Abstract
MtrC and OmcA are cell surface-exposed lipoproteins important for reducing solid metal oxides. Deletions of type II secretion system (T2SS) genes reduced their extracellular release and their accessibility to the proteinase K treatment, demonstrating the direct involvement of T2SS in translocation of MtrC and OmcA to the bacterial cell surface.
Decaheme c-type cytochromes (c-Cyts) MtrC (also known as OmcB) and OmcA of the gram-negative bacterium Shewanella oneidensis are lipoproteins anchored at extracellular side of the outer membrane (OM) (20, 21), where they are hypothesized as the terminal reductases of the insoluble form of metals, such as iron [Fe(III)] and manganese [Mn(IV)] (hydr)oxide (for reviews, see references 10, 13, 23, and 26). Consistent with this hypothesis, MtrC and OmcA are predicted to possess a putative binding site for solid metal oxide (17) and purified MtrC and OmcA bind and directly transfer electrons to the oxide (10). The MtrC and OmcA also are found in the extracellular polymeric substance produced by the bacterial cells under anaerobic, uranyl-reducing conditions, where they are spatially colocated with nano-domain uraninite (UO2) (18). However, it is still unclear how MtrC and OmcA are translocated across the OM to the extracellular environment.
Type II secretion system (T2SS) is involved in bacterial reduction of solid Fe(III)/Mn(IV) oxides. The Shewanella mutants without key components of T2SS, such as GspD, GspE, or GspG, had impaired ability to reduce Fe(III) or Mn(IV) oxide and resulted in absence of a heme-containing protein with apparent molecular mass of 91 kDa in the KCl cell extracts or absence of c-Cyts in the protruding structures or domains formed on the bacterial surface when the bacteria were cultured under the electron acceptor-limited conditions (1, 2, 6, 12). Deletion of the oxpG gene (a homolog of xcmT/xcpT/gspG family) from one of the T2SSs of Geobacter sulfurreducens also impaired the bacterial ability to reduce Fe(III) oxide. The T2SS of Geobacter involved in reducing metals differed from that of S. oneidensis, whose system contains all the core components (i.e., GspCDEFGHIJKLMO) (4), since (i) it was a Xcm-like T2SS and (ii) the exoproteins translocated by this type T2SS, such as OmpB of G. sulfurreducens and CumA of Pseudomonas putida GB-1, were multicopper proteins in which CumA was an extracellular Mn(II) oxidase (3, 5, 19). OmpB is required for reduction of insoluble metal oxides, but whether it is a functional metal reductase has yet to be determined (19). Despite its involvements in reducing metals, the role of T2SS in extracellular translocation of MtrC and OmcA has never been experimentally investigated.
Translocation of exoproteins by T2SS is a two-step process. The exoproteins are first translocated from the cytoplasm across the inner membrane (IM) to the periplasm via either the Sec or Tat secretion pathway and then across the OM via T2SS (for reviews, see references 4, 8, 15, 22, and 24). Both MtrC and OmcA possess the signal peptides that target them to the periplasm through the Sec pathway. Inside the periplasm, MtrC and OmcA undergo an extensive maturation process that includes (i) acylation at the cysteine residue of their N termini and (ii) covalent insertion of 10 heme groups into each of their polypeptides (14, 16, 21, 25, 27). After maturation, MtrC and OmcA that lack the +2 Asp sorting signal for their IM retention are subsequently repositioned to the extracellular side of OM via a previously uncharacterized mechanism (18, 20). The focus of this research, thus, was to investigate the role of T2SS in the extracellular translocation of MtrC and OmcA.
GspD and GspG affect extracellular release of native MtrC and OmcA.
