Abstract
The microaerophilic food-borne pathogen Campylobacter jejuni experiences variable oxygen concentrations during its life cycle, especially during transitions between the external environment and the avian or mammalian gut. Single knockout mutations in either one of two related thiol peroxidase genes, tpx and bcp, resulted in normal microaerobic growth (10% [vol/vol] oxygen) but poorer growth than that of the wild type under high-aeration conditions (21% [vol/vol] oxygen). However, a tpx/bcp double mutant had a severe microaerobic growth defect and did not grow at high aeration in shake flasks. Although the single mutant strains were no more sensitive than the wild-type strains in disc diffusion assays with hydrogen peroxide, organic peroxides, superoxide, or nitrosative stress agents, in all cases the double mutant was hypersensitive. Quantitative cell viability and cellular lipid peroxidation assays indicated some increased sensitivity of the single tpx and bcp mutants to peroxide stress. Protein carbonylation studies revealed that the tpx/bcp double mutant had a higher degree of oxygen- and peroxide-induced oxidative protein damage than did either of the single mutants. An analysis of the peroxidase activity of the purified recombinant enzymes showed that, surprisingly, Tpx reduced only hydrogen peroxide as substrate, whereas Bcp also reduced organic peroxides. Immunoblotting of wild-type cell extracts with Tpx- or Bcp-specific antibodies showed increased abundance of both proteins under high aeration compared to that under microaerobic growth conditions. Taken together, the results suggest that Tpx and Bcp are partially redundant antioxidant enzymes that play an important role in protection of C. jejuni against oxygen-induced oxidative stress.
Campylobacter jejuni, a gram-negative bacterium with a microaerobic growth requirement, is a major food-borne pathogen in both the developing and the developed world (26, 40). C. jejuni is a commensal in chickens, and contaminated chicken meat is a major source of infection (18). The genome sequence of C. jejuni strain NCTC 11168 was published in 2000 (27), and several other strains have been recently sequenced including strains 81116 (28) and 81-176 (15). However, despite the prevalence of C. jejuni-mediated illness, the physiology of the bacterium is relatively poorly understood.
Oxidative stress is a major problem for any organism that uses oxygen as a terminal electron acceptor, as incomplete reduction of oxygen to water can yield reactive oxygen species (ROS) such as the superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (HO·) (39). Buildup of these highly reactive species can lead to damage to proteins, nucleic acids, and membranes. ROS are also produced by the immune system to kill invading microbes. Therefore, an ability to combat these compounds is key to the survival of bacterial pathogens in the environment and the host (16, 39). Bacteria have evolved a wide variety of mechanisms to combat ROS-mediated stress. These include superoxide dismutase (29), catalase (7, 39), cytochrome c peroxidases (1), and alkyl hydroperoxide-reductase (AhpC) (2). AhpC belongs to a large family of enzymes known as the peroxiredoxins, which are present in both eukaryotes and prokaryotes (31, 45), with AhpC being the most widely studied. In bacteria, AhpC confers resistance to a broad range of oxidative stress agents including hydrogen peroxide, organic peroxides, and lipid peroxides (10, 14, 35, 42) as well as stress caused by reactive nitrogen species in the form of peroxynitrite (3). C. jejuni contains an AhpC homologue, which in strain 81116 has been shown in previous work to be important for aerotolerance and organic peroxide stress resistance but which, from mutant phenotype data, does not appear to contribute to hydrogen peroxide resistance (2).
Two other peroxiredoxins are widespread in bacteria. These are thiol peroxidase (Tpx) and the bacterioferritin comigratory protein (Bcp). Tpx and Bcp appear to be able to use a wide variety of peroxides as substrates in vitro, such as hydrogen peroxide, organic peroxides, and lipid peroxides (6, 32, 43). Tpx has been reported to be periplasmic in Escherichia coli (4) but has been shown to use the cytoplasmic thioredoxin system as an electron donor (46), so the location of the protein remains unclear. An E. coli mutant lacking Tpx activity is more sensitive to various oxidative stresses (5), and studies with a Helicobacter pylori tpx mutant suggested that Tpx plays a significant role in defense against both superoxide- and peroxide-mediated stress, as well as high oxygen concentrations (9, 25). The H. pylori tpx mutant also showed a deficiency in mouse colonization compared to the wild type, implicating Tpx as an important virulence factor in this bacterium (25). E. coli mutants lacking Bcp are also more sensitive to stress induced by hydrogen peroxide and organic peroxides (17). H. pylori bcp mutants were somewhat more sensitive to peroxide stress than was the wild type (9, 43). In general, however, Bcp has been less well studied than Tpx.
C. jejuni contains both Tpx and Bcp homologues, which in strain NCTC 11168 are encoded by Cj0779 and Cj0271, respectively. In this study we have characterized the roles of these enzymes through phenotypic analysis of single and double mutants with mutations in the tpx and bcp genes and by overexpression and purification of both enzymes in order to determine their preferred substrates. The results show that Tpx and Bcp are partially redundant but together play important roles in resistance to a number of oxidative and nitrosative stress agents, as well as to molecular oxygen. Peroxidase assays with the purified proteins showed that Tpx is a dedicated hydrogen peroxide reductase, whereas Bcp is able to act on a wider variety of peroxide substrates. Finally, evidence from cellular fractionation studies suggests that both Tpx and Bcp are cytoplasmic enzymes in C. jejuni.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Campylobacter jejuni strain NCTC 11168 was routinely grown at 37°C in a microaerobic atmosphere (10% [vol/vol] O2, 5% [vol/vol] CO2, 85% [vol/vol] N2) in a MACS-VA500 growth cabinet (Don Whitley Scientific, Shipley, United Kingdom) on Columbia agar (Oxoid, Basingstoke, United Kingdom) containing 5% (vol/vol) lysed horse blood and amphotericin B and vancomycin (10 μg ml−1 each). Kanamycin and chloramphenicol were added to plates at final concentrations of 50 and 30 μg ml−1, respectively, to select for C. jejuni tpx and bcp mutants where relevant. Microaerobic liquid cultures of C. jejuni were grown in 25- to 100-ml batches of brain heart infusion broth supplemented with 5% (vol/vol) fetal bovine serum (BHI-FBS) contained in 250-ml shake flasks in the above-described microaerobic atmosphere and orbitally shaken at 100 rpm. For fully aerobic growth of C. jejuni, microaerobically grown overnight cultures were used to inoculate 50 ml BHI-FBS medium contained in 250-ml baffled conical flasks. These cultures were grown in normal atmospheric oxygen conditions at 37°C with 300 rpm shaking in order to aerate the cells as fully as possible.
Escherichia coli was routinely grown at 37°C with shaking at 200 rpm in Luria-Bertani (LB) medium supplemented with appropriate antibiotics. Growth of C. jejuni and E. coli was monitored by measuring the optical density at 600 nm (OD600) using a Pharmacia Biotech Ultrospec 2000 spectrophotometer.
DNA isolation and manipulation.
Plasmid DNA was isolated from E. coli using the QIAquick miniprep spin kit (Qiagen). C. jejuni chromosomal DNA was isolated using the Wizard Genomic DNA purification kit (Promega). Standard techniques were employed for the cloning, transformation, preparation, and restriction analysis of plasmid DNA extracted from E. coli (34).
Construction of C. jejuni tpx and bcp mutants.
The tpx gene was PCR amplified in two fragments (Tpx1, encompassing part of the upstream peb2 gene and the 5′ end of tpx, and Tpx2, encompassing the 3′ end of tpx and part of the downstream napA gene) to create a unique internal deletion flanked by HpaI sites, using the following primers (HpaI sites underlined): Tpx 1F (5′-GGACTTTATGAAGATATGGC-3′), Tpx 1R (5′-ACAGGTTAACCATACTTACTACAATCACTTC-3′), Tpx 2F (5′-ACAGGTTAACGCTATGGGAAGATTTTGCAGT-3′), and Tpx 2R (5′-GATCTTAGCATTAAAATAACC-3′). Both fragments, each of 800 bp, were independently cloned into the EcoRI site of plasmid pCR2.1 and transformed into E. coli TOPO-TA (Invitrogen, The Netherlands). Transformants were recovered by selection plating onto ampicillin (50 μg ml−1), and clones with inserts of the correct size were identified.
The two tpx fragments were removed from the pCR2.1 plasmids by digestion with EcoRI and HpaI and then ligated and cloned into the pUC-based vector pMTL20. The tpx gene already having an internal deletion was disrupted by the insertion of a chloramphenicol resistance gene isolated from plasmid pAV35 (41) into the unique HpaI site of pMTL20 to produce p-tpx-CAT.
