Abstract
Homeostasis of Zn2+ and Mn2+ is important for the physiology and virulence of the human pathogen Streptococcus pneumoniae. Here, transcriptome analysis was used to determine the response of S. pneumoniae D39 to a high concentration of Zn2+. Interestingly, virulence genes encoding the choline binding protein PcpA, the extracellular serine protease PrtA, and the Mn2+ uptake system PsaBC(A) were strongly upregulated in the presence of Zn2+. Using random mutagenesis, a previously described Mn2+-responsive transcriptional repressor, PsaR, was found to mediate the observed Zn2+-dependent derepression. In addition, PsaR is also responsible for the Mn2+-dependent repression of these genes. Subsequently, we investigated how these opposite effects are mediated by the same regulator. In vitro binding of purified PsaR to the prtA, pcpA, and psaB promoters was stimulated by Mn2+, whereas Zn2+ destroyed the interaction of PsaR with its target promoters. Mutational analysis of the pcpA promoter demonstrated the presence of a PsaR operator that mediates the transcriptional effects. In conclusion, PsaR is responsible for the counteracting effects of Mn2+ and Zn2+ on the expression of several virulence genes in S. pneumoniae, suggesting that the ratio of these metal ions exerts an important influence on pneumococcal pathogenesis.
The human pathogen Streptococcus pneumoniae is a commensal of the nasopharynx, but it can become virulent and infect the lungs, middle ear, brain, and bloodstream, causing severe diseases such as pneumonia, otitis media, meningitis, or sepsis (see references 10 and 61 for reviews). Little is known about the specific environmental changes that S. pneumoniae encounters during infection of the human body and the way in which these affect virulence. One important environmental factor is probably the concentration of metal ions, which form a class of nutrients that are important for bacteria in small amounts but are often toxic in larger amounts (9, 20, 57, 58).
An important metal ion that S. pneumoniae could face is Zn2+, which is present in the human body in concentrations ranging from a few μM to over 100 μM (81). In the host, Zn2+ is of great importance for immunity, as it is necessary for proper functioning of immune cells (28), and mild Zn2+ deficiency severely affects immune function (72). Zn2+ levels are elevated during inflammation (53), and Zn2+ administration reduces airway infections in children in developing countries (71). Moreover, Zn2+ deficiency results in an increased risk of pneumococcal infection and death in mice (75) and in a lower immune response to the pneumococcal antigen PspA (76). Interestingly, several studies suggest that the use of and response to metal ions, such as Zn2+, Mn2+, and Fe2+, is important for the virulence and physiology of pathogenic streptococci (3, 11, 12, 18, 30, 32, 39, 40, 46, 50, 54, 55, 63, 64, 66, 67).
In S. pneumoniae several systems dedicated to the acquisition of specific metal ions have been studied. These are the PsaBCA Mn2+ transporter (18, 44, 50), the AdcCBA Zn2+ uptake system (18, 19), and three iron uptake loci (piaABCD, piuBCDA, and pit) (11, 12). The PsaBCA complex is involved in virulence, oxidative stress, penicillin stress, competence, and adhesion via interaction with human E-cadherin (2, 6, 18, 32, 48, 50, 59, 60, 79). Expression of the psaBCA genes is repressed by the DtxR family regulator PsaR in response to high Mn2+ concentrations (31). S. pneumoniae also contains systems involved in cation efflux (58), such as czcD, which has recently been shown to be responsible for Zn2+ resistance (39). The presence in S. pneumoniae of this Zn2+ efflux system together with the AdcCBA Zn2+ uptake system indicates that this pathogen has to deal with fluctuating Zn2+ levels in the human body.
Therefore, we explored the response of S. pneumoniae to a high Zn2+concentration by means of transcriptome analysis. Interestingly, Zn2+ was found to increase expression of several genes involved in virulence, namely, prtA, pcpA, and psaBC, which could be counteracted by Mn2+. Subsequent research showed that the transcriptional regulator PsaR is directly responsible for the Mn2+-dependent repression and the Zn2+-dependent derepression of these genes.
MATERIALS AND METHODS
DNA techniques, β-galactosidase assays, bacterial strains, and growth conditions.
All DNA manipulation techniques, growth conditions, and media were the same as described previously (37, 38) unless indicated otherwise. β-Galactosidase assays were performed as described previously (38).
Chelex-treated M17 was prepared by autoclaving 2% Chelex 100 resin (Bio-Rad) with M17, followed by 2 h of stirring. After removal of the resin, 50 μM CaCl2, 50 μM MgCl2, 5 μM FeCl3, and 0.25% glucose were added, and the resulting medium, designated GM17chel, was used as growth medium as specified in Results. Metal ions were added as the salts ZnSO4, MnCl2, MgCl2, CaCl2, NiCl2, CuSO4, and FeSO4. The strains and plasmids used or constructed in this study are listed in Table 1. Primers are listed in Table 2. Since most work presented in this paper was carried out before publication of the D39 genome sequence (43), primer sequences are based on the R6 genome sequence (27).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| S. pneumoniae | ||
| D39 | Serotype 2 strain, cps2 | 4, 43; laboratory of P. Hermans |
| R6 | D39 (cps2 2538-9862) with increased transformation efficiency | 27 |
| D39nisRK | D39 ΔbgaA::nisRK; Trmpr | 37 |
| MP102 | D39 ΔczcD | This work |
| RW100 | D39 ΔpsaR | This work |
| RW101 | D39nisRK ΔpsaR | This work |
| RW102 | D39 ΔbgaA::PpcpA-lacZ; Tetr | This work |
| RW103 | D39 ΔbgaA::PnrdD-lacZ; Tetr | This work |
| RW104 | D39nisRK prtA-lacZ; Ermr | This work |
| RW105 | D39 ΔbgaA::Pspr0276-lacZ; Tetr | This work |
| RW106 | D39 ΔbgaA::PpsaR-lacZ; Tetr | This work |
| RW107 | RW100 ΔbgaA::PpcpA-lacZ; Tetr | This work |
| RW108 | RW100 ΔbgaA::PnrdD-lacZ; Tetr | This work |
| RW109 | RW101 prtA-lacZ; Ermr | This work |
| RW110 | RW100 ΔbgaA::Pspr0276-lacZ; Tetr | This work |
| RW111 | RW100 ΔbgaA::PpsaR-lacZ; Tetr | This work |
| RW112 | D39 ΔbgaA::PpsaB-lacZ; Tetr | This work |
| RW113 | RW100 ΔbgaA::PpsaB-lacZ; Tetr | This work |
| RW114 | RW121 ΔbgaA::PpcpA-lacZ; Tetr | This work |
| RW120 | RW103 with mariner insertion in psaR; Specr | This work |
| RW121 | D39 ΔpsaCA::ermR; Ermr | This work |
| RW122 | MP102 ΔbgaA::PpcpA-lacZ; Tetr | This work |
| RW131 | D39 ΔbgaA::PpcpA-3-lacZ; Tetr | This work |
| RW132 | D39 ΔbgaA::PpcpA-3a-lacZ; Tetr | This work |
| RW133 | D39 ΔbgaA::PpcpA-4-lacZ; Tetr | This work |
| RW134 | D39 ΔbgaA::PpcpA-5-lacZ; Tetr | This work |
| RW135 | D39 ΔbgaA::PpcpA-rev3-lacZ; Tetr | This work |
| RW136 | D39 ΔbgaA::PpcpA-rev4-lacZ; Tetr | This work |
| RW141 | RW100 ΔbgaA::PpcpA-3-lacZ; Tetr | This work |
| RW142 | RW100 ΔbgaA::PpcpA-3a-lacZ; Tetr | This work |
| RW143 | RW100 ΔbgaA::PpcpA-4-lacZ; Tetr | This work |
| RW144 | RW100 ΔbgaA::PpcpA-5-lacZ; Tetr | This work |
| RW145 | RW100 ΔbgaA::PpcpA-rev3-lacZ; Tetr | This work |
| RW146 | RW100 ΔbgaA::PpcpA-rev4-lacZ; Tetr | This work |
| E. coli EC1000 | Kmr; MC1000 derivative carrying a single copy of the pWV01 repA gene in glgB | 45 |
| L. lactis NZ9000 | MG1363 ΔpepN::nisRK | 41 |
| Plasmids | ||
| pR412T7 | Specr; derivative of pR412 (49) | 8 |
| pORI13 | Ermr; ori+repA−; promoterless lacZ, for single-copy chromosomal lacZ fusions. | 70 |
| pORI280 | Ermr; ori+repA−; deletion derivative of pWV01; constitutive lacZ expression from P32 promoter | 45 |
| pPP2 | Ampr Tetr; promoterless lacZ; for replacement of bgaA (spr0565) with promoter-lacZ fusions; derivative of pPP1 | 22 |
| pNZ8048 | Cmr; nisin-inducible PnisA | 16 |
| PNG8048E | Cmr Ermr; nisin-inducible PnisA, pNZ8048 derivative containing Ermr gene to facilitate cloning | Laboratory collection |
| pRW1 | pORI13::prtA-lacZ | This work |
| pRW2 | pPP2 Pspr0276 | This work |
| pRW3 | pPP2 PnrdD | This work |
| pRW4 | pPP2 PpsaR | This work |
| pRW5 | pPP2 PpcpA | This work |
| pRW6 | pPP2 PpsaB | This work |
| pRW11 | pPP2 PpcpA-3 | This work |
| pRW12 | pPP2 PpcpA-3a | This work |
| pRW13 | pPP2 PpcpA-4 | This work |
| pRW14 | pPP2 PpcpA-5 | This work |
| pRW15 | pPP2 PpcpA-rev3 | This work |
| pRW16 | pPP2 PpcpA-rev4 | This work |
| pRW20 | pORI280 ΔpsaR | This work |
| pRW21 | pNG8048E containing a 64-bp fragment comprising the PsaR binding site | This work |
| pRW22 | pRW12 containing a point mutation (T→G, bp −186b) in the PsaR binding box | This work |
| pRW23 | pRW12 containing a point mutation (A→G, bp −184b) in the PsaR binding box | This work |
| pRW24 | pRW12 containing two point mutations (A→G, bp −184b; A→C, bp −185b) in the PsaR binding box | This work |
| pRW25 | pNG8048E carrying psaR-strep downstream of PnisA | This work |
Ermr, erythromycin resistance; Tetr, tetracycline resistance; Cmr, chloramphenicol resistance; Specr, spectimomycin resistance.
