Abstract
Integrating conjugative elements (ICEs) are self-transmissible, mobile elements that are widespread among bacteria. Following their excision from the chromosome, ICEs transfer by conjugation, a process initiated by a single-stranded DNA break at a specific locus called the origin of transfer (oriT). The SXT/R391 family of ICEs includes SXTMO10, R391, and more than 25 related ICEs found in gammaproteobacteria. A previous study mapped the oriT locus of SXTMO10 to a 550-bp intergenic region between traD and s043. We suspected that this was not the correct oriT locus, because the identical traD-s043 region in R391 and other SXT/R391 family ICEs was annotated as a gene of an unknown function. Here, we investigated the location and structure of the oriT locus in the ICEs of the SXT/R391 family and demonstrated that oriTSXT corresponds to a 299-bp sequence that contains multiple imperfect direct and inverted repeats and is located in the intergenic region between s003 and rumB′. The oriTSXT locus is well conserved among SXT/R391 ICEs, like R391, R997, and pMERPH, and cross-recognition of oriTSXT and oriTR391 by R391 and SXTMO10 was demonstrated. Furthermore, we identified a previously unannotated gene, mobI, located immediately downstream from oriTSXT, which proved to be essential for SXTMO10 transfer and SXTMO10-mediated chromosomal DNA mobilization. Deletion of mobI did not impair the SXTMO10-dependent transfer of the mobilizable plasmid CloDF13, suggesting that mobI has no role in the assembly of the SXTMO10 mating pair apparatus. Instead, mobI appears to be involved in the recognition of oriTSXT.
Integrating conjugative elements (ICEs) are a large family of self-transmissible mobile genetic elements that are widespread among bacteria (8, 12). These elements confer a range of properties upon the host bacteria, including resistance to antibiotics and heavy metals, virulence, symbiosis establishment, and alternative metabolic pathways. ICEs are made of a core set of genes ensuring their maintenance, mobility, and regulation (8, 12, 44, 50).
Following their excision from the chromosome of the host cell as a circular intermediate, ICEs transfer by conjugation, using a mechanism similar to that of conjugative plasmids (8, 15, 19, 23, 32, 39). Integration into and excision from the chromosome occur by recombination mediated by an ICE-encoded site-specific recombinase called integrase (Int) (10). Several ICEs are able to mobilize nonconjugative plasmids in cis and in trans as well as chromosomal DNA in an Hfr-like manner (27).
Conjugative DNA transfer takes place in two key steps: (i) biochemical processing of the DNA molecule for transfer and (ii) assembly of a mating apparatus bridging the donor and recipient cells to allow DNA transfer. Typically conjugative DNA transfer is initiated at a specific cis-acting site called the origin of transfer (oriT), required for efficient translocation of the DNA to the recipient cell (23, 39). A DNA relaxase encoded by the conjugative element recognizes the oriT locus and cleaves one strand within the oriT locus at a specific site called nic, forming a single-strand DNA break. The relaxase remains covalently attached to the 5′ end of the nicked DNA strand (22) that is translocated into the recipient cell through the mating bridge (34, 43, 51). Within the recipient cell, host enzymes convert the transferred single-stranded DNA into double-stranded DNA that can be recircularized and/or recombined into the recipient chromosome.
In most documented cases, auxiliary proteins of both host and plasmid origins assist the relaxase, allowing the formation of a DNA-protein complex known as the relaxosome (14, 42). Plasmid oriTs generally contain features, such as inverted repeats and A tracts, that are located near the plasmid strand cleavage site and form hairpins when in single-stranded form (19).
In contrast to plasmid oriTs, little is known about initiation of conjugative transfer of ICEs. To date, the oriTs from only two ICEs, Tn916 from Enterococcus faecalis and ICEBs1 from Bacillus subtilis, have been identified and characterized (30, 31). Like conjugative plasmids, ICEs encode their own relaxases, able to recognize and bind to the oriT, and cut at the nic site. Auxiliary proteins were also described for Tn916, which requires the transposon-encoded integrase, conferring both strand and sequence specificities to the endonucleolytic cleavage activity of the relaxase Orf20 (45).
SXTMO10 is a 99.5-kb ICE initially identified in clinical strains of Vibrio cholerae O139 from India. It confers resistance to chloramphenicol, streptomycin, sulfamethoxazole, and trimethoprim (49). R391 is an ICE derived from a clinical isolate of Providencia rettgeri from South Africa (16), conferring kanamycin and mercury resistance (41). Both genetic studies and DNA sequence analyses revealed that R391 and SXTMO10 are functionally and genetically related (4, 6, 26). They share a highly conserved genetic backbone, coding for their regulation, excision/integration, and conjugative transfer (3). Genes specific to each ICE are interspersed in the conserved sequence, and hot spots have been identified as targets for different insertions. SXTMO10, R391, and more than 25 related ICEs (1, 9, 29, 38) were recently grouped within the SXT/R391 family (7); all these ICEs are characterized by a highly conserved integrase, IntSXT, and by the ability to integrate into the 5′ end of prfC, a nonessential gene involved in the termination of translation (28).
Beaber et al. (4) reported that the DNA-processing region of SXTMO10 and R391 encompasses three genes: (i) traI, which encodes a putative relaxase; (ii) traD, which encodes a putative coupling protein that is part of the mating pore and acts as a receptor for the relaxase attached to the region encoded by the 5′ end of the transferred single-stranded DNA strand; and (iii) s043 (traJ), encoding another putative conjugation coupling factor. Deletion of any of these three genes abolished SXTMO10 transfer (4, 6). Beaber et al. (4) mapped the oriT locus of SXTMO10 (oriTSXT) to a 550-bp intergenic region between traD and traJ. While no open reading frame (ORF) between traD and traJ in SXTMO10 has been annotated (4), orf35, a putative ORF that encodes a protein of unknown function, has been annotated to occur at the same locus in R391, with no evidence for the presence of an oriT locus (6). The presence of an ORF in this region of R391 led us to question whether oriTSXT had been properly assigned. In the present study, we determined that oriTSXT is actually located in the intergenic region between s003 and rumB′ and characterized the minimal oriT regions of several ICEs belonging to the family SXT/R391. We also identified a new gene, mobI, which is required for SXTMO10 transfer as well as for cis-mobilization of chromosomal DNA in an Hfr-like manner but not for trans-mobilization of the broad-host-range mobilizable plasmid CloDF13.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains used in this study are described in Table 1. The strains were routinely grown in Luria-Bertani (LB) broth at 37°C in an orbital shaker/incubator and were maintained at −80°C in LB broth containing 15% (vol/vol) glycerol. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol, 20 μg/ml; kanamycin, 50 μg/ml; nalidixic acid (Nx), 40 μg/ml; rifampin (Rf), 100 μg/ml; spectinomycin, 50 μg/ml; streptomycin (Sm), 50 μg/ml; sulfamethoxazole (Su), 160 μg/ml; tetracycline (Tc), 12 μg/ml; and trimethoprim (Tm), 32 μg/ml.
