Abstract
The catabolism of 4-hydroxyacetophenone in Pseudomonas fluorescens ACB is known to proceed through the intermediate formation of hydroquinone. Here, we provide evidence that hydroquinone is further degraded through 4-hydroxymuconic semialdehyde and maleylacetate to β-ketoadipate. The P. fluorescens ACB genes involved in 4-hydroxyacetophenone utilization were cloned and characterized. Sequence analysis of a 15-kb DNA fragment showed the presence of 14 open reading frames containing a gene cluster (hapCDEFGHIBA) of which at least four encoded enzymes are involved in 4-hydroxyacetophenone degradation: 4-hydroxyacetophenone monooxygenase (hapA), 4-hydroxyphenyl acetate hydrolase (hapB), 4-hydroxymuconic semialdehyde dehydrogenase (hapE), and maleylacetate reductase (hapF). In between hapF and hapB, three genes encoding a putative intradiol dioxygenase (hapG), a protein of the Yci1 family (hapH), and a [2Fe-2S] ferredoxin (hapI) were found. Downstream of the hap genes, five open reading frames are situated encoding three putative regulatory proteins (orf10, orf12, and orf13) and two proteins possibly involved in a membrane efflux pump (orf11 and orf14). Upstream of hapE, two genes (hapC and hapD) were present that showed weak similarity with several iron(II)-dependent extradiol dioxygenases. Based on these findings and additional biochemical evidence, it is proposed that the hapC and hapD gene products are involved in the ring cleavage of hydroquinone.
Acetophenones are widely found in the environment as degradation products of industrial chemicals. Ring-chlorinated acetophenones originate from the microbial degradation of insecticides (6-8), polychlorobiphenyls (3-5), and chloroxanthones (83). Nonchlorinated acetophenones have been identified as intermediates in the microbial degradation of ethylbenzene (16, 85), 1-phenylethanol (18), 4-ethylphenol (42), and the flame-retardant tetrabromobisphenol A (55, 73).
Several aerobic microorganisms are capable of utilizing acetophenones for their growth (17-19, 32, 34, 35). 4-Hydroxyacetophenone can be converted in two different ways to 4-hydroxybenzoate (28, 35, 48). The latter compound then enters the β-ketoadipate pathway via the formation of the ring cleavage substrate 3,4-dihydroxybenzoate (42). For Pseudomonas fluorescens ACB (34), Pseudomonas putida JD1 (19), and Aspergillus fumigatus ATCC 28282 (41), it was shown that the initial steps of 4-hydroxyacetophenone mineralization comprise a Baeyer-Villiger oxidation to 4-hydroxyphenyl acetate and subsequent ester hydrolysis to hydroquinone (1.4-dihydroxybenzene) (19, 34, 41, 44). However, the subsequent degradation of hydroquinone, a potential human nephrocarcinogen (54), remained elusive.
The aerobic degradation of hydroquinone may proceed in two different ways. Hydroquinone can be hydroxylated to hydroxyhydroquinone (1,2,4-trihydroxybenzene) (24, 87), followed by ring fission by a intradiol hydroxyhydroquinone 1,2-dioxygenase (39, 53, 72). Alternatively, direct ring fission of hydroquinone to 4-hydroxymuconic semialdehyde by a hydroquinone 1,2-dioxygenase may occur (10, 19, 59, 77). Hydroquinone 1,2-dioxygenases have been indicated to be involved in the degradation of 4-ethylphenol (19), p-nitrophenol (12, 34, 70), 4-nitrocatechol (12, 13), and the insecticide fenitrothion (33). However, none of these enzymes has been characterized in much detail.
19F nuclear magnetic resonance (NMR) studies have given some clues about the further degradation of hydroquinone in P. fluorescens ACB (60, 61). Whole cells converted 4-fluoroacetophenone to 4-fluorophenol. Prolonged incubations showed that 4-fluorophenol is not further degraded, suggesting that 4-hydroxyacetophenone-grown cells of P. fluorescens ACB lack phenol hydroxylase activity (60, 61). Without such monooxygenase activity, hydroquinone cannot be converted to hydroxyhydroquinone (24).
In this paper we have addressed the further degradation of hydroquinone in the catabolism of 4-hydroxyacetophenone in P. fluorescens ACB. To that end, we studied the conversion of (fluorinated) hydroquinones by (dialyzed) cell extracts and sequenced the hap gene cluster involved in 4-hydroxyacetophenone utilization. The biochemical and genetic characterization of the degradation pathway revealed that hydroquinone is metabolized through 4-hydroxymuconic semialdehyde and maleylacetate to β-ketoadipate. Two novel genes were found whose functions are linked to the ring cleavage of hydroquinone.
MATERIALS AND METHODS
Chemicals.
NADH and NAD+ were from Boehringer. Phenylmethylsulfonyl fluoride was from Merck. N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES) was from Sigma. 2,3-Difluoro-4-hydroxybenzoic acid was synthesized from 2,3-difluorophenol according to Komiyama and Hirai (52) and purified by straight-phase high-performance liquid chromatography (HPLC) (Kieselgel 60 μm; Merck) with a 1 to 40% linear gradient of ethanol in petroleum ether (60 to 80°C). The identity of the product was demonstrated by 1H NMR. Other fluorinated 4-hydroxybenzoic acids were synthesized and purified as described before (23, 38). All other chemicals were of analytical grade.
Bacterial strains and culture conditions.
P. fluorescens ACB (34) was grown on 4-hydroxyacetophenone as reported before (44). Cell extract was prepared as described previously (44).
Enzymatic synthesis and transformation of difluorohydroquinones.
2,3-Difluoro-, 2,5-difluoro-, and 3,5-difluorohydroquinone were prepared from the corresponding difluorinated 4-hydroxybenzoates by incubation with 4-hydroxybenzoate 1-hydroxylase from Candida parapsilosis CBS604 (23). The reaction mixtures contained 50 mM air-saturated potassium phosphate (pH 7.0), 10 μM flavin adenine dinucleotide, 1 mM ascorbate, 0.3 mM difluoro-4-hydroxybenzoate, 0.25 mM NADH, and 80 μl of 4-hydroxybenzoate 1-hydroxylase in a total volume of 2.0 ml. The reactions were carried out at 25°C. After 2 h of incubation, the reaction mixtures were frozen in liquid nitrogen. Completion of the reactions was checked with 19F NMR. Next, the difluorohydroquinones (1,800 μl) were incubated for 2 h in an orbital shaker at 30°C in the presence of 20 μl of P. fluorescens ACB cell extract. Formation of products was followed with 19F NMR.
Enzyme purification.
Partial purification of hydroquinone dioxygenase (HQDO) was performed at 4°C. P. fluorescens ACB cells (5 g, wet weight) were suspended in 5 ml of 20 mM BisTris [bis(2-hydroxyethyl)imino-tris(hydroxymethyl)-methane] chloride, pH 7.0, containing 0.1 mM phenylmethylsulfonyl fluoride (buffer A). Immediately after the addition of 1 mM EDTA, 2 mM MgCl2, and 1 mg of DNase, cells were disrupted three times through a precooled French press at 10,000 lb/in2. After centrifugation (27,000 × g for 30 min), the clarified cell extract was adjusted to 25% ammonium sulfate saturation. The precipitate thus formed was removed by centrifugation (27,000 × g for 30 min), and the supernatant was loaded onto a phenyl-Sepharose column (1.6 × 11 cm) equilibrated in buffer A containing 25% ammonium sulfate. After a washing step with 3 column volumes of starting buffer, the HQDO activity was eluted with a 100-ml linear gradient of 25 to 0% ammonium sulfate in buffer A. Active fractions were pooled and adjusted to 60% saturation with pulverized ammonium sulfate. The precipitate was collected by centrifugation (27,000 × g for 30 min), dissolved in 1 ml of buffer A, and stored at −20°C.
Analytical methods.
