Abstract
The Streptomyces coelicolor absB gene encodes an RNase III family endoribonuclease and is normally essential for antibiotic biosynthesis. Here we report that AbsB controls its own expression by sequentially and site specifically cleaving stem-loop segments of its polycistronic transcript. Our results demonstrate a ribonucleolytic regulatory role for AbsB in vivo.
Streptomycetes are a family of prokaryotic soil-dwelling microorganisms whose morphologically and developmentally complex life cycle involves mycelial growth and spore formation (8). These eubacteria also are notable for their production of pharmaceutically useful compounds, including antitumor agents, immunosuppressants, and more than two-thirds of all natural antibiotics (6, 9). Streptomyces coelicolor, which has been the most widely studied streptomycete, synthesizes four identified antibiotics (9), whose production is controlled during the S. coelicolor life cycle by the combined actions of a series of regulatory and biosynthetic-pathway genes (4).
The absB gene was discovered in a screen for S. coelicolor mutants that show defective antibiotic synthesis but normal morphological development (1); recently, however, AbsB has been found also to be required for the proper formation of sporulation septa (14). Subsequent sequence analysis revealed that the N-terminal domain of the AbsB protein contains a motif characteristic of RNase III family endoribonucleases (13). Members of this enzyme family, which has been implicated in the processing of pre-rRNA, rRNA, polycistronic mRNAs, and small regulatory RNAs (3, 10), normally cleave duplex segments of RNAs configured as stem-loop structures and are ubiquitous among prokaryotes, eukaryotes, and archaea (10). The originally isolated point mutation in AbsB substitutes a proline for a leucine in the first α-helix component of the putative RNA binding domain located near the C-terminal end of the protein (13). That AbsB may, in fact, have ribonucleolytic activity was first suggested by the accumulation of 30S rRNA precursors in absB mutant bacteria (13); later work has shown that purified AbsB protein can cleave transcripts encoded by the S. coelicolor rpsO-pnp operon in vitro (7). However, there has been no direct evidence in vivo of RNase function carried out by the AbsB protein.
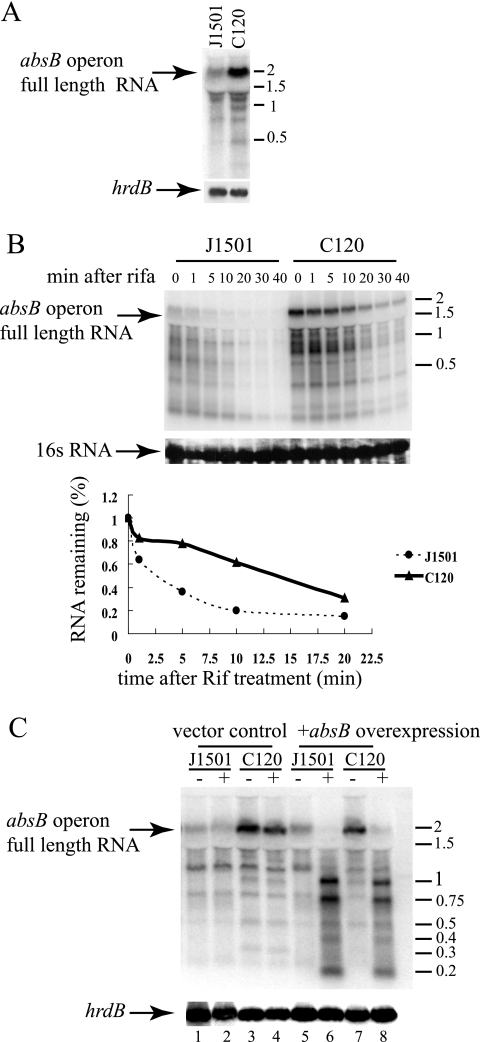
The S. coelicolor absB gene is predicted to be the terminal open reading frame (ORF) of a three-gene operon that also encodes a hypothetical protein (SC7A1.14, SCO5570) and RmpF, a homologue of ribosomal protein L32 (SCO5571) (13, 14). DNA microarray results showed increased absB transcript abundance in bacteria mutated in absB (11), suggesting that the AbsB protein may be targeting its own transcript ribonucleolytically. Consistent with this notion, Northern blot analysis shows the accumulation of an ∼2,000-nucleotide (nt) RNA band corresponding in length to the full-length SC7A1.14-rmpF-absB transcript in S. coelicolor strain C120 ( Fig. 1A), which contains a point mutation that substitutes a proline for the leucine at amino acid position 120 of the AbsB protein (13). Moreover, elevation of the steady-state level of 2,000-nt SC7A1.14-rmpF-absB transcripts was accompanied by a decreased rate of decay of these transcripts, as measured by the analysis of RNA samples taken at different times after rifampin addition, which prevents the initiation of new transcripts and consequently enables the determination of the rate of loss of previously synthesized mRNA. As shown in Fig. 1B, the half-life of SC7A1.14-rmpF-absB-encoded