TABLE 1.
E. coli K-12-derivative strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| CAG18439 | MG1655 lacZU118 lacI42::Tn10 (Tcr) | 46 |
| CAG18420 | MG1655 lacZU118 lacI42::Tn10kan (Knr) | 46 |
| VB111 | MG1655 Nxr | This study |
| VB112 | MG1655 Rfr | This study |
| HW220 | CAG18439 prfC::SXTMO10 (Cmr Smr Sur Tmr Tcr) | 28 |
| VB4 | CAG18439 prfC::SXTMO10 Δint | V. Burrus, unpublished results |
| VB82 | CAG18420 prfC::SXTMO10 (Cmr Smr Sur Tmr Knr) | This study |
| VB95 | CAG18420 prfC::SXTMO10 Δint | This study |
| VB154 | CAG18420 prfC::SXTMO10 ΔmobI | This study |
| DC5 | VB111 prfC::SXTMO10 (Cmr Smr Sur Tmr Nxr) | This study |
| DC16 | VB111 prfC::SXTMO10 ΔoriT1 | This study |
| DC17 | VB111 prfC::SXTMO10 ΔoriT2 | This study |
| DC6 | VB111 prfC::R391 (Knr Nxr) | This study |
| DC59 | VB111 prfC::R391 ΔoriT1 | This study |
| DC60 | VB111 prfC::R391 ΔoriT2 | This study |
| Plasmids | ||
| pACYC184 | Tcr Cmr | New England Biolabs |
| pSU4628 | CloDF13::TnAΔEcoRV (Apr) | 13 |
| pACYC184 Δcat | pACYC184 Δcat (ΔMscI-PvuII; Tcr) | This study |
| pMRA | pACYC184 Δcat s003-rumB′ (1,141-bp fragment) | This study |
| pMRB | pACYC184 Δcat rumA-s024 (829-bp fragment) | This study |
| pMRC | pACYC184 Δcat traD-traJ (690-bp fragment) | This study |
| pMRD | pACYC184 Δcat traA-s052 (581-bp fragment) | This study |
| pMRE | pACYC184 Δcat s071-s072 (579-bp fragment) | This study |
| pMRA-LM | pACYC184 Δcat oriTSXT (LM fragment) | This study |
| pMRA-M | pACYC184 Δcat oriTSXT | This study |
| pMRA-MR | pACYC184 Δcat oriTSXT-mobI | This study |
| pMRA-Ra | pACYC184 Δcat mobI (first TTG) | This study |
| pMRA-Rb | pACYC184 Δcat mobI (second TTG) | This study |
| pDC-oR391 | pACYC184 Δcat oriTR391 | This study |
| pDC-oR997 | pACYC184 Δcat oriTR997 | This study |
| pDC-oMERPH | pACYC184 Δcat oriTpMERPH | This study |
| pMobI-B | pBAD-TOPO mobI (first TTG codon) | This study |
| pMobI-D | pBAD-TOPO mobI (second TTG codon) | This study |
| pVI36 | pKD13 Δkan::aad7 (Spr) PCR template for one-step chromosomal gene inactivation | 17; V. Burrus, unpublished results |
Bacterial conjugation.
Conjugation assays were used to transfer SXTMO10, R391, or plasmids into Escherichia coli strains. Mating assays were performed by mixing equal volumes of overnight cultures of donor and recipient strains. The cells were harvested by centrifugation and resuspended in a 1/20 volume of LB broth. Cell suspensions were poured onto LB agar plates and incubated at 37°C for 6 h. The cells were then resuspended in 1 ml of LB medium, and serial dilutions were plated onto appropriate selective media to determine the numbers of donors, recipients, and exconjugants. Frequency of transfer was expressed as the number of exconjugant cells per donor cell in the mating mixture at the time of plating. Nx- or Rf-resistant derivative strains of E. coli MG1655 (VB111 and VB112, respectively) were used as recipients in conjugation experiments. To induce expression of pMobI-B or pMobI-D in complementation assays, mating experiments were carried out with LB agar plates supplemented with 0.02% arabinose.
Plasmid and strain construction.
The plasmids used in this study are described in Table 1. Plasmid pACYC184 Δcat was constructed by digestion with MscI/PvuII of pACYC184 DNA harvested from the dam dcm strain E. coli ER2925 (New England Biolabs). After digestion, the 2,424-bp fragment was religated using T4 DNA ligase (New England Biolabs). All of the pACYC184 Δcat-derived plasmids that were used in mobilization experiments were constructed by cloning of XbaI-flanked PCR products into the XbaI site. Plasmids pMRA, pMRB, pMRC, pMRD, and pMRE were created using the primer pairs MELR1/MELR2, MELR3/MELR4, MELR5/MELR6, MELR7/MELR8, and MELR9/MELR10, respectively, and genomic DNA of E. coli HW220 as a template (Tables 1 and 2). Plasmids pMRA-LM, pMRA-M, pMRA-MR, pMRA-Ra, and pMRA-Rb were constructed using the primer pairs oriT1F/oriT1R, oriT2F/oriT1R, oriT2F/MELR2, orfX2F/MELR2, and orfX1F/MELR2, respectively, and genomic DNA of E. coli HW220 as a template (Tables 1 and 2). pDC-oR391, pDC-oR997, and pDC-oMERPH were constructed using the primer pair oriT2F/oriT1R and genomic DNA of E. coli DC6, AB1157 R997, and AB1157 pMERPH (36), respectively, as templates. PCR products were purified from a 1% agarose gel by using a QIAquick gel extraction kit (Qiagen), digested by XbaI, and ligated to XbaI-digested DNA of pACYC184 Δcat, using T4 DNA ligase (New England Biolabs). The inserts of pMRA and all the pACYC184 Δcat-derivative plasmids containing fragments that overlap the s003-rumB′ intergenic region were in the same orientation. Plasmids pMobI-D and pMobI-B, used for complementation assays, were constructed by cloning the short (426-bp) or long (444-bp) version of mobI. The gene mobI was amplified by PCR using primer pair orfX-F1d/orfX-R or orfX-F2d/orfX-R, respectively, into the TA cloning expression vector pBAD-TOPO (Invitrogen) according to the manufacturer's instructions.
TABLE 2.