Where the first base of the pcpA start codon (ATG) is +1.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequence (5′ to 3′) | Restriction site |
|---|---|---|
| PBMrIRPi | AGACCGGGGACTTATCAGCC | |
| PBMrTn1 | CTAGCGACGCCATCTATGTG | |
| TMr_1 | TGCATTTAATACTAGCGACGCCATCTATGTGTC | |
| TMr_4 | GGATCCATTCGCGTCAATTCGAGGGG | |
| PpsaB-1-lacZ | CGGAATTCTTCCAAGTTTTTTACACTTG | EcoRI |
| PpsaB-2-lacZ | CGGGATCCATTGTTGGTCCATGGAGCAC | BamHI |
| pspr1945-1 | CGGAATTCCCTTCAAATTTTAAGTCC | EcoRI |
| pspr1945-2 | CGGGATCCGTTAATGATAATATTGTAG | BamHI |
| PpcpA_f_2 | CGGAATTCTAATTTCTTTTTTAACCCAC | EcoRI |
| PpcpA_f_3 | CGGAATTCGTGGGTTAATTTTCCTTGAC | EcoRI |
| PpcpA_f_3a | CGGAATTCTAAAAAAGAAATTAAAGTGG | EcoRI |
| PpcpA_f_4 | CGGAATTCAATGTACTACTCTATTCTAC | EcoRI |
| PpcpA_rev_1 | CGGGATCCAGGATTGGTTCATTAGGGAC | BamHI |
| PpcpA_rev_3 | CGGGATCCGTCAAGGAAAATTAACCCAC | BamHI |
| PpcpA_rev_4 | CGGGATCCCTAGTAGAATAGAGTAGTAC | BamHI |
| marR-lacZ1 | CGGAATTCGCTATTTTCGTCATATCC | EcoRI |
| marR-lacZ2 | CGGGATCCCATTTTAGATAGTCTTCTTTG | BamHI |
| marR-del1 | TGCTCTAGACAATTGCCCACCAGTCCCG | XbaI |
| marR-del2 | TTCTTTGTTTGGGGTCATTC | |
| marR-del3 | GACCCCAAACAAAGAAGTCGAGAAAATCAACTAAT | |
| marR-del4 | GAAGATCTCTTTTGTCAGCTGAACGA | BglII |
| PprtA-1 | CGGAATTCTAGCGCTGATATTTCATC | |
| PprtA-2 | CGGGATCCGTTAGTGACAATACTGTG | |
| spr0561-1 | CGGAATTCGATACGGGAGAGGTAAGTG | EcoRI |
| spr0561-2 | CGGGATCCGAAATTGTCTCATCACCTC | BamHI |
| PSpr183-1.2 | CGGAATTCCAACCTAAGGTGATTGTGG | EcoRI |
| PSpr183-2.2 | CGGGATCCGAATTTCTGTAATAATTCGC | BamHI |
| Pspr0276-1 | CGGAATTCGAATTAGAATTATTGTGAG | BamHI |
| Pspr0276-2 | CGGGATCCGAGCAGCAACAGCACCACC | XbaI |
| Ery-for | TAACGATTATGCCGATAACT | |
| Ery-rev | GCATGCATCGATTAGATCTC | |
| psaCA-KO-1 | AAATCCTTACACCGAATTGC | |
| psaCA-KO-2 | GAGATCTAATCGATGCATGCTTCTGCAATCATAGGTCACCTCC | |
| psaCA-KO-3 | AGTTATCGGCATAATCGTTAGACAAGATTGCTGAAGGATTGG | |
| psaCA-KO-4 | TGCGCGTGCTAATAGGTGCC | |
| P-pcpA-box1 | CATGTCTTTTTTAACACGGGTTAAAAAAGAAATTAAAGTGGGTTAATTTTCCTTGACTTAAAATTTAA | |
| P-pcpA-box2 | CTAGTTAAATTTTAAGTCAAGGAAAATTAACCCACTTTAATTTCTTTTTTAACCCGTGTTAAAAAAGA | |
| PcpA_mut1.1 | GATTTCTTTTTTAACCCGTG | |
| PcpA_mut1.2 | AAAGTGGGTTAATTTTCCTTG | |
| PpcpA_mut2.1 | GTAATTTCTTTTTTAACCCGTG | |
| PpcpA_mut2.2 | AGTGGGTTAATTTTCCTTGAC | |
| PpcpA_mut3.1 | GCAATTTCTTTTTTAACCCGTG | |
| PpcpA_mut3.2 | AGTGGGTTAATTTTCCTTGAC | |
| Spr1480OX-2 | TGCTCTAGATTAGTTGATTTTCTCGACATAG | XbaI |
| spr1480-OX-new | CGAGCCATCATGAAACATCATCATCATCATCATACCCCAAACAAAGAAGACTATC | RcaI |
| Spr1480-Ctermstrep_OX | TGCTCTAGATTATTTTTCAAATTGTGGATGGCTCCAAGCGCTGTTGATTTTCTCGACATAGAGTTG | XbaI |
| Spr1480OX-1 | CGAGCCATCATGACCCCAAACAAAGAAGAC | RcaI |
Construction of transcriptional lacZ fusions.
Ectopic lacZ fusions to the pcpA (spr1945), psaR (spr1480), psaB (spr1492), nrdD (spr0183), and spr0276 promoters were made in pPP2 with primer pairs pspr1945-1/pspr1945-2, marR-lacZ1/marR-lacZ2, PpsaB-1-lacZ/PpsaB-2-lacZ, Pspr183-1.2/Pspr183-2.2, and Pspr0267-1/Pspr0276-2, yielding plasmids pRW5, pRW4, pRW6, pRW3, and pRW2. A chromosomal lacZ fusion to the 3′ end of prtA (spr0561) was made with primer pair spr0561-1/spr0561-2 in plasmid pORI13, leading to plasmid pRW1. In all cases Escherichia coli EC1000 was used as the cloning host. The lacZ fusion constructs were introduced into wild-type D39 and D39 ΔpsaR (RW100) in the case of pPP2 (integration via double crossover in the bgaA gene) and into D39nisRK and its isogenic psaR mutant (RW101) in the case of pORI13 (integration by single crossover), giving strains RW102 to RW113. The PpcpA-lacZ fusion was also introduced into the psaCA (RW121) and czcD (MP102) deletion strains, giving strains RW114 and RW122. All plasmid constructs were checked by sequencing, and new loci created with these plasmids were verified by PCR.
Random mutagenesis screen.
Random mutagenesis using the Himar1 MarC9 mariner transposon was performed essentially as described previously (42, 49). pR412-T7 (8), a derivative of pR412, was used as the source of the spec mariner transposon. Mutated R6 chromosomal DNA was transformed into strain R6, yielding a mutant library of approximately 20,000 CFU. Chromosomal DNA of this library was used to perform random mutagenesis in strain D39 ΔbgaA::PpcpA-lacZ. Mutants were selected on GM17 with 1% sheep blood, 0.006% X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), 1.5 μg/ml tetracycline, and 130 μg/ml spectinomycin. Transposon insertion sites in mutants showing derepression of expression of the PpcpA-lacZ transcriptional fusion were identified by the direct sequencing method as described previously (34), using primer PBMrTn1 with PBMrIRPi (nested).
