should interact immediately with GSH (42), leading to the generation of GSSG and Cr(III). This process may produce free radicals but detoxify chromate, which otherwise may interfere with the sulfate metabolism. The absence of GSH increased chromate toxicity (Fig. 4). Thus, the beneficial effect of GSH-mediated chromate detoxification should compensate for the production of free radicals.
Cadmium.
Similar to the findings for chromate, GSH-free cells suffered increased toxicity from cadmium. The deletion of the gene for the cadmium efflux pump ZntA had a much stronger effect on cadmium toxicity (Fig. 6) than the absence of GSH. Thus, GSH protects the cytoplasm by preventing the binding of Cd2+ to toxicity targets, but decreasing the absolute amount of cytoplasmic cadmium cations by efflux was more effective than decreasing the concentration of available cadmium by GSH-mediated sequestration.
GSH and transition metals.
The cytoplasmic metal cation concentration is tightly controlled by the transport flow backbone of metal homeostasis. However, it is unclear what the cytoplasmic metal concentration is. With respect to the cytoplasmic concentration of GSH and the affinity of most transition metals for sulfur (48), every divalent transition metal cation entering an E. coli cell may form a bis-glutathionato complex immediately. The complex binding constants rely heavily on the pH and the buffer composition. Robust numbers are 1042 for the Hg2+-bis-glutathionato complex (54) and 1039 for the Cu+-monoglutathionato complex (55), which easily explains the sensitivity of the copper resistance regulator CueR to zeptomolar levels of copper (6).
The values for other transition metals should be below the copper and mercury values due to the lower affinity of these metals for sulfur. If a zinc quota of 200 μM zinc, a GSH concentration of 5 mM, and a free zinc concentration of 4 × 10−16 M (between the half-maximum-induction points for Zur and ZntR regulation) (56) are assumed, the complex binding constant of the Zn2+-bis-glutathionato complex should be 2 × 1016, which fits with the data for copper and mercury, again considering the different affinities for sulfur. The rate constant for the formation of a Zn2+-monoglutathionato complex is 3,900 M−1 s−1 (10). Thus, 200 μM free zinc with 5 mM GSH should form the monoglutathionato complexes as a first step with an initial velocity of 3.9 mmol/s, leading to the rapid formation of zinc-bis-glutathionato complexes.
Thus, the absence of GSH should lead to increased metal sensitivity because target sites for metal toxicity are now readily available. However, this was not the case for the metal cations of the first transition period, like zinc and copper. The absence of GSH had no effect (Fig. 2 and 3) in wild-type cells. However, in the absence of the respective P-type ATPase efflux systems for Zn2+ and Cu+ (ZntA and CopA, respectively), the absence of GSH clearly decreased metal resistance. Thus, these transport systems complemented the absence of GSH and their detoxification activities were sufficient to prevent the binding of Zn2+ or Cu+ to cytoplasmic target sites.
So, cells needed GSH for full resistance to elements with high affinities for thiol groups, like Cd2+ and chromate, while metal cations like Zn2+ were sufficiently detoxified by efflux even in the absence of GSH. Cu+ was also sufficiently detoxified by efflux, although Cu+ has an even higher affinity for sulfur-containing ligands than Cd2+ (48). However, copper toxicity in E. coli seems to be a periplasmic rather than a cytoplasmic effect (38), and a copper uptake system in E. coli has not been identified yet.
If GSH detoxifies metal cations by sequestration and chromate by reduction, γEC should be able to perform similar tasks. Cells of the ΔgshB mutant strain contained levels of γEC higher than the levels of GSH in wild-type cells (Fig. 1). Nevertheless, ΔgshB mutant cells were less resistant to copper, cadmium, zinc, and chromate than ΔgshA mutant cells that did not contain considerable amounts of any cellular thiol compound. Even in the absence of efflux systems like ZntA and CopA, γEC was not able to provide any degree of protection.