DNA sequences and positions in SXTMO10 of the primers used in this study
| Primer name | Nucleotide sequence (5′ to 3′)a | Positions (GenBank accession no. AY055428) | Use in this study |
|---|---|---|---|
| MELR1 | CGCTCTAGAATACGATCCGCAGGA | 3199-3220 | Cloning of the intergenic region s003-rumB′ |
| MELR2 | TGCTCTAGAAGGTACGAAGAAAGATTG | 3311-3336 | Cloning of the intergenic region s003-rumB′ |
| MELR3 | CCTCTAGAAACACGTTCCATGAACA | 24143-24167 | Cloning of the intergenic region rumA-s004 |
| MELR4 | GTTTCTAGAATACGCTCCATGCAATCT | 24945-24969 | Cloning of the intergenic region rumA-s004 |
| MELR5 | AGCTCTAGACAGGCAGTATAAGGA | 49176-49198 | Cloning of the intergenic region traD-traJ |
| MELR6 | TTCTCTAGACTAATGACCCGACCACTCCA | 49842-49864 | Cloning of the intergenic region traD-traJ |
| MELR7 | GTCTCTAGAATACGCCAACAGTGCTTG | 55176-55201 | Cloning of the intergenic region traA-s052 |
| MELR8 | TGATCTAGAACGCCCAATTGCACA | 55732-55754 | Cloning of the intergenic region traA-s052 |
| MELR9 | ACTTCTAGATATTCGAACGCTTGA | 80654-80676 | Cloning of the intergenic region s071-s072 |
| MELR10 | AATTCTAGAAGCCATTGGTCGTTAA | 81208-81231 | Cloning of the intergenic region s071-s072 |
| oriT1F | GCTCTAGAGGTTTTCAATCAATCAACCG | 3347-3370 | See Fig. 3A |
| oriT1R | TTTCTAGAAAACCAATTTCCCCA | 3792-3814 | See Fig. 3A |
| oriT2F | GCTCTAGATGGCGGCGGATGA | 3516-3536 | See Fig. 3A |
| orfX1F | TTATCTAGATGGGGAAATTGGTTTGG | 3785-3810 | See Fig. 3A |
| orfX2F | GGTTCTAGATTTTGGGGTTAATTGGA | 3768-3793 | See Fig. 3A |
| orfX-F1d | TGATAGGGGGTTAATTGGAT | 3775-3795 | Complementation of ΔmobI with orfX-R (short ORF) |
| orfX-F2d | TGATAGGGGAAATTGGTTTG | 3790-3810 | Complementation of ΔmobI with orfX-R (long ORF) |
| orfX-R | GAAGTAGCGCAGTTGACTGA | 4250-4230 | |
| orfXWF | TTTGGCTTTTGGGGTTAATTGGATGGGGAAAT TGGTGTGTAGGCTGGAGCTGCTTCG | 3771-3802 | Deletion of mobI in SXTMO10 |
| orfXWR | GGGACCAGTTACCACGAGTGAAGTAGCGCAG TTGACATTCCGGGGATCCGTCGACC | 4269-4233 | Deletion of mobI in SXTMO10 |
| DAN1-F | AGGCTCTGTTTGGCGGCGGATGACCTAGTCAAAAAAGTGTAGGCTGGAGCTGCTTCG | 3516-3553 | Deletion of oriT1 and oriT2 in SXTMO10 |
| DAN1-R | CCCATCCAATTAACCCCAAAAGCCAAAACCACTATCATTCCGGGGATCCGTCGACC | 3762-3798 | Used with DAN1-F for deletion of oriT2 in SXTMO10 |
| DAN1-R2 | TGCCGTAACAATCACTGTTTGGCGTCTCGATATAAAATTCCGGGGATCCGTCGACC | 3690-3726 | Used with DAN1-F for deletion of oriT1 in SXTMO10 |
| DAN2-F | AGGCTCTGTTTGGCGGTGGATGACTGAGCCAAAAAAGTGTAGGCTGGAGCTGCTTCG | 5426-5463 | Deletion of oriT1 and oriT2 in R391 |
| DAN2-R | CCCATCCAATTAACCCCAAAAACCAAAACTACTATCATTCCGGGGATCCGTCGACC | 5708-5672 | Used with DAN1-F for deletion of oriT2 in R391 |
| DAN2-R2 | TGCCGTAACAATCACTGTTTGGCGTCTCGATATAAAATTCCGGGGATCCGTCGACC | 5636-5600 | Used with DAN2-F for deletion of oriT1 in R391 |
Underlined nucleotides indicate the XbaI site introduced for cloning purposes.
ΔmobI, ΔoriT1, and ΔoriT2 mutations were introduced into SXTMO10 by using the one-step chromosomal gene inactivation technique (17) with primers orfXWF/orfXWR, DAN1-F/DAN1-R2, and DAN1-F/DAN1-R, respectively, and pVI36 as the template (Tables 1 and 2) as previously described (4). Similarly, ΔoriT1 and ΔoriT2 mutations were introduced into R391 by using the same technique with primers and DAN2-F/DAN2-R2 and DAN2-F/DAN2-R, respectively, and pVI36 as the template.
Molecular biology techniques.
Plasmid DNA was prepared with a QIAprep spin mini prep kit (Qiagen) or a QIAfilter plasmid midi kit (Qiagen), as described in the manufacturer's instructions. All the enzymes used in this study were provided by New England Biolabs and were used according to manufacturer's instructions. The PCR assays for amplifying fragments cloned into the pACYC184 Δcat vector were performed with the primers described in Table 2 with 50-μl PCR mixtures with 1 U of Taq DNA polymerase (New England Biolabs). The PCR conditions were as follows: (i) 3 min at 94°C; (ii) 30 cycles of 30 s at 94°C, 30 s at a suitable annealing temperature, and 30 s to 90 s at 72°C; and (iii) 2 min at 72°C. When needed, PCR products were purified using a QIAquick PCR purification kit (Qiagen) according to the manufacturer's instructions. The purified PCR products or inserts of constructed plasmids were sequenced by DNA LandMarks, Inc. (Saint-Jean-sur-Richelieu, Quebec, Canada). The DNA sequences were compared with the GenBank DNA sequence database by using the BLASTN program (2). E. coli was transformed by electroporation according to Dower et al. (18), using a Bio-Rad GenePulser Xcell apparatus set at 25 μF, 200 Ω, and 2.5 kV.
RESULTS
Location of oriTSXT revisited.