Construction of deletion strains.
An in-frame marker-free deletion of psaR (spr1480) was constructed with plasmid pORI280 essentially as described previously (37), using primer pairs marR-del1/marR-del2 and marR-del3/marR-del4 and E. coli EC1000 as the cloning host. The mutant was checked by PCR and DNA sequencing.
A deletion strain of psaCA was made with primer pairs psaCA-KO-1/psaCA-KO-2 and psaCA-KO-1/psaCA-KO-4 by overlap extension PCR (73) and allelic replacement with an erythromycin resistance cassette, yielding strain RW121.
Construction of pcpA promoter subclones in pPP2.
The following promoter subclones of the pcpA promoter were made in pPP2 (primer pairs are in parentheses): PpcpA-3 (PpcpA_f_2 and PpcpA_rev_1), PpcpA-3a (PpcpA_f_3a and PpcpA_rev_1), PpcpA-4 (PpcpA_f_3 and PpcpA_rev_1), PpcpA-5 (PpcpA_f_4 and PpcpA_rev_1), PpcpA-rev3 (spr1945-1and PpcpA_rev_3), and PpcpA-rev4 (spr1945-1 and PpcpA_rev_4). All fragments were cloned as EcoRI/BamHI fragments in the same sites of pPP2, yielding plasmids pRW11 to pRW16. The constructs were sequenced and introduced into strains D39 and D39ΔpsaR (RW100), giving strains RW131 to RW136 and RW141 to RW146.
Construction of point mutations in the PsaR binding site in PpcpA.
A pNG8048E derivative containing a 64-bp fragment of the pcpA promoter comprising the PsaR binding site was constructed by annealing oligonucleotides P-pcpA-box1/P-pcpA-box2 and cloning them into NcoI/XbaI-digested pNG8048E, giving plasmid pRW21. This plasmid was used as a template for a PCR with Phusion DNA polymerase using the primer pairs PcpA_mut1.1/PcpA_mut1.2, PpcpA_mut2.1/PpcpA_mut2.2, and PpcpA_mut3.1/PpcpA_mut3.2, giving rise to plasmids pRW22 and pRW23, each with one point mutation in the predicted PsaR binding site, and pRW24, with two point mutations in the predicted PsaR binding site, respectively. The constructs were checked by DNA sequencing.
Microarray analyses.
For microarray analysis, the transcriptome of wild-type D39 grown in four biological replicates in GM17 was compared to the transcriptome of the same strain grown in four biological replicates in GM17 plus 0.25 mM ZnSO4. Growth, RNA isolation, labeling, hybridization, and analysis of slides were done essentially as described previously (38). Since the analysis was performed by interslide comparisons (i.e., the wild-type transcriptome analyzed with four slides was compared to the wild-type transcriptome in GM17 plus Zn2+ analyzed with four different slides), scaled signals from PostPrep of the slides hybridized to the GM17 samples were pairwise compared with the scaled signals of the slides hybridized to the GM17-Zn2+ samples. The resultant table with the scaled (and normalized) signals was used as input for the CyberT variant for statistical analysis of control versus experimental data, using a local running copy of the CyberT algorithm for paired data (http://molgen.biol.rug.nl/cgi-bin/cybert/CyberT-8.0.form.pl?DATATYPE=CE). Genes were considered differentially expressed when the Bayesian P value was <0.001 and the false discovery rate was <0.01, unless otherwise stated (80).
Overexpression and purification of Strep-tagged PsaR.
For the overexpression of a C-terminally Strep-tagged variant of PsaR, psaR was amplified from D39 chromosomal DNA using primers Spr1480OX-1 and Spr1480-Ctermstrep_OX. The resulting PCR product was digested with RcaI/XbaI and cloned into the NcoI/XbaI sites of pNG8048E, yielding plasmid pRW25. Overexpression in Lactococcus lactis NZ9000 was done essentially as described previously (41). Purification of PsaR-Strep from L. lactis was performed using the Streptactin column from IBA according to the supplier's instructions. Buffers without EDTA were used, and the purified protein was stored at a concentration of 0.15 mg/ml in the elution buffer (100 mM Tris-HCl [pH 8], 150 mM NaCl, 2.5 mM desthiobiotin, 1 mM β-mercaptoethanol) with 10% glycerol at −80°C.
EMSAs.
Electrophoretic mobility shift assays (EMSAs) were performed with [γ-33P]ATP-labeled probes in buffer containing 20 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 0.1 mM dithiothreitol, 8.7% (wt/vol) glycerol, 62.5 mM KCl, 25 μg/ml bovine serum albumin, 25 μg/ml poly(dI-dC), and 3000 cpm of [γ-33P]ATP-labeled PCR product. Various metal ions were added in concentrations as specified in Results. As probes, PCR products comprising the promoter regions of psaB (primers PpsaB-1-lacZ/PpsaB-2-lacZ), pcpA (PpcpA_f_2/PpcpA_rev_1), and prtA (PprtA-1/PprtA-2) were used. As a control, a PCR fragment of the pcpA promoter without the intact PsaR operator was used (PpcpA_f_4/PpcpA_rev_1). Reaction mixtures were incubated at 37°C for 10 min before loading on gels. Gels were run in 0.44 M Tris-borate buffer (pH 8.3) at 100 V for 90 min.
Measurement of concentrations of Mn2+ and Zn2+ in growth media.
Using atomic absorption spectroscopy on a Vista AX-CCD simultaneous ICP-AES spectrometer, the concentrations of Mn2+ and Zn2+ in GM17 and GM17chel were determined at wavelengths (nm) of 257.610/259.372 (Mn) and 202.548/213.857 (Zn).
Microarray accession number.
Microarray data have been deposited to the Gene Expression Omnibus (GEO) and can be accessed via http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11438. In addition, slide images and raw and processed data are available at http://molgen.biol.rug.nl/publication/zinc_data/.
RESULTS
Identification of Zn2+-regulated genes in S. pneumoniae.
To identify genes that are regulated by a high level of Zn2+ in S. pneumoniae, we compared the transcriptome of wild-type D39 grown in GM17 with that of the same strain grown in GM17 with 0.25 mM ZnSO4 (Table 3). With this concentration of Zn2+, growth is not affected (39). The most upregulated genes were pcpA, encoding a choline binding protein (69) involved in virulence (31), and Zn2+ resistance gene czcD (39). Also upregulated were the serine protease gene prtA and the Mn2+ ABC transporter genes psaBC, which are both involved in virulence (6, 7, 32, 50), and an operon (SP0202 to SP0207) encoding, among other proteins, the NrdDG ribonucleoside triphosphate reductase, which is involved in synthesis of deoxyribonucleoside triphosphates (33, 78). An operon consisting of genes involved in cellobiose metabolism (SP0303 to SP0310) (52) was strongly downregulated. Thus, expression of genes with a variety of functions is affected by growth of S. pneumoniae in medium with an elevated Zn2+ concentration.
TABLE 3.