Zn2+ binds primarily to the thiol group of cysteine, followed by the imidazole ring of histidine and the carboxyl groups of aspartate and glutamate, but only if these groups are deprotonated (75). Cd2+ should bind in a similar manner with a stronger emphasis on thiols. GSH contains a thiol group and two carboxyl groups, which should be able to bind to both divalent cations, forming a six-ligand “cage” around them in the respective bis-glutathionato complex. In γEC, the second carboxyl group of glycine is missing, leaving the cage open, allowing other molecules to attack and displace γEC much more easily than GSH. Since the main reason behind cadmium toxicity in E. coli is the sequestration of sulfide (originating during assimilatory sulfate reduction or genesis or the disturbance of iron-sulfur clusters [25]), γEC may not prevent this toxic action sufficiently in E. coli.
Acknowledgments
This study was supported by grant Ni262/3 of the Deutsche Forschungsgemeinschaft (DFG) and by the DFG graduate college 416.
We thank Cornelia Grosse, Gregor Grass, and Dirk Wesenberg for helpful comments.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Apontoweil, P., and W. Berends. 1975. Glutathione biosynthesis in Escherichia coli K 12. Properties of the enzymes and regulation. Biochim. Biophys. Acta 3991-9. [DOI] [PubMed] [Google Scholar]
- 2.Aslund, A., and J. Beckwith. 1999. Bridge over troubled waters: sensing stress by disulfide formation. Cell 96751-753. [DOI] [PubMed] [Google Scholar]
- 3.Bae, W., W. Chen, A. Mulchandani, and R. K. Mehra. 2000. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 70518-524. [DOI] [PubMed] [Google Scholar]
- 4.Brocklehurst, K. R., J. L. Hobman, B. Lawley, L. Blank, S. J. Marshall, N. L. Brown, and A. P. Morby. 1999. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol. Microbiol. 31893-902. [DOI] [PubMed] [Google Scholar]
- 5.Carmel-Harel, O., and G. Storz. 2000. Roles of glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae response to oxidative stress. Annu. Rev. Microbiol. 54439-461. [DOI] [PubMed] [Google Scholar]
- 6.Changela, A., K. Chen, Y. Xue, J. Holschen, C. E. Outten, T. V. O'Halloran, and A. Mondragon. 2003. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 3011383-1387. [DOI] [PubMed] [Google Scholar]
- 7.Chesney, J. A., J. W. Eaton, and J. R. J. Mahoney. 1996. Bacterial glutathione: a sacrificial defense against chlorine compounds. J. Bacteriol. 1782131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobbett, C., and P. Goldsbrough. 2002. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 53159-182. [DOI] [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominey, L. A., and K. Kustin. 1983. Kinetics and mechanism of Zn(II) complexation with reduced glutathione. J. Inorg. Biochem. 18153-160. [DOI] [PubMed] [Google Scholar]
- 11.Ernst, W. H. O., G.-J. Krauss, J. A. C. Verkleij, and D. Wesenberg. 2008. Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant Cell Environ. 31123-143. [DOI] [PubMed] [Google Scholar]
- 12.Fahey, R. C. 2001. Novel thiols of prokaryotes. Annu. Rev. Microbiol. 55333-356. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, G. P., and I. R. Booth. 1998. Importance of glutathione for growth and survival of Escherichia coli cells: detoxification of methylglyoxal and maintenance of intracellular K+. J. Bacteriol. 1804314-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes, A. P., M. Fladvad, C. Berndt, C. Andrésen, C. H. Lillig, P. Neubauer, M. Sunnerhagen, A. Holmgren, and A. Vlamis-Gardikas. 2005. A novel monothiol glutaredoxin (Grx4) from Escherichia coli can serve as a substrate for thioredoxin reductase. J. Biol. Chem. 28024544-24552. [DOI] [PubMed] [Google Scholar]
- 15.Figueira, E., I. G. Lima, and S. I. A. Pereira. 2005. Cadmium tolerance plasticity in Rhizobium leguminosarum bv. viciae: glutathione as a detoxifying agent. Can. J. Microbiol. 517-14. [DOI] [PubMed] [Google Scholar]