For construction of a bcp mutant strain, primers bcp-F (5′-ACG TGA ATT CAA TTT TAT CTG CTG AGA TCA T-3′) and bcp-R (5′-ACG TGA ATT CTC ACA TTC TTT TTG TAA AGC-3′) were used to amplify a 1,400-bp fragment of the C. jejuni NCTC 11168 genome containing the full-length bcp gene plus 5′ and 3′ flanking regions by PCR, and the gene was cloned into cloning vector pGEM 3Zf (−) to produce plasmid pGEM-Bcp. The ahpAIII kanamycin resistance cassette from Campylobacter coli (41, 44) was cloned into a unique ClaI restriction site in the center of the bcp gene to produce plasmid pGEM-bcp-KAN. Transformation of C. jejuni NCTC 11168 with plasmids p-tpx-CAT and pGEM-bcp-KAN was carried out by electroporation, and transformants were selected on Columbia blood agar plates supplemented with chloramphenicol at a final concentration of 30 μg ml−1 or kanamycin at a final concentration of 50 μg ml−1. Colonies were restreaked onto Columbia blood agar plates, and correct insertion of the antibiotic resistance cassettes into the target genes was verified by extraction of chromosomal DNA by MicroLYSIS (Web Scientific Ltd., Crewe, United Kingdom) according to the manufacturer's instructions. PCR using gene-specific primers for tpx (forward primer, T-tpx-F, 5′-CAC CAT GAG TAT AGT AAA TTT TAA AGG AAA-3′; reverse primer, T-tpx-R, 5′-ATG GCA ACC ACA ACC ACC G-3′) and bcp (forward primer, T-bcp-F, 5′-CAC CAT GAG TTT AAA TAT AGG AGA TAA GG-3′; reverse primer, T-bcp-R, 5′-AAG ACT TTC AAG CAC TTT TAA AG-3′) confirmed allelic exchange by double crossover, as demonstrated by an increase in PCR product of approximately 0.8 or 1.4 kb for the chloramphenicol or kanamycin cassette insertion, respectively. A tpx/bcp double mutant was constructed by electroporation of the C. jejuni 11168 tpx mutant with pGEM-bcp-KAN and selection on Columbia blood agar plates containing both chloramphenicol and kanamycin.
Disc diffusion assays.
Fifty-milliliter cultures of C. jejuni NCTC 11168 and isogenic mutant strains were grown microaerobically at 37°C to early stationary phase, and the OD600 values were adjusted to 1.0 with BHI. Twenty milliliters of each culture was added separately to 400-ml cooled Mueller-Hinton agar (Oxoid, United Kingdom) containing amphotericin and vancomycin at final concentrations of 10 μg ml−1. Following pouring of plates, a sterile 8-mm paper disc made from Whatman no. 1 paper (Whatman, Brentford, United Kingdom) was placed in the center of the plate and 5 μl of the agent being tested was added to the disc. The agents and concentrations used were as follows: 500 mM H2O2, 10% (vol/vol) cumene hydroperoxide, 400 mM tert-butyl-hydroperoxide, 50 mM methyl viologen, and 200 mM sodium nitroprusside. Plates were incubated microaerobically at 37°C for 3 days, and the diameters of the zones of inhibition created around the discs were measured.
Viability assays.
To determine the effect of peroxides on viability, 50-ml cultures of C. jejuni NCTC 11168 and isogenic mutant strains were grown microaerobically at 37°C to early stationary phase and the OD600 values were adjusted to 1.0 with BHI. The agents being tested were added to final concentrations as follows: H2O2 (1 or 2 mM), cumene hydroperoxide (0.1 mM), and tert-butyl-hydroperoxide (0.1 mM). At time points 0, 60, 120, 180, and 240 min, 20-μl aliquots were removed in triplicate and diluted from 10−1 to 10−8 in 200-μl volumes. Five-microliter aliquots of each dilution were plated in triplicate onto blood agar plates and incubated at 37°C for 3 days, after which time the colonies were counted. For aerobic cell viability assays, 50-ml cultures of the relevant strains in 250-ml flasks were grown overnight microaerobically in the growth cabinet as described previously and then transferred to standard atmospheric incubation conditions at 37°C with 300-rpm shaking to aerate the cells as fully as possible.
Detection of protein carbonylation.
Cell extracts (CEs) were prepared from wild-type C. jejuni NCTC 11168 and the three isogenic mutant strains by growing cells microaerobically in 50 ml BHI-FBS with standard antibiotics for 16 h until cells reached early stationary phase. For CEs of stressed cells, stress agents (H2O2 at a 1 mM or cumene hydroperoxide at a 0.1 mM final concentration) were added and cells were grown for a further 2 h. For aerobic stress, stationary-phase cells were transferred to an aerobic environment and shaken at 300 rpm at 37°C for 2 h. Cells were pelleted and resuspended in 1/10 of the original volume of 10 mM Tris-HCl, pH 7.5, and disrupted by sonication. Cell debris and unbroken cells were then removed by centrifugation at 8,000 × g for 15 min at 4°C. The method for estimation of protein carbonyl content was based on dinitrophenylhydrazine (DNPH) derivatization, as described by Shacter et al. (36). This method is highly specific for protein carbonyls (19, 20, 36). Samples were added to an equal volume of 12% (wt/vol) sodium dodecyl sulfate (SDS) to give a final concentration of 6% (wt/vol) SDS. To this solution an equal volume of 10 mM DNPH in 10% (vol/vol) trifluoroacetic acid was added, and the mixture was incubated at room temperature for 15 min to allow derivatization to form protein carbonyl-DNP groups. The sample was prepared for loading onto SDS-polyacrylamide gels by adding an equal volume of 2× sample buffer. SDS-15% polyacrylamide gels were run at 150 mV until the dye front had reached the bottom of the gel, and gels were electroblotted onto a polyvinylidene difluoride membrane. DNP groups were detected immunologically using primary anti-DNP antibody raised in rabbit (Sigma). Anti-rabbit-horseradish peroxidase antibody was used as the secondary antibody (Sigma). Primary and secondary antibodies were both used at a dilution of 1:2,000. Detection was carried out using an enhanced chemiluminescence kit (Amersham).
Lipid peroxidation assays.
Detection of total cellular lipid peroxides was carried out using the FOX II reagent, which provides a sensitive colorimetric assay for peroxides when measured spectrophotometrically at 560 nm. The method used was adapted from that described by Master et al. (22). The FOX II reagent contained 90% methanol, 25 mM H2SO4, 250 μM ferrous sulfate, and 100 μM xylene orange. Fifty-milliliter cultures of C. jejuni NCTC 11168 and isogenic mutant strains were grown microaerobically at 37°C to early stationary phase. For stressing the cells, 1 mM hydrogen peroxide was added to stationary-phase cultures and these were grown microaerobically for a further 2 h. The OD600 of each culture was adjusted to 0.5 using sterile BHI. A 100-μl aliquot of cell culture was added to a cuvette containing 900 μl FOX II reagent and incubated at room temperature for 30 min. The absorbance of each sample at 560 nm was then measured and adjusted to allow for any absorbance caused by the cells by measuring a blank of just methanol plus 100 μl cell suspension. Each culture was measured in triplicate, and results were quantified using a standard curve produced using a range of hydrogen peroxide concentrations.
Overexpression and purification of C. jejuni His-tagged Tpx and Bcp.
Primers T-tpx-F, T-tpx-R, T-bcp-F, and T-bcp-R were used to amplify the full-length C. jejuni NCTC 11168 tpx and bcp genes minus the stop codons, by PCR. These fragments were then cloned into the T7 overexpression vector pET-101/D-TOPO (Invitrogen), part of the Invitrogen Champion TOPO cloning kit, according to the manufacturer's instructions, to produce plasmids (designated pET-Tpx and pET-Bcp, respectively) that express Tpx or Bcp with a C-terminal six-His tag. E. coli BL21 Star(DE3) cells transformed with either of these plasmids were grown at 37°C to an OD600 of ∼0.5 in 100 ml LB medium containing 50 μg ml−1 carbenicillin. Expression of Tpx or Bcp was induced by addition of 1 mM (final concentration) IPTG (isopropyl-β-d-thiogalactopyranoside) into the medium followed by growth at 37°C for a further 3 h, and the cells were harvested by centrifugation (6,000 × g for 20 min at 4°C). Cells were resuspended in 5 ml binding buffer (20 mM sodium phosphate, pH 7.4, 0.5 M NaCl, 20 mM imidazole), and cells were disrupted by sonication in an MSE Soniprep sonicator. Cell debris was removed by centrifugation as described above and by filtration through a 0.2-μm-pore-size filter. The supernatant was applied to a HisTrap column (GE Healthcare) and purified using the His-tag linear gradient program with the AKTA Prime Plus station (Amersham) according to the manufacturer's instructions. Buffers used contained 20 mM imidazole (binding buffer; described above) or 500 mM imidazole (elution buffer). These were applied in a linear gradient from 0 to 100% elution buffer to elute His-tagged proteins from the column. Fractions containing purified Tpx or Bcp were pooled; desalted using size-exclusion chromatography into 10 mM Tris-HCl, pH 8, to remove NaCl and imidazole from the elution buffer; and concentrated using Vivaspin centrifugal concentrators (Sigma). Protein concentrations were determined using the Bradford assay. N-terminal sequencing of purified proteins (Arthur Moir, University of Sheffield) gave sequences of SIVNFKG and SLNIGDK, which correspond to the first seven residues of the expected deduced Tpx and Bcp proteins, respectively, minus the N-terminal methionine residues.
Nitrate reductase assays.