Summary of transcriptome comparison of S. pneumoniae strain D39 grown in GM17 and in GM17 with addition of 0.25 mM Zn2+
| TIGR4 locus tag | Function (TIGR annotation) | Ratioa |
|---|---|---|
| SP0202 | Anaerobic ribonucleoside triphosphate reductase NrdD | 2.5 |
| SP0204 | Predicted acetyltransferase, GNAT family | 2.4 |
| SP0205 | Anaerobic ribonucleoside triphosphate reductase activating protein NrdG | 1.8b |
| SP0206 | Hypothetical protein; predicted uridine kinase | 1.8b |
| SP0207 | Hypothetical protein; predicted uridine kinase | 2.2 |
| SP0303 | 6-Phospho-β-glucosidase BglA | −7.6 |
| SP0305 | Phosphotransferase system cellobiose-specific component IIB | −4.1 |
| SP0306 | Putative transcriptional regulator; possible antiterminator BglG | −14.8 |
| SP0307 | Phosphotransferase system, IIA component | −6.3b |
| SP0308 | Phosphotransferase system cellobiose-specific component IIA | −3.0 |
| SP0309 | Hypothetical protein | −7.6 |
| SP0310 | Phosphotransferase system cellobiose-specific component IIC | −6.0 |
| SP0338 | Putative ATP-dependent clp protease, ATP binding subunit ClpL | −2.0 |
| SP0515 | Heat-inducible transcription repressor HrcA | −2.0 |
| SP0516 | Heat shock protein GrpE | −1.6 |
| SP0517 | Chaperone protein DnaK (heat shock protein 70) | −1.4 |
| SP0518 | Hypothetical protein | −2.1 |
| SP0519 | Chaperone protein DnaJ | −1.4 |
| SP0640 | Hypothetical protein | 1.5b |
| SP0641 | Cell wall-associated serine proteinase precursor PrtA | 2.7 |
| SP0645 | Putative phosphotransferase system IIA component | 2.8 |
| SP0646 | Phosphotransferase system, IIB component, putative | 2.4 |
| SP0879 | Hypothetical protein | −2.1 |
| SP1648 | Manganese (and/or zinc) ABC transporter, ATP binding protein PsaB | 2.3 |
| SP1649c | Manganese (and/or zinc) ABC transporter, permease protein PsaC | 2.6 |
| SP1762 | Hypothetical protein | 2.7 |
| SP1855 | Alcohol dehydrogenase, zinc-containing AdhB | 1.7 |
| SP1856 | Transcriptional regulator, MerR family | 2.0b |
| SP1857 | Cation efflux system protein CzcD | 7.2 |
| SP1935 | Hypothetical protein | 2.8 |
| SP2136 | Choline binding protein; surface protein PcpA | 8.5 |
Ratios of >2.0 or <−2.0 (wild-type D39 compared to wild-type D39 plus 0.25 mM Zn2+) are shown; in some cases neighboring genes with lower fold changes are also indicated.
Ratio with a false discovery rate of >0.01 but <0.1.
For SP1650 (psaA), the number of observations (replicates) was too low and hence no significance was obtained.
Zn2+-dependent expression of pcpA is mediated by PsaR.
To investigate in more detail the transcriptional regulation of pcpA, the most Zn2+-induced gene found in the microarray experiment, a transcriptional lacZ fusion to the pcpA promoter was constructed by use of plasmid pPP2, which integrates into the bgaA locus (22). Using strain D39 containing this PpcpA-lacZ transcriptional fusion, it was demonstrated that only elevated Zn2+ concentrations lead to high expression from the pcpA promoter, although also some weak induction could be seen for Co2+ and Fe2+ (Table 4).
TABLE 4.
Expression of PpcpA specifically in the presence of various metal cationsa
| Addition to GM17 (mM) | Mean (SD) β-galactosidase activity (Miller units) |
|---|---|
| None | 4 (1) |
| Zn2+ (0.2) | 54 (12) |
| Zn2+ (0.4) | 388 (45) |
| Cu2+ (0.05) | 3 (1) |
| Cu2+ (0.1) | 3 (1) |
| Co2+ (0.05) | 8 (3) |
| Co2+ (0.1) | 16 (3) |
| Ni2+ (0.1) | 4 (1) |
| Ni2+ (0.4) | 5 (2) |
| Fe2+ (0.1) | 9 (2) |
| Fe2+ (0.4) | 21 (6) |
| Mg2+ (1.0) | 4 (1) |
| Mg2+ (10) | 2 (1) |
β-Galactosidase of a PpcpA-lacZ transcriptional fusion was measured in the wild-type strain D39 (strain RW102) grown in GM17 with the indicated metal ions. For Zn2+, Ni2+, Co2+, and Cu2+ the concentrations used have similar effects on growth (see also reference 39). Values are from three experiments.
To identify the factor repressing transcription of pcpA under normal growth conditions, strain D39 PpcpA-lacZ (RW102) was randomly mutagenized with the mariner transposon (42, 49) and blue colonies were screened for on GM17 agar plates with X-Gal but without Zn2+ supplementation. Among 7,200 CFU, one blue clone was found, containing a transposon insertion in the gene encoding the MarR family transcriptional regulator PsaR (spr1480, SPD_1450, SP1638). The insertion in psaR gave rise to high expression of the PpcpA-lacZ fusion, which was independent of Zn2+ (data not shown). We constructed a markerless deletion mutant of psaR and found that in this mutant expression of PpcpA-lacZ is highly derepressed independent of Zn2+ (Table 5), suggesting that PsaR is responsible for Zn2+-dependent derepression of pcpA expression.
TABLE 5.
β-Galactosidase activities of the wild-type D39 and the ΔpsaR, ΔpsaCA, and ΔczcD strains, all containing the PpcpA-lacZ transcriptional fusion, grown in GM17 and GM17chel supplemented with metal ionsa
| Medium and addition(s) (mM) | Mean (SD) β-galactosidase activity (Miller units)b in:
|
|||
|---|---|---|---|---|
| Wild type | ΔpsaR mutant | ΔpsaCA mutant | ΔczcD mutant | |
| GM17 | ||||
| None | 6 (3) | 1,204 (67) | 594 (55) | 18 (5) |
| Zn2+ (0.1) | 17 (4) | ND | ND | 69 (12) |
| Zn2+ (0.2) | 66 (21) | 1,102 (277) | ND | ND |
| Zn2+ (0.4) | 417 (113) | 1,189 (115) | 446 (37) | ND |
| Zn2+ (0.4) + Mn2+ (0.01) | 33 (12) | 1,119 (113) | 237 (22) | ND |
| Zn2+ (0.4) + Mn2+ (0.05) | 11 (4) | 1,219 (203) | 7 (4) | ND |
| Zn2+ (0.4) + Fe2+ (0.05) | 269 (22) | ND | ND | ND |
| GM17chel | ||||
| None | 315 (47) | 1,217 (222) | ND | ND |
| Zn2+ (0.2) | 424 (35) | 1,260 (102) | ND | ND |
| Mn2+ (0.05) | 6 (2) | 1,315 (78) | ND | ND |
The wild-type D39 and the ΔpsaR, ΔpsaCA, and ΔczcD strains are strains RW102, RW107, RW114, and RW122, respectively.
Values are from three experiments. ND, not determined.
PsaR regulates prtA and psaBCA in a Zn2+-dependent way.
To test whether PsaR is also responsible for the Zn2+-dependent expression of the other genes identified in the microarray analysis, transcriptional lacZ fusions to prtA, PpsaB, and PnrdD (Pspr0183) were constructed and introduced in both the wild type and the psaR mutant. As expected, in the wild type expression of prtA and PpsaB increased upon addition of Zn2+ to the GM17 growth medium (Tables 6 and 7). In the psaR mutant expression was derepressed in GM17, showing that PsaR mediates the Zn2+-dependent expression of prtA and psaBCA as well. Transcription from PnrdD was two- to threefold higher in cells grown in GM17 with Zn2+ compared to GM17, but this was not affected by the psaR mutation (data not shown). We also tested the effect of metal ions on the expression of psaR itself. A PpsaR-lacZ transcriptional fusion was highly expressed in GM17 but was not influenced by Zn2+ and Mn2+ or by the psaR mutation (data not shown).
TABLE 6.
β-Galactosidase activities of the wild-type D39 and the ΔpsaR strain, containing the prtA-lacZ transcriptional fusion, grown in GM17 and GM17chel supplemented with metal ionsa
| Medium and addition(s) (mM) | Mean (SD) β-galactosidase activity (Miller units)b in:
|
|
|---|---|---|
| Wild type | ΔpsaR mutant | |
| GM17 | ||
| None | 0.2 (0.06) | 2.2 (0.3) |
| Zn2+ (0.2) | 0.6 (0.17) | 2.3 (0.5) |
| Zn2+ (0.4) | 1.2 (0.05) | 2.1 (0.4) |
| Zn2+ (0.4) + Mn2+ (0.01) | 0.5 (0.09) | 2.1 (0.2) |
| Zn2+ (0.4) + Mn2+ (0.05) | 0.2 (0.08) | 2.1 (0.2) |
| GM17chel | ||
| None | 0.5 (0.03) | 2.2 (0.1) |
| Zn2+ (0.2) | 0.9 (0.05) | 2.0 (0.4) |
| Mn2+ (0.05) | 0.2 (0.04) | 2.1 (0.4) |
The wild-type D39 and the ΔpsaR strain are strains RW104 and RW109, respectively. lacZ was fused to the 3′ end of prtA on the native chromosomal location, using plasmid pORI13. This might explain the much lower β-galactosidase activity compared to the values for the lacZ fusions with PpcpA and PpsaB (Tables 5 and 7).
Values are from three experiments.
TABLE 7.