Absorption spectra were recorded using a Hewlett-Packard 8453 diode array spectrophotometer. HQDO activity was routinely determined by monitoring the formation of 4-hydroxymuconic semialdehyde at 320 nm (ɛ320 = 11.0 mM−1 cm−1) (77). The assay mixture (1.0 ml) typically contained 50 μl of cell extract and 10% (wt/vol) glycerol in 20 mM BES, pH 7.0. Reactions were started by the addition of 10 μl of 50 mM hydroquinone in dimethylformamide. For activity tests with iron(II)-treated enzyme, partially purified HQDO preparations were freshly incubated for at least 1 min with 100 μM iron(II) sulfate and desalted over a Biogel P-6DG column (1 × 10 cm), running in 20 mM BisTris, pH 7.0, containing 10% glycerol. One unit of HQDO activity is defined as the amount of enzyme that forms 1 μmol of semialdehyde product per minute. 4-Hydroxymuconic semialdehyde dehydrogenase activity was measured by the addition of 1 mM NAD+ to the above-produced semialdehyde and following the decrease of absorbance at 320 nm (77).
19F NMR measurements were performed on a Bruker DPX 400 NMR spectrometer, essentially as described elsewhere (88). The sample temperature and sample volume were 7°C and 1.6 ml, respectively. For calibration an insert containing D2O and a known amount of 4-fluorobenzoate was used, which also served as a deuterium lock for locking the magnetic field. Chemical shifts are reported relative to CFCl3. The resonance of the 4-fluorobenzoate internal standard was set at −114.2 ppm with respect to CFCl3. The detection limit of an overnight 19F NMR measurement (60,000 scans) is 1 μM. The sample volume was 1.6 ml. 19F NMR chemical shift values of the various fluorine-containing compounds were identified on the basis of authentic reference compounds (68) or as described in the present study.
HPLC analysis was performed on a Waters 600 controller system equipped with a reversed-phase Alltima C18 column (150 by 4.6 mm) running in 10% (vol/vol) acetonitrile and containing either 1, 0.1, or 0% acetic acid. Products were detected with a Waters 996 photodiode array detector.
LC-mass spectrometry (LC-MS) experiments were carried out on a LCQ Classic mass spectrometer (Thermo-Finnigan, San Jose, CA). Reaction mixtures containing 20 μl of 50 mM hydroquinone and 20 μl of desalted cell extract in 940 μl of sodium bicarbonate, pH 7.8, were incubated for 7 min at 25°C. Separation was achieved on a 150- by 2-mm Alltima C18 (Alltech, Breda, The Netherlands) column running in 10% (vol/vol) acetonitrile. MS analysis was performed in the negative atmospheric pressure chemical ionization mode using a vaporizer temperature of 450°C, a discharge voltage of 2 kV, and a capillary temperature of 150°C with nitrogen as a sheath gas (20%) and auxiliary gas (5%). Tandem MS (MS/MS) scans were recorded in the data-dependent mode when an MS base peak signal higher than 106 was obtained with a normalized collision energy of 35% and a 2-Da isolation width.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out with 15% Tris-glycine gels (91). An Amersham Pharmacia Biotech low-molecular-mass calibration kit containing phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa) served as a reference. Proteins were stained with Coomassie brilliant blue G250.
N-terminal sequencing.
The N-terminal sequence of HQDO was determined by Edman degradation. The contents of a 15% SDS-PAGE gel (140 by 120 by 1.5 mm) loaded with 50 μg of partially purified enzyme were blotted onto a polyvinylidene difluoride Immobilon-P support (Millipore) in 10 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid], pH 11.0, containing 10% ethanol. After the gel was stained with 0.1% Coomassie R-250 in 50% methanol, the main band corresponding to a relative molecular mass of 38 kDa was excised. Gas phase sequencing of the polypeptide on the Immobilon support was carried out at the sequencing facility of Leiden University, The Netherlands.
Cloning of the hap gene cluster.
The hap gene cluster from P. fluorescens ACB was sequenced from a genomic cosmid library as described previously (44). In brief, by using degenerated primers based on the N-terminal amino acid sequences of 4-hydroxyphenyl acetate esterase and 4-hydroxyacetophenone monooxygenase, a PCR product was obtained that contained the esterase gene (hapB). This gene was used as a probe to screen the cosmid library, which yielded two positive clones (44). Sequencing of these cosmid clones resulted in a contiguous segment of 14.8 kbp consisting of 14 complete open reading frames (ORFS).
Sequence comparison.
Protein sequence similarity searches were performed using the BLASTP option at www.ncbi.nlm.nih.gov. Multiple sequence alignments were made with the CLUSTAL W program at the European Bioinformatics Institute (www.ebi.ac.uk/clustalw) (81). BioEdit (29) was used to calculate the pairwise identity and similarity scores (PAM250 matrix) from the aligned sequences and for the display of the alignment.
Nucleotide sequence accession number.
The nucleotide and amino acid sequence data reported in this paper have been deposited in the DDBJ/EMBL/GenBank sequence databases under accession number AF355751.
RESULTS
Conversion of hydroquinone by desalted cell extract of P. fluorescens ACB.
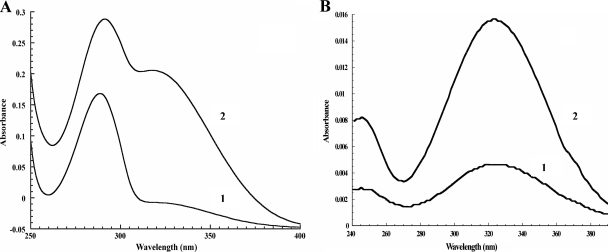
To study the conversion of hydroquinone in the degradation pathway of 4-hydroxyacetophenone in P. fluorescens ACB, 0.5 mM hydroquinone was incubated with desalted cell extract in 20 mM BES, pH 7.0, at 25°C. Absorption spectral analysis revealed that hydroquinone with a maximum absorption at 290 nm was converted into a product with a maximum absorption at 320 nm (Fig. 1A).
FIG. 1.
Product analysis of the conversion of hydroquinone by desalted cell extract of P. fluorescens ACB. (A) Absorption spectrum of hydroquinone (1) and after conversion (2). (B) HPLC diode array spectra of the 4-hydroxymuconic semialdehyde product with (1) and without (2) 0.1% acetic acid in the eluent.
HPLC analysis of the reaction mixture showed a spectrum (Fig. 1B) indicative for the formation of 4-hydroxymuconic semialdehyde (77). HPLC separation of substrate and product was achieved at neutral pH because acidic mobile phase conditions resulted in vanishing of the absorbance at 320 nm, a feature observed with maleyl-substituted ring fission products (11). The identity of the reaction product of the conversion of hydroquinone by P. fluorescens ACB was confirmed by LC-MS. The product eluted at 2.65 min, and its mass spectrum gave an m/z of 141.0 in the negative mode. Collision-induced defragmentation showed an m/z of 96.9, corresponding to the decarboxylation of 4-hydroxymuconic semialdehyde (77). Addition of NAD+ to the incubation mixture resulted in a decrease of absorbance at 320 nm, most likely due to conversion of 4-hydroxymuconic semialdehyde to maleylacetate. During this reaction, no increase at 340 nm was observed, suggesting that maleylacetate was rapidly further converted by an NADH-dependent maleylacetate reductase.
Degradation of difluorinated hydroquinones by P. fluorescens ACB.
To get more information about the degradation of hydroquinones by P. fluorescens ACB, several difluorinated hydroquinones were prepared from the corresponding difluorinated 4-hydroxybenzoates by the action of 4-hydroxybenzoate 1-hydroxylase from C. parapsilosis CBS604. Table 1 gives an overview of the 19F NMR chemical shift values of the fluorinated 4-hydroxybenzoates and hydroquinones at pH 8.0. It should be mentioned here that difluorinated hydroquinones were selected for analysis because the oxidative ring opening of monofluorinated hydroquinones might result in nonfluorinated products that cannot be detected by 19F NMR.
TABLE 1.
19F NMR chemical shift values of fluorinated substrates and products
| Compound | Chemical shift(s) (ppm) |
|---|---|
| 2,3-Difluoro-4-hydroxybenzoate | −143.9 (F2), −167.4 (F3) |
| 2,3-Difluorohydroquinone | −163.8 |
| 2,3-Difluoro-4-hydroxymuconic | |
| semialdehyde | −129.7 (F2), −154.4 (F3) |
| enol-2,3-Difluoromaleylacete | −130.3 (F2), −154.0 (F3) |
| keto-2,3-Difluoromaleylacete | −129.0 (F2), −142.1 (F3) |
| 2,5-Difluoro-4-hydroxybenzoate | −121.4 (F2), −146.9 (F5) |
| 2,5-Difluorohydroquinone | −144.6 |
| 2,5-Difluoro-4-hydroxymuconic | |
| semialdehydea | −110.7 (F2), −174.9 (F5) |
| 3,5-Difluoro-4-hydroxybenzoate | −139.2 |
| 3,5-Difluorohydroquinone | −136.8 |
Or enol-2,5-difluoromaleylacetate.