- 16.Franke, S., G. Grass, C. Rensing, and D. H. Nies. 2003. Molecular analysis of the copper-transporting CusCFBA efflux system from Escherichia coli. J. Bacteriol. 1853804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs, J. A., and H. R. Warner. 1975. Isolation of an Escherichia coli mutant deficient in glutathione synthesis. J. Bacteriol. 124140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharieb, M. M., and G. M. Gadd. 2004. Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 17183-188. [DOI] [PubMed] [Google Scholar]
- 19.Gralnick, J., E. Webb, B. Beck, and D. Downs. 2000. Lesions in gshA (encoding l-glutamyl-l-cysteine synthetase) prevent aerobic synthesis of thiamine in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 1825180-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grass, G., B. Fan, B. P. Rosen, S. Franke, D. H. Nies, and C. Rensing. 2001. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J. Bacteriol. 1834664-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grass, G., S. Franke, N. Taudte, D. H. Nies, L. M. Kucharski, M. E. Maguire, and C. Rensing. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 1871604-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grass, G., M. Otto, B. Fricke, C. J. Haney, C. Rensing, D. H. Nies, and D. Munkelt. 2005. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch. Microbiol. 1839-18. [DOI] [PubMed] [Google Scholar]
- 23.Grass, G., and C. Rensing. 2001. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 286902-908. [DOI] [PubMed] [Google Scholar]
- 24.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 1832145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helbig, K., C. Grosse, and D. H. Nies. 2008. Cadmium toxicity in glutathione mutants of Escherichia coli. J. Bacteriol. 1905439-5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochgräfe, F., J. Mostertz, D.-C. Pöther, D. Becher, J. D. Helmann, and M. Hecker. 2007. S-Cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J. Biol. Chem. 28225981-25985. [DOI] [PubMed] [Google Scholar]
- 27.Holmgren, A. 1989. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 26413963-13966. [PubMed] [Google Scholar]
- 28.Kang, S. H., S. Singh, J. Y. Kim, W. Lee, A. Mulchandani, and W. Chen. 2007. Bacteria metabolically engineered for enhanced phytochelatin production and cadmium accumulation. Appl. Environ. Microbiol. 736317-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirlin, W. G., J. Cai, S. A. Thompson, D. Diaz, T. J. Kavanagh, and D. P. Jones. 1999. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic. Biol. Med. 271208-1218. [DOI] [PubMed] [Google Scholar]
- 30.Kosower, N. S., and E. M. Kosower. 1978. The glutathione status of cells. Int. Rev. Cytol. 54109-160. [DOI] [PubMed] [Google Scholar]
- 31.Kuanyu, L., S. Hein, W. Zou, and G. Klug. 2004. The glutathione-glutaredoxin system in Rhodobacter capsulatus: part of a complex regulatory network controlling defense against oxidative stress. J. Bacteriol. 1866800-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latinwo, L. M., C. Donald, C. Ikediobi, and S. Silver. 1998. Effects of intracellular glutathione on sensitivity of Escherichia coli to mercury and arsenite. Biochem. Biophys. Res. Commun. 24267-70. [DOI] [PubMed] [Google Scholar]
- 33.Lee, S. M., G. Grass, C. J. Haney, B. Fan, B. P. Rosen, A. Anton, D. H. Nies, and C. Rensing. 2002. Functional analysis of the Escherichia coli zinc transporter ZitB. FEMS Microbiol. Lett. 215273-278. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y., J. Hugenholtz, T. Abee, and D. Molenaar. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 695739-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lima, A. I. G., S. C. Corticeiro, and E. Figueira. 2006. Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzyme Microb. Technol. 39763-769. [Google Scholar]