Benzyl viologen-linked nitrate reductase assays with intact cells were carried out as described by Pittman et al. (30) in a 1-ml volume using a Shimadzu UV-2401 PC spectrophotometer. The final assay mixture consisted of 10 mM Tris-HCl (pH 7.5), 100 μM benzyl viologen (Sigma), and 5 mM sodium nitrate, contained in a screw-top quartz cuvette (Hellma) fitted with a silicone seal. After addition of cells, buffer, and benzyl viologen the mixture was sparged with nitrogen gas for approximately 10 min, and then aliquots of a freshly prepared sodium dithionite (Sigma) solution were injected into the cuvette until the absorbance at 578 nm was stable at approximately 1.5 units. The assay was started by the injection of anaerobic sodium nitrate into the cuvette. Rates of reductase activity were calculated using an extinction coefficient (ɛ578) for benzyl viologen of 8,600 M−1 cm−1. For this and the other enzyme assays described below, protein concentrations were determined using the method of Markwell et al. (21).
Catalase assay.
Catalase activity in CEs was measured by directly monitoring the breakdown of hydrogen peroxide at 240 nm (42), using an extinction coefficient (ɛ240) of 39.4 M−1 cm−1 in a 1-ml assay mixture containing 10 mM Tris-HCl, pH 7, and 50 mM hydrogen peroxide.
DTT-linked peroxidase assays.
The activity of purified Tpx or Bcp linked to dithiothreitol (DTT) oxidation was determined by monitoring the absorbance at 310 nm in a Shimadzu UV-2401 PC spectrophotometer. This assay was modified from the work of Hillas et al. (14). The reaction mixture contained 50 mM HEPES-NaOH (pH 7), 1 mM EDTA, 1 μM pure Tpx or Bcp, 2 mM DTT, and 2 mM peroxides (hydrogen peroxide, tert-butyl-hydroperoxide, or cumene hydroperoxide) in a total volume of 1 ml at 37°C. The reaction was started by the addition of the substrate. All buffers and water used were Chelex treated per the manufacturer's instructions (Sigma).
NADPH-linked peroxidase activity assays.
The peroxidase activity of purified Tpx and Bcp linked to NADPH oxidation via the thioredoxin reductase-thioredoxin system was determined by monitoring the decrease in absorbance at 340 nm in a Shimadzu UV-2401 PC spectrophotometer. Pure thioredoxin (Trx) and thioredoxin reductase (TrxR) from E. coli were obtained from Sigma-Aldrich (United Kingdom). The reaction mixture contained 50 mM HEPES-NaOH (pH 7.0), 20 μM NADPH, 20 μg Trx, 6.25 μg TrxR, 1 μM pure enzyme (Tpx or Bcp), and various concentrations of peroxides (hydrogen peroxide, tert-butyl-hydroperoxide, cumene hydroperoxide, or linoleic acid hydroperoxide). Reactions were carried out in a total volume of 1 ml at 37°C. The reaction was started by the addition of NADPH. Linoleic acid hydroperoxide was generated by incubating 100 μM linoleic acid (Sigma) with 10 μg ml−1 soybean lipoxidase in 50 mM HEPES-NaOH (pH 7.0) at room temperature for 60 min. The concentration of linoleic acid hydroperoxide was determined spectrophotometrically using an extinction coefficient (ɛ234) for linoleic acid hydroperoxide of 25,000 M−1 cm−1 at 234 nm.
Cellular fractionation and detection of Tpx and Bcp by immunoblotting.
For determination of the cellular location of Bcp and Tpx, C. jejuni periplasm and cytoplasm were isolated by polymyxin B treatment of whole cells, as described by Sommerlad and Hendrixson (37). Cross-contamination of the periplasmic and cytoplasmic fractions was assayed using markers for each fraction (cytochrome c content as the periplasmic marker and isocitrate dehydrogenase [ICDH] activity as the cytoplasmic marker), using the assays described by Myers and Kelly (24). For detection of Tpx and Bcp in cells, 1-ml samples were taken at defined time points throughout the growth curve, and cells were pelleted and then prepared for loading onto SDS gels by being boiled in reduced sample buffer for 5 min. Loadings were normalized through OD600 readings carried out at the same time as the samples were pelleted. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using the Mini-Protean 3 apparatus (Bio-Rad, California). Transfer of proteins was carried out using a Mini Trans-Blot cell (Bio-Rad). The gel blot sandwich was constructed according to the manufacturer's instructions, and the proteins were transferred to a nitrocellulose membrane (Hybond-C Extra; Amersham Biosciences) at a current of 11 mA for 14 h at 4°C. All immunodetection steps were carried out at room temperature with constant agitation. TBS-T (25 mM Tris-HCl, pH 7.4, 130 mM NaCl, 0.1% Tween 20) was used both as a base for blocking agent (5% bovine serum albumin dissolved in TBS-T) and for washing. Primary polyclonal antibodies (anti-Tpx and anti-Bcp, raised in rabbit from purified C. jejuni Tpx and Bcp, respectively, produced by Simon Smith, Antibody Resource Centre, University of Sheffield) were diluted in blocking agent (1:2,000) and applied to the membrane. Membranes were reacted for approximately 1 h and washed in TBS-T before the secondary antibody (monoclonal anti-rabbit immunoglobulin G; Sigma) was diluted (1:2,000) and applied to the membrane. Antibody binding was visualized by means of enhanced chemiluminescence (ECL kit; Amersham Biosciences, United Kingdom), according to the manufacturer's instructions. No cross-reaction between anti-Tpx and Bcp or between anti-Bcp and Tpx was observed at the antibody titers used in this work. Total Lab 100 software (Nonlinear Dynamics Ltd., United Kingdom) was used to determine the area and intensity of each band on the resulting ECL film. The product of these values was then used to calculate the band density (arbitrary units).
Real-time PCR.
Wild-type C. jejuni NCTC 11168 and 11168 bcp BHI-FBS cultures (25 ml) were harvested directly into an equal volume of prechilled 5% (vol/vol) phenol made up in 100% ethanol to stabilize the RNA and then centrifuged at 6,000 × g for 10 min at 4°C. Total RNA was then purified from cell pellets using the RNeasy minikit (Qiagen, United Kingdom) according to the manufacturer's instructions. The RNA concentration and purity were determined using an Eppendorf BioPhotometer. cDNA synthesis was carried out using 4 μg of DNase-treated RNA primed with 0.5 μg random hexamer primers (Promega, United Kingdom). Reaction mixtures (20 μl) containing 0.5 mM dATP, dCTP, dGTP, and dTTP were incubated for 2 h at 42°C with 200 units BioScript reverse transcriptase (Bioline). Following synthesis, cDNA was purified using the PCR purification kit (Qiagen) to remove unincorporated deoxynucleoside triphosphates and primers. Gene-specific primers were designed to amplify internal fragments of Cj0272 (gene downstream of bcp; Cj0271) and a control gene, gyrA, using PRIMER 3 software (33). A Sybr green mix was made in the ratio of 13 μl Quantace Sensimix (Bioline), 0.5 μl Sybr green, and 4.5 μl nuclease-free water (Sigma). Each reaction was carried out in a total volume of 25 μl on a 96-well optical reaction plate (Applied Biosystems). Each well contained 16 μl of the Sybr green mix (above), 12.5 pmol each primer pair, and 5 μl of cDNA sample. PCR amplification was carried out in an ABI 7700 Thermocycler (PE Applied Biosystems) with the thermal cycling conditions of 50°C for 2 min followed by 95°C for 10 min followed by 40 cycles of 95°C for 15 seconds and 65°C for 1 min. The data were analyzed using the Sequence Detector System software (PE Applied Biosystems) and further processed in Microsoft Excel. A standard curve was established for each gene studied by using genomic DNA to confirm that primers were amplified at the same rate and to validate the experiments. Reactions without template were carried out as negative controls.
RESULTS
Construction and verification of mutants.
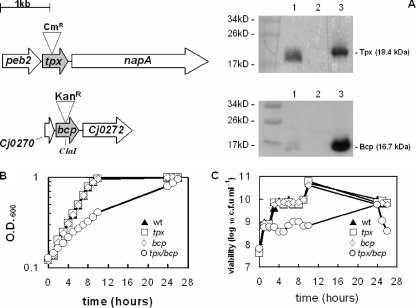
To investigate the physiological role of Tpx and Bcp, we first created single tpx and bcp insertion mutants, and a tpx/bcp double mutant, in C. jejuni strain NCTC 11168, using antibiotic resistance cassettes. Directly downstream of tpx and transcribed in the same direction is the napA gene, encoding nitrate reductase (Fig. 1). Nitrate reductase activity assays using wild-type and tpx mutant strains showed that there was no loss of NapA activity in the tpx mutant, with values of 4.0 ± 0.2 μmol min−1 mg protein−1 and 4.0 ± 0.1 μmol min−1 mg protein−1 for the wild type and tpx mutant, respectively, whereas in a napA mutant (30) the nitrate reductase activity was zero. This shows that there is no effect on the expression of the napA gene caused by insertion of the chloramphenicol resistance cassette in tpx. Directly downstream of the bcp gene is Cj0272, encoding a conserved hypothetical protein (Fig. 1). Reverse transcription-PCR analysis of Cj0272 expression in the bcp mutant showed a 2.1- ± 0.7-fold downregulation compared to the wild-type parent, indicating a minimal downstream effect caused by insertion of the kanamycin resistance cassette into the bcp gene. The amplified Cj0272 cDNA from reverse transcription-PCRs of both wild-type and bcp mutant RNA was also visible on an agarose gel. Immunoblotting of cell extracts of the wild-type and tpx and bcp mutant strains, using anti-Tpx or anti-Bcp polyclonal antibodies raised against the purified proteins, clearly showed the expected absence of Tpx and Bcp proteins in the respective mutants (Fig. 1A).