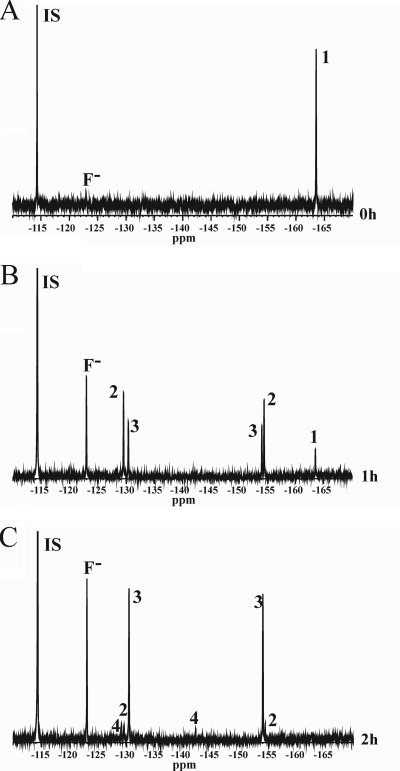
Figure 2 shows the 19F NMR spectra of the incubation of 2,3-difluorohydroquinone with cell extract of P. fluorescens ACB. After 1 h, about 95% of the fluorinated aromatic substrate was converted (Fig. 2B), whereas all substrate was converted after 2 h of incubation (Fig. 2C). Formation of new signals at −129.0, −129.7, −130.3, −142.1, −154.0 and −154.4 ppm was observed to an extent that accounts for the decrease in the amount of 2,3-difluorohydroquinone. The resonance at −123.0 ppm originates from fluoride anions, indicating that some defluorination has occurred. Based on their intensity, the other six signals could be attributed to the formation of three difluorinated products. The first product with chemical shift values at −129.7 and −154.4 ppm was assigned to 2,3-difluoro-4-hydroxymuconic semialdehyde. This assignment is based on the following considerations. The NMR spectra show no formation of monofluorinated products. Furthermore, possible hydroxylation by a hydroquinone monooxygenase can be excluded because the chemical shift values of the newly formed peaks are not consistent with the formation of 5,6-difluoro-1,2,4-trihydroxybenzene (−162.0 ± 1.2 and −174.8 ± 1.7 ppm) (51). 2,3-Difluorohydroquinone can be split by HQDO between C-1 and C-2 or between C-1 and C-6. If ring fission occurred between C-1 and C-2, this would result in the formation of an acyl halide, as described for the conversion of 2-chlorohydroquinone by chlorohydroquinone dioxygenase (10, 67, 94). The acyl fluoride will readily react with water, yielding a monofluorinated maleylacetate. From this we conclude that ring fission occurred between C-1 and C-6 yielding 2,3-difluoro-4-hydroxymuconic semialdehyde. Upon 2,3-difluoro-4-hydroxymuconic semialdehyde formation, the chemical surrounding of the C-2 fluorine atom of 2,3-difluorohydroquinone changes more than that of the C-3 fluorine atom. Thus, the chemical shift of −129.7 ppm is assigned to the 2-fluoro substituent, whereas the chemical shift of −154.4 ppm is assigned to the 3-fluoro substituent of the semialdehyde (Table 1).
FIG. 2.
19F NMR spectra of the conversion of 2,3-difluorohydroquinone by P. fluorescens ACB before the addition of cell extract (A), after 1 h of incubation (B), and after 2 h of incubation (C). Substrate and products are numbered as follows: 1, 2,3-difluorohydroquinone; 2, 2,3-difluoro-4-hydroxymuconic semialdehyde; 3, enol-2,3-difluoromaleylacetate; and 4, keto-2,3-difluoromaleylacetate. The internal standard 4-fluorobenzoate (IS) and the formed fluoride anion (F−) are also indicated.
The second difluorinated product which is already formed after 1 h but is most abundantly present after 2 h of incubation (Fig. 2C) is assigned to 2,3-difluoromaleylacetate. This assignment is based on the fact that 4-hydroxymuconic semialdehydes are readily converted by 4-hydroxymuconic semialdehyde dehydrogenases to the corresponding maleylacetates (77, 96). Maleylacetates can exist in the keto and enol tautomeric forms (76). Conversion of the 2,3-difluoro-4-hydroxymuconic semialdehyde to enol-2,3-difluoromaleylacetate does not change the direct environment of the two fluorine atoms, and only small differences in chemical shift values will occur. Therefore, the chemical shift values of −130.3 and −154.0 ppm are assigned to enol-2,3-difluoromaleylacetate. Figure 2C also shows the formation of a minor compound with resonances at −129.0 and −142.1 ppm. This compound is assigned to keto-2,3-difluoromaleylacetate. The keto tautomer of the maleylacetate drastically changes the chemical shift value of the 3-fluoro substituent, whereas the surrounding environment of the 2-fluoro substituent does not significantly change. Thus, the chemical shift value of −129.0 ppm is assigned to the 2-fluoro substituent, whereas the chemical shift value of −142.1 ppm is assigned to the 3-fluoro substituent of keto-2,3-difluoromaleylacetate. Halogenated maleylacetates generally are further degraded by reductive dehalogenation to β-ketoadipate (10, 46, 90). Therefore, the strong increase in fluoride anion observed in Fig. 2B and C most likely results from maleylacetate reductase activity present in P. fluorescens ACB.
A 2-h incubation of 2,5-difluorohydroquinone with cell extract of P. fluorescens ACB resulted in the conversion of about 80% of the aromatic substrate (results not shown). Formation of about 25% fluoride anions (−123.0 ppm) and one main difluorinated aromatic product with resonances at −110.7 and −174.9 ppm was observed, showing ring splitting between C-1 and C-6. This compound is tentatively assigned to 2,5-difluoro-4-hydroxymuconic semialdehyde or enol-2,5-difluoromaleylacetate (Table 1).
A 2-h incubation of 3,5-difluorohydroquinone with cell extract of P. fluorescens ACB did not show accumulation of fluorinated compounds (results not shown). Under the conditions applied, nearly 100% of the fluorinated aromatic substrate was converted into fluoride anions.
Partial purification of HQDO.
Purification of HQDO by ammonium sulfate fractionation and hydrophobic interaction chromatography resulted in an enzyme preparation with a specific activity of about 0.5 U mg−1. The partially purified enzyme was rather unstable and rapidly lost activity. Part of the HQDO activity could be restored by incubation of the enzyme with iron(II) salts. The partially purified enzyme showed two main bands on SDS-PAGE around 38 kDa and 70 kDa. The N-terminal sequence of the 70-kDa protein (SAFNTTLPSLDY) corresponded to that of residues 2 to 13 of 4-hydroxyacetophenone monooxygenase (44). The N-terminal sequence of the 38-kDa protein was found to be AMLEAVETEN. Except for the starting methionine, this sequence corresponds to the N-terminal part of orf2 of the hap gene cluster (see below).
Cloning and sequencing of the hap gene cluster.
We have sequenced a 15-kb fragment of the P. fluorescens ACB genome containing the 4-hydroxyphenyl acetate hydrolase gene (44). Analysis revealed that the DNA sequence contains 14 complete ORFs (Table 2). Except for orf11, encoding a putative outer membrane channel protein, all ORFs have the same direction. The upstream region of the sequenced DNA (orf1 to orf9) contains a cluster of genes involved in the degradation of 4-hydroxyacetophenone (hapCDEFGHIBA). Downstream of the hap genes, three genes encoding putative regulatory proteins (orf10, orf12, and orf13) and two genes coding for proteins possibly involved in a membrane efflux pump (orf11 and orf14) were found. The sequences of the orf genes have been deposited in the DDBJ/EMBL/GenBank databases (see Materials and Methods).
TABLE 2.