transcripts in the absB mutant C120 strain was approximately three times the half-life of such transcripts in the parental absB+ strain, J1501. Accumulation of SC7A1.14-rmpF-absB transcripts in absB mutant bacteria was reversed by adventitious expression of the cloned wild-type absB ORF under the control of the thiostrepton-inducible tipA promoter (Fig. 1C), as was the defect in antibiotic production. Such expression resulted in the production of an ∼1,000-nt transcript corresponding in length to the absB ORF (Fig. 1C, lanes 6 and 8). Additionally, the appearance of two smaller RNA transcripts (750 and 200 nt) in cells overexpressing the absB ORF sequence suggested that the ORF transcript contains a site targeted by the AbsB protein.
FIG. 1.
absB mRNA abundance in the J1501 (wild-type) and C120 (absB mutant) strains of S. coelicolor. (A) Mycelia were collected after 48 h of incubation at 30°C of spore suspensions spread on cellophane membranes placed on R5 plates (11), and 20 μg of total RNA isolated with an RNeasy mini kit (Qiagen) was electrophoresed on denatured agarose gels. Following transfer to Zeta-Probe (Bio-Rad) membrane, RNA was analyzed by Northern blotting (NorthernMax kit; Ambion) with randomly radiolabeled absB ORF DNA as the probe. Phosphorimaging (Typhoon; GE) was used for RNA detection and quantification. A band corresponding in length to the full-length SCA7A1.14-rmpF-absB operon transcript is indicated by an arrow. hrdB transcripts which encode a constitutively expressed sigma factor (5) were used as internal loading controls. (B) J1501 and C120 spores germinated for 6 h in 2× YT medium (11) were inoculated into R5 liquid medium at an optical density at 450 nm of 0.05. After growth at 30°C for 16 h to an optical density at 450 nm of 1.6, 500 μg/ml rifampin (rifa or Rif) was added and Northern blotting analysis of total RNA which was isolated from aliquots of the cultures at the times indicated was performed as described above. 16S rRNA transcripts served as an internal loading control. (C) Strains J1501 and C120 expressing AbsB adventitiously from the inducible tipA promoter were constructed by introducing apramycin resistance plasmid pIJ6902 containing the intact absB ORF (11). RNA was isolated from strains harboring the empty pIJ6902 vector (J1501, lanes 1 and 2; C120, lanes 3 and 4) or pIJ6902-absB (J1501, lanes 5 and 6; C120, lanes 7 and 8) that were grown on R5-aparamycin plates containing (+) or lacking (−) thiostrepton for 48 h. hrdB transcripts which encode a constitutively expressed sigma factor (5) were the internal control. The values beside the gels are molecular lengths of RNA markers in thousands.
To elucidate more precisely the sites of ribonucleolytic cleavage of SC7A1.14-rmpF-absB transcripts by AbsB, we produced hexahistidine-tagged wild-type and mutant AbsB proteins in protease-deficient Escherichia coli strain BL21 by cloning the absB ORF in expression plasmid pET28a and purified these proteins by Ni column chromatography (2). While both proteins were produced in and purified from E. coli under identical conditions, Western blot analysis, using anti-His antibody, of the Ni column eluates obtained resulted in a fivefold greater yield of the mutant protein (data not shown). Additionally, we observed that the 1,000-nt transcript encoding the wild-type AbsB protein was cleaved in E. coli (Fig. 2A) to yield an ∼750-nt fragment whereas the transcript encoding the mutant AbsB protein remained intact, suggesting that the AbsB protein acts ribonucleolytically in vivo on absB mRNA synthesized in that microorganism.