FIG. 1.
Growth phenotypes of tpx and bcp mutants. (A) Mutation strategy for the tpx and bcp genes of C. jejuni NCTC 11168 and the loss of Tpx and Bcp proteins in the respective mutants, as shown by immunoblotting with anti-Tpx antibodies (upper blot) or anti-Bcp antibodies (lower blot). Lane 1 contains wild-type CE, and lane 2 contains CE from either the tpx (upper blot) or the bcp (lower blot) mutant. Lane 3 contains purified Tpx (upper blot) or Bcp (lower blot). (B) Growth curve of the wild-type (wt) and tpx, bcp, and tpx/bcp mutant strains under microaerobic conditions. (C) Viable counts corresponding to the growth curve in panel B.
Tpx and Bcp are necessary for hyperoxic stress resistance.
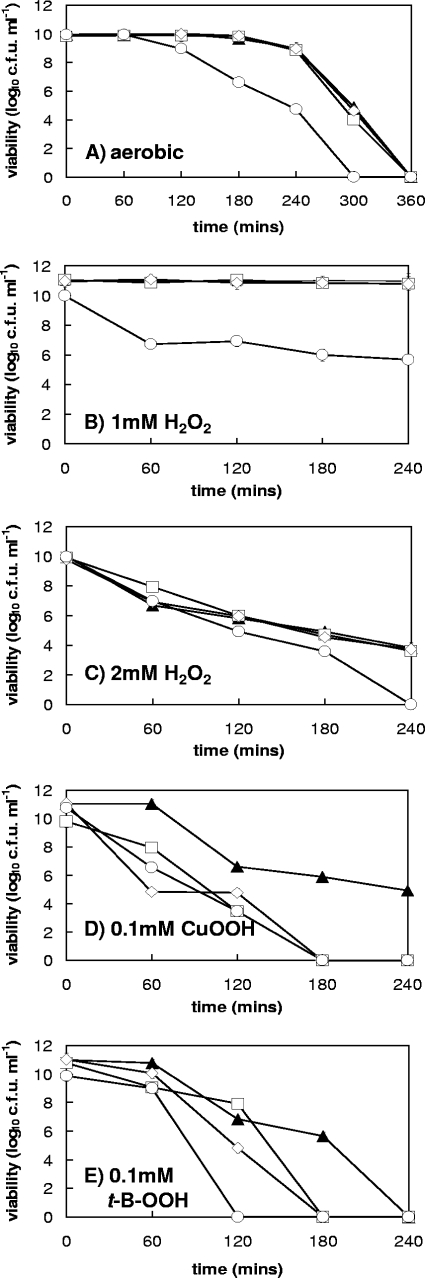
Growth curves of wild-type C. jejuni 11168 and the three isogenic mutants were obtained under standard microaerobic conditions (Fig. 1B and C). The tpx and bcp single mutants grew at rates comparable to that of wild-type C. jejuni 11168, with a turbidity doubling time of approximately 3 h. However, the tpx/bcp double mutant strain showed a significant growth defect during microaerobic growth in BHI-FBS, with a doubling time of over 7 h, but it reached a cell density similar to that of the other strains after 24 h of growth. Viable counts of these growth curves showed the same overall pattern (Fig. 1C), but with the double mutant losing viability markedly in stationary phase. These strains were also grown under highly aerobic conditions to examine the effects of hyperoxic stress. As expected for the microaerophilic C. jejuni wild type, this resulted in growth inhibition and a low final cell density compared to that for microaerobic growth. The tpx and bcp single mutants initially showed some growth, similar to that of wild-type cells, but their growth was retarded after just a few hours and the final cell densities reached were lower than that of the wild type. No significant growth of the tpx/bcp double mutant occurred under these conditions (data not shown). The effect of increased oxygen on the viability of C. jejuni was also assessed by the transfer of early-stationary-phase microaerobically grown cells to highly aerobic conditions (Fig. 2A). The single tpx and bcp mutants lost viability at the same rate as did wild-type cells, with viability being below the level of detection after 6 h. However, the tpx/bcp double mutant lost viability much more rapidly. These data highlight an important and partially redundant role for Tpx and Bcp in the protection of C. jejuni against oxidative stress caused by molecular oxygen.
FIG. 2.
Effect of oxidative stress on the viability of early-stationary-phase cells. Strains were grown overnight in BHI-FBS, and viable counts were determined in triplicate on individual cell suspensions after the initial OD600 was adjusted to approximately 1.0, as described in Materials and Methods. In most cases error bars are too small to be seen. (A) Aerobic conditions; (B) 1 mM hydrogen peroxide; (C) 2 mM hydrogen peroxide; (D) 0.1 mM cumene hydroperoxide; (E) 0.1 mM tert-butyl-hydroperoxide. Symbols: closed triangles, wild type; open squares, tpx mutant; open diamonds, bcp mutant; open circles, tpx/bcp mutant. The data shown are from one representative experiment, but it was repeated twice with similar results.
Redundant roles of Tpx and Bcp in peroxidative and nitrosative stress resistance.
Disc diffusion assays (Table 1) and cell viability assays (Fig. 2) were used to determine the roles of Tpx and Bcp in protection against peroxides, superoxide, and nitrosative stress. By either method, the single tpx and bcp mutant strains appeared to be no more sensitive to hydrogen peroxide than was the wild type, but the double tpx/bcp mutant was much more sensitive to this type of stress, showing a statistically significantly larger zone of inhibition than that of the wild type in disc diffusion assays, and with a large loss of viability in the presence of 1 mM hydrogen peroxide (Fig. 2B). At 2 mM hydrogen peroxide, the double mutant showed a total loss of viability after 240 min of incubation, although the wild type and single mutants showed higher rates of viability loss than they did at 1 mM (Fig. 2C). Both the tpx and bcp single mutants and the tpx/bcp double mutant lost viability more rapidly than did wild-type cells when exposed to the organic peroxides cumene hydroperoxide (Fig. 2D) and tert-butyl-hydroperoxide (Fig. 2E). However, when these stress agents were used in disc diffusion assays, only the tpx/bcp double mutant showed a statistically significant increase in the size of the zone of inhibition observed (Table 1). Similarly, single tpx and bcp mutants were not more sensitive to superoxide stress mediated by methyl viologen than were wild-type cells (Table 1), but the tpx/bcp double mutant had a significantly increased sensitivity compared to the wild-type strain. An increased sensitivity to nitrosative stress with the nitrosating agent sodium nitroprusside was again also observed only in the tpx/bcp double mutant (Table 1).
TABLE 1.
Effects of oxidative and nitrosative stress agents on wild-type and mutant strains measured by disc diffusion assays
| Stress agent on disc | Mean diam (mm) of inhibition zone ± SD (n = 10) for strain:
|
|||
|---|---|---|---|---|
| Wild type | tpx mutant | bcp mutant | tpx/bcp mutanta | |
| 500 mM H2O2 | 26.7 ± 1.7 | 25.4 ± 1.2 | 25.6 ± 1.3 | 30.1 ± 1.9 |
| 10% (vol/vol) cumene hydroperoxide | 34.0 ± 1.8 | 32.4 ± 1.1 | 33.8 ± 1.6 | 43.1 ± 3.2 |
| 400 mM tert-butyl-hydroperoxide | 36.8 ± 3.6 | 36.2 ± 2.1 | 39.0 ± 3.5 | 45.9 ± 3.9 |
| 50 mM methyl viologen | 13.7 ± 1.3 | 14.1 ± 1.2 | 14.0 ± 0.8 | 27.8 ± 2.9 |
| 200 mM sodium nitroprusside | 16.3 ± 1.6 | 16.3 ± 1.8 | 15.9 ± 0.7 | 20.9 ± 2.6 |
The difference between the tpx/bcp mutant and all other strains with each stress agent is significant at P < 0.005 using Student's t test. The zones of inhibition of the single tpx and bcp mutants are not significantly different from that of the wild type or each other with any of the stress agents used.
Peroxide sensitivity assays may be affected by changes in other peroxide-degrading enzyme activities, particularly catalase. In order to investigate this, we assayed catalase activity in early-stationary-phase CEs. The following high specific activities were found in all the strains, even when they were grown in the absence of hydrogen peroxide: wild type, 15 ± 2 μmol min−1 mg protein−1; tpx mutant, 12 ± 2 μmol min−1 mg protein−1; bcp mutant, 12 ± 2 μmol min−1 mg protein−1; tpx/bcp mutant, 14 ± 3 μmol min−1 mg protein−1. Thus, differences in catalase activity cannot explain the difference in peroxide sensitivity between the single and double mutants.