ORFs of a 14-kb DNA fragment of P. fluorescens ACB
| ORF | Frame | Position in sequence (nt) | Gene product
|
Function | Closest characterized/uncharacterized homolog(s)b
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of residues | Mass (Da) | Description | GenBank accession no. | Sequence identity (%) | Reference | Organismc | ||||
| hapC | +3 | 360-857 | 165 | 17,899 | Hypothetical protein | ZP_01296719 | 78 | P. aeruginosa PA7 | ||
| hapD | +1 | 904-1929 | 341 | 38,345 | Hydroquinone dioxygenase | Hypothetical protein | ZP_01296720 | 80 | P. aeruginosa PA7 | |
| hapE | +1 | 2020-3483 | 487 | 52,400 | 4-Hydroxymuconic semialdehyde | p-Cumic aldehyde dehydrogenase | AAB62298 | 47 | 22 | P. putida F1 |
| dehydrogenase | Hypothetical protein | ZP_01296721, | 85 | P. aeruginosa PA7 | ||||||
| hapF | +3 | 3492-4559 | 355 | 37,612 | Maleylacetate reductase | Maleylacetate reductase | Q45072 | 56 | 20 | B. cepacia AC1100 |
| hypothetical protein | ZP_01296722 | 76 | P. aeruginosa PA7 | |||||||
| hapG | +1 | 4603-5478 | 291 | 32,159 | Hydroxyhydroquinone dioxygenase | Hydroxyquinol 1,2-dioxygenase | BAF44523 | 49 | 95 | Rhizobium sp. MTP-10005 |
| Hypothetical protein | ZP_01296723 | 67 | P. aeruginosa PA7 | |||||||
| hapH | +3 | 5475-5786 | 103 | 11,828 | Protein YciI family | Putative protein | ZP_01296724 | 72 | P. aeruginosa PA7 | |
| hapI | +3 | 5790-6179 | 129 | 13,866 | Ferredoxin | Chloroplast-type ferredoxin XylT | P23103, | 17 | 31 | P. putida |
| Hypothetical protein | ZP_01296725 | 58 | P. aeruginosa PA7 | |||||||
| hapB | +1 | 6169-7086 | 305 | 33,262 | 4-Hydroxyphenyl acetate esterase | Lipase | AAC38151 | 42 | 14 | Pseudomonas sp. strain B11-1 |
| Esterase/lipase | YP_299651 | 45 | R. eutropha JMP134 | |||||||
| hapA | +2 | 7139-9061 | 640 | 71,957 | 4-Hydroxyacetophenone | Steroid monooxygenase | JC7158 | 21 | 63 | R. rhodochrous |
| monooxygenase | Hypothetical protein | YP_639903 | 39 | Mycobacterium sp. strain MCS | ||||||
| orf10 | +3 | 9162-10094 | 310 | 35,222 | Regulatory protein (LysR family) | HTH-type transcript. regulator ptxR | P72131 | 24 | 30 | P. aeruginosa PA01 |
| Hypothetical protein | ZP_01296718 | 67 | P. aeruginosa PA7 | |||||||
| orf11 | −3 | 10166-11626 | 486 | 53,006 | Outer membrane channel protein | Outer membrane channel protein ttgF | CAB72260 | 55 | 64 | P. putida |
| Hypothetical efflux system | ZP_00898203 | 54 | P. syringae | |||||||
| orf12 | +2 | 11774-12382 | 202 | 22,221 | Regulatory protein (TetR family) | HTH-type transcriptional regulator srpR | Q9R9T9 | 57 | 92 | P. putida S12 |
| Hypothetical transcript. regulator, TetR | ZP_01499416 | 27 | B. phymatum STM815 | |||||||
| orf13 | +3 | 12450-13241 | 263 | 28,189 | Regulatory protein (Ic1R family) | HTH-type transcriptional regulator srpS | Q9R9U0 | 57 | 92 | P. putida S12 |
| Hypothetical regulatory protein | ZP_00898206 | 55 | P. putida F1 | |||||||
| orf14 | +1 | 13801-14736 | 311 | 34,623 | Efflux pump (EmrA family) | Multidrug resistance protein A | P27303 | 21 | 56 | E. coli |
| Hypothetical secretion protein | YP_574727 | 48 | C. salexigens | |||||||
ant, nucleotides.
Characterized homologs are shown in boldface.
R. eutropha, Ralstonia eutropha; P. syringae, Pseudomonas syringae; B. phymatum, Burkholderia phymatum; E. coli, Escherichia coli; C. salexigens, Chromohalobacter salexigens.
The hapA gene product, 4-hydroxyacetophenone monooxygenase (EC 1.14.13.84), is the first enzyme in the degradation pathway. HapA (formerly called HapE) (44) converts 4-hydroxyacetophenone to 4-hydroxyphenyl acetate and is active with a wide range of aromatic and aliphatic ketones (43-45). HapA belongs to the family of flavin adenine dinucleotide-containing Baeyer-Villiger monooxygenases (26, 58, 86) and shows the highest sequence identity with steroid monooxygenase from Rhodococcus rhodochrous (63) (Table 2).
HapB (formerly called HapD) (44) is the second enzyme of the 4-hydroxyacetophenone degradation pathway. This esterase (EC 3.1.1.2) efficiently converts 4-hydroxyphenyl acetate to hydroquinone (44). The N-terminal sequence of purified HapB (44) exactly matches the amino acid sequence derived from the hapB gene. The HapB amino acid sequence shows significant similarity with several lipases and esterases and contains a consensus motif, GXSXG, that includes the conserved active-site serine (9). HapB shares the highest sequence identity (38%) with a lipase from Pseudomonas sp. strain B11-1 (Table 2). This hydrolytic enzyme has been shown to be active with a range of p-nitrophenyl esters (14).
The reaction of hydroquinone to 4-hydroxymuconic semialdehyde is catalyzed by an extradiol-type of dioxygenase (19, 77). This conclusion is supported by the fact that the enzymatic activity of partially purified HQDO is stimulated by iron(II) ions. Based on the identification of the N-terminal sequence, the function of HQDO is linked to the hapD gene. HapD showed weak sequence identity (6 to 12%) with known nonheme-iron(II)-dependent dioxygenases and no significant sequence similarity with any other characterized protein. A BLASTP search in the current bacterial genome database (848 genomes) revealed that only nine homologous ORFs can be found that are highly similar in sequence (>59% sequence identity). All of these hapD homologs are upstream, flanked by a gene that shows significant sequence homology (>49% sequence identity) with hapC. In the accompanying paper (62), biochemical evidence is provided that hapC and hapD encode the α- and β-subunits of HQDO.
The deduced amino acid sequence of the fourth protein in the degradation pathway, HapE, shows 45% sequence identity with CymC, a p-cumic aldehyde dehydrogenase, from P. putida (21) (Table 2). Based on the hydroquinone degradation experiments reported above, HapE is assigned to be an NAD+-dependent dehydrogenase (EC 1.2.1.61) that converts 4-hydroxymuconic semialdehyde to maleylacetate. Interestingly, HapE shows 37 to 43% sequence identity with 2-hydroxymuconic semialdehyde dehydrogenases, which are involved in the meta-cleavage pathways of catechol degradation (66, 80). There is no other sequence of a 4-hydroxymuconic semialdehyde dehydrogenase known. However, genome sequencing projects have revealed putative proteins that have up to 85% sequence identity with HapE from P. fluorescens ACB (Table 2).
HapF shows the highest sequence identity (56%) with the characterized maleylacetate reductase (EC 1.3.1.32) from Pseudomonas cepacia (20). This enzyme converts maleylacetate to β-ketoadipate, a well-known step in many degradation pathways, and it is present in the degradation of both hydroquinone and hydroxyhydroquinone (47).
The hap gene cluster contains three genes that are not directly involved in the degradation of 4-hydroxyacetophenone. The hapG gene product shows 43% sequence identity with 6-chlorohydroxyhydroquinone 1,2-dioxygenase from Wautersia eutropha JMP134, an iron(III)-dependent intradiol ring cleavage enzyme that converts 6-chlorohydroxyhydroquinone to chloromaleylacetate (47, 57). HapG is also closely related to other hydroxyhydroquinone 1,2-dioxygenases (1, 25, 50, 65).From this and the crystal structure of hydroxyhydroquinone dioxygenase from Nocardioides simplex 3E (Protein Data Bank code 1TMX) (25), it can be inferred that the strictly conserved residues Tyr160, Tyr194, His218, and His220 of HapG are involved in coordination of the active-site ferric ion.
hapH codes for a conserved protein of the Yci1 family (pfam03795), but its function is unknown. hapI encodes a ferredoxin that might be involved in reduction of the iron-metal cofactor of extradiol dioxygenases (27, 36, 37, 69, 84).