FIG. 2.
AbsB cleavage of absB-containing transcripts. (A) Total RNA was isolated from E. coli strains producing either wild-type or mutant absB transcripts after 4 h of treatment with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and analyzed by Northern blotting with random radiolabeled absB ORF DNA as the probe. (B) His-tagged wild-type or mutant AbsB protein was purified as previously described (2). DNA corresponding to the full-length SC7A1.14-rmpF-absB operon was amplified from S. coelicolor genomic DNA with 5′ primers that included the sequence of the bacteriophage T7 promoter. The primers are RNA A-forward (5′-TAATACGACTCACTATAGGGCCGGATTGGGCCGAGAGCGAATGG-3′), RNA A-reverse (5′-GCGGTCTGGGCCGGTGGATGAGCG-3′), RNA B-forward (5′-TAATACGACTCACTATAGGGGGCTCTTCGGTCGCGTGTATGCC-3′), and RNA B-reverse (same as RNA A-reverse). RNA substrates were synthesized with a MEGAscript T7 kit (Ambion) and purified. A 5-μg RNA sample was incubated with 100 ng purified protein in a 20-μl reaction mixture (30 mM Tris-Cl [pH 8.0], 160 mM NaCl, 0.01 μg/μl tRNA, 0.1 mM EDTA, 0.1 mM dithiothreitol, 10 mM MgCl2) as previously described (2). Purified cleavage products were electrophoresed in a denatured 1.5% agarose gel as described in the legend to Fig. 1A. Visualization of the reaction products was carried out by UV light. The arrows indicate the positions of uncleaved RNA and the cleavage products. (C) RNA A was treated with AbsB protein for the times indicated. (D) Approximate positions of cleavage sites in the absB operon transcript determined by this analysis. tsp, transcription start point; ter, terminus. The values beside the gels are molecular lengths of RNA markers in thousands.
To identify the sites targeted by the AbsB protein, we amplified two segments of S. coelicolor genomic DNA corresponding to the full-length absB operon by PCR with forward primers that included the E. coli bacteriophage T7 promoter sequence and used the resulting amplicons as templates for the in vitro production of RNAs (RNA A, 1,877 nt; RNA B, 1,842 nt; Fig. 2D) generated by T7 RNA polymerase; these RNAs have identical 3′ termini but differ at their 5′ ends. As shown in Fig. 2B, treatment of either RNA A or RNA B with purified wild-type His-tagged AbsB yielded two prominent bands (II-1 and II-2) corresponding, respectively, to RNA species approximately 1,200 and 500 nt in length and two less prominent bands (I-1 and I-2) corresponding to 1,700- and 200-nt species (Fig. 2B, lanes 2 and 5). In contrast, only faint bands of various lengths were detected following treatment of the RNA with the mutant AbsB protein (Fig. 2B, lanes 3 and 6), consistent with the defective ribonucleolytic activity observed in vivo in absB mutant bacteria. Additional experiments in which RNA A was incubated for various lengths of time with the wild-type enzyme (Fig. 2C) showed (i) that cleavage of RNA A was time dependent, (ii) that the abundance of band I-1 reached a maximum after 10 min of incubation and decreased subsequently, and (iii) that RNA bands II-1 and II-2 first appeared after 10 to 15 min of incubation and reached maximal abundance after 60 min of incubation. Taken together, these results argue strongly that SC7A1.14-rmpF-absB transcripts are cleaved sequentially at two separate sites and that RNA species I-1 is a decay intermediate that is subsequently cleaved to generate the shorter RNA species, which is not degraded further by AbsB.