β-Galactosidase activities of the wild-type D39 and the ΔpsaR strain, containing the PpsaB-lacZ transcriptional fusion, grown in GM17 and GM17chel supplemented with metal ionsa
| Medium and addition(s) (mM) | Mean (SD) β-galactosidase activity (Miller units)b in:
|
|
|---|---|---|
| Wild type | ΔpsaR mutant | |
| GM17 | ||
| None | 134 (34) | 1,167 (121) |
| Zn2+ (0.2) | 448 (110) | 970 (78) |
| Zn2+ (0.4) | 760 (124) | 1,089 (98) |
| Zn2+ (0.4) + Mn2+ (0.01) | 651 (18) | 1,205 (144) |
| Zn2+ (0.4) + Mn2+ (0.05) | 142 (11) | 1,072 (262) |
| GM17chel | ||
| None | 583 (41) | 1,107 (220) |
| Zn2+ (0.2) | 901 (33) | 1,180 (102) |
| Mn2+ (0.05) | 111 (27) | 1,023 (45) |
The wild-type D39 and the ΔpsaR strain are strains RW112 and RW113, respectively.
Values are from three experiments.
The expression of the cellobiose utilization operon SP303 to SP310 (spr0276 to spr0282, R6 annotation) was further analyzed using a Pspr0276-lacZ transcriptional fusion. Surprisingly, in GM17 the expression was hardly measurable, and no effect of Zn2+ was observed (data not shown). However, in the absence of glucose as well as in the presence of cellobiose, Pspr0276 was highly expressed (data not shown). It is not immediately clear what the reason is for the downregulation of this operon in the transcriptome analysis.
Regulation of PpsaB, PpcpA, and prtA depends on the balance between Mn2+ and Zn2+.
In an earlier study, expression of pcpA and psaBCA was demonstrated to be repressed by PsaR in the presence of Mn2+ (31). To specify how Mn2+ and Zn2+ influence PsaR activity, we analyzed the expression of transcriptional lacZ fusions to PpcpA, PpsaB, and prtA in the presence of various concentrations of both Mn2+ and Zn2+ in GM17 medium and in GM17 medium treated with the metal ion chelator Chelex 100 resin (GM17chel). GM17 contains 2.0 μM Mn2+ and 8.1 μM Zn2+ and GM17chel contains 0.02 μM Mn2+ and 0.00 μM Zn2+, showing that the Chelex treatment effectively removed these cations. In GM17, upregulation of the expression of all three lacZ fusions in the presence of a high concentration of Zn2+ was nullified by the addition of Mn2+ when added in a concentration of 0.01 to 0.05 mM, which is about 10 to 40 times lower than the concentration of Zn2+ (Tables 5, 6, and 7). This repressive effect was specific for Mn2+, since 0.05 mM Ni2+, Cu2+, and Co2+ had no effect on the Zn2+-dependent expression of the PpcpA-lacZ transcriptional fusion (data not shown); for Fe2+, there was only a weak repressive effect (Table 5). In GM17chel, expression of all three lacZ fusions was derepressed compared to that in GM17 (Tables 5, 6, and 7), which was expected because of the much lower concentration of Mn2+ after Chelex treatment. Addition of Zn2+ increased the derepression even more, while Mn2+ led to repression of expression again, which is in agreement with the observations made in GM17 (Tables 5, 6, and 7).
To investigate whether the opposite effects of Zn2+ and Mn2+ are the result of the competition for uptake of these cations, expression of the PpcpA-lacZ fusion in czcD and psaCA deletion mutants was measured. In a czcD deletion mutant, which is, as a consequence of impaired Zn2+ efflux, likely to accumulate higher intracellular levels of this metal ion (39), expression of PpcpA-lacZ in both GM17 and GM17 with 0.1 mM Zn2+ (the highest possible concentration for the czcD deletion mutant) was higher than that in the wild type (Table 5). In a psaCA deletion mutant, which is impaired in uptake of Mn2+ into the cell (50) the expression of PpcpA-lacZ was also highly derepressed, However, addition of Mn2+, albeit at a higher concentration than with the wild type, still led to repression (Table 5). These results suggest that the observed regulatory effects on expression of pcpA, prtA, and psaBCA that are induced by Mn2+ and Zn2+ converge at the level of transcriptional regulation by PsaR.
Identification of a PsaR operator in the promoters of pcpA, prtA, and psaB.
Using Gibbs Motif Sampler (77), a palindromic sequence (Fig. 1A and B), located just upstream of (PprtA and PpcpA) or overlapping with (PpsaB) the predicted core promoter regions, that might serve as the PsaR operator was uncovered.
FIG. 1.
Identification of a putative PsaR operator. (A) Weight matrix of the identified PsaR operator as present in the promoter regions of pcpA, prtA, and psaB. (B) Positions of the PsaR operator (shaded) in the promoter regions of pcpA, prtA, and psaB. Putative core promoter sequences are in bold. The ribosome binding sites are in bold and underlined. Start codons are in italic.
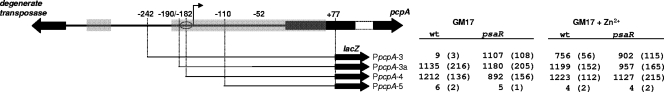
To dissect the promoter of pcpA experimentally, we performed a promoter subcloning experiment where the pcpA promoter was truncated from the 5′ end and fused to lacZ in the reporter plasmid pPP2 (Fig. 2). Expression of a promoter fragment truncated upstream of the predicted operator (PpcpA-3) was Zn2+ dependent, but as expected, deletion of half of the identified operator (PpcpA-4) led to fully derepressed, Zn2+-independent expression (Fig. 2). In the presence of 0.05 mM Mn2+, the β-galactosidase activity of PpcpA-4 in wild-type D39 was 1,197 ± 78 Miller units, which is similar to the values for PpcpA-4 in GM17 and GM17 with Zn2+ (Fig. 2), indicating that the effects of Zn2+ and Mn2+ are mediated by the same PsaR operator site. To determine if the operator sequence was fully identified, a truncation of the promoter region 8 bp upstream of the operator sequence (PpcpA-3a) was constructed, which gave rise to full derepression, suggesting that additional bases 5′ to the operator are also important for PsaR-mediated repression of the pcpA promoter. For subclone PpcpA-5, expression was close to zero under all conditions. Deletion of the same region as in PpcpA-5 but now from the 3′ side (PpcpA-rev3 versus PpcpA-rev4; strains RW135, RW136, and RW145, RW146) confirmed that promoter activity locates exclusively to this area (data not shown). This demonstrates that the core promoter sequence is located in the region between the 5′ base pair positions of subclones PpcpA-4 and PpcpA-5 (Fig. 2 and 1B).
FIG. 2.
Subcloning of PpcpA. A schematic overview of the PpcpA truncations is shown. Numbers indicate the positions of the truncations, which were fused to lacZ, relative to the putative pcpA start site (+1). The flag indicates the position of the core promoter, and the oval indicates the putative PsaR operator. Gray-shaded areas indicate regions of similarity with PprtA (89% identity for the short stretch and 82% identity for the long stretch). The table on the right gives β-galactosidase activities (Miller units) of the promoter truncations in wild-type D39 (wt, strains RW131 to RW134) and the ΔpsaR mutant (psaR, strains RW141 to RW144) grown in GM17 and in GM17 plus 0.5 mM Zn2+. Standard deviations of three measurements are given in parentheses.
To further show that the predicted PsaR operator is functional, a 64-bp DNA fragment of the pcpA promoter comprising the PsaR operator was put into plasmid pNG8048E, which replicates in S. pneumoniae (37). Subsequently, several point mutations in the first half of the motif were introduced (Table 8). By putting the wild-type construct into D39 containing the PpcpA-lacZ transcriptional fusion, transcription from PpcpA was strongly derepressed, showing that this 64-bp stretch of DNA titrates away the repressive effect of PsaR on the expression of pcpA (Table 8). However, with the constructs containing the mutated PsaR boxes, this derepressive effect was not present (Table 8). This shows clearly that the bases in the predicted PsaR binding box are required for PsaR-dependent repression of pcpA. The entire S. pneumoniae R6, D39, and TIGR4 sequences were searched with a weight matrix of the PsaR operator sequence (Fig. 1A) using Genome2D (5), but the motif was not found in additional promoter regions (data not shown). In conclusion, a PsaR regulatory element in the promoters of pcpA, psaB, and prtA was identified.
TABLE 8.
Mutational analysis of the PsaR operator
| PsaR box | Sequence (5′→3′)a | Mean (SD) β-galactosidase activity (Miller units) for the following strain and mediumb:
|
|||
|---|---|---|---|---|---|
| Wild type
|
ΔpsaR mutant
|
||||
| GM17 | GM17 + Zn2+ (0.4 mM) | GM17 | GM17 + Zn2+ (0.4 mM) | ||
| Wild type | AAATTAAAGTGGGTTAATTT | 733 (123) | 853 (122) | 1,288 (170) | 1,331 (167) |
| mut 1 | AAATGAAAGTGGGTTAATTT | 74 (15) | 376 (78) | 1,160 (135) | 1,271 (122) |
| mut 2 | AAATTAGAGTGGGTTAATTT | 121 (25) | 416 (75) | 1,300 (101) | 1,278 (69) |
| mut 3 | AAATTCGAGTGGGTTAATTT | 58 (12) | 464 (16) | 1,389 (111) | 1,378 (178) |
Sequences of wild-type and mutant PsaR boxes of the pcpA promoter; point mutations are in bold.