Besides the hap gene cluster and orf5-orf7, there are five additional ORFs in the 15-kb DNA fragment of P. fluorescens ACB. Sequence similarity searches suggest that orf10, orf12, and orf13 code for putative regulatory proteins while orf11 encodes a putative outer membrane channel protein and orf14 codes for a putative efflux pump.
DISCUSSION
This paper describes the biochemical and genetic characterization of the 4-hydroxyacetophenone catabolic pathway in P. fluorescens ACB. Earlier studies revealed that catabolism of 4-hydroxyacetophenone is initiated by a Baeyer-Villiger oxidation to 4-hydroxyphenyl acetate and then proceeds through the formation of hydroquinone (34, 44). Here, we showed that P. fluorescens ACB converts hydroquinone to 4-hydroxymuconic semialdehyde and that difluorinated hydroquinones are converted via difluorinated 4-hydroxymuconic semialdehydes to the corresponding difluoromaleylacetates. Formation of significant amounts of fluoride anion pointed at the activity of maleylacetate reductase (46, 47, 90).
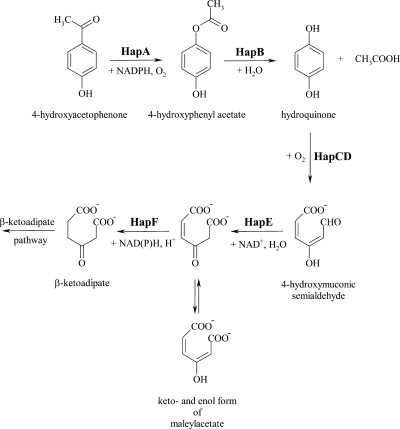
Cloning and sequence analysis of the genes involved in the catabolism of 4-hydroxyacetophenone revealed a gene cluster (hapCDEFGHIBA) involved in 4-hydroxyacetophenone degradation: 4-hydroxyacetophenone monooxygenase (HapA), 4-hydroxyphenyl acetate hydrolase (HapB), HQDO (HapD), 4-hydroxymuconic semialdehyde dehydrogenase (HapE), and maleylacetate reductase (HapF). Based on these results, we propose that P. fluorescens ACB degrades 4-hydroxyacetophenone to β-ketoadipate by the pathway depicted in Fig. 3.
FIG. 3.
Proposed degradation pathway of 4-hydroxyacetophenone by P. fluorescens ACB.
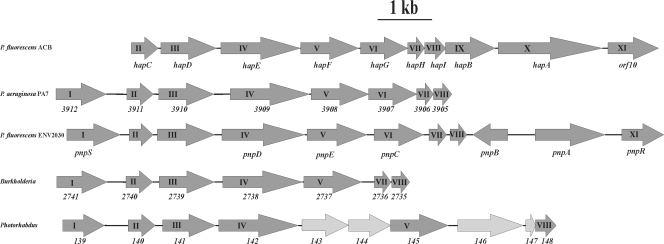
In the hapCDEFGHIBA cluster, the function of hapD is linked to the conversion of hydroquinone to 4-hydroxymuconic semialdehyde. This reaction presumably is catalyzed by an iron(II)-dependent extradiol dioxygenase (93). These enzymes generally contain a His2-carboxylate triad involved in binding the iron atom (49, 75, 78, 79, 82, 89). A BLASTP search for HapD sequence homologs in the microbial genome database revealed a small number of proteins (in Pseudomonas aeruginosa PA7, Photorhabdus luminescens subsp. laumondii TTO1, and seven Burkholderia genomes) that are highly similar in sequence (sequence identity of >59%). In the above-mentioned microbial genomes, the hapD gene is flanked by a hapC-like gene (Fig. 4). Biochemical studies presented in the accompanying paper (62) unambiguously showed that hapC is also required for the oxygenolytic ring fission of hydroquinone.
FIG. 4.
Organization of the hap gene cluster and surrounding ORFs of a 14.8-kb DNA fragment of P. fluorescens ACB. For an explanation, see Table 2. The hap gene cluster is aligned with the pnp gene cluster from P. fluorescens ENV2030 (2, 96) and with gene clusters found in the genomes of P. aeruginosa PA7 (NC_009656 3912 corresponds to PSPA7_3912, e.g.), Burkholderia cepacia 18194 also called Burkholderia sp. strain 383 (NC_007511; chromosome 2; 2741 corresponds to Bcep18194_B2741, e.g.), and Photorhabdus luminescens subsp. laumondii TTO1 (NC_005126; gene number 139 corresponds to plu139, e.g.). Corresponding Roman numbers belong to comparable genes. The genes indicated with I and XI code for members of the family of LysR-type transcriptional regulators (15, 71, 74).
Sequence homology indicates that hapI encodes a [2Fe-2S] ferredoxin. In several related microorganisms, this electron transfer protein is required for the reductive reactivation of catechol 2,3-dioxygenases (27, 36, 37, 69, 84). This leads us to suggest that in P. fluorescens ACB, HapI might be involved in the reactivation of HapCD.
HapG has many structural properties in common with the iron(III)-dependent intradiol dioxygenases. This suggests that this enzyme is not involved in the catabolic pathway of 4-hydroxyacetophenone. HapG might be responsible for the observed activities of P. fluorescens ACB with catechols (60) and hydroxyhydroquinone. Why hapG is embedded in the hap cluster is not clear. However, there are more examples of the presence of a hydroxyhydroquinone dioxygenase in an organism where an HQDO serves as a key enzyme in the degradation pathway of aromatic compounds. Such a situation has been reported for the 4-nitrophenol pathways of P. fluorescens ENV2030 (96) and a Moraxella strain (77). A hydroxyhydroquinone dioxygenase is also present in the degradation pathway of 2,4-dinitrotoluene in Burkholderia cepacia R34, but another extradiol dioxygenase carries out the ring fission of 2,4,5-trihydroxytoluene (40). It seems that there is a progression in the organization of the pathway genes toward a compact region encoding the entire pathway. In that progression, remnants from previous assembly events (like hapG) persist. The presence of hapG in the hap gene cluster might indicate an intermediate point in the evolution of an optimal system for 4-hydroxyacetophenone degradation (40). Embedded in the hap gene cluster, hapG might be available to assist in other degradation pathways for the intradiol splitting of hydroxyhydroquinone or other catechols.
The hap gene cluster of P. fluorescens ACB shows similarities with the pnp gene cluster of P. fluorescens ENV2030 involved in 4-nitrophenol utilization (Fig. 4) (96). The hapEFG genes have the same size and orientation as the pnpDEC genes. Bang and Zylstra reported that pnpD (corresponding to hapE) codes for a 4-hydroxymuconic semialdehyde dehydrogenase showing homology with both eukaryotic and prokaryotic aldehyde dehydrogenases and that pnpE (corresponding to hapF) codes for a maleylacetate reductase (2). Furthermore, it was concluded that pnpC codes for the dioxygenase involved in the formation of 4-hydroxymuconic semialdehyde from hydroquinone (2). The pnpC gene corresponds to the hapG gene, and the amino acid sequence of the PnpC protein has a high level of sequence similarity with hydroxyhydroquinone dioxygenases (2, 96). Thus, the question arises whether PnpC is involved in the biodegradation of 4-nitrophenol in P. fluorescens ENV2030. From a gene cluster comparison (Fig. 4), we propose that an enzyme similar to HapCD is involved in the degradation of p-nitrophenol in P. fluorescens ENV2030.
It is striking that in the sequenced genome of P. aeruginosa PA7 an orthologous partial hap gene cluster is found (Table 2 and Fig. 4). This cluster contains, in the same orientation as in P. fluorescens ACB, all hapCDEFGHI orthologs. This observation suggests that P. fluorescens ACB has acquired the hapAB genes in a recent genetic event and has thereby become efficient in utilizing acetophenones as energy and carbon sources.
Acknowledgments
We thank Ludwin van Aart and Bep van Veldhuizen for assistance in NMR experiments.
This work was supported by the Council for Chemical Sciences of The Netherlands Organization for Scientific Research through the division Procesvernieuwing voor een Schoner Milieu.
Footnotes
Published ahead of print on 23 May 2008.