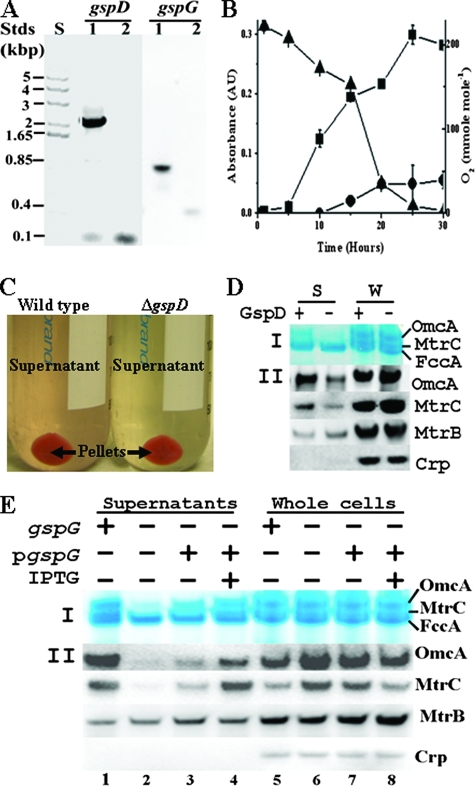
We first measured the release of MtrC and OmcA into the growth medium by the mutants with an in-frame deletion of gspD (ΔgspD) or gspG (ΔgspG) (Fig. 1A) in comparison to the wild type (wt) of S. oneidensis. Cultures were grown in 200-ml sealed serum bottles with air in the headspace and 69 ml of chemically defined medium in which lactate (20 mM) was used as the sole carbon and energy source (12). As the cultures grew, the O2 in the headspace of the bottles decreased from 225 to 2.7 mmol of O2 per mol of air (mmol mol−1) during the 30-h incubation period (11). When O2 in the headspace decreased to 153 mmol mol−1 (usually after 15 h of culture), the wt culture became pink and exhibited significant absorbance at 525 nm (Fig. 1B). After the cultures from four bottles were pooled and centrifuged (6,000 × g, 15 min, 4°C), the supernatant of the wt remained pink (Fig. 1C). In contrast, the supernatant of the ΔgspD or ΔgspG culture was pale yellow (only that of ΔgspD is shown in Fig. 1C). The color of the ΔgspG supernatant, which was complemented with the gspG cloned in a plasmid where the expression of gspG was controlled by an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter, was also pale yellow in the absence of IPTG but was pink when IPTG was added to the medium at a final concentration of 1 mM (data not shown). All of these results suggested that deletion of gspD or gspG was preventing the release of pigmented proteins to the medium. No major difference was observed between the wt and the mutants at the end of 16 h of culture in terms of (i) their cell densities, since the CFU/ml values were 2.3 ± 0.4 × 108 for the wt, 2.1 ± 0.5 × 108 for the ΔgspD mutant, and 2.0 ± 0.4 × 108 for the ΔgspG mutant (n = 6), and (ii) their cell colors (only those of the wt and the ΔgspD mutant are shown in Fig. 1C). These results indicate that deletion of gspD or gspG has little or no effect on cell growth and pigmentation under the conditions used in this study. Filtration of the supernatant through 0.2-μm-pore-size filters to remove any remaining cells did not change the supernatant color.
FIG. 1.
Influence of deletion of gspD or gspG on extracellular release of native MtrC and OmcA. (A) Agarose gel showing size of DNA standards (Stds) in kilobase pairs (lane S) and PCR products of the gspD or gspG amplified from the wt (lanes 1) and the respective mutants (lanes 2). (B) Bacterial culture of wt. The optical density at 600 nm of cell culture (▪), the optical density at 525 nm of the filtrate of cell culture (•), and the O2 concentration (▴) in the headspace of serum bottle during the time course of the study were measured. For points without an error bar, the error was smaller than the symbol (n = 3). (C) Colors of the supernatants of wt and ΔgspD cultures under O2-limited growth condition. (D and E) Heme staining and Western blot analyses of the effects of deleting gspD (D) or gspG (E). After 2 μg of concentrated supernatant proteins (lanes S [D] and lanes 1 to 4 [E]) or 10 μg (D) or 5 μg (E) of whole-cell lysate proteins (lanes W [D] and lanes 5 to 8 [E]) from wt (lanes +) and respective mutant (lanes -) were separated by SDS-PAGE, they were either visualized by heme staining (I panel) or probed with the respective antibodies (II panels).