To characterize further the 550-bp region of SXTMO10 that was reported as containing oriT (4) and determine the minimal segment required for efficient transfer, we amplified by PCR a 690-bp fragment overlapping the traD-traJ intergenic region and cloned it into pACYC184 Δcat. This plasmid is a Tc resistance-conferring vector derived from the low-copy-number, nonmobilizable plasmid pACYC184. We aimed to use the resulting plasmid, pMRC, as a positive control to compare the efficiency of mobilization by SXTMO10 of plasmids harboring a range of shorter fragments overlapping the insert of pMRC.
pMRC was introduced into E. coli VB95, a strain that contains a Δint mutant of SXTMO10 able to produce the Tra proteins required for conjugative transfer but unable to excise from the chromosome. The use of SXTMO10 Δint ensures that mobilization of the plasmid does not occur by homologous recombination-mediated cointegration with and subsequent transfer of SXTMO10 to the recipient (Fig. 1). A recA strain could not be considered for these experiments, since the presence of recA is essential for an adequate expression of the tra genes in SXTMO10 (5).
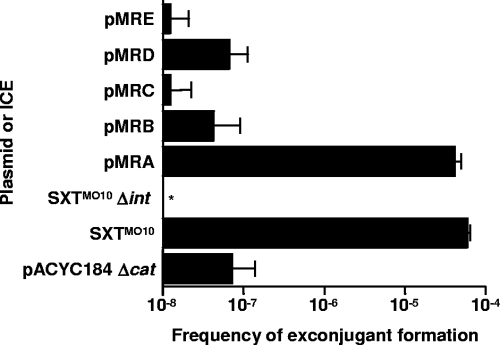
FIG. 1.
Identification of the cis-acting region of SXTMO10 required for efficient transfer of a nonmobilizable vector. Mobilization of plasmids containing various intergenic regions of SXTMO10 was assessed using a conjugation assay. The inserts in these plasmids, all derivatives of the nonmobilizable vector pACYC184 Δcat, were intergenic regions s003-rumB′ (pMRA), rumA-s024 (pMRB), traD-traJ (pMRC), traA-s052 (pMRD), and s071-s072 (pMRE). The frequency of exconjugant formation was obtained by dividing the number of exconjugants (Tcr Nxr CFU) by the number of donors (Knr CFU). Mobilization assays were carried out using E. coli CAG18420 SXTMO10 Δint (VB95) as the donor strain. The frequency of transfer of SXTMO10 was determined by using E. coli VB82 as a donor strain. E. coli VB111, a Nxr-derivative strain of E. coli MG1655 was used as the recipient strain in all the assays. The bars represent the mean and standard deviation values obtained from three independent experiments. The asterisk indicates that the frequency of exconjugant formation was below the limit of detection (1 × 10−8).
VB95 pMRC was used as a donor in mating experiments involving a Nx-resistant derivative of E. coli MG1655 as a recipient strain (Fig. 1). Nxr Tcr exconjugants normally formed at a very low frequency when pACYC184 Δcat was used as a negative control. Indeed, this plasmid cannot be mobilized unless its insert contains a genuine oriT locus that is recognized by the transfer proteins encoded by SXTMO10. Surprisingly, we observed that the frequency of transfer of pMRC was lower than that of the control and more than 3 orders of magnitude lower than that of wild-type SXTMO10 (Fig. 1). This observation was in marked contrast with the Beaber et al. report (4) in which the 550-bp traD-traJ intergenic region was found to allow the mobilization by SXTMO10 of a high-copy-number, nonmobilizable vector at a frequency that was similar to that of SXTMO10. To determine the reason for this discrepancy, we took a closer look at the traD-traJ region of SXTMO10.
Our analysis of the sequence of the fragment of SXTMO10 (GenBank accession no. AY055428) that encompasses traD and traJ (positions 45,042 to 51,360) revealed three sequence mismatches and one +1 frameshift mutation compared to the sequence of the same locus (positions 64,662 to 58,345) in the whole genome of V. cholerae MO10 (GenBank accession no. NZ_AAKF00000000), i.e., the original host of SXTMO10 (data not shown). Resequencing of this region based on PCR products amplified from HW220 (Table 1), an E. coli CAG18439 exconjugant containing SXTMO10, revealed 100% identity at the nucleotide level with the sequence of MO10. Taking into account these four sequence alterations in the SXTMO10 genome, the traD-traJ intergenic region of SXTMO10 in V. cholerae MO10 actually contains a 561-bp ORF that was overlooked in the previous annotation and codes for an hypothetical protein designated VchoM_02001241 (GenBank accession no. NZ_AAKF02000011, nucleotides 59939 to 60499).
Nucleotide BLAST analysis of VchoM_02001241 against the GenBank database revealed 94% identity with orf35 of R391 from P. rettgeri (AY090559.1) and orf1755 of ICESpuPO1 from Shewanella sp. strain W3-18-1 (CP000503) as well as 95% identity with a hypothetical ORF of ICEPdaSpa1 from the fish pathogen Photobacterium damselae subsp. piscicida (AJ870986). orf35 and orf1755 code for hypothetical proteins of unknown functions (6, 40). Our analysis did not reveal any domain or molecular arrangement typical of oriTs in VchoM_02001241, orf35, orf1755, or the hypothetical ORF in ICEPdaSpa1.
The presence of an ORF between traD and traJ and our inability to mobilize pMRC conflicted with previously published data (4) and led us to reconsider the localization of oriT in the ICEs of the SXT/R391 family.
oriTSXT is located within the intergenic region s003-rumB′.
We adopted a comparative genomics approach that facilitated rapid identification of the region of SXTMO10 that contained oriT. We reasoned that this functional fragment was likely to be (i) an intergenic sequence and (ii) a region conserved in the core set of sequences shared by SXTMO10, R391, and the other ICEs of the SXT/R391 family. We identified four intergenic regions that met these criteria: s003-rumB′, rumA-s024, traA-s052, and s071-s072, here named A, B, D, and E, respectively.
The four intergenic regions were amplified by PCR (see Table 2 for primer details) and cloned into pACYC184 Δcat, yielding the plasmids pMRA, pMRB, pMRD, and pMRE. Each plasmid was introduced into E. coli VB95. The results of mating experiments involving E. coli VB95 donors harboring each plasmid are shown in Fig. 1. All plasmids but pMRA exhibited transfer frequencies comparable to or below that of the empty vector, i.e., 3 to 4 orders of magnitude below that of SXTMO10. In fact, phenotypic and molecular analysis of apparent Nxr Tcr exconjugants indicated that most colonies were spontaneous Nxr mutants of donor cells and not genuine exconjugants (data not shown), confirming the absence of cotransfer of SXTMO10 Δint with the pACYC184 Δcat derivatives. In contrast, pMRA transferred at a rate that was comparable to the rate of transfer of SXTMO10 (Fig. 1).
These data demonstrate that oriTSXT was located not in the traD-traJ region but in the intergenic region between s003 and rumB′ instead.
The intergenic region s003-rumB′ is conserved in SXT/R391 ICEs and contains a new ORF.