The lengths of the cleavage products observed following the treatment of RNA A and RNA B with the AbsB protein suggest that the site of initial cleavage of the polycistronic SC7A1.14-rmpF-absB transcript is within the absB ORF, whereas the second cleavage event occurs within SC7A1.14 (Fig. 2D). As the 3′ termini of RNA A and RNA B are identical but RNA A is initiated 36 nt 5′ to RNA B, the generation of similarly sized 200-nt cleavage products from both RNAs implies that the first cleavage event in these RNAs occurs 200 nt from their 3′ termini; the generation of a slightly larger 500-nt RNA (II-2) from RNA A implies that the second cleavage event occurs 500 nt from their 5′ termini. In additional in vitro experiments, truncated RNAs that individually contain sequences of each proposed AbsB target site were cleaved at corresponding locations (data not shown), indicating that the site specificity of AbsB-mediated cleavages is determined by the RNA sequence in the region of cleavage rather than by interactions between distant regions of the full-length operon transcript.
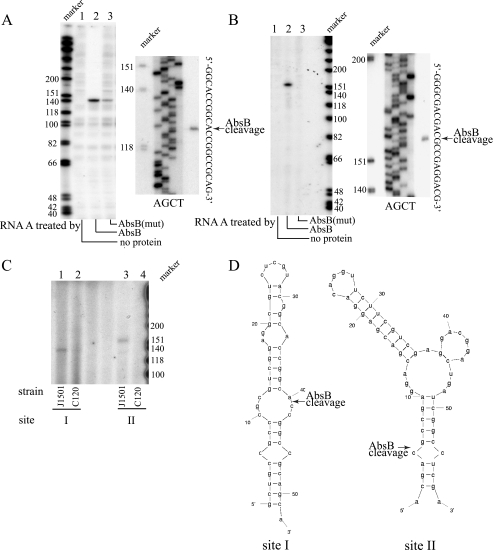
Primer extension analyses of intact, AbsB-cleaved SC7A1.14-rmpF-absB transcripts precisely mapped the two cleavage sites identified by the above in vitro experiments (Fig. 3A for site I and B for site II). The detection of faint bands representing apparent cleavage products following treatment of the substrate with the mutant protein suggests that the leucine-to-proline substitution at amino acid position 120 does not totally abolish ribonucleolytic activity (Fig. 3A, lane 3). Specific primer extension products were observed only after the treatment of substrates with the wild-type AbsB protein (Fig. 3A and B, lanes 2). Additional primer extension analyses with total RNA isolated from the wild-type strain (J1501) as the template showed bands of the same lengths as those generated by AbsB cleavage of its transcript in vitro (Fig. 3C, lanes 1 and 3), whereas no such bands were detected in absB mutant strain C120 (Fig. 3C, lanes 2 and 4), confirming that AbsB cleaves at the same two sites in vivo and also confirming that the in vivo ribonucleolytic action of the mutant AbsB protein is dramatically impaired. Cleavages at both sites occurred in regions predicted to form stem-loop structures by Mfold analysis (Fig. 3D) (15), as is characteristic of cleavages by RNase III family enzymes (10).
FIG. 3.
Mapping of SC7A1.14-rmpF-absB operon cleavage sites by primer extension. Substrates were either 20 ng RNA synthesized as described above and treated with AbsB protein in vitro or 100 μg total S. coelicolor RNA. Primer I (5′-CGCCTCGTCGGGGTCCGCGT-3′), a synthetic deoxyribonucleotide corresponding to the sequence ∼150 nt 3′ to site I (as shown in Fig. 2D) and primer II (5′-GGTCGTCCGACAGGCGCAC-3′), located ∼150 nt 3′ to site II, were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase. A Primer Extension System (Promega) and a 7-deaza-dGTP sequencing kit (USB) were used for the extension reactions (left) and the generation of sequencing ladders (right). (A) Uncleaved RNA A (lane 1), RNA A treated with AbsB (lane 2), and RNA A treated with mutant AbsB protein (lane 3) were reverse transcribed with primer I. The products were separated on 10% denatured polyacrylamide gel and exposed to a PhosphorImager. (B) Primer extension analysis of cleavage site II. RNA A treated as described for panel A was reverse transcribed with primer II. (C) A 100-μg sample of RNA from the wild-type (lanes 1 and 3) or absB mutant (lanes 2 and 4) strain was reverse transcribed with the primers for sites I and II. No RNA was added to the gel lanes located between lanes 2 and 3. (D) Secondary structures of the RNA sequences of these two cleavage sites identified. The positions where AbsB cleaves are indicated. The molecular marker sizes beside the gels are in nucleotide bases.