Overall, as with the effect of molecular oxygen, the data in Table 1 and Fig. 2B to E suggest a high degree of redundancy in the functions of Tpx and Bcp, such that a pronounced oxidative stress phenotype that is severe enough to significantly affect growth and viability is observed only in a strain lacking both enzymes. However, more subtle effects of the absence of either enzyme may not be apparent in such experiments. The contribution of each enzyme to the protection of lipids and proteins, two key classes of cellular macromolecules that are prone to oxidative damage, was therefore investigated.
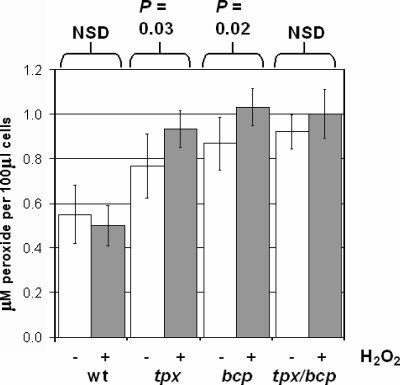
Mutants lacking Tpx and Bcp have increased lipid peroxide levels.
ROS can react with unsaturated membrane lipid fatty acids to form several species of lipid peroxides, which can lead to damage to both membranes and associated proteins. Comparison of the accumulation of lipid peroxides in wild-type and mutant strains, measured using the sensitive FOX II reagent, revealed that single tpx or bcp mutants have increased lipid peroxidation levels compared to those of wild-type cells (Fig. 3). When cells were grown in the presence of 1 mM hydrogen peroxide, the wild-type peroxidation level did not change significantly (P > 0.05), whereas small but statistically significant increases occurred in the single tpx (P = 0.03) and bcp (P = 0.02) mutants. The overall lipid peroxide content in the tpx/bcp double mutant was also considerably higher than that in wild-type cells, although the increase in peroxide content in cells grown in the presence over the content of cells grown in the absence of hydrogen peroxide could not be demonstrated to be statistically significant in this case (P > 0.05; Fig. 3).
FIG. 3.
tpx and bcp mutants have increased lipid peroxide contents. Lipid peroxide contents of wild-type (wt) C. jejuni NCTC 11168 and the three mutant strains were determined under unstressed (white bars) and 1 mM hydrogen peroxide-stressed (gray bars) conditions. Cells were grown to early stationary phase, and lipid peroxide contents were measured using the FOX II reagent as described in Materials and Methods. NSD, no significant difference.
Evidence for increased protein carbonylation in tpx and bcp mutants.
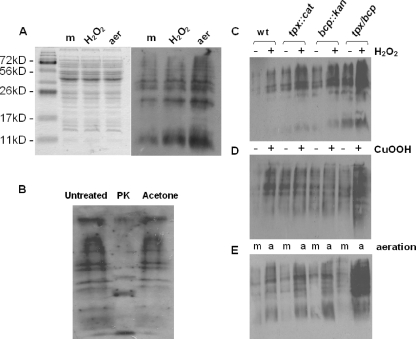
Protein carbonylation is an irreversible modification that occurs when cellular proteins are oxidized by reactive oxygen and nitrogen species. As such, the level of carbonylation of proteins can be used as a useful indicator of the degree of oxidative stress which the cells have experienced (20, 38). The assay used here exploits the ability of DNPH to form an adduct with protein carbonyl groups (19). The DNP-carbonyl groups can then be detected via standard immunoblotting procedures using an anti-DNP antibody, resulting in a characteristic banding pattern (Fig. 4A). That these bands represent carbonylated proteins rather than lipid or lipopolysaccharide is demonstrated by the controls in Fig. 4B. Treatment with proteinase K resulted in the disappearance or alteration of molecular weight of the majority of the bands compared to the untreated control, whereas acetone precipitation to remove lipids caused no change in the profile.
FIG. 4.
Effect of tpx and bcp mutations on protein carbonyl content. (A) Relative levels of protein carbonylation in wild-type cells grown microaerobically to early stationary phase with no further treatment (m), exposed to 1 mM hydrogen peroxide for a further 2 h (H2O2), or incubated with vigorous shaking at high aeration (21% [vol/vol] oxygen) for 2 h (aer). (Left panel) Coomassie blue-stained 12% (wt/vol) SDS-polyacrylamide gel containing equivalent amounts of wild-type CE protein. (Right panel) Corresponding immunoblot with anti-DNP antibodies. (B) Control immunoblot assay to confirm that the detected bands are carbonylated proteins. Aliquots of a CE from aerated cells were either left untreated, incubated with 200 μg ml−1 proteinase K (PK) at 37°C for 16 h, or treated with 2 volumes of −20°C acetone to precipitate protein and remove lipid. Samples were then derivatized as described in Materials and Methods, and equal loadings were applied to a 12% (wt/vol) SDS-polyacrylamide gel and immunoblotted with anti-DNP antibodies. (C to E) Levels of protein carbonylation detected on anti-DNP immunoblots in wild-type (wt) and mutant strains in the presence (+) and absence (−) of 1 mM hydrogen peroxide (C) or 0.1 mM cumene hydroperoxide (D) or after growth at high aeration (E), under 21% (vol/vol) oxygen (a) versus microaerobically (m).
We found that when wild-type cells are grown under standard microaerobic conditions in the presence of 1 mM hydrogen peroxide, there is an increase in the carbonyl content of a number of cellular proteins compared to that of unstressed microaerobically grown cells, as evidenced by increased band intensities on the immunoblot (Fig. 4A). Interestingly, the level of carbonylation is much higher when cells are grown aerobically than when they are grown microaerobically (Fig. 4A).
There is an obvious increase in the carbonyl content of cellular proteins in the single tpx and bcp mutant strains and in the double mutant, compared to the wild type, when cells are grown under standard microaerobic conditions (Fig. 4C and 4D, lanes marked with a minus sign). In the presence of 1 mM hydrogen peroxide, the level of carbonylation clearly increased in wild-type cells and in both single mutants, with a further increase in the tpx/bcp double mutant strain (Fig. 4C). The level of carbonylation also increased in the wild-type and two single mutant strains when 0.1 mM cumene hydroperoxide was used to stress the cells (Fig. 4D), but again the peroxide-induced level of carbonylation was clearly highest in the tpx/bcp double mutant (Fig. 4D). Finally, under highly aerobic conditions (Fig. 4E) a more pronounced pattern is seen, with raised carbonylation levels in the single mutants compared to the wild type, but a much more significant effect of high oxygenation in the double mutant (Fig. 4E, last lane). Thus, aerobic conditions appear to cause significant oxidative damage to proteins in C. jejuni and this is particularly exacerbated in strains lacking both Tpx and Bcp.
Peroxidase activity of purified C. jejuni Tpx and Bcp.
In order to correlate the enzymatic activity of Tpx and Bcp with the phenotypes observed in the above studies with mutant strains, we determined the range of peroxide substrates that were used by the purified overexpressed enzymes. Optimal overexpression of C. jejuni Tpx and Bcp from pET plasmids was achieved heterologously in E. coli BL21* after 3 h of growth in the presence of 1 mM IPTG. The C-terminal His-tagged Tpx and Bcp proteins were purified to homogeneity using nickel-chelate affinity chromatography. Purity was confirmed by SDS-PAGE and immunoblotting and through N-terminal sequencing. The activities of the purified Tpx and Bcp were assayed in two systems, one using the nonphysiological electron donor DTT, monitored directly by DTT oxidation, and the other using the proposed physiological electron donor system, thioredoxin plus thioredoxin reductase, linked to NADPH oxidation.
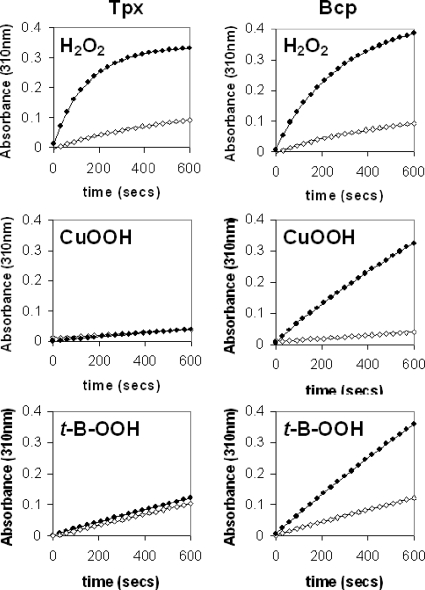
DTT-linked assays showed that Tpx and Bcp were both able to catalyze hydrogen peroxide-dependent DTT oxidation (Fig. 5, top panels), with the initial rates being approximately the same for the two enzymes. Surprisingly however, only Bcp was able to oxidize DTT in the presence of the organic peroxide cumene hydroperoxide or tert-butyl-hydroperoxide (Fig. 5, middle and lower panels, right column). No measurable rate of cumene hydroperoxide or tert-butyl-hydroperoxide-dependent DTT oxidation was observed when Tpx was used in the assay system (Fig. 5, middle and lower panels, left column).
FIG. 5.