- 36.Loftin, I. R., S. Franke, S. A. Roberts, A. Weichsel, A. Heroux, W. R. Montfort, C. Rensing, and M. M. McEvoy. 2005. A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry 4410533-10540. [DOI] [PubMed] [Google Scholar]
- 37.Lu, M., and D. Fu. 2007. Structure of the zinc transporter YiiP. Science 3171746-1748. [DOI] [PubMed] [Google Scholar]
- 38.Macomber, L., C. Rensing, and J. A. Imlay. 2007. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 1891616-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaggan, D., T. M. Logan, D. G. Lynn, and W. Epstein. 1990. Involvement of gamma-glutamyl peptides in osmoadaptation of Escherichia coli. J. Bacteriol. 1723631-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendoza-Cozatl, D., H. Loza-Tavera, A. Hernandez-Navarro, and R. Moreno-Sanchez. 2005. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 29653-671. [DOI] [PubMed] [Google Scholar]
- 41.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messer, J., M. Reynolds, L. Stoddard, and A. Zhitkovich. 2006. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic. Biol. Med. 401981-1992. [DOI] [PubMed] [Google Scholar]
- 43.Miller, S., L. S. Ness, C. M. Wood, B. C. Fox, and I. R. Booth. 2000. Identification of an ancillary protein, YabF, required for activity of the KefC glutathione-gated potassium efflux system in Escherichia coli. J. Bacteriol. 1826536-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra, K., A. B. Banerjee, S. Ray, and M. Ray. 1995. Glyoxalase III from Escherichia coli: a single novel enzyme for the conversion of methylglyoxal into d-lactate without reduced glutathione. Biochem. J. 305999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton, G. L., K. Arnold, M. S. Price, C. Sherrill, S. B. Delcardayre, Y. Aharonowitz, G. Cohen, J. Davies, R. C. Fahey, and C. Davis. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 1781990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton, G. L., and R. C. Fahey. 1995. Determination of biothiols by bromobimane labeling and high-performance liquid chromatography. Methods Enzymol. 251148-166. [DOI] [PubMed] [Google Scholar]
- 47.Newton, G. L., and R. C. Fahey. 2002. Mycothiol biochemistry. Arch. Microbiol. 178388-394. [DOI] [PubMed] [Google Scholar]
- 48.Nies, D. H. 2007. Bacterial transition metal homeostasis, p. 118-142. In D. H. Nies and S. Silver (ed.), Molecular microbiology of heavy metals, vol. 6. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 49.Nies, D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27313-339. [DOI] [PubMed] [Google Scholar]
- 50.Nies, D. H. 1999. Microbial heavy metal resistance. Appl. Microbiol. Biotechnol. 51730-750. [DOI] [PubMed] [Google Scholar]
- 51.Nies, D. H., S. Koch, S. Wachi, N. Peitzsch, and M. H. J. Saier. 1998. CHR, a novel family of prokaryotic proton motive force-driven transporters probably containing chromate/sulfate transporters. J. Bacteriol. 1805799-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nies, D. H., and S. Silver. 1989. Metal ion uptake by a plasmid-free metal-sensitive Alcaligenes eutrophus strain. J. Bacteriol. 1714073-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtake, H., C. Cervantes, and S. Silver. 1987. Decreased chromate uptake in Pseudomonas fluorescens carrying a chromate resistance plasmid. J. Bacteriol. 1693853-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oram, P. D., X. Fang, Q. Fernando, P. Letkeman, and D. Letkeman. 1996. The formation constants of mercury(II)-glutathione complexes. Chem. Res. Toxicol. 9709-712. [DOI] [PubMed] [Google Scholar]
- 55.Osterberg, R., R. Ligaarden, and D. Persson. 1979. Copper(I) complexes of penicillamine and glutathione. J. Inorg. Biochem. 10341-355. [DOI] [PubMed] [Google Scholar]
- 56.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2922488-2492. [DOI] [PubMed] [Google Scholar]
- 57.Outten, C. E., F. W. Outten, and T. V. O'Halloran. 1999. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J. Biol. Chem. 27437517-37524. [DOI] [PubMed] [Google Scholar]