To determine whether MtrC and OmcA were the pigmented proteins whose secretion was affected by deleting gspD or gspG gene, we concentrated the filtered supernatants from the wt, ΔgspD mutant, ΔgspG mutant, ΔgspG mutant plus complement, and ΔgspG mutant plus complement plus IPTG with Millipore concentrators with a molecular mass cutoff of 7 kDa and analyzed the proteins in the supernatants and in the whole-cell lysates by heme staining and Western blotting after they were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The intensity of OmcA bands of heme staining from the supernatants of both mutants was much weaker compared to the wt. Uninduced, complemented ΔgspG mutant only showed a slight increase in the intensity of OmcA heme-staining band on an SDS-PAGE gel, while the addition of IPTG significantly increased its intensity. The amount of MtrC in the supernatants, however, was too low to be visualized by heme staining (Fig. 1D, panel I, lanes S; Fig. 1E, lanes 1 to 4). Western blot analysis showed not only that OmcA and MtrC in the supernatants were less abundant in the mutants than in the wt (Fig. 1D, panels II, lanes S; Fig. 1E, lanes 1 to 2) but also that they were more abundant in the whole cells of the mutants than in those of wt (Fig. 1D, panels II, lanes W; Fig. 1E, lanes 5 to 6). Complementation of ΔgspG mutant with functional GspG reversed the effects of deleting gspG on the relative abundances of OmcA and MtrC in both supernatants and whole cells, demonstrating that the effect of gspG gene deletion on MtrC and OmcA is nonpolar (Fig. 1E). The reason for the observed difference between the gspD and gspG deletions on MtrC and OmcA (Fig. 1D and E) is unclear, but this result indicates that the elimination of GspD or GspG might have different impacts on localization of these c-Cyts. The abundances of an integral OM protein MtrB and a cytoplasmic protein Crp, meanwhile, were present in approximately equal abundance in the supernatants and/or whole cells of both the wt and the mutants, suggesting that the deletion of gspD or gspG had little effect on the abundances of these proteins (Fig. 1D and E). Likewise, no apparent difference was observed between the mutants and wt on the intensities of the periplasmic fumarate reductase FccA bands stained for hemes from the supernatants and the whole cells, demonstrating integrity of the OM in the T2SS mutants with regard to their permeability to the periplasmic proteins (Fig. 1D and E). Thus, the absence of either GspD or GspG, two key components of the T2SS, significantly decreases the extracellular release of native MtrC and OmcA. The release of MtrC and OmcA serves as a convenient assay system to test the role of T2SS in extracellular translocation of MtrC and OmcA, even though the nature and mechanisms of their release remain obscure.
GspD and GspG affect the extracellular release of recombinant MtrC and OmcA.