Peroxidase activities of purified enzymes using DTT as reductant. Each panel is an absorbance time course at 310 nm (due to oxidized DTT) in the absence (open symbols) or the presence (closed symbols) of the peroxide substrate shown, using purified Tpx (left column) or Bcp (right column). The reaction mixture contained 50 mM HEPES-NaOH (pH 7.0), 1 mM EDTA, 1 μM pure Tpx or Bcp, 2 mM DTT, and 2 mM peroxide (hydrogen peroxide, cumene hydroperoxide, or tert-butyl-hydroperoxide) in a total volume of 1 ml at 37°C. The reaction was started by the addition of the substrate. All buffers and water used were Chelex treated per the manufacturer's instructions (Sigma) to minimize nonspecific DTT oxidation.
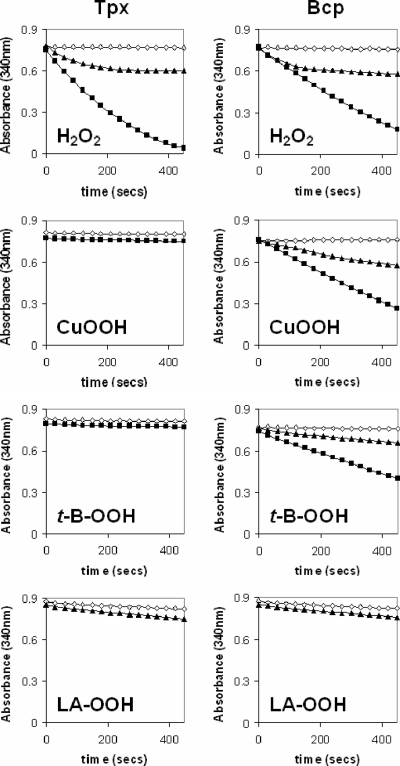
One possibility to explain the unexpected lack of Tpx activity with organic peroxides concerns the use of the artificial electron donor DTT. This assay also suffers from a variable background rate of substrate-independent DTT oxidation (apparent in Fig. 5). We therefore employed the physiological electron donor thioredoxin, reduced by thioredoxin reductase and NADPH. This assay is more specific and more sensitive, but the thioredoxin system had to be comprised of commercially available enzymes derived from E. coli, as these enzymes are not available from C. jejuni. Nevertheless, both Tpx and Bcp were found to be able to reduce hydrogen peroxide linked to the oxidation of NADPH, as evidenced by a large decrease in the absorbance at 340 nm (Fig. 6). This activity was absolutely dependent on the presence of the substrate peroxide, thiol peroxidase, thioredoxin, and thioredoxin reductase, and the rate of NADPH oxidation was higher with 100 μM H2O2 than with 20 μM H2O2 (Fig. 6). However, no NADPH oxidation occurred when purified Tpx was incubated in this assay with the organic peroxide cumene hydroperoxide or tert-butyl-hydroperoxide (Fig. 6). Only purified Bcp was able to use these organic peroxides as substrates (Fig. 6), and the rate of NADPH oxidation was proportional to the peroxide concentration (Fig. 6). Finally, we also synthesized linoleic acid hydroperoxide (a mimic for cellular lipid peroxides) for use in this assay system but found that only a very slightly higher rate of NADPH oxidation occurred with either enzyme when 20 μM linoleic acid hydroperoxide was provided as a substrate (Fig. 6).
FIG. 6.
Peroxidase activities of purified enzymes using the thioredoxin system as reductant. Each panel is an absorbance time course at 340 nm (due to NADPH oxidation) in the absence (open diamonds) or the presence (closed symbols) of the peroxide substrate shown, using purified Tpx (left column) or Bcp (right column). The reaction mixture contained 50 mM HEPES-NaOH (pH 7.0), 20 μM NADPH, 20 μg E. coli Trx, 6.25 μg E. coli TrxR, 1 μM pure thiol peroxidase enzyme (Tpx or Bcp), and either 20 μM (closed triangles) or 100 μM (closed squares) peroxide substrate. Reactions were carried out in a total volume of 1 ml at 37°C. The reaction was started by the addition of NADPH. LA, linoleic acid.
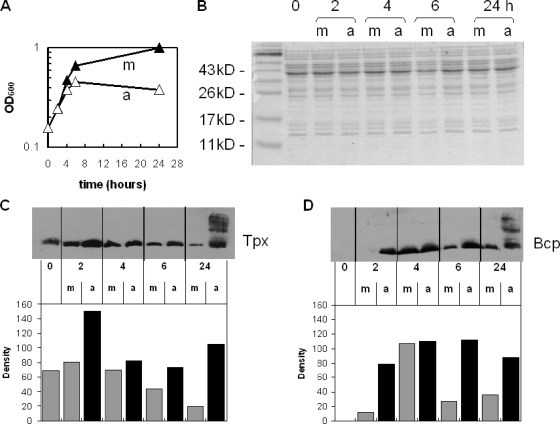
Aerobic growth increases the expression of Tpx and Bcp in C. jejuni.
The purified Tpx and Bcp proteins were also used to raise polyclonal antibodies in rabbits for use in expression studies. Cultures of wild-type C. jejuni were grown either under standard microaerobic conditions or under highly aerated conditions (Fig. 7A). Total cellular proteins in samples of cells taken throughout the growth cycle were separated by SDS-PAGE (Fig. 7B) and immunoblotted using either anti-Tpx or anti-Bcp antibodies (Fig. 7C and D). There was virtually no Bcp produced up until 2 to 4 h of microaerobic growth (Fig. 7D). The densitometry of the blots showed that Tpx and Bcp expression increased in both microaerobic control cultures and aerobically grown cultures as cells entered exponential phase, with maximal levels in both conditions after 2 h of growth for Tpx and 4 h for Bcp (Fig. 7C and D, respectively). The amounts of the two proteins in aerobically grown cells were consistently higher than those in microaerobically grown cells, this being particularly evident for Bcp, which was barely detectable in early-exponential-phase microaerobic cultures (Fig. 7D). Levels of both Tpx and Bcp appeared to decrease somewhat after 4 to 6 h of microaerobic growth but remained high in cells growing aerobically. After 24 h of growth, both Tpx and Bcp abundance decreased further still in microaerobically grown cells but remained high in aerobically grown cells. Interestingly, there also appeared to be significant amounts of oligomerization or aggregation of both Tpx and Bcp in aerobically grown cell samples at 24 h, as indicated by higher-molecular-weight bands present on the blots at this time point (Fig. 7C and D, respectively). The major higher-molecular-weight band for each protein appeared to be equivalent to a dimer. This apparent SDS-resistant aggregation did not occur at 24 h in microaerobically grown cells. When C. jejuni wild-type cells were grown with 1 mM H2O2, no increase in expression of either Tpx or Bcp occurred throughout the growth cycle (data not shown), unlike the results above for aerobic conditions.
FIG. 7.
Effect of oxygen on the synthesis of Tpx and Bcp in wild-type cells. (A) Growth curves of microaerobically (m; closed triangles) and aerobically (a; open triangles) grown wild-type C. jejuni used to measure Tpx and Bcp expression. (B) Coomassie blue-stained SDS-polyacrylamide gel showing normalized protein loadings of CEs from each time point for microaerobic (m) and aerobic (a) cells. (C and D) Immunoblots (upper panels) and corresponding densitometry histograms (lower panels; arbitrary units) of gels identical to panel B, using anti-Tpx (C) or anti-Bcp (D) as the primary antibody. Note that for the 24-h samples only the density of the major Tpx or Bcp band was measured.
Evidence that Tpx and Bcp are cytoplasmic proteins in C. jejuni.
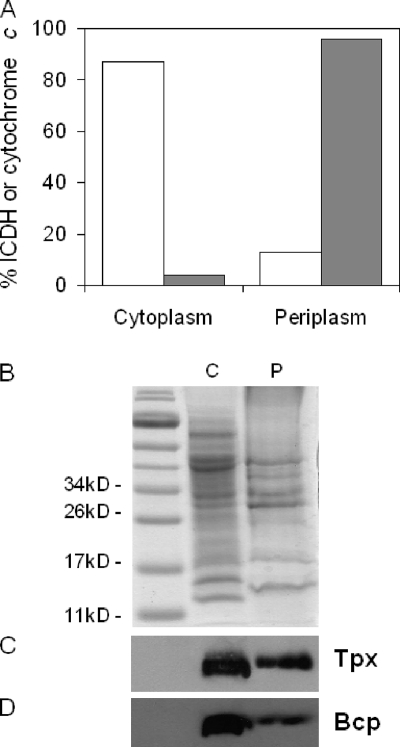
Tpx has been previously reported to be periplasmic in E. coli (4) but has been shown to use the cytoplasmic thioredoxin system as an electron donor. Moreover, neither Tpx nor Bcp from a range of bacteria, including C. jejuni, has obvious N-terminal signal sequences that might direct it to the periplasm. To determine the cellular location of the Tpx and Bcp proteins in C. jejuni, periplasmic and cytoplasmic fractions were prepared by a gentle procedure involving outer membrane disruption with the antibiotic polymyxin B (37). The fractions were then separated by SDS-PAGE (Fig. 8B), and immunoblots were probed with anti-Tpx or anti-Bcp antibodies (Fig. 8C and D, respectively). Analysis of the cytochrome c content (periplasmic specific marker) and ICDH activity (cytoplasmic specific marker) in the fractions showed that there was an inevitable but small amount of cross-contamination; the cytoplasmic fraction contained 4% of the total cytochrome c content, and the periplasm contained 13% of the total ICDH activity (Fig. 8A). Nevertheless, immunoblots clearly showed a much stronger signal for both Tpx and Bcp in the cytoplasmic fraction than in the periplasmic fraction (Fig. 8C and D, respectively). The signal present in the periplasmic fraction can almost certainly be attributed to cytoplasmic cross-contamination.