- 58.Patzer, S. I., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 281199-1210. [DOI] [PubMed] [Google Scholar]
- 59.Pittman, M. S., H. C. Robinson, and R. K. Poole. 2005. A bacterial glutathione transporter (Escherichia coli CydDC) exports reductant to the periplasm. J. Biol. Chem. 28032254-32261. [DOI] [PubMed] [Google Scholar]
- 60.Prinz, W. A. F., A. Aslund, A. Holmgren, and J. Beckwith. 1997. The role of thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds. J. Biol. Chem. 27215661-15667. [DOI] [PubMed] [Google Scholar]
- 61.Ranquet, C., S. Ollagnier-de-Choudens, L. Loiseau, F. Barras, and M. Fontecave. 2007. Cobalt stress in Escherichia coli. J. Biol. Chem. 28230442-30451. [DOI] [PubMed] [Google Scholar]
- 62.Rensing, C., B. Fan, R. Sharma, B. Mitra, and B. P. Rosen. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 97652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rensing, C., B. Mitra, and B. P. Rosen. 1997. The zntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 9414326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riccillo, P. M., C. I. Muglia, F. J. de Bruijn, A. J. Roe, I. R. Booth, and O. M. Aguilar. 2000. Glutathione is involved in environmental stress responses in Rhizobium tropici, including acid tolerance. J. Bacteriol. 1821748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritz, D., and J. Beckwith. 2007. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 5521-48. [DOI] [PubMed] [Google Scholar]
- 66.Rost, J., and S. Rapoport. 1964. Reduction-potential of gutathione. Nature 201185-187. [DOI] [PubMed] [Google Scholar]
- 67.Saby, S., P. Leroy, and J.-C. Block. 1999. Escherichia coli resistance to chlorine and glutathione synthesis in response to oxygenation and starvation. Appl. Environ. Microbiol. 655600-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 69.Sauge-Merle, S., S. Cuine, P. Carrier, C. Lecomte-Pradines, D. T. Luu, and G. Peltier. 2003. Enhanced toxic metal accumulation in engineered bacterial cells expressing Arabidopsis thaliana phytochelatin synthase. Appl. Environ Microbiol. 69490-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma, R., C. Rensing, B. P. Rosen, and B. Mitra. 2000. The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J. Biol. Chem. 2753873-3878. [DOI] [PubMed] [Google Scholar]
- 71.Sherrill, C., and R. C. Fahey. 1998. Import and metabolism of glutathione by Streptococcus mutans. J. Bacteriol. 1801454-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sriprang, R., M. Hayashi, H. Ono, M. Takagi, K. Hirata, and Y. Murooka. 2003. Enhanced accumulation of Cd2+ by a Mesorhizobium sp. transformed with a gene from Arabidopsis thaliana coding for phytochelatin synthase. Appl. Environ. Microbiol. 691791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, H., T. Koyanagi, S. Izuka, A. Onishi, and H. Kumagai. 2005. The yliA, -B, -C, and -D genes of Escherichia coli K-12 encode a novel glutathione importer with an ATP-binding cassette. J. Bacteriol. 1875861-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorgersen, M. P., and D. M. Downs. 2007. Cobalt targets multiple metabolic processes in Salmonella enterica. J. Bacteriol. 1897774-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trzaskowski, B., L. Adamowicz, and P. A. Deymier. 2008. A theoretical study of zinc(II) interactions with amino acid models and peptide fragments. J. Biol. Inorg. Chem. 13133-137. [DOI] [PubMed] [Google Scholar]
- 76.Vatamaniuk, O. K., S. Mari, Y. P. Lu, and P. A. Rea. 2000. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. J. Biol. Chem. 27531451-31459. [DOI] [PubMed] [Google Scholar]
- 77.Vergauwen, B., F. Pauwels, M. Vaneechoutte, and J. J. Van Beeumen. 2003. Exogenous glutathione completes the defense against oxidative stress in Haemophilus influenzae. J. Bacteriol. 1851572-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]