REFERENCES
- 1.Armengaud, J., K. N. Timmis, and R. M. Wittich. 1999. A functional 4-hydroxysalicylate/hydroxyquinol degradative pathway gene cluster is linked to the initial dibenzo-p-dioxin pathway genes in Sphingomonas sp. strain RW1. J. Bacteriol. 1813452-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang, S.-W., and G. J. Zylstra. 1997. Cloning and sequencing of the hydroquinone 1,2-dioxygenase, γ-hydroxymuconic semialdehyde dehydrogenase, maleylacetate reductase genes from Pseudomonas fluorescens ENV2030, abstr. Q-383, p.519. Abstr. 97th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 3.Barton, M. R., and R. L. Crawford. 1988. Novel biotransformations of 4-chlorobiphenyl by a Pseudomonas sp. Appl. Environ. Microbiol. 54594-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedard, D. L., and M. L. Haberl. 1990. Influence of chlorine substitution pattern on the degradation of polychlorinated biphenyls by eight bacterial strains. Microb. Ecol. 2087-102. [DOI] [PubMed] [Google Scholar]
- 5.Bedard, D. L., M. L. Haberl, R. J. May, and M. J. Brennan. 1987. Evidence for novel mechanisms of polychlorinated biphenyl metabolism in Alcaligenes eutrophus H850. Appl. Environ. Microbiol. 531103-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beynon, K. I., D. H. Hutson, and A. N. Wright. 1973. The metabolism and degradation of vinyl phosphate insecticides. Residue Rev. 4755-142. [DOI] [PubMed] [Google Scholar]
- 7.Beynon, K. I., and A. N. Wright. 1967. The breakdown of 14-C-chlorfenvinphos in soils and in crops grown in the soils. J. Sci. Food Agric. 18143-150. [DOI] [PubMed] [Google Scholar]
- 8.Beynon, K. I., and A. N. Wright. 1969. Breakdown of the insecticide, Gardona, on plants and in soils. J. Sci. Food Agric. 20250-256. [Google Scholar]
- 9.Brady, L., A. M. Brzozowski, Z. S. Derewenda, E. Dodson, G. Dodson, S. Tolley, J. P. Turkenburg, L. Christiansen, B. Huge-Jensen, L. Norskov, L. Thim, and U. Menge. 1990. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature 343767-770. [DOI] [PubMed] [Google Scholar]
- 10.Cai, M., and L. Xun. 2002. Organization and regulation of pentachlorophenol-degrading genes in Sphingobium chlorophenolicum ATCC 39723. J. Bacteriol. 1844672-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman, P. J., and D. W. Ribbons. 1976. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J. Bacteriol. 125985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan, A., A. K. Chakraborti, and R. K. Jain. 2000. Plasmid-encoded degradation of p-nitrophenol and 4-nitrocatechol by Arthrobacter protophormiae. Biochem. Biophys. Res. Commun. 270733-740. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan, A., S. K. Samanta, and R. K. Jain. 2000. Degradation of 4-nitrocatechol by Burkholderia cepacia: a plasmid-encoded novel pathway. J. Appl. Microbiol. 88764-772. [DOI] [PubMed] [Google Scholar]
- 14.Choo, D.-W., T. Kurihara, T. Suzuki, K. Soda, and N. Esaki. 1998. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol. 64486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colmer, J. A., and A. N. Hamood. 2001. Molecular analysis of the Pseudomonas aeruginosa regulatory genes ptxR and ptxS. Can. J. Microbiol. 47820-828. [PubMed] [Google Scholar]
- 16.Cox, D. P., and C. D. Goldsmith. 1979. Microbial conversion of ethylbenzene to 1-phenethanol and acetophenone by Nocardia tartaricans ATCC 31190. Appl. Environ. Microbiol. 38514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cripps, R. E. 1975. The microbial metabolism of acetophenone. Metabolism of acetophenone and some chloroacetophenones by an Arthrobacter species. Biochem. J. 152233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cripps, R. E., P. W. Trudgill, and J. G. Whateley. 1978. The metabolism of 1-phenylethanol and acetophenone by Nocardia T5 and an Arthrobacter species. Eur. J. Biochem. 86175-186. [DOI] [PubMed] [Google Scholar]
- 19.Darby, J. M., D. G. Taylor, and D. J. Hopper. 1987. Hydroquinone as the ring-fission substrate in the catabolism of 4-ethylphenol and 4-hydroxyacetophenone by Pseudomonas putida JD1. J. Gen. Microbiol. 1332137-2146. [Google Scholar]
- 20.Daubaras, D. L., C. D. Hershberger, K. Kitano, and A. M. Chakrabarty. 1995. Sequence analysis of a gene cluster involved in metabolism of 2,4,5-trichlorophenoxyacetic acid by Burkholderia cepacia AC1100. Appl. Environ. Microbiol. 611279-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton, R. 1997. p-Cymene catabolic pathway in Pseudomonas putida F1: cloning and characterization of DNA encoding conversion of p-cymene to p-cumate. J. Bacteriol. 1793171-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton, R. W. 1996. p-Cumate catabolic pathway in Pseudomonas putida Fl: cloning and characterization of DNA carrying the cmt operon. J. Bacteriol. 1781351-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eppink, M. H. M., S. A. Boeren, J. Vervoort, and W. J. H. van Berkel. 1997. Purification and properties of 4-hydroxybenzoate 1-hydroxylase (decarboxylating), a novel flavin adenine dinucleotide-dependent monooxygenase from Candida parapsilosis CBS604. J. Bacteriol. 1796680-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppink, M. H. M., E. Cammaart, D. van Wassenaar, W. J. Middelhoven, and W. J. H. van Berkel. 2000. Purification and properties of hydroquinone hydroxylase, a FAD-dependent monooxygenase involved in the catabolism of 4-hydroxybenzoate in Candida parapsilosis CBS604. Eur. J. Biochem. 2676832-6840. [DOI] [PubMed] [Google Scholar]
- 25.Ferraroni, M., J. Seifert, V. M. Travkin, M. Thiel, S. Kaschabek, A. Scozzafava, L. Golovleva, M. Schlömann, and F. Briganti. 2005. Crystal structure of the hydroxyquinol 1,2-dioxygenase from Nocardioides simplex 3E, a key enzyme involved in polychlorinated aromatics biodegradation. J. Biol. Chem. 28021144-21154. [DOI] [PubMed] [Google Scholar]
- 26.Fraaije, M. W., N. M. Kamerbeek, W. J. H. van Berkel, and D. B. Janssen. 2002. Identification of a Baeyer-Villiger monooxygenase sequence motif. FEBS Lett. 51843-47. [DOI] [PubMed] [Google Scholar]
- 27.Göbel, M., O. H. Kranz, S. R. Kaschabek, E. Schmidt, D. H. Pieper, and W. Reineke. 2004. Microorganisms degrading chlorobenzene via a meta-cleavage pathway harbor highly similar chlorocatechol 2,3-dioxygenase-encoding gene clusters. Arch. Microbiol. 182147-156. [DOI] [PubMed] [Google Scholar]
- 28.Gunter, S. E. 1953. The enzymic oxidation of p-hydroxymandelic acid p-hydroxybenzoic acid. J. Bacteriol. 66341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 4195-98. [Google Scholar]
- 30.Hamood, A. N., J. A. Colmer, U. A. Ochsner, and M. L. Vasil. 1996. Isolation and characterization of a Pseudomonas aeruginosa gene, ptxR, which positively regulates exotoxin A production. Mol. Microbiol. 2197-110. [DOI] [PubMed] [Google Scholar]
- 31.Harayama, S., A. Polissi, and M. Rekik. 1991. Divergent evolution of chloroplast-type ferredoxins. FEBS Lett. 28585-88. [DOI] [PubMed] [Google Scholar]