The 918-bp s003-rumB′ intergenic region was further analyzed. Nucleotide BLAST analysis revealed that this region shares 95%, 97%, 100%, and 96% identity with corresponding regions of R391 (GenBank accession no. AY090559.1), ICESpuPO1 (GenBank accession no. NC_008750.1), ICEPdaSpa1 (GenBank accession no. AJ870986), and the incompletely characterized ICEVchVie1 from V. cholerae V21 (GenBank accession no. AB114188), respectively. This intergenic region also shares 96% identity with segments of the genomes of V. cholerae B33 (GenBank accession no. NZ_AAWE01000040), V. cholerae MZO-3 (GenBank accession no. NZ_AAUU01000001), and the uropathogenic strain Proteus mirabilis HI4320 (GenBank accession no. AM942759). These three strains harbor uncharacterized ICEs of the SXT/R391 family that we named here ICEVchB33, ICEVchMZO3, and ICEPmiHI4320, respectively. A ClustalW alignment of these sequences showed that the intergenic region that contains the SXTMO10 oriT locus is extremely well conserved among the SXT/R391 ICEs (299 bp of this intergenic region are shown in Fig. 2).
FIG. 2.
ClustalW alignment of the 299-bp M fragment located within the s003-rumB′ intergenic region of SXTMO10, with the corresponding sequences from nine different SXT/R391 ICEs. The SXTMO10 sequence from the whole genome of V. cholerae MO10 (GenBank accession no. NZ_AAKF00000000) is used as the reference. Identical nucleotides in other ICEs are represented by dots, whereas variations of nucleotide sequence are indicated at the corresponding positions. Underlined nucleotides indicate the positions of primers DAN1-F, DAN1-R2, and DAN1-R, used for oriTSXT deletions. In the same positions are the primers DAN2-F, DAN2-R2, and DAN2-R, used for oriTR391 deletions. The imperfect direct and indirect repeats are shown by arrows. The two possible TTG initiation codons of mobI are shown in bold. The putative ribosome binding site upstream from the second TTG of mobI is shown in italic.
Further analysis of the 918-bp intergenic region s003-rumB′ revealed the presence of a set of five imperfect direct and inverted repeats located 222 bp upstream from the initiation codon of s003 (Fig. 2). Direct and inverted repeats are a common feature found in oriT regions in conjugative plasmids (39). These repeats might be involved in the specific recognition of the oriT locus by the relaxase and/or accessory proteins, allowing the single-strand, site-specific cleavage reaction to take place at the nic site (21, 24, 39).
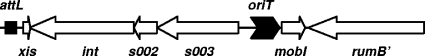
An ORF of 444 nucleotides, here named mobI, directed toward rumB′ was identified 13 bp downstream from the rumB′ stop codon by using the gene prediction program GeneMark.hmm (33) (Fig. 3A). mobI was overlooked in the previous annotation of the SXTMO10 genome, likely due to its anomalous initiation codon TTG. Two alternative TTG initiation codons were identified at positions 1 and 19 of this ORF, producing two possible translation products of 146 and 140 amino acids, respectively. Protein BLAST analysis (BlastP) of both putative proteins did not reveal any similarity with existing proteins or conserved domains in the GenBank database. However, we found that mobI was also present in R391, ICESpuPO1, ICEPdaSpa1, ICEVchVie1, ICEVchB33, ICEVchMZO3, and ICEPmiHI4320. In each ICE, mobI contained the two alternative TTG initiation sites and encoded putative proteins that shared between 97 and 100% identity with the two alternative hypothetical proteins encoded by mobI in SXTMO10. The conservation of mobI suggests that this ORF could play a significant role as a protein-encoding gene in the transfer or maintenance of SXT/R391 ICEs.
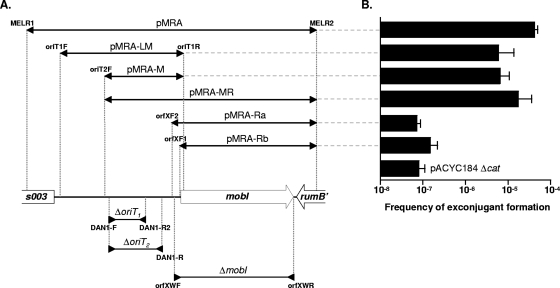
FIG. 3.
Identification of the oriT locus of SXTMO10. Panel A is a schematic representation of the region of SXTMO10 encompassing the beginning of s003 and the end of rumB′. The positions of s003, mobI, and rumB′ are indicated. The inserts of the plasmids used in mobilization experiments, all of which were derived from the low-copy-number, nonmobilizable vector pACYC184 Δcat, are represented above the genetic map by overlapping segments delimited by arrows pointing outwards. Deletions within the region are depicted below the genetic map by overlapping segments delimited by arrows pointing inwards. The positions of the oligonucleotides used for amplification and cloning or construction of the deletions are indicated. Panel B corresponds to the results of experiments determining the mobilization of the different plasmids by SXTMO10 Δint. The frequency of exconjugant formation was obtained by dividing the number of exconjugants (Rfr Tcr CFU) by the number of donors (Knr CFU). In all the cases, the donor was E. coli VB95 and the recipient strain was E. coli VB112. The bars indicate the mean values and standard deviations of results from three independent experiments.
oriT is a 299-bp segment located upstream from mobI.
To further localize the site of oriTSXT within the 918-bp s003-rumB′ region, five distinct overlapping fragments of 468 bp (LM), 299 bp (M), 821 bp (MR), 550 bp (Rb), and 571 bp (Ra) were amplified by PCR and cloned into pACYC184 Δcat, yielding pMRA-LM, pMRA-M, pMRA-MR, pMRA-Ra, and pMRA-Rb, respectively (Fig. 3A). Inserts Ra and Rb both contain mobI and differ only by the forward primers used to amplify the region, containing the first and the second TTG initiation codons of mobI, respectively. Insert M contains the DNA sequence overlapping the five imperfect direct and inverted repeats described above. The five resulting plasmids were introduced into E. coli VB95, and mobilization assays were carried out as described above. The results of these experiments are shown in Fig. 3B.
The frequency of exconjugant formation for the plasmids containing insert Ra or Rb did not differ significantly from that of the empty vector. This result indicates that neither of these two inserts contained oriTSXT as they were not mobilized by SXTMO10 Δint. Therefore, mobI does not correspond to the functional cis-acting oriT locus. In marked contrast, all of the plasmids harboring inserts overlapping the segment M, such as LM and MR, were found to be mobilizable by SXTMO10 Δint. In addition, insert M itself was sufficient to allow SXTMO10 Δint-mediated mobilization of the plasmid, suggesting that oriTSXT was located within this 299-bp fragment. Interestingly, the frequency of exconjugant formation for pMRA-LM and pMRA-M was slightly lower than that of pMRA (Fig. 3B). Such a reduction of the frequency of transfer might result from missing cis-acting auxiliary sequences that could enhance the efficiency of transfer. Indeed, the presence of mobI on the plasmid seemed to slightly increase the efficiency of transfer as shown in Fig. 3B.