β-Galactosidase activities of wild-type D39 and the ΔpsaR mutant harboring the PpcpA-lacZ transcriptional fusion (strains RW102 and RW107) containing plasmid pRW21 (wild-type PsaR box), pRW22 (PsaR box mut1), pRW23 (PsaR box mut2), or pRW24 (PsaR box mut3).
Binding of PsaR-Strep to the pcpA, psaB, and prtA promoters in the presence of Mn2+ is counteracted by Zn2+.
To find out whether the observed Zn2+- and Mn2+-dependent effects on expression of the PsaR targets are caused by direct modulation of the DNA binding activity of the PsaR protein, EMSAs were performed with purified Strep-tagged PsaR (PsaR-Strep). PsaR-Strep alone did not shift the promoter regions of pcpA, psaB, and prtA (Fig. 3A to C, lanes 2). However, in the presence of Mn2+, PsaR-Strep did bind to the promoter regions of pcpA, psaB, and prtA (Fig. 3A to C, lanes 3). PsaR-Strep did not bind under any condition to a truncated pcpA promoter lacking the PsaR binding box (Fig. 3D). Besides Mn2+, 0.05 mM Co2+ was also able to stimulate the binding of PsaR-Strep to the promoter fragments, whereas 0.05 mM Fe2+, Cu2+, Ni2+, and Zn2+ did not (data not shown). These data show that the PsaR-Strep-DNA interaction was specific and indicates that PsaR directly functions as a Mn2+-dependent repressor of its target genes. Based on the lacZ expression studies, we hypothesized that Zn2+ should somehow impair PsaR binding to fulfill its function as an Mn2+-dependent repressor. Therefore, experiments addressing the influence of Zn2+ on the in vitro PsaR-Strep-DNA interaction in the presence of Mn2+ were performed (Fig. 3A, B, and C, lanes 4 to 8). These demonstrated that the stimulatory effect of Mn2+ on the binding of PsaR-Strep to all three promoter fragments was counteracted by the addition of Zn2+. There was also a weaker counteracting effect of Cu2+ for the pcpA and prtA promoters (Fig. 3A and C, lanes 8). Thus, Mn2+ stimulates PsaR binding to the operators in the pcpA, psaB, and prtA promoters, whereas in the presence of Zn2+, PsaR binding is abolished, indicating that Mn2+ and Zn2+ exert their regulatory effects on pcpA, prtA, and psaBCA expression directly through PsaR.
FIG. 3.
In vitro interaction of PsaR-Strep with the pcpA (A), psaB (B), and prtA (C) promoter regions and with a truncated PpcpA fragment lacking the PsaR operator (D). Purified PsaR-Strep was added at concentrations of 5 nM (PpcpA), 11 nM (PprtA), and 25 nM (PpsaB). Metal ions were added as indicated above the lanes at a concentration of 50 μM. X, free probe. The horizontal bar above lanes 3 to 8 indicates the presence of Mn2+, and the horizontal bar above lanes 2 to 8 indicates the presence of PsaR-Strep. Arrows indicate the positions of the shifted probes, and asterisks indicate the position of the free probe. The presence of weaker bands which run higher than the free probe in the gels is a phenomenon that has also been seen by others in similar experiments. These bands may represent unspecific PCR products or single-stranded DNA (1, 15).
DISCUSSION
In this study, we analyzed the transcriptome change of the human pathogen S. pneumoniae in response to a high level of Zn2+. Expression of several genes and operons with diverse functions was affected by Zn2+, including pcpA, prtA, and psaBCA. The observed Zn2+-dependent expression of these virulence genes was shown to be directly mediated by the Mn2+-responsive repressor PsaR (31). We further demonstrate that this is caused by Mn2+-dependent binding of PsaR to and Zn2+-dependent release from the promoters of these genes. Thus, these data represent an intriguing insight in the opposite regulatory effects of two metal cations on the expression of a set of virulence genes, mediated by a single transcriptional repressor.
The concentrations as well as the ratio of Mn2+ and Zn2+ may vary greatly between different sites in the human body. For example, in lung tissue the total concentration of Mn2+ is approximately 0.2 μg/g (wet weight) (3.6 μM) and that of Zn2+ is 15 μg/g (wet weight) (229 μM), whereas in the blood serum concentrations of Mn2+ and Zn2+ are 0.5 ng/ml (9 nM) and 1.0 μg/ml (15.3 μM), respectively (81). Control of availability of both ions is of importance to the host, as a recent study showed that the human immune system employs chelation of Mn2+ and Zn2+ by calprotectin as a way to inhibit bacterial growth in tissue abscesses (13). On the other hand, sufficiently high levels of Zn2+ are required for proper functioning of the immune system (72). Thus, it is likely that the concentrations of Mn2+ and Zn2+ fluctuate greatly in the environment, which will lead to varying concentrations of these metal ions in the cytoplasm of S. pneumoniae. However, virulence studies that have been carried out so far with respect to pcpA, prtA, and psaBCA do not point to a specific site where the proteins encoded by these genes are needed (7, 26, 31, 47, 50, 51, 62). Interestingly, pcpA is also regulated by the nutritional regulator CodY (26), meaning that the concentrations of both metal ions as well as amino acids affect pcpA expression.
Homologs of PsaR in other organisms seem to have slightly different functions. In Streptococcus gordonii, ScaR is an Mn2+-dependent repressor of the Sca (Mn2+) permease (29). In Streptococcus pyogenes, MtsR regulates the Mn2+-specific ABC transporter MtsABC in response to Mn2+, while the heme-specific HtsABC transporter is repressed by MtsR in response to Fe3+ (23). The Streptococcus mutans SloR regulates several genes involved in biofilm formation, genetic competence, oxidative stress tolerance, and adherence in response to Mn2+ and, to a lesser extent, Fe3+ (35, 63, 67, 74).
The EMSAs performed in this study are in line with the transcriptional data and suggest that a direct effect of Zn2+ and Mn2+ on the PsaR-promoter interaction causes the observed transcriptional effects. However, in the EMSAs Mn2+ does not overcome the Zn2+ effect at equimolar concentration, whereas in vivo, only low (but repressive) concentrations of Mn2+ are counteracted by Zn2+. This might be because of different intracellular concentrations/availabilities of these metal ions compared to the extracellular concentrations and indicates that in vivo Mn2+ is the principal effector. It will be interesting to know why Zn2+ and Mn2+ have these opposite effects on the DNA binding properties and activity of PsaR.
Clues about this could come from recent structural studies on DtxR from Corynebacterium diphtheriae (14) and MntR, a DtxR family protein from Bacillus subtilis that responds to Mn2+ (65). Both DtxR and MntR contain two metal binding sites per monomer: a low-affinity site and a high-affinity site (14, 21). MntR binds metal ions with affinities that roughly follow the Irving-Williams series, where Mn2+ displays the lowest affinity for MntR and Zn2+ the highest (21). As MntR has a much higher affinity for Zn2+ than Mn2+ but only very poorly activates DNA binding of MntR, the specificity of MntR is not correlated with the metal binding affinity (21). The conformation of Mn2+-bound MntR differs from the Zn2+-bound conformation with respect to the occupancy of the metal binding sites: Mn2+ binds to two sites, whereas only one Zn2+ ion binds to MntR, which does not allow binding of a second one (36). Metal binding at the second site is proposed to be required for DNA binding, as it promotes a disorder-to-order transition of MntR structure (17). PsaR shares 25% and 15% sequence identity with DtxR and MntR, respectively. Moreover, sequence alignment shows that most residues that constitute the metal ion binding sites in DtxR and MntR are conserved in PsaR (data not shown). Therefore, it might be that Zn2+ prevents Mn2+ binding to PsaR, rendering PsaR in a monomeric or destabilized state, and in this way counteracts Mn2+-induced DNA binding and transcriptional repression.
Interference with the effect of one metal ion on a metal-sensory protein by another metal ion has been reported recently for CzrA in B. subtilis (24). CzrA normally is activated for DNA release in the presence of Zn2+, but Cu2+ inhibits the Zn2+-induced allostery, since in vitro the protein preferentially binds Cu2+. However, these effects are not seen in vivo. High levels of Cu2+ in the growth medium induces the Fur regulon in B. subtilis (56). Thus, opposite effects of metals on regulation of gene expression seems to occur with other classes of metalloregulatory proteins as well.