- 32.Havel, J., and W. Reineke. 1993. Microbial degradation of chlorinated acetophenones. Appl. Environ. Microbiol. 592706-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayatsu, M., M. Hirano, and S. Tokuda. 2000. Involvement of two plasmids in fenitrothion degradation by Burkholderia sp. strain NF100. Appl. Environ. Microbiol. 661737-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higson, F. K., and D. D. Focht. 1990. Bacterial degradation of ring-chlorinated acetophenones. Appl. Environ. Microbiol. 563678-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopper, D. J., H. G. Jones, E. A. Elmorsi, and M. E. Rhodes-Roberts. 1985. The catabolism of 4-hydroxyacetophenone by an Alcaligenes sp. J. Gen. Microbiol. 1311807-1814. [Google Scholar]
- 36.Hugo, N., J. Armengaud, J. Gaillard, K. N. Timmis, and Y. Jouanneau. 1998. A novel [2Fe-2S] ferredoxin from Pseudomonas putida mt2 promotes the reductive reactivation of catechol 2,3-dioxygenase. J. Biol. Chem. 2739622-9629. [DOI] [PubMed] [Google Scholar]
- 37.Hugo, N., C. Meyer, J. Armengaud, J. Gaillard, K. N. Timmis, and Y. Jouanneau. 2000. Characterization of three XylT-like [2Fe-2S] ferredoxins associated with catabolism of cresols or naphthalene: evidence for their involvement in catechol dioxygenase reactivation. J. Bacteriol. 1825580-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadan, A. P., M. J. H. Moonen, S. A. Boeren, L. A. Golovleva, I. M. C. M. Rietjens, and W. J. H. van Berkel. 2004. Biocatalytic potential of p-hydroxybenzoate hydroxylase from Rhodococcus rhodnii 135 and Rhodococcus opacus 557. Adv. Synth. Catal. 346367-375. [Google Scholar]
- 39.Jain, R. K., J. H. Dreisbach, and J. C. Spain. 1994. Biodegradation of p-nitrophenol via 1,2,4-benzenetriol by an Arthrobacter sp. Appl. Environ. Microbiol. 603030-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, G. R., R. K. Jain, and J. C. Spain. 2002. Origins of the 2,4-dinitrotoluene pathway. J. Bacteriol. 1844219-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, K. H., P. W. Trudgill, and D. J. Hopper. 1994. 4-Ethylphenol metabolism by Aspergillus fumigatus. Appl. Environ. Microbiol. 601978-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones, K. H., P. W. Trudgill, and D. J. Hopper. 1993. Metabolism of p-cresol by the fungus Aspergillus fumigatus. Appl. Environ. Microbiol. 591125-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamerbeek, N. M., D. B. Janssen, W. J. H. van Berkel, and M. W. Fraaije. 2003. Baeyer-Villiger monooxygenases, an emerging family of flavin-dependent biocatalysts. Adv. Synth. Catal. 345667-678. [Google Scholar]
- 44.Kamerbeek, N. M., M. J. H. Moonen, J. G. M. van der Ven, W. J. H. van Berkel, M. W. Fraaije, and D. B. Janssen. 2001. 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB. A novel flavoprotein catalyzing Baeyer-Villiger oxidation of aromatic compounds. Eur. J. Biochem. 2682547-2557. [DOI] [PubMed] [Google Scholar]
- 45.Kamerbeek, N. M., A. J. Olsthoorn, M. W. Fraaije, and D. B. Janssen. 2003. Substrate specificity and enantioselectivity of 4-hydroxyacetophenone monooxygenase. Appl. Environ. Microbiol. 69419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaschabek, S. R., and W. Reineke. 1992. Maleylacetate reductase of Pseudomonas sp. strain B13: dechlorination of chloromaleylacetates, metabolites in the degradation of chloroaromatic compounds. Arch. Microbiol. 158412-417. [DOI] [PubMed] [Google Scholar]
- 47.Kaschabek, S. R., and W. Reineke. 1995. Maleylacetate reductase of Pseudomonas sp. strain B13: specificity of substrate conversion and halide elimination. J. Bacteriol. 177320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy, S. I. T., and C. A. Fewson. 1968. Enzymes of the mandelate pathway in Bacterium NCIB 8250. Biochem. J. 107497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kita, A., S. Kita, I. Fujisawa, K. Inaka, T. Ishida, K. Horiike, M. Nozaki, and K. Miki. 1999. An archetypical extradiol-cleaving catecholic dioxygenase: the crystal structure of catechol 2,3-dioxygenase (metapyrocatechase) from Pseudomonas putida mt-2. Structure 725-34. [DOI] [PubMed] [Google Scholar]
- 50.Kitagawa, W., N. Kimura, and Y. Kamagata. 2004. A novel p-nitrophenol degradation gene cluster from a gram-positive bacterium, Rhodococcus opacus SAO101. J. Bacteriol. 1864894-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koerts, J., M. M. Velraeds, A. E. Soffers, J. Vervoort, and I. M. Rietjens. 1997. Influence of substituents in fluorobenzene derivatives on the cytochrome P450-catalyzed hydroxylation at the adjacent ortho aromatic carbon center. Chem. Res. Toxicol. 10279-288. [DOI] [PubMed] [Google Scholar]
- 52.Komiyama, M., and H. Hirai. 1984. Selective synthesis using cyclodextrins as catalyst. 2. The para-oriented carboxylation of phenols. J. Am. Chem. Soc. 106174-178. [Google Scholar]
- 53.Latus, M., H. Seitz, J. Eberspacher, and F. Lingens. 1995. Purification and characterization of hydroxyquinol 1,2-dioxygenase from Azotobacter sp. strain GP1. Appl. Environ. Microbiol. 612453-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau, S. S., T. J. Monks, J. I. Everitt, E. Kleymenova, and C. L. Walker. 2001. Carcinogenicity of a nephrotoxic metabolite of the “nongenotoxic” carcinogen hydroquinone. Chem. Res. Toxicol. 1425-33. [DOI] [PubMed] [Google Scholar]
- 55.Lobos, J. H., T. K. Leib, and T. M. Su. 1992. Biodegradation of bisphenol A and other bisphenols by a gram-negative aerobic bacterium. Appl. Environ. Microbiol. 581823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lomovskaya, O., and K. Lewis. 1992. emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 898938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louie, T. M., C. M. Webster, and L. Xun. 2002. Genetic and biochemical characterization of a 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J. Bacteriol. 1843492-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malito, E., A. Alfieri, M. W. Fraaije, and A. Mattevi. 2004. Crystal structure of a Baeyer-Villiger monooxygenase. Proc. Natl. Acad. Sci. USA 10113157-13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyauchi, K., Y. Adachi, Y. Nagata, and M. Takagi. 1999. Cloning and sequencing of a novel meta-cleavage dioxygenase gene whose product is involved in degradation of γ-hexachlorocyclohexane in Sphingomonas paucimobilis. J. Bacteriol. 1816712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moonen, M. J. H., I. M. C. M. Rietjens, and W. J. H. van Berkel. 2001. 19F NMR study on the biological Baeyer-Villiger oxidation of acetophenones. J. Ind. Microbiol. Biotechnol. 2635-42. [DOI] [PubMed] [Google Scholar]
- 61.Moonen, M. J. H., I. M. C. M. Rietjens, and W. J. H. van Berkel. 1999. Purification and some properties of acetophenone monooxygenase, p.375-378. Abstr. 13th Int. Cong. Flavins Flavoproteins, Konstanz, Germany, 29 August to 4 September 1999.