Among the T2SS exoproteins that have been investigated to date, pullulanase (PulA) of Klebsiella oxytoca is the only lipoprotein whose secretion by T2SS has been well characterized. Recombinant PulA with addition of six histidine residues (His6 tag) in its C terminus and lacking a lipid-anchor (i.e., nonacylated) in its N terminus was still efficiently translocated to the extracellular environment by the T2SS of K. oxytoca (9). To investigate whether recombinant MtrC and OmcA behaved similarly to recombinant PulA with respect to their extracellular secretion, we tested the effects of deletions of gspD or gspG gene on the secretion of the recombinant MtrC and OmcA that contained a V5/His6 tag in their C termini and were either acylated or not in their N termini. The procedures for making these constructs were described previously (7, 25) and the strains, plasmids, and oligonucleotide primers used in this study are listed in Table 1.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant genotype or sequence (5′-3′) | Purpose | Source or reference |
|---|---|---|---|
| Strains | |||
| MR-1 | Wild type | ATCC | |
| ΔSO0166 | ΔgspD | D. Saffarini | |
| ΔSO0169 | ΔgspG | D. Saffarini | |
| ΔSO0169 complement | ΔgspG + pgspG | D. Saffarini | |
| MR-1 + pLS132 | Wild type + pomcA | This study | |
| MR-1 + pLS138 | Wild type + pmtrC | This study | |
| MR-1 + pLS146 | Wild type + pmtrC | This study | |
| MR-1 + pLS147 | Wild type + pomcA | This study | |
| ΔSO0166 + pLS132 | ΔgspD + pomcA | This study | |
| ΔSO0166 + pLS138 | ΔgspD + pmtrC | This study | |
| ΔSO0166 + pLS146 | ΔgspD + pmtrC | This study | |
| ΔSO0166 + pLS147 | ΔgspD + pomcA | This study | |
| ΔSO0169 + pLS132 | ΔgspG + pomcA | This study | |
| ΔSO0169 + pLS138 | ΔgspG + pmtrC | This study | |
| ΔSO0169 + pLS146 | ΔgspG + pmtrC | This study | |
| ΔSO0169 + pLS147 | ΔgspG + pomcA | This study | |
| Plasmids | |||
| pLS132 | Coding sequence for omcA | 25 | |
| pLS138 | Coding sequence for mtrC | 28 | |
| pLS146 | Coding sequence for the signal peptide of mtrB and the mature mtrC that lacks a lipid anchor | This study | |
| pLS147 | Coding sequence for the signal peptide of mtrB and the mature omcA that lacks a lipid anchor | 7 | |
| Primers | |||
| SO0166F | CAATAACACGCGTTAGGGG | Validation of ΔgspD | |
| SO0166R | CCTCGACTTGGGATACTTG | Validation of ΔgspD | |
| SO0169F | GTTACGCTCGCCCTCGG | Validation of ΔgspG | |
| SO0169R | TACGGTTTCATCGAGCAC | Validation of ΔgspG | |
| SO1776EF1 | CACCTAAGAAGGAGATATACATCCCATGAAATTTAAACTCAATTTGATCAC | Construction of pLS146 | |
| SO1776SR | ATTACCATCGCTTCCACCATCAGCAGCGACGGCCAAG | Construction of pLS146 | |
| SO1778EF3 | GGTGGAAGCGATGGTAATAAC | Construction of pLS146 | |
| SO1778ER3 | CTTGCAACAACCAGGGCAACATGCAGCAAGCTTGTCATCGTCATCCATTTTCACTTTAGTGTGATCTGC | Construction of pLS146 |
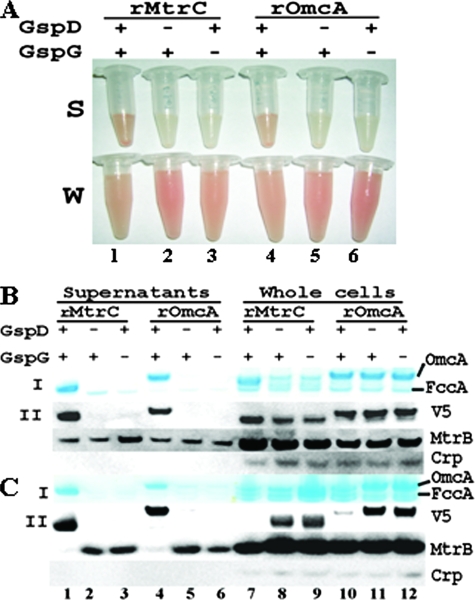
Similar to the results with native MtrC and OmcA, the supernatants of wt (with respect to the T2SS) containing recombinant MtrC or OmcA were pink, in sharp contrast to the pale yellow supernatants from the ΔgspD or ΔgspG mutants. The cultures containing whole cells, meanwhile, were red (only those of recombinant/acylated are shown in panels S and W of Fig. 2A). Because they were overexpressed by addition of l-arabinose at a final concentration of 1 mM, the recombinant MtrC and OmcA were the predominant heme proteins in the supernatants (Fig. 2B and C, I panels). The FccA band was very faint or undetectable in the samples containing recombinant MtrC or OmcA (Fig. 2B and C, panels I, lanes 1 and 4), in contrast to earlier observations with