The identified PsaR binding site is similar to operator sequences of PsaR homologs in other streptococcal species. S. gordonii ScaR binds to a similar region in the scaA promoter but also to a second inverted repeat (29). This second repeat is present in the promoter region of psaB in S. pneumoniae but not in the promoters of pcpA and prtA. SloR in S. mutans also likely exerts its repressive effect on sloABC through a larger palindromic sequence that includes the conserved region that we identify (35). Apart from the promoters of prtA, pcpA, and psaBCA, no others that contain the identified PsaR operator in their promoter regions could be found in the R6 and TIGR4 genomes. This suggests that the PsaR regulon consists of only these genes in S. pneumoniae, in contrast to the case for S. mutans, where SloR directly regulates a large number of genes (67). It is very likely that also in TIGR4 the activity of PsaR is dependent on both Zn2+ and Mn2+, since, apart from one amino acid difference (Asn161→Ser), TIGR4 PsaR is identical to the R6 and D39 PsaR sequences (data not shown). Johnston et al. (31) also found a repressive effect of Mn2+ and PsaR on the expression of the rlrA pathogenicity islet (SP0461 to SP0465). The rlrA locus is not present in the genomes of D39 and R6, making the effect of PsaR on rlrA and the downstream genes a strain-dependent phenomenon. There is a possible PsaR operator in the rlrA promoter region with a perfectly conserved first half-site, but a very degenerate second half-site, 5′-AAATTAAAACAACTTCCATC-3′ (consensus bases are in bold). Point mutations in the conserved bases of the first half of the operator destroyed PsaR-dependent regulation for the pcpA promoter (Table 8). However, we did not test the effect of mutations in the second half-site. Therefore, it cannot be excluded that an operator consisting of an intact first repeat and a degenerate inverted repeat, as is the case for the putative operator in the rlrA promoter, is still able to confer some weak PsaR-dependent regulation. As RlrA activates expression of the genes downstream of rlrA, namely, rrgA, rrgB, rrgC, and srtB (25), weak repression of rlrA by PsaR likely explains the upregulation of the rlrA locus in the psaR mutant (31, 68).
In conclusion, this study indicates that the relative availabilities of Zn2+ and Mn2+ in the human body could modulate the expression of several virulence genes and in this way affect the outcome of infection by S. pneumoniae.
Acknowledgments
We thank D. Morrison for the generous gift of competence-stimulating peptide. We thank Anne de Jong for help with the DNA microarray production and analysis.
T.G.K. and J.J.E.B. are supported by IOP Genomics grant IGE03002 from the Dutch Ministry of Economic Affairs.
Footnotes
Published ahead of print on 30 May 2008.
REFERENCES
- 1.Albano, M., W. K. Smits, L. T. Ho, B. Kraigher, I. Mandic-Mulec, O. P. Kuipers, and D. Dubnau. 2005. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J. Bacteriol. 1872010-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderton, J. M., G. Rajam, S. Romero-Steiner, S. Summer, A. P. Kowalczyk, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2007. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42225-236. [DOI] [PubMed] [Google Scholar]
- 3.Ando, M., Y. C. Manabe, P. J. Converse, E. Miyazaki, R. Harrison, J. R. Murphy, and W. R. Bishai. 2003. Characterization of the role of the divalent metal ion-dependent transcriptional repressor MntR in the virulence of Staphylococcus aureus. Infect. Immun. 712584-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery, O. T., C. M. Macleod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. Mol. Med. 1344-365. [PMC free article] [PubMed] [Google Scholar]
- 5.Baerends, R. J. S., W. K. Smits, A. de Jong, L. W. Hamoen, J. Kok, and O. P. Kuipers. 2004. Genome2D: a visualization tool for the rapid analysis of bacterial transcriptome data. Genome Biol. 5R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 645255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethe, G., R. Nau, A. Wellmer, R. Hakenbeck, R. R. Reinert, H. P. Heinz, and G. Zysk. 2001. The cell wall-associated serine protease PrtA: a highly conserved virulence factor of Streptococcus pneumoniae. FEMS Microbiol. Lett. 20599-104. [DOI] [PubMed] [Google Scholar]
- 8.Bijlsma, J. J., P. Burghout, T. G. Kloosterman, H. J. Bootsma, A. de Jong, P. W. Hermans, and O. P. Kuipers. 2007. Development of genomic array footprinting for identification of conditionally essential genes in Streptococcus pneumoniae. Appl. Environ. Microbiol. 731514-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blencowe, D. K., and A. P. Morby. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 27291-311. [DOI] [PubMed] [Google Scholar]
- 10.Bogaert, D., R. de Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4144-154. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40572-585. [DOI] [PubMed] [Google Scholar]
- 12.Brown, J. S., S. M. Gilliland, J. Ruiz-Albert, and D. W. Holden. 2002. Characterization of pit, a Streptococcus pneumoniae iron uptake ABC transporter. Infect. Immun. 704389-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbin, B. D., E. H. Seeley, A. Raab, J. Feldmann, M. R. Miller, V. J. Torres, K. L. Anderson, B. M. Dattilo, P. M. Dunman, R. Gerads, R. M. Caprioli, W. Nacken, W. J. Chazin, and E. P. Skaar. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319962-965. [DOI] [PubMed] [Google Scholar]
- 14.D'Aquino, J. A., J. Tetenbaum-Novatt, A. White, F. Berkovitch, and D. Ringe. 2005. Mechanism of metal ion activation of the diphtheria toxin repressor DtxR. Proc. Natl. Acad. Sci. USA 10218408-18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.den Hengst, C. D., S. A. F. T. van Hijum, J. M. Geurts, A. Nauta, J. Kok, and O. P. Kuipers. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 28034332-34342. [DOI] [PubMed] [Google Scholar]
- 16.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 623662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeWitt, M. A., J. I. Kliegman, J. D. Helmann, R. G. Brennan, D. L. Farrens, and A. Glasfeld. 2007. The conformations of the manganese transport regulator of Bacillus subtilis in its metal-free state. J. Mol. Biol. 3651257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25727-739. [DOI] [PubMed] [Google Scholar]
- 19.Dintilhac, A., and J. P. Claverys. 1997. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res. Microbiol. 148119-131. [DOI] [PubMed] [Google Scholar]
- 20.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300931-936. [DOI] [PubMed] [Google Scholar]
- 21.Golynskiy, M. V., W. A. Gunderson, M. P. Hendrich, and S. M. Cohen. 2006. Metal binding studies and EPR spectroscopy of the manganese transport regulator MntR. Biochemistry 4515359-15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halfmann, A., R. Hakenbeck, and R. Bruckner. 2007. A new integrative reporter plasmid for Streptococcus pneumoniae. FEMS Microbiol. Lett. 268217-224. [DOI] [PubMed] [Google Scholar]
- 23.Hanks, T. S., M. Liu, M. J. McClure, M. Fukumura, A. Duffy, and B. Lei. 2006. Differential regulation of iron- and manganese-specific MtsABC and heme-specific HtsABC transporters by the metalloregulator MtsR of group A Streptococcus. Infect. Immun. 745132-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvie, D. R., C. Andreini, G. Cavallaro, W. Meng, B. A. Connolly, K. Yoshida, Y. Fujita, C. R. Harwood, D. S. Radford, S. Tottey, J. S. Cavet, and N. J. Robinson. 2006. Predicting metals sensed by ArsR-SmtB repressors: allosteric interference by a non-effector metal. Mol. Microbiol. 591341-1356. [DOI] [PubMed] [Google Scholar]
- 25.Hava, D. L., C. J. Hemsley, and A. Camilli. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J. Bacteriol. 185413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriksen, W. T., H. J. Bootsma, S. Estevao, T. Hoogenboezem, A. de Jong, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190590-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 1835709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibs, K. H., and L. Rink. 2003. Zinc-altered immune function. J. Nutr. 1331452S-1456S. [DOI] [PubMed] [Google Scholar]
- 29.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38140-153. [DOI] [PubMed] [Google Scholar]
- 30.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 712656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston, J. W., D. E. Briles, L. E. Myers, and S. K. Hollingshead. 2006. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 741171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 725858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jordan, A., and P. Reichard. 1998. Ribonucleotide reductases. Annu. Rev. Biochem. 6771-98. [DOI] [PubMed] [Google Scholar]