There is no obvious sequence homology between these two AbsB cleavage sites in the SC7A1.14-rmpF-absB transcript and the site in the transcript of the intergenic region of the rpoS-pnp operon found previously to be cleaved by the AbsB protein in vitro (7). However, both AbsB cleavages in SCA7A1.14-rmpF-absB occurred within the internal single-stranded regions of stem-loop structures, as did the cleavage of the rpoS-pnp transcript by AbsB, which resembles cleavage by Bacillus subtilis RNase III rather than by E. coli RNase III, which normally cleaves within a base-paired hairpin segment (10, 12).
Our results show that AbsB autoregulates its expression by cleaving its own transcript and that an important consequence of the mutational ablation of absB activity, which results in the absence of antibiotic biosynthetic functions, is the loss of cleavage ability. The loss of cleavage ability in absB mutant bacteria implies that the previously observed decrease in the steady-state level of the multiple mRNAs in these bacteria (11) is a secondary rather than a primary effect of AbsB cleavage.
Acknowledgments
We thank Christine Miller, Kai Bao, and Johan Kers for strains and advice and Roberta Peterson for assistance with the preparation of the manuscript.
These studies were supported by NIH grants AI08619 and GM54158 to S.N.C.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Adamidis, T., and W. Champness. 1992. Genetic analysis of absB, a Streptomyces coelicolor locus involved in global antibiotic regulation. J. Bacteriol. 1744622-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarasinghe, A. K., I. Calin-Jageman, A. Harmouch, W. Sun, and A. W. Nicholson. 2001. Escherichia coli ribonuclease III: affinity purification of hexahistidine-tagged enzyme and assays for substrate binding and cleavage. Methods Enzymol. 342143-158. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409363-366. [DOI] [PubMed] [Google Scholar]
- 4.Bibb, M. 1996. 1995 Colworth Prize Lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 1421335-1344. [DOI] [PubMed] [Google Scholar]
- 5.Buttner, M. J., K. F. Chater, and M. J. Bibb. 1990. Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2). J. Bacteriol. 1723367-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challis, G. L., and D. A. Hopwood. 2003. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 100(Suppl. 2)14555-14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, S. A., P. Bralley, and G. H. Jones. 2005. The absB gene encodes a double strand-specific endoribonuclease that cleaves the read-through transcript of the rpsO-pnp operon in Streptomyces coelicolor. J. Biol. Chem. 28033213-33219. [DOI] [PubMed] [Google Scholar]
- 8.Chater, K. F. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47685-713. [DOI] [PubMed] [Google Scholar]
- 9.Chater, K. F., and M. J. Bibb. 1997. Regulation of bacterial antibiotic production. In products of secondary metabolism, p. 59-105. In H. Kleinkauf and H. Von Dohren (ed.), Biotechnology, 7th ed. VCH, Weinheim, Germany.
- 10.Drider, D., and C. Condon. 2004. The continuing story of endoribonuclease III. J. Mol. Microbiol. Biotechnol. 8195-200. [DOI] [PubMed] [Google Scholar]
- 11.Huang, J., J. Shi, V. Molle, B. Sohlberg, D. Weaver, M. J. Bibb, N. Karoonuthaisiri, C. J. Lih, C. M. Kao, M. J. Buttner, and S. N. Cohen. 2005. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 581276-1287. [DOI] [PubMed] [Google Scholar]
- 12.Mitra, S., and D. H. Bechhofer. 1994. Substrate specificity of an RNase III-like activity from Bacillus subtilis. J. Biol. Chem. 26931450-31456. [PubMed] [Google Scholar]
- 13.Price, B., T. Adamidis, R. Kong, and W. Champness. 1999. A Streptomyces coelicolor antibiotic regulatory gene, absB, encodes an RNase III homolog. J. Bacteriol. 1816142-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sello, J. K., and M. J. Buttner. 2008. The gene encoding RNase III in Streptomyces coelicolor is transcribed during exponential phase and is required for antibiotic production and for proper sporulation. J. Bacteriol. 1904079-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 313406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]