native MtrC and OmcA (Fig. 1D and E). Compared to that in wt, little or no recombinant MtrC or OmcA was detected in the supernatants of ΔgspD or ΔgspG by heme staining (Fig. 2B and C, I panels, lanes 2, 3, 5, and 6) or Western blot (Fig. 2B and C, II panels, lanes 2, 3, 5, and 6) under the conditions tested. There was significant accumulation of recombinant/nonacylated MtrC and OmcA in the cells of the T2SS mutants relative to the wt (Fig. 2C, lanes 7 to 12). In contrast to these results, as well as those with native MtrC and OmcA, there was no apparent excess accumulation of recombinant/acylated MtrC or OmcA in the cells of ΔgspD or ΔgspG relative to the wt. Heme staining showed overexpression of the MtrC in the cells with a functional T2SS but little in either ΔgspD or ΔgspG cells. It also showed that the abundance of OmcA was nearly identical among wt, ΔgspD, and ΔgspG cells. While the results of Western blot analysis of the recombinant/acylated OmcA were consistent with that of heme staining, they also showed the presence of recombinant/acylated MtrC in all cell types. After normalizing against MtrB, the difference in MtrC abundance between wt and ΔgspD or ΔgspG mutant was much less apparent than that from heme staining (Fig. 2B, lanes 7 to 12). These results demonstrated that recombinant/acylated MtrC was expressed in ΔgspD and ΔgspG mutants. Given the profound differences in the abundance of the recombinant/acylated MtrC and OmcA in the supernatants of the wt and either ΔgspD or ΔgspG mutant in concert with similar abundances in the cells, we conclude that T2SS is involved in the extracellular release of the recombinant/acylated forms of MtrC and OmcA. The reason for no apparent accumulation of recombinant/acylated MtrC or OmcA in the cells of T2SS mutants is currently unknown.
FIG. 2.
Influence of deletion of gspD or gspG on extracellular release of recombinant MtrC and OmcA. (A) The concentrated supernatants (panel S) and whole cells (panel W) of wt (lanes 1 and 4), ΔgspD mutant (lanes 2 and 5), and ΔgspG mutant (lanes 3 and 6) in which recombinant (r)/acylated MtrC and OmcA were overexpressed. (B and C) Heme staining and Western blot analyses of the gene deletion effects on recombinant/acylated MtrC and OmcA (B) and recombinant/nonacylated MtrC and OmcA (C). A total of 1 μg (B) or 0.5 μg (C) of concentrated supernatant proteins (lanes 1 to 6) or 5 μg of whole-cell lysate proteins (lanes 7 to 12) from wt (lanes 1, 4, 7, and 10), ΔgspD mutant (lanes 2, 5, 8, and 11), and ΔgspG mutant (lanes 3, 6, 9, and 12) were separated by SDS-PAGE and were then either visualized by heme staining (I panels) or probed with the respective antibodies (II panels).
Because they are not membrane associated (7; L. Shi, unpublished data), the extracellular release of recombinant/nonacylated MtrC and OmcA into the growth medium should be a single-step process: their translocation across the OM, similar to that of recombinant/nonacylated PulA from K. oxytoca (9). The effects of GspD and GspG on the recombinant/nonacylated c-Cyts therefore suggest that T2SS plays a direct role in extracellular secretion of MtrC and OmcA by translocating them across the OM.
GspD and GspG affect accessibility of MtrC and OmcA to proteinase K.