In an attempt to narrow the size of the functional oriTSXT sequence, we constructed two deletion mutants in the 299-bp M region. In the first mutant, SXTMO10 ΔoriT1 (DC16), a 139-bp segment containing the repeated sequence R1 was deleted (Fig. 2 and 3A). In the second mutant, SXTMO10 ΔoriT2 (DC17), a 211-bp segment including the repeated sequences R1 to R4 was deleted. Both deletions were designed to avoid affecting the first putative initiation codon of mobI (Fig. 2 and 3A). E. coli DC16 and DC17 were used as donor strains in mating assays to assess the effect of each deletion on SXTMO10 transfer. Both mutations had a marked effect: the ΔoriT1 mutation decreased 120-fold the rates of transfer of the Su and Tm markers compared to the wild type, whereas the ΔoriT2 mutation nearly abolished the transfers of these two markers (Fig. 4).
FIG. 4.
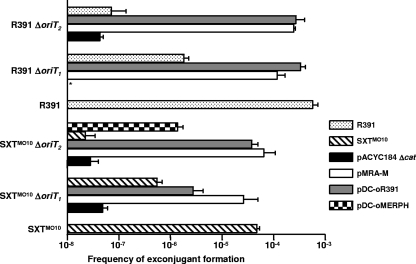
Cross-recognition and activity of SXTMO10 and R391 on their cognate oriTs and on the oriT locus of pMERPH. The plasmids used in mobilization experiments, all of which were derived from the low-copy-number, nonmobilizable vector pACYC184 Δcat, contained the oriT fragment from SXTMO10 (pMRA-M), R391 (pDC-oR391), or pMERPH (pDC-oMERPH). pACYC184 Δcat was used as a negative control. All donors were derivatives of E. coli VB111 containing SXTMO10 or R391 or the ΔoriT1 or ΔoriT2 mutants of these two ICEs. In all the cases, the recipient strain was E. coli VB112. Frequency of exconjugant formation was determined by dividing the number of exconjugants (Rfr Tcr CFU for the pACYC184 Δcat derivatives, Rfr Knr CFU for R391 mutants, or Rfr Sur Tmr CFU for SXTMO10 mutants) by the number of donors (Nxr CFU). The bars indicate the mean values and standard deviations of results from three independent experiments. The asterisk indicates that the frequency of exconjugant formation was below the limit of detection (1 × 10−8).
We also constructed the same 139-bp ΔoriT1 and 211-bp ΔoriT2 mutations in R391 as in SXTMO10, assuming that oriTR391 was located at the same locus as in SXTMO10 and that these deletions would affect R391 transfer as they did for SXTMO10. Indeed, while the overall rate of transfer of R391 was about 10-fold higher than that of SXTMO10, as observed for SXTMO10, the deletion mutants R391 ΔoriT1 (DC59) and R391 ΔoriT2 (DC60) exhibited low frequencies of transfer compared to the wild type: the ΔoriT1 mutation strongly decreased the rate of transfer of the Kn marker, and the ΔoriT2 mutation nearly abolished it (Fig. 4).
These data clearly indicated that the region overlapping the repeats R1 to R4, absent in the ΔoriT2 mutants, was necessary for efficient transfer of both SXTMO10 and R391. Interestingly, while deletion of the first 139 bp starting at the repeat R1 strongly affected the transfer of both SXTMO10 and R391, it did not completely abolish it.
Cross-recognition of different oriT alleles between the members of the SXT/R391 family.
Although the alignment of oriT sequences of the ICEs of the SXT/R391 family revealed that this region is highly conserved (Fig. 2), differences exist. R391 is one of the ICEs that exhibit the highest divergence in this particular segment. Furthermore, while all the Tra proteins that are encoded by SXTMO10 and R391 share from 98 to 100% identity, TraISXT, the putative relaxase encoded by SXTMO10, is only 94.3% identical to TraIR391, the putative relaxase encoded by R391. We wondered whether this divergence could confer specificity to the relaxase for the recognition of its cognate oriT locus; for instance, TraIR391 might not recognize or act at oriTSXT as efficiently as oriTR391 and vice and versa.
To assess whether this divergence was conferring to SXTMO10 and R391 any specificity for their cognate oriT loci, we compared the rate of mobilization by each ICE of plasmids containing one or the other oriT. First, the 299-bp M fragment of R391 was amplified by PCR and cloned into pACYC184 Δcat, giving pDC-oR391. We verified that pDC-oR391 was mobilizable by R391 by introducing it into and mobilizing it from E. coli DC59 and DC60, two strains containing, respectively, R391 ΔoriT1 and R391 ΔoriT2 to avoid cointegration and cotransfer of pDC-oR391 with R391. As shown in Fig. 4, pDC-oR391 was mobilized by both mutants at a frequency that was comparable to that of wild-type R391, clearly demonstrating the presence of oriTR391 in the insert of this plasmid.
We then investigated the specificity of SXTMO10 and R391 for their cognate oriT loci by comparing the frequencies of mobilization of pMRA-M (oriTSXT) and pDC-oR391 (oriTR391) from E. coli VB111 harboring ΔoriT mutants of either SXTMO10 or R391. We found that SXTMO10 ΔoriT1 and SXTMO10 ΔoriT2 were able to mobilize both pMRA-M and pDC-oR391 at comparable rates (Fig. 4); yet, SXTMO10 ΔoriT1 mobilized pMRA-M slightly more efficiently than pDC-oR391. Similar results were observed with R391 ΔoriT1 and R391 ΔoriT2 for both plasmids, but without any significant difference with either of these two plasmids.
We extended our study to the oriT loci of two other ICEs belonging to the SXT/R391 family: R997 from Proteus mirabilis and pMERPH from Shewanella putrefaciens. Since the complete sequences of these two ICEs were not available, we amplified the 299-bp M region by using the same primer pair as for amplification of oriTSXT and oriTR391 (Table 2). The amplicons were cloned into pACYC184 Δcat, yielding plasmids pDC-oR997 and pDC-oMERPH, and sequenced (Fig. 2). Interestingly, ClustalW alignment of the cloned fragment revealed that the regions encompassing oriT are virtually identical in SXTMO10, ICEPdaSpa1, and R997. Since oriTR997 and oriTSXT are identical and mobilization of pDC-oR997 (oriTR997) was expected to yield results identical to that of pMRA-M, only mobilization of pDC-oMERPH (oriTpMERPH) was carried out (Fig. 4). We observed that SXTMO10 ΔoriT2 was able to mobilize the plasmid containing oriTpMERPH to the recipient. However, the rate of transfer of pDC-oMERPH was more than 10 times lower that the rate of transfer of pMRA-M. This defect could be attributed to the insertion of a single nucleotide into the R1 repeat in pDC-oMERPH (Fig. 2). This hypothesis is consistent with the observation that the absence of the repeat R1 in the ΔoriT1 mutants strongly diminishes the transfers of both SXTMO10 and R391.