- 34.Karlyshev, A. V., M. J. Pallen, and B. W. Wren. 2000. Single-primer PCR procedure for rapid identification of transposon insertion sites. BioTechniques 281078-1082. [DOI] [PubMed] [Google Scholar]
- 35.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 684441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kliegman, J. I., S. L. Griner, J. D. Helmann, R. G. Brennan, and A. Glasfeld. 2006. Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis. Biochemistry 453493-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloosterman, T. G., J. J. E. Bijlsma, J. Kok, and O. P. Kuipers. 2006. To have neighbour's fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology 152351-359. [DOI] [PubMed] [Google Scholar]
- 38.Kloosterman, T. G., W. T. Hendriksen, J. J. Bijlsma, H. J. Bootsma, S. A. van Hijum, J. Kok, P. W. Hermans, and O. P. Kuipers. 2006. Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J. Biol. Chem. 28125097-25109. [DOI] [PubMed] [Google Scholar]
- 39.Kloosterman, T. G., M. M. van der Kooi-Pol, J. J. Bijlsma, and O. P. Kuipers. 2007. The novel transcriptional regulator SczA mediates protection against Zn(2+) stress by activation of the Zn-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 651049-1063. [DOI] [PubMed] [Google Scholar]
- 40.Kolenbrander, P. E., R. N. Andersen, R. A. Baker, and H. F. Jenkinson. 1998. The adhesion-associated sca operon in Streptococcus gordonii encodes an inducible high-affinity ABC transporter for Mn2+ uptake. J. Bacteriol. 180290-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuipers, O. P., P. G. Ruyter, M. Kleerebezem, and W. M. Vos. 1998. Quorum sensing controlled gene expression in lactic acid bacteria. J. Biotechnol. 6415-21. [Google Scholar]
- 42.Lampe, D. J., M. E. Churchill, and H. M. Robertson. 1996. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 155470-5479. [PMC free article] [PubMed] [Google Scholar]
- 43.Lanie, J. A., W. L. Ng, K. M. Kazmierczak, T. M. Andrzejewski, T. M. Davidsen, K. J. Wayne, H. Tettelin, J. I. Glass, and M. E. Winkler. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 18938-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 61553-1561. [DOI] [PubMed] [Google Scholar]
- 45.Leenhouts, K., G. Buist, A. Bolhuis, A. ten Berge, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253217-224. [DOI] [PubMed] [Google Scholar]
- 46.Loo, C. Y., K. Mitrakul, I. B. Voss, C. V. Hughes, and N. Ganeshkumar. 2003. Involvement of the adc operon and manganese homeostasis in Streptococcus gordonii biofilm formation. J. Bacteriol. 1852887-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marra, A., J. Asundi, M. Bartilson, S. Lawson, F. Fang, J. Christine, C. Wiesner, D. Brigham, W. P. Schneider, and A. E. Hromockyj. 2002. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect. Immun. 701422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marra, A., S. Lawson, J. S. Asundi, D. Brigham, and A. E. Hromockyj. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 1481483-1491. [DOI] [PubMed] [Google Scholar]
- 49.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38867-878. [DOI] [PubMed] [Google Scholar]
- 50.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53889-901. [DOI] [PubMed] [Google Scholar]
- 51.McCluskey, J., J. Hinds, S. Husain, A. Witney, and T. J. Mitchell. 2004. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol. Microbiol. 511661-1675. [DOI] [PubMed] [Google Scholar]
- 52.McKessar, S. J., and R. Hakenbeck. 2007. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J. Bacteriol. 1891342-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milanino, R., M. Marrella, R. Gasperini, M. Pasqualicchio, and G. Velo. 1993. Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions 39195-209. [DOI] [PubMed] [Google Scholar]
- 54.Mitrakul, K., C. Y. Loo, C. Gyurko, C. V. Hughes, and N. Ganeshkumar. 2005. Mutational analysis of the adcCBA genes in Streptococcus gordonii biofilm formation. Oral Microbiol. Immunol. 20122-127. [DOI] [PubMed] [Google Scholar]
- 55.Montanez, G. E., M. N. Neely, and Z. Eichenbaum. 2005. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology 1513749-3757. [DOI] [PubMed] [Google Scholar]
- 56.Moore, C. M., A. Gaballa, M. Hui, R. W. Ye, and J. D. Helmann. 2005. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 5727-40. [DOI] [PubMed] [Google Scholar]
- 57.Moore, C. M., and J. D. Helmann. 2005. Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 8188-195. [DOI] [PubMed] [Google Scholar]
- 58.Nies, D. H. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27313-339. [DOI] [PubMed] [Google Scholar]
- 59.Novak, R., J. S. Braun, E. Charpentier, and E. Tuomanen. 1998. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol. Microbiol. 291285-1296. [DOI] [PubMed] [Google Scholar]
- 60.Novak, R., E. Tuomanen, and E. Charpentier. 2000. The mystery of psaA and penicillin tolerance in Streptococcus pneumoniae. Mol. Microbiol. 361504-1505. [DOI] [PubMed] [Google Scholar]
- 61.Obaro, S., and R. Adegbola. 2002. The pneumococcus: carriage, disease and conjugate vaccines. J. Med. Microbiol. 5198-104. [DOI] [PubMed] [Google Scholar]
- 62.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 725582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paik, S., A. Brown, C. L. Munro, C. N. Cornelissen, and T. Kitten. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J. Bacteriol. 1855967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panina, E. M., A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA 1009912-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 351454-1468. [DOI] [PubMed] [Google Scholar]
- 66.Ricci, S., R. Janulczyk, and L. Bjorck. 2002. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect. Immun. 704968-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rolerson, E., A. Swick, L. Newlon, C. Palmer, Y. Pan, B. Keeshan, and G. Spatafora. 2006. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. J. Bacteriol. 1885033-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosch, J. W., B. Mann, J. Thornton, J. Sublett, and E. Tuomanen. 2008. Convergence of regulatory networks on the pilus locus of Streptococcus pneumoniae. Infect. Immun. 763187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-Beato, A. R., R. Lopez, and J. L. Garcia. 1998. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol. Lett. 164207-214. [DOI] [PubMed] [Google Scholar]
- 70.Sanders, J. W., G. Venema, J. Kok, and K. Leenhouts. 1998. Identification of a sodium chloride-regulated promoter in Lactococcus lactis by single-copy chromosomal fusion with a reporter gene. Mol. Gen. Genet. 257681-685. [DOI] [PubMed] [Google Scholar]
- 71.Sazawal, S., R. E. Black, S. Jalla, S. Mazumdar, A. Sinha, and M. K. Bhan. 1998. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics 1021-5. [DOI] [PubMed] [Google Scholar]
- 72.Shankar, A. H., and A. S. Prasad. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68447S-463S. [DOI] [PubMed] [Google Scholar]
- 73.Song, J. H., K. S. Ko, J. Y. Lee, J. Y. Baek, W. S. Oh, H. S. Yoon, J. Y. Jeong, and J. Chun. 2005. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol. Cells 19365-374. [PubMed] [Google Scholar]
- 74.Spatafora, G., M. Moore, S. Landgren, E. Stonehouse, and S. Michalek. 2001. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology 1471599-1610. [DOI] [PubMed] [Google Scholar]
- 75.Strand, T. A., D. E. Briles, H. K. Gjessing, A. Maage, M. K. Bhan, and H. Sommerfelt. 2001. Pneumococcal pulmonary infection, septicaemia and survival in young zinc-depleted mice. Br. J. Nutr. 86301-306. [DOI] [PubMed] [Google Scholar]
- 76.Strand, T. A., S. K. Hollingshead, K. Julshamn, D. E. Briles, B. Blomberg, and H. Sommerfelt. 2003. Effects of zinc deficiency and pneumococcal surface protein a immunization on zinc status and the risk of severe infection in mice. Infect. Immun. 712009-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thijs, G., K. Marchal, M. Lescot, S. Rombauts, M. B. De, P. Rouze, and Y. Moreau. 2002. A Gibbs sampling method to detect overrepresented motifs in the upstream regions of coexpressed genes. J. Comput. Biol. 9447-464. [DOI] [PubMed] [Google Scholar]
- 78.Torrents, E., G. Buist, A. Liu, R. Eliasson, J. Kok, I. Gibert, A. Graslund, and P. Reichard. 2000. The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and allosteric regulation of the pure enzyme system. J. Biol. Chem. 2752463-2471. [DOI] [PubMed] [Google Scholar]
- 79.Tseng, H. J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 701635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Hijum, S. A. F. T., A. de Jong, R. J. S. Baerends, H. A. Karsens, N. E. Kramer, R. Larsen, C. D. den Hengst, C. J. Albers, J. Kok, and O. P. Kuipers. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Versieck, J. 1985. Trace elements in human body fluids and tissues. Crit. Rev. Clin. Lab. Sci. 2297-184. [DOI] [PubMed] [Google Scholar]