FIG. 8.
Cellular location of Tpx and Bcp proteins in C. jejuni. (A) Distribution of marker proteins in cytoplasm and periplasm after fractionation of wild-type C. jejuni cells according to the method of Sommerlad and Hendrixson (37). Open bars represent the activity of the cytoplasmic enzyme ICDH, and gray bars represent cytochrome c content determined spectroscopically at 550 nm after dithionite reduction (24). (B) Coomassie blue-stained 12% (wt/vol) SDS-polyacrylamide gel showing normalized loadings of cytoplasm (C) and periplasm (P). (C) Immunoblot assay of an identical gel using anti-Tpx antibodies. (D) Immunoblot assay using anti-Bcp antibodies.
DISCUSSION
In this study, we have obtained evidence for a role for the closely related peroxiredoxins Tpx and Bcp in the protection of C. jejuni against oxidative stress, particularly that caused by excess oxygen and exogenous peroxides. Single mutations in the tpx or bcp genes resulted in even poorer growth than that of the wild type under high-aeration conditions. When the mutations were combined in the double mutant, growth was dramatically reduced under microaerobic conditions and was prevented almost completely under high aeration. Thus, AhpC (2) is not the only peroxiredoxin in C. jejuni that has a role in aerotolerance; all three such proteins are important. Our data do suggest, however, a significant degree of redundancy in the function of Tpx and Bcp, which is reinforced by the data from the disc diffusion assays using a variety of oxidative-stress-inducing agents. Viability assays did indicate some increased sensitivity of the single mutants to hydrogen peroxide and organic peroxides, but there are several alternative H2O2 defense systems in C. jejuni, such as catalase (13), and two cytochrome c peroxidases (1); our assays showed all the strains to have high catalase activities which may be able to partially compensate for the individual loss of Tpx or Bcp. However, the related peroxiredoxin AhpC is the main hydrogen peroxide scavenger in E. coli, with low μM Km values (35). Although ahpC mutants of C. jejuni are apparently not hypersensitive to hydrogen peroxide (2), perhaps the function of this peroxiredoxin needs to be revisited. tpx and bcp single mutants in H. pylori and E. coli are hypersensitive to both H2O2 and organic peroxides (5, 9, 17, 43), and the H. pylori mutants were also sensitive to high oxygen conditions, but no studies have been carried out with double mutants. The superoxide stress phenotypes of the C. jejuni mutants also point to redundancy in the roles of Tpx and Bcp, whereas single tpx and bcp mutants in the closely related H. pylori were found to be more sensitive to superoxide generators than was the wild type (9), and tpx mutants in E. coli are also more sensitive to superoxide-mediated killing (5). The double tpx/bcp mutant was also more sensitive to the nitrosating agent sodium nitroprusside (an NO+ donor), most likely reflecting an indirect increase in ROS, including peroxides, under conditions of nitrosative stress. However, some peroxiredoxins like AhpC from H. pylori and Mycobacterium tuberculosis can act as peroxynitrite reductases and may therefore protect directly against nitrosative stress (3). C. jejuni contains at least two NO-detoxifying enzymes, the hemoglobin Cgb (11, 12) and the nitrite reductase NrfA (30).
The increase in lipid peroxidation and protein carbonylation in the tpx and bcp single mutants compared to those of wild-type cells grown under microaerobic conditions indicates the individual importance of both Tpx and Bcp in defending against ROS during normal cell growth. Lipid peroxidation has not been extensively documented in bacteria, largely because their lipid fatty acids tend not be polyunsaturated and thus are not so susceptible to peroxidation, but an increase in lipid peroxide levels has been shown to occur in tpx mutants of E. coli (6) and in ahpC mutants of Mycobacterium tuberculosis (22). However, the polyunsaturated host cell membrane lipids will become oxidized during the inflammatory response to infection and so bacterial peroxiredoxins may also be a useful defense against host-derived lipid peroxides. This may be particularly relevant in a mucosal pathogen like C. jejuni.
The results from the protein carbonylation experiments indicated that losing both Tpx and Bcp results in a particularly dramatic increase in the level of protein oxidation, particularly after challenge with oxygen or peroxides. Studies with single thiol peroxidase mutations in E. coli (6) have shown a similar pattern of increased carbonylation. We do not know the identity of those proteins which are most heavily carbonylated, but in other bacteria like Bacillus subtilis (23) the elongation factors EF-G, TufA, and EF-Ts are particularly affected, which could cause global effects on protein synthesis and cell growth if their activity is compromised, thus contributing to the poor growth of the tpx/bcp double mutant.
The substrate specificity of Bcp appears to be similar to that found in other organisms (17, 43). In C. jejuni, NADPH is oxidized fastest when H2O2 is used as the substrate. The rates of NADPH oxidation achieved for both cumene hydroperoxide and tert-butyl-hydroperoxide are very similar. Thus, the C. jejuni Bcp acts as a general peroxidase enzyme with a broad substrate range. This is also the case for the Bcp enzymes from H. pylori and E. coli (17, 43). Rates of NADPH oxidation with linoleic acid hydroperoxide were virtually undetectable with the C. jejuni Bcp or Tpx enzyme, although in both H. pylori and E. coli, Bcp was shown to preferentially use linoleic acid hydroperoxide as a substrate (17, 43). The fact that both single mutants exhibit an increase in lipid peroxide content suggests that Tpx and Bcp from C. jejuni do use lipid peroxides as substrates. Other peroxiredoxins, for example, the AhpC from M. tuberculosis (14), can detoxify a wide variety of lipid peroxides. However, the increase in lipid peroxides could also be indirectly due to the loss of enzymes involved in hydrogen peroxide removal, the buildup of hydrogen peroxide leading to an increase in lipid peroxides.
Tpx in C. jejuni appears to be a dedicated hydrogen peroxide-detoxifying enzyme, as no DTT or NADPH oxidation occurred at observable rates when organic peroxides were provided as substrates. This is in contrast to Tpx enzymes from other organisms, which appear to have a wider substrate range. Tpx in E. coli is able to use H2O2, organic peroxides, and lipid peroxides as substrates (4, 6), with the enzymes from H. pylori and M. tuberculosis able to use cumene hydroperoxide in vitro (25, 32) and the M. tuberculosis enzyme also being able to use H2O2 and tert-butyl-hydroperoxide (32). In these organisms, Tpx appears to act as a general peroxide detoxification enzyme, much like Bcp from C. jejuni. Tpx may therefore be an important scavenger of hydrogen peroxide in C. jejuni, as has been seen for the related peroxiredoxin AhpC in E. coli (35). The apparent substrate specificity for Tpx and the phenotypic results of the tpx mutant for sensitivity to organic peroxides are apparently in contradiction with each other, but this could be explained by the formation of some intracellular hydrogen peroxide after treatment of cells with the organic peroxides.
This study has provided evidence that the synthesis of both Tpx and Bcp is increased by atmospheric oxygen, but it is not known if this is a direct effect of molecular oxygen or an indirect effect, which could be attributed to a general increase in levels of ROS caused by aerobic growth. However, the fact that neither Tpx nor Bcp increased in abundance if cells were grown in the presence of external hydrogen peroxide does suggest that there is a specific oxygen effect. Interestingly, Tpx from E. coli is also not induced by peroxide-mediated oxidative stress but is the primary peroxiredoxin produced under anaerobic—rather than aerobic—conditions (6). After prolonged aerobic growth, aggregation of Tpx and Bcp was observed in cell samples by immunoblotting. This effect has also been reported for the peroxiredoxin AhpC of H. pylori and may have a protective role distinct from the antioxidant function of the protein (8). Tpx and Bcp may be important in exponential growth, with levels decreasing as cells are entering stationary phase. The growth inhibition seen in the three mutant strains compared to wild-type cells when grown aerobically also provides further evidence for the importance of Tpx and Bcp when cells are actively dividing, particularly under nonoptimal, aerobic conditions.
The localization results strongly suggest that Tpx and Bcp are both cytoplasmic enzymes in C. jejuni. A cytoplasmic location is in apparent contradiction to that of Tpx in E. coli, which was reported to be periplasmic (4), but would be consistent with the lack of a signal sequence and use of the cytoplasmic thioredoxin system as the source of reductant for catalysis; Tpx and Bcp from E. coli, M. tuberculosis, H. pylori, and now C. jejuni have all been reported to be thioredoxin dependent (4, 6, 17, 25, 32, 43). Perhaps the location of these enzymes in other bacteria, particularly E. coli, should be reexamined.