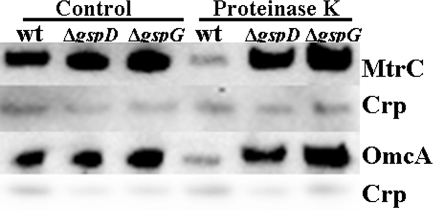
To confirm the findings that T2SS directly affects the translocation of MtrC and OmcA across the OM, we treated the cells of wt, ΔgspD, and ΔgspG, individually, with proteinase K (New England Biolabs, Ipswich, MA) in the buffer (100 mM Tris [pH 7.4], 150 mM NaCl, 1 mM CaCl2, 50 μM MgCl2) that contained 0.5 mg of the protease/ml and 109 cells/ml at room temperature for 60 min. Compared to those in controls without the treatment, the abundances of MtrC and OmcA in wt cells were much lower after treatment with proteinase K under the conditions tested. In contrast to wt, the treatment of ΔgspD and ΔgspG mutants with proteinase K had little effect on the abundance of MtrC and OmcA (Fig. 3). These results are not only consistent with the previous findings that MtrC and OmcA are cell surface exposed (20) but also confirm that T2SS indeed regulates directly the translocation of MtrC and OmcA across the OM.
FIG. 3.
Proteinase K accessibility of MtrC and OmcA in wt, ΔgspD mutant, and ΔgspG mutant cells. The preparations of cells were as described in the text. The whole-cell lysates (∼106 cells) were separated by SDS-PAGE and then probed with the respective antibodies.
Conclusions.
Although the nature or the mechanisms of extracellular release of acylated MtrC and OmcA into the growth medium under the condition tested in this study are currently unknown, the results of this research demonstrate for the first time the direct role of the T2SS in translocating MtrC and OmcA across the OM, since deletion of T2SS genes (i) impairs the ability of S. oneidensis to release of MtrC and OmcA to the growth medium and (ii) makes the MtrC and OmcA resistant to the proteinase K treatment. Furthermore, the effects of T2SS mutations on MtrC and OmcA are very similar to those on PulA of K. oxytoca. Based on these and previous findings, the sequential steps of extracellular translocation of MtrC and OmcA in S. oneidensis should include their (i) biosyntheses in the cytoplasm, (ii) translocation across the IM via the Sec pathway, (iii) maturation in the periplasm, and (iv) translocation across the OM via the T2SS.
Acknowledgments
This work was supported by the U.S. Department of Energy (DOE) Office of Biological and Environmental Sciences Program (BERP) under the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL) Biogeochemistry Grand Challenge Project. A portion of this work was performed at EMSL, a national scientific user facility sponsored by the DOE BERP and located at Pacific Northwest National Laboratory. Pacific Northwest National Laboratory is operated for the U.S. DOE by Battelle Memorial Institute under contract DE-AC05-76RLO1830.
We thank Daad Saffarini (University of Wisconsin-Milwaukee) for providing the T2SS mutant strains.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Biju, V., D. Pan, Y. A. Gorby, J. Fredrickson, J. McLean, D. Saffarini, and H. P. Lu. 2007. Combined spectroscopic and topographic characterization of nanoscale domains and their distributions of a redox protein on bacterial cell surfaces. Langmuir 231333-1338. [DOI] [PubMed] [Google Scholar]
- 2.Bretschger, O., A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson, and K. H. Nealson. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 737003-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouwers, G. J., J. P. de Vrind, P. L. Corstjens, P. Cornelis, C. Baysse, and E. W. de Vrind-de Jong. 1999. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 651762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13581-588. [DOI] [PubMed] [Google Scholar]
- 5.De Vrind, J., A. De Groot, G. J. Brouwers, J. Tommassen, and E. De Vrind-De Jong. 2003. Identification of a novel Gsp-related pathway required for secretion of the manganese-oxidizing factor of Pseudomonas putida strain GB-1. Mol. Microbiol. 47993-1006. [DOI] [PubMed] [Google Scholar]
- 6.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J. Bacteriol. 184142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggleston, C. M., J. Voros, L. Shi, B. H. Lower, T. C. Droubay, and P. J. S. Colberg. 2007. Binding and direct electrochemistry of OmcA, an outer-membrane cytochrome from an iron reducing bacterium, with oxide electrodes: a candidate biofuel cell system. Inorg. Chim. Acta 361769-777. [Google Scholar]
- 8.Filloux, A. 2004. The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694163-179. [DOI] [PubMed] [Google Scholar]
- 9.Francetic, O., and A. P. Pugsley. 2005. Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J. Bacteriol. 1877045-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredrickson, J. K., and J. M. Zachra. 2008. Electron transfer at the microbe-mineral interface: a grand challenge in biogeochemistry. Geobiology doi: 10.1111/j.1472-4669.2008.00146. [DOI] [PubMed]
- 11.Gillespie, R. J., D. A. Humphreys, N. C. Baird, and E. A. Robinson. 1989. The atmosphere and the gas laws, p. 99-150. In E. Ober (ed.), Chemistry, 2nd ed. Allyn and Bacon, Inc., Needham Heights, MA.