Together, these data indicate that the divergences observed among the members of the SXT/R391 family, between the TraI proteins, and between the oriT loci do not seem to constitute a barrier to the initiation of transfer at the oriT locus of another ICE of the SXT/R391 family.
mobI is required for SXTMO10 transfer and acts in trans.
To investigate the role of mobI in the transfer of SXTMO10, we constructed the deletion mutant E. coli CAG18420 SXTMO10 ΔmobI (VB154). The frequency of transfer of SXTMO10 ΔmobI from CAG18420 was more than 3 orders of magnitude lower than that of wild-type SXTMO10 (Fig. 5). This observation indicated that mobI is necessary for efficient transfer of SXTMO10 by conjugation. Moreover, the necessity of mobI for efficient transfer of oriTSXT from VB154 was confirmed in pMRA-M mobilization assays. Very few Rfr Tcr exconjugants (4.5 × 10−8) could be recovered from a mating experiment involving VB154 pMRA-M as a donor strain and E. coli VB112 as a recipient strain, indicating that, unlike wild-type SXTMO10, SXTMO10 ΔmobI is not able to mobilize a plasmid harboring oriTSXT.
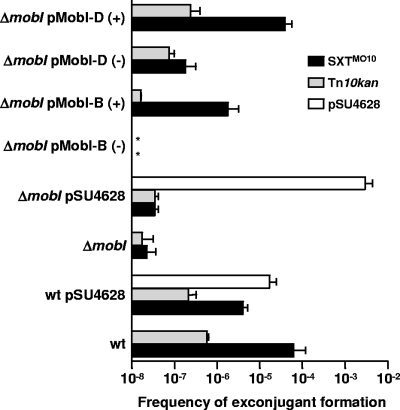
FIG. 5.
Role of mobI in conjugative transfer of SXTMO10 and mobilization of chromosomal DNA or of a broad-host-range mobilizable plasmid. The donors were either E. coli VB154, which harbors SXTMO10 ΔmobI (ΔmobI), or E. coli VB82, which contains SXTMO10 (wt), and the recipient strain was E. coli VB112. When indicated, the donor strains also harbored pSU4628, a derivative of the mobilizable plasmid CloDF13, or pMobI-D or pMobI-B, which contained, respectively, the short (426-bp) or the long (444-bp) version of mobI under the control of the arabinose-inducible promoter PBAD. (+) and (−) indicate the presence or absence of arabinose during mating for induction of mobI expression. The frequencies of exconjugant formation were determined by dividing the number of exconjugants (Rfr Sur Tmr CFU for SXTMO10 ΔmobI, Rfr Knr CFU for lacI42::Tn10kan, or Rfr Apr CFU for pSU4628) by the number of donors (Knr CFU). The bars show the mean and standard deviation values obtained from three independent experiments. The asterisk indicates that the frequency of exconjugant formation was below the limit of detection (1 × 10−8).
Complementation analyses demonstrated that mobI acts in trans and confirmed that it is not part of the cis-acting oriT locus. As shown in Fig. 5, introduction into VB154 of pMobI-D or pMobI-B, two plasmids containing the short (426-bp) or long (444-bp) version of mobI under the control of PBAD, restored the transfer of SXTMO10 ΔmobI in the presence of arabinose. In contrast, in the absence of arabinose, very little or no complementation was observed. Interestingly, the 426-bp mobI sequence was sufficient to complement the transfer defect of SXTMO10 ΔmobI, suggesting that the second TTG codon is the genuine start codon for mobI. pMobI-B was unable to restore the transfer of SXTMO10 ΔmobI to the wild-type level, suggesting that the distance between the PBAD promoter and the second initiation codon of mobI was not ideal in the long version of the gene. This hypothesis is also supported by the presence of a suitable ribosome binding site (GGGAAA), located 5 bp upstream from the second TTG initiation codon. In ICESpuPO1, this sequence is even closer to the consensus ribosome binding site motif (AGGAAA) (Fig. 2). No such translation initiation signal can be found upstream from the first TTG codon of mobI. Together, these findings indicate that mobI is a trans-acting gene required for the conjugative transfer of SXTMO10; it is likely that mobI is also required for transfer of all ICEs of the SXT/R391 family, given its conservation.
mobI is required for SXTMO10-mediated transfer of chromosomal DNA.
Mobilization of chromosomal DNA in an Hfr-like manner by SXTMO10 has been previously described (26). Commonly, antibiotic resistance-conferring transposons located at defined positions in the E. coli genome are used as a selectable marker in Hfr mating experiments to monitor their mobilization and subsequent integration into the recipient chromosome by homologous recombination (27, 46). We took advantage of this ability to study whether mobI could be involved in the transfer of chromosomal DNA mediated by SXTMO10.
In E. coli CAG18420, Tn10kan is inserted into lacI (7.9 min), which is located downstream from prfC (99.3 min), the integration site of SXTMO10 (27, 46). SXTMO10 has been shown to mobilize in cis the chromosomal locus lacI::Tn10kan at a frequency of 10−7 (27). Since deletion of mobI abolished transfer of SXTMO10, we expected that this mutation would also abolish transfer of chromosomal DNA. We used E. coli CAG18420 SXTMO10 (VB82) and CAG18420 SXTMO10 ΔmobI (VB154) as donor strains in mating experiments to compare the frequency of mobilization of the chromosomal marker lacI::Tn10kan. As expected, deletion of mobI markedly reduced the transfer of lacI::Tn10kan (Fig. 5). Complementation of ΔmobI with pMobI-D restored transfer of this chromosomal marker to nearly wild-type levels (Fig. 5), and again, complementation with pMobI-B resulted in a weak restoration of mobilization of lacI::Tn10kan. Since SXTMO10-mediated mobilization of lacI::Tn10kan was driven from oriTSXT, the experiment described above did not allow us to determine whether mobI was involved in oriT recognition or mating pair formation; yet it emphasized the importance of mobI for conjugative transfer processes mediated by SXTMO10.
mobI is not required for SXTMO10-mediated transfer of CloDF13.