In summary, Tpx and Bcp from C. jejuni are enzymes with a key role in oxidative stress defense, particularly against molecular oxygen during exponential-phase growth. Bcp appears to be a general peroxide reductase able to act on a wide variety of compounds, whereas Tpx is a specific H2O2 detoxification enzyme. Both enzymes also have a more minor indirect role in defending against superoxide and nitrosative stress.
Acknowledgments
This work was supported by a United Kingdom Biotechnology and Biological Sciences Research Council studentship to J.M.A.
Footnotes
Published ahead of print on 30 May 2008.
REFERENCES
- 1.Atack, J. M., and D. J. Kelly. 2006. Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv. Microb. Physiol. 5273-106. [DOI] [PubMed] [Google Scholar]
- 2.Baillon, M.-L. A., A. H. M. van Vliet, J. M. Ketley, C. Constantinidou, and C. W. Penn. 1999. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J. Bacteriol. 1814798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryk, R., P. Griffin, and C. Nathan. 2000. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407211-215. [DOI] [PubMed] [Google Scholar]
- 4.Cha, M., H. Kim, and I. Kim. 1995. Thioredoxin-linked “thiol peroxidase” from periplasmic space of Escherichia coli. J. Biol. Chem. 27028635-28641. [DOI] [PubMed] [Google Scholar]
- 5.Cha, M., H. Kim, and I. Kim. 1996. Mutation and mutagenesis of thiol peroxidase of Escherichia coli and a new type of thiol peroxidase family. J. Bacteriol. 1785610-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha, M., W. Kim, C. Lim, K. Kim, and I. Kim. 2004. Escherichia coli periplasmic thiol peroxidase acts as lipid hydroperoxide peroxidase and the principal antioxidative function during anaerobic growth. J. Biol. Chem. 2798769-8778. [DOI] [PubMed] [Google Scholar]
- 7.Chelikani, P., I. Fita, and P. C. Loewen. 2004. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang, M.-H., M.-S. Wu, W.-L. Lo, J.-T. Lin, C.-H. Wong, and S.-H. Chiou. 2006. The antioxidant protein alkylhydroperoxide reductase of Helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc. Natl. Acad. Sci. USA 1032552-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comtois, S. L., M. D. Gidley, and D. J. Kelly. 2003. Role of the thioredoxin system and the thiol-peroxidases Tpx and Bcp in mediating resistance to oxidative and nitrosative stress in Helicobacter pylori. Microbiology 149121-129. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, H. R., and L. B. Poole. 1997. Roles for the two cysteine residues of AhpC in catalysis of peroxide reduction by alkyl hydroperoxide reductase from Salmonella typhimurium. Biochemistry 3613349-13356. [DOI] [PubMed] [Google Scholar]
- 11.Elvers, K. T., G. Wu, N. J. Gilberthorpe, R. K. Poole, and S. F. Park. 2004. Role of an inducible single-domain hemoglobin in mediating resistance to nitric oxide and nitrosative stress in Campylobacter jejuni and Campylobacter coli. J. Bacteriol. 1865332-5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elvers, K. T., S. M. Turner, L. M. Wainwright, G. Marsden, J. Hinds, J. A. Cole, R. K. Poole, C. W. Penn, and S. F. Park. 2005. NssR, a member of the Crp-Fnr superfamily from Campylobacter jejuni, regulates a nitrosative stress-responsive regulon that includes both a single-domain and a truncated haemoglobin. Mol. Microbiol. 57735-750. [DOI] [PubMed] [Google Scholar]
- 13.Grant, K., and S. Park. 1995. Molecular characterization of KatA from Campylobacter jejuni and generation of a catalase-deficient mutant of Campylobacter coli by interspecific allelic exchange. Microbiology 1411369-1376. [DOI] [PubMed] [Google Scholar]
- 14.Hillas, P. J., F. S. del Alba, J. Oyarzabal, A. Wilks, and P. R. Ortiz de Montellano. 2000. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J. Biol. Chem. 27518801-18809. [DOI] [PubMed] [Google Scholar]
- 15.Hofreuter, D., J. Tsai, R. O. Watson, V. Novik, B. Altman, M. Benitez, C. Clark, C. Perbost, T. Jarvie, L. Du, and J. E. Galan. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 744694-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imlay, J. A. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57395-418. [DOI] [PubMed] [Google Scholar]
- 17.Jeong, W., M.-K. Cha, and I.-H. Kim. 2000. Thioredoxin-dependent hydroperoxide peroxidase activity of bacterioferritin comigratory protein (BCP) as a new member of the thiol-specific antioxidant protein (TSA)/alkyl hydroperoxide peroxidase C (AhpC) family. J. Biol. Chem. 2752924-2930. [DOI] [PubMed] [Google Scholar]
- 18.Ketley, J. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 1435-21. [DOI] [PubMed] [Google Scholar]
- 19.Levine, R. 1983. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J. Biol. Chem. 25811823-11827. [PubMed] [Google Scholar]
- 20.Levine, R., D. Garland, C. Oliver, A. Amici, I. Climent, A. Lenz, B. Ahn, S. Shaltiel, and E. Stadtman. 1990. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186464-478. [DOI] [PubMed] [Google Scholar]
- 21.Markwell, M. A. K., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87206-210. [DOI] [PubMed] [Google Scholar]
- 22.Master, S. S., B. Springer, P. Sander, E. C. Boettger, V. Deretic, and G. S. Timmins. 2002. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology 1483139-3144. [DOI] [PubMed] [Google Scholar]
- 23.Mostertz, J., and M. Hecker. 2003. Patterns of protein carbonylation following oxidative stress in wild-type and sigB Bacillus subtilis cells. Mol. Genet. Genomics 269640-648. [DOI] [PubMed] [Google Scholar]
- 24.Myers, J. D., and D. J. Kelly. 2005. A sulphite respiration system in the chemoheterotrophic human pathogen Campylobacter jejuni. Microbiology 151233-242. [DOI] [PubMed] [Google Scholar]
- 25.Olczak, A. A., R. W. Seyler, Jr., J. W. Olson, and R. J. Maier. 2003. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect. Immun. 71580-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, S. F. 2002. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 74177-188. [DOI] [PubMed] [Google Scholar]
- 27.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, A. H. M. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403665-668. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, B. M., D. J. H. Gaskin, R. P. A. M. Segers, J. M. Wells, P. J. M. Nuijten, and A. H. M. van Vliet. 2007. The complete genome sequence of Campylobacter jejuni strain 81116 (NCTC11828). J. Bacteriol. 1898402-8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pesci, E. C., D. L. Cottle, and C. L. Pickett. 1994. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect. Immun. 622687-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman, M. S., K. T. Elvers, L. Lee, M. A. Jones, R. K. Poole, S. F. Park, and D. J. Kelly. 2007. Growth of Campylobacter jejuni on nitrate and nitrite: electron transport to NapA and NrfA via NrfH and distinct roles for NrfA and the globin Cgb in protection against nitrosative stress. Mol. Microbiol. 63575-590. [DOI] [PubMed] [Google Scholar]
- 31.Poole, L. B. 2005. Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases. Arch. Biochem. Biophys. 433240-254. [DOI] [PubMed] [Google Scholar]
- 32.Rho, B.-S., L.-W. Hung, J. M. Holton, D. Vigil, S.-I. Kim, M. S. Park, T. C. Terwilliger, and J.-D. Pedelacq. 2006. Functional and structural characterization of a thiol peroxidase from Mycobacterium tuberculosis. J. Mol. Biol. 361850-863. [DOI] [PubMed] [Google Scholar]
- 33.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 1837173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shacter, E., J. E. Williams, M. Lim, and R. L. Levine. 1994. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic. Biol. Med. 17429-437. [DOI] [PubMed] [Google Scholar]
- 37.Sommerlad, S. M., and D. R. Hendrixson. 2007. Analysis of the roles of FlgP and FlgQ in flagellar motility of Campylobacter jejuni. J. Bacteriol. 189179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtman, E. 1992. Protein oxidation and aging. Science 2571220-1224. [DOI] [PubMed] [Google Scholar]
- 39.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2188-194. [DOI] [PubMed] [Google Scholar]
- 40.Tauxe, R. V. 1992. Epidemiology of Campylobacter infections in the United States and other industrialized nations, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 41.van Vliet, A. H. M., K. G. Wooldridge, and J. M. Ketley. 1998. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 1805291-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, G., R. C. Conover, S. Benoit, A. A. Olczak, J. W. Olson, M. K. Johnson, and R. J. Maier. 2004. Role of a bacterial organic hydroperoxide detoxification system in preventing catalase inactivation. J. Biol. Chem. 27951908-51914. [DOI] [PubMed] [Google Scholar]
- 43.Wang, G., A. A. Olczak, J. P. Walton, and R. J. Maier. 2005. Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect. Immun. 73378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 9423-28. [DOI] [PubMed] [Google Scholar]
- 45.Wood, Z. A., E. Schroder, J. R. Harris, and L. B. Poole. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2832-40. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, Y., X.-Y. Wan, H.-L. Wang, Z.-Y. Yan, Y. De Hou, and D.-Y. Jin. 1997. Bacterial scavengase p20 is structurally and functionally related to peroxiredoxins. Biochem. Biophys. Res. Commun. 233848-852. [DOI] [PubMed] [Google Scholar]