- 12.Gorby, Y. A., S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 10311358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gralnick, J. A., and D. K. Newman. 2007. Extracellular respiration. Mol. Microbiol. 651-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartshorne, R. S., B. N. Jepson, T. A. Clarke, S. J. Field, J. Fredrickson, J. Zachara, L. Shi, J. N. Butt, and D. J. Richardson. 2007. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J. Biol. Inorg. Chem. 121083-1094. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, T. L., J. Abendroth, W. G. Hol, and M. Sandkvist. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255175-186. [DOI] [PubMed] [Google Scholar]
- 16.Kranz, R., R. Lill, B. Goldman, G. Bonnard, and S. Merchant. 1998. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29383-396. [DOI] [PubMed] [Google Scholar]
- 17.Lower, B. H., R. D. Lins, Z. Oestreicher, T. P. Straatsma, M. F. Hochella, Jr., L. Shi, and S. K. Lower. 2008. In vitro evolution of a peptide with a hematite binding motif that may constitute a natural metal-oxide binding archetype. Environ. Sci. Technol. doi: 10.1021/es702688c. [DOI] [PubMed]
- 18.Marshall, M. J., A. S. Beliaev, A. C. Dohnalkova, D. W. Kennedy, L. Shi, Z. Wang, M. I. Boyanov, B. Lai, K. M. Kemner, J. S. McLean, S. B. Reed, D. E. Culley, V. L. Bailey, C. J. Simonson, D. A. Saffarini, M. F. Romine, J. M. Zachara, and J. K. Fredrickson. 2006. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 4e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta, T., S. E. Childers, R. Glaven, D. R. Lovley, and T. Mester. 2006. A putative multicopper protein secreted by an atypical type II secretion system involved in the reduction of insoluble electron acceptors in Geobacter sulfurreducens. Microbiology 1522257-2264. [DOI] [PubMed] [Google Scholar]
- 20.Myers, C. R., and J. M. Myers. 2003. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett. Appl. Microbiol. 37254-258. [DOI] [PubMed] [Google Scholar]
- 21.Myers, C. R., and J. M. Myers. 2004. The outer membrane cytochromes of Shewanella oneidensis MR-1 are lipoproteins. Lett. Appl. Microbiol. 39466-470. [DOI] [PubMed] [Google Scholar]
- 22.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 5750-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson, D. J. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146(Pt. 3)551-571. [DOI] [PubMed] [Google Scholar]
- 24.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40271-283. [DOI] [PubMed] [Google Scholar]
- 25.Shi, L., B. Chen, Z. Wang, D. A. Elias, M. U. Mayer, Y. A. Gorby, S. Ni, B. H. Lower, D. W. Kennedy, D. S. Wunschel, H. M. Mottaz, M. J. Marshall, E. A. Hill, A. S. Beliaev, J. M. Zachara, J. K. Fredrickson, and T. C. Squier. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 1884705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi, L., T. C. Squier, J. M. Zachara, and J. K. Fredrickson. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol. Microbiol. 6512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokuda, H., and S. Matsuyama. 2004. Sorting of lipoproteins to the outer membrane in Escherichia coli. Biochim. Biophys. Acta 16935-13. [DOI] [PubMed] [Google Scholar]
- 28.Wigginton, N. S., K. M. Rosso, B. H. Lower, L. Shi, and M. F. Hochella. 2007. Electron tunneling properties of outer-membrane decaheme cytochromes from Shewanella oneidensis. Geochim. Cosmochim. Acta 71543-555. [Google Scholar]