- 62.Moonen, M. J. H., S. A. Synowsky, W. A. M. van den Berg, A. H. Westphal, A. J. R. Heck, R. H. H. van den Heuvel, M. W. Fraaije, and W. J. H. van Berkel. 2008. Hydroquinone dioxygenase from Pseudomonas fluorescens ACB: a novel member of the family of nonheme-iron(II)-dependent dioxygenases. J. Bacteriol. 1905199-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morii, S., S. Sawamoto, Y. Yamauchi, M. Miyamoto, M. Iwami, and E. Itagaki. 1999. Steroid monooxygenase of Rhodococcus rhodochrous: sequencing of the genomic DNA, and hyperexpression, purification, and characterization of the recombinant enzyme. J. Biochem. 126624-631. [DOI] [PubMed] [Google Scholar]
- 64.Mosqueda, G., and J.-L. Ramos. 2000. A set of genes encoding a second toluene efflux system in Pseudomonas putida DOT-T1E is linked to the tod genes for toluene metabolism. J. Bacteriol. 182937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murakami, S., T. Okuno, E. Matsumura, S. Takenaka, R. Shinke, and K. Aoki. 1999. Cloning of a gene encoding hydroxyquinol 1,2-dioxygenase that catalyzes both intradiol and extradiol ring cleavage of catechol. Biosci. Biotechnol. Biochem. 63859-865. [DOI] [PubMed] [Google Scholar]
- 66.Nordlund, I., and V. Shingler. 1990. Nucleotide sequences of the meta-cleavage pathway enzymes 2-hydroxymuconic semialdehyde dehydrogenase and 2-hydroxymuconic semialdehyde hydrolase from Pseudomonas CF600. Biochim. Biophys. Acta 1049227-230. [DOI] [PubMed] [Google Scholar]
- 67.Ohtsubo, Y., K. Miyauchi, K. Kanda, T. Hatta, H. Kiyohara, T. Senda, Y. Nagata, Y. Mitsui, and M. Takagi. 1999. PcpA, which is involved in the degradation of pentachlorophenol in Sphingomonas chlorophenolica ATCC39723, is a novel type of ring-cleavage dioxygenase. FEBS Lett. 459395-398. [DOI] [PubMed] [Google Scholar]
- 68.Peelen, S., I. M. C. M. Rietjens, M. G. Boersma, and J. Vervoort. 1995. Conversion of phenol derivatives to hydroxylated products by phenol hydroxylase from Trichosporon cutaneum. A comparison of regioselectivity and rate of conversion with calculated molecular orbital substrate characteristics. Eur. J. Biochem. 227284-291. [DOI] [PubMed] [Google Scholar]
- 69.Polissi, A., and S. Harayama. 1993. In vivo reactivation of catechol 2,3-dioxygenase mediated by a chloroplast-type ferredoxin: a bacterial strategy to expand the substrate specificity of aromatic degradative pathways. EMBO J. 123339-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prakash, D., A. Chauhan, and R. K. Jain. 1996. Plasmid-encoded degradation of p-nitrophenol by Pseudomonas cepacia. Biochem. Biophys. Res. Commun. 224375-381. [DOI] [PubMed] [Google Scholar]
- 71.Putrins, M., A. Tover, R. Tegova, U. Saks, and M. Kivisaar. 2007. Study of factors which negatively affect expression of the phenol degradation operon pheBA in Pseudomonas putida. Microbiology 1531860-1871. [DOI] [PubMed] [Google Scholar]
- 72.Rieble, S., D. K. Joshi, and M. H. Gold. 1994. Purification and characterization of a 1,2,4-trihydroxybenzene 1,2-dioxygenase from the basidiomycete Phanerochaete chrysosporium. J. Bacteriol. 1764838-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ronen, Z., and A. Abeliovich. 2000. Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl. Environ. Microbiol. 662372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothmel, R. K., T. L. Aldrich, J. E. Houghton, W. M. Coco, L. N. Ornston, and A. M. Chakrabarty. 1990. Nucleotide sequencing and characterization of Pseudomonas putida catR: a positive regulator of the catBC operon is a member of the LysR family. J. Bacteriol. 172922-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato, N., Y. Uragami, T. Nishizaki, Y. Takahashi, G. Sazaki, K. Sugimoto, T. Nonaka, E. Masai, M. Fukuda, and T. Senda. 2002. Crystal structures of the reaction intermediate and its homologue of an extradiol-cleaving catecholic dioxygenase. J. Mol. Biol. 321621-636. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt, S., and G. W. Kirby. 2001. Dioxygenative cleavage of C-methylated hydroquinones and 2,6-dichlorohydroquinone by Pseudomonas sp. HH35. Biochim. Biophys. Acta 156883-89. [DOI] [PubMed] [Google Scholar]
- 77.Spain, J. C., and D. T. Gibson. 1991. Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57812-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Straganz, G. D., and B. Nidetzky. 2006. Variations of the 2-His-1-carboxylate theme in mononuclear non-heme FeII oxygenases. Chembiochem 71536-1548. [DOI] [PubMed] [Google Scholar]
- 79.Sugimoto, K., T. Senda, H. Aoshima, E. Masai, M. Fukuda, and Y. Mitsui. 1999. Crystal structure of an aromatic ring opening dioxygenase LigAB, a protocatechuate 4,5-dioxygenase, under aerobic conditions. Structure 7953-965. [DOI] [PubMed] [Google Scholar]
- 80.Takeo, M., T. Fujii, K. Takenaka, and Y. Maeda. 1998. Cloning and sequencing of a gene cluster for the meta-cleavage pathway of aniline degradation in Acinetobacter sp. strain YAA. J. Ferment. Bioeng. 85514-517. [Google Scholar]
- 81.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Titus, G. P., H. A. Mueller, J. Burgner, S. Rodriguez De Cordoba, M. A. Penalva, and D. E. Timm. 2000. Crystal structure of human homogentisate dioxygenase. Nat. Struct. Biol. 7542-546. [DOI] [PubMed] [Google Scholar]
- 83.Tomasek, P. H., and R. L. Crawford. 1986. Initial reactions of xanthone biodegradation by an Arthrobacter sp. J. Bacteriol. 167818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tropel, D., C. Meyer, J. Armengaud, and Y. Jouanneau. 2002. Ferredoxin-mediated reactivation of the chlorocatechol 2,3-dioxygenase from Pseudomonas putida GJ31. Arch. Microbiol. 177345-351. [DOI] [PubMed] [Google Scholar]
- 85.Utkin, I. B., M. M. Yakimov, L. N. Matveeva, E. I. Kozlyak, I. S. Rogozhin, Z. G. Solomon, and A. M. Bezborodov. 1991. Degradation of styrene and ethylbenzene by Pseudomonas species Y2. FEMS Microbiol. Lett. 77237-242. [Google Scholar]
- 86.van Berkel, W. J., N. M. Kamerbeek, and M. W. Fraaije. 2006. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 124670-689. [DOI] [PubMed] [Google Scholar]
- 87.van Berkel, W. J. H., M. H. M. Eppink, W. J. Middelhoven, J. Vervoort, and I. M. C. M. Rietjens. 1994. Catabolism of 4-hydroxybenzoate in Candida parapsilosis proceeds through initial oxidative decarboxylation by a FAD-dependent 4-hydroxybenzoate 1-hydroxylase. FEMS Microbiol. Lett. 121207-215. [DOI] [PubMed] [Google Scholar]
- 88.Vervoort, J., P. A. de Jager, J. Steenbergen, and I. M. C. M. Rietjens. 1990. Development of a 19F-n.m.r. method for studies on the in vivo and in vitro metabolism of 2-fluoroaniline. Xenobiotica 20657-670. [DOI] [PubMed] [Google Scholar]
- 89.Vetting, M. W., L. P. Wackett, L. Que, Jr., J. D. Lipscomb, and D. H. Ohlendorf. 2004. Crystallographic comparison of manganese- and iron-dependent homoprotocatechuate 2,3-dioxygenases. J. Bacteriol. 1861945-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vollmer, M. D., K. Stadler-Fritzsche, and M. Schlömann. 1993. Conversion of 2-chloromaleylacetate in Alcaligenes eutrophus JMP134. Arch. Microbiol. 159182-188. [DOI] [PubMed] [Google Scholar]
- 91.Weber, K., and M. Osborn. 1969. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 2444406-4412. [PubMed] [Google Scholar]
- 92.Wery, J., B. Hidayat, J. Kieboom, and J. A. de Bont. 2001. An insertion sequence prepares Pseudomonas putida S12 for severe solvent stress. J. Biol. Chem. 2765700-5706. [DOI] [PubMed] [Google Scholar]
- 93.Xu, L., K. Resing, S. L. Lawson, P. C. Babbitt, and S. D. Copley. 1999. Evidence that pcpA encodes 2,6-dichlorohydroquinone dioxygenase, the ring cleavage enzyme required for pentachlorophenol degradation in Sphingomonas chlorophenolica strain ATCC 39723. Biochemistry 387659-7669. [DOI] [PubMed] [Google Scholar]
- 94.Xun, L., J. Bohuslavek, and M. Cai. 1999. Characterization of 2,6-dichloro-p-hydroquinone 1,2-dioxygenase (PcpA) of Sphingomonas chlorophenolica ATCC 39723. Biochem. Biophys. Res. Commun. 266322-325. [DOI] [PubMed] [Google Scholar]
- 95.Yoshida, M., T. Oikawa, H. Obata, K. Abe, H. Mihara, and N. Esaki. 2007. Biochemical and genetic analysis of the gamma-resorcylate (2,6-dihydroxybenzoate) catabolic pathway in Rhizobium sp. strain MTP-10005: identification and functional analysis of its gene cluster. J. Bacteriol. 1891573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zylstra, G. J., S.-W. Bang, L. M. Newman, and L. L. Perry. 2000. Microbial degradation of mononitrophenols and mononitrobenzoates, p. 145-160. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. CRC Press, Boca Raton, FL.