To investigate whether mobI functions in mating pair formation or in DNA processing, we took advantage of the ability of SXTMO10 to mobilize in trans plasmids such as the broad-host-range mobilizable plasmid CloDF13 (27). CloDF13 encodes its own mobilization proteins that recognize and act at its cognate oriT locus (13). We expected that if mobI was involved in mating pair formation, the ΔmobI mutation would affect both the transfer of SXTMO10 and the mobilization of CloDF13. We transformed the wild-type SXTMO10 (VB82) and the SXTMO10 ΔmobI (VB154) E. coli strains with pSU4628, an Ap-resistant derivative of plasmid CloDF13 (Table 1), and used these strains as donors in mating experiments (Fig. 5).
Interestingly, the ΔmobI mutation did not abolish the mobilization in trans of pSU4628 (Fig. 5), highlighting the independence of pSU4628 transfer from SXTMO10-encoded mobI. This observation strongly suggests that mobI encodes a protein that specifically recognizes SXTMO10 DNA, likely acting at oriTSXT but not playing any role in the formation of a functional mating bridge. On the contrary, the presence of mobI in the donor cells seemed to impair the transfer of CloDF13 since we observed that the rate of transfer of pSU4628 was about 2 orders of magnitude higher when mobilization was mediated by SXTMO10 ΔmobI than when it was mediated by wild-type SXTMO10 (Fig. 5). Since CloDF13 encodes its own mobilization proteins, we speculate that mobI competes with CloDF13-encoded activity or that transfer-proficient SXTMO10 and CloDF13 compete for the mating bridge when both are present in the same cell. This hypothesis is reinforced by the slight reductions of transfer of SXTMO10 and lacI::Tn10kan observed when pSU4628 was present in the wild-type donor strain (Fig. 5), as already described by Hochhut et al. (27).
DISCUSSION
Besides generating genetic diversity (20), conjugation is the major mechanism involved in the dissemination of antibiotic resistance (35). Despite the importance of the SXT/R391 family in the dissemination of antibiotic resistance among clinically important pathogens, such as V. cholerae, our knowledge of the genetic determinants required for initiation and termination of the conjugative transfer of this family of ICEs is still very limited (7). Here, we have defined a fundamental property of this family, the origin of transfer. We corrected a previous study (4) and found that the SXTMO10 oriT locus is contained in a 918-bp intergenic region located between s003 and rumB′. A 299-bp fragment internal to the 918-bp region was sufficient to allow mobilization of a low-copy-number, nonmobilizable plasmid by a mutant of SXTMO10 deficient for excision and integration. In fact, our observations indicate that this 299-bp fragment corresponds to the oriT loci of SXTMO10, R391, R997, pMERPH, and very likely several if not all of the other ICEs of the SXT/R391 family.
Our findings contradict a previous report which suggested that oriTSXT was located within a 550-bp intergenic region located between traD and traJ (4). Beaber et al. reported that this fragment was sufficient to enable the mobilization by SXTMO10 of the high-copy-number, nonmobilizable vector pCR2.1 (4). However, Beaber et al. did not demonstrate that the mobility of this plasmid was independent of SXTMO10 transfer. Homologous recombination between SXTMO10 and the cloned fragment could have led to the cointegration of SXTMO10 with the plasmid and its subsequent mobilization to the recipient. In our hands, the 550-bp traD-traJ intergenic fragment did not enable mobilization of a low-copy-number, nonmobilizable vector by the excision-deficient SXTMO10, whereas a 299-bp segment internal to the s003-rumB′ fragment did so. Moreover, deletion of most of this region from SXTMO10 nearly abolished its transmissibility without altering the mobilization of a plasmid containing the 299-bp segment, underscoring the importance of this sequence as a cis-acting element necessary for transfer and mobilization.
Beaber et al. (3) previously reported that the genes and sequences encoding the conjugative machinery of SXTMO10 and R391 were divided in four functional clusters separated by ICE-specific insertions: the DNA-processing cluster traID-oriT-traJ and the three mating pore formation clusters traLEKBVA, traC-trsF-traWUN, and traFHG. The true origin of transfer is located upstream from s003 and the promoter that likely drives the expression of the integrase and upstream from mobI, a previously unannotated gene that is necessary for transfer of SXTMO10 (Fig. 6). We showed that mobI is required for mobilization of a nonmobilizable plasmid harboring oriTSXT. Since this gene is not required for SXTMO10-mediated mobilization of the broad-host-range mobilizable plasmid CloDF13, we suggest that it specifically recognizes and acts at the oriT loci of the ICEs of the SXT/R391 family. The absence of proteins homologous to the putative MobI protein in the GenBank database suggests that MobI belongs to a new class of mobilization proteins. Therefore, the DNA-processing functions are themselves divided in two clusters in the SXT/R391 family, the traID-VchoM_02001241-traJ cluster and the oriT-mobI cluster. The gene mobI is orientated in the same direction as all the other tra genes. This organization likely reflects an ancestral structure where all these genes were organized as an operon, later disrupted by insertions of foreign DNA (3, 9, 38, 40).
FIG. 6.
Schematic representation of the oriT locus and surrounding genes. The arrow representing the oriT locus indicates the polarity of transfer as reported in Hochhut et al. (27).
The locations of oriT and mobI within SXTMO10 likely have functional consequences. They are found directly upstream from the putative promoter region driving the expression of the genes s003, s002, and int, which are likely organized as an operon. s002 and s003 encode proteins of unknown functions, and int encodes the tyrosine recombinase Int (integrase) that catalyzes the integration and excision of the SXT/R391 ICEs (10, 28). A previous report described a functional interaction between the integrase IntTn916 and oriTTn916 of the conjugative transposon Tn916 (25). IntTn916 binds specifically to oriTTn916, providing stability to the relaxase-DNA complex (45). The location of oriTSXT could reflect a possible role for Int in the regulation of excision and transfer of the ICE. For instance, similar to what was found for Tn916, binding of Int to oriTSXT could serve as a signal indicating that Int has been produced and that SXTMO10 has likely excised and is ready for transfer. In this scenario, binding of Int to oriTSXT would prevent the premature transfer of the integrated ICE, restricting transfer of only intact copies of the element to the recipient cells. However, previously published data indicated that neither int nor s002 and s003 are required for SXTMO10-mediated transfer of chromosomal markers (10, 27). We also showed here that pMRA, which contains oriTSXT, is mobilizable to the recipient by SXTMO10 Δint, indicating that excision of the ICE, and therefore expression of Int, is not a requisite prior to initiation of transfer at oriTSXT.
It is worth noting that according to the new position of oriT and the polarity of DNA transfer that has been discovered by Hochhut et al. (27), the first gene that enters into the recipient cell is mobI, followed by the rumAB operon, which encodes a functional polV-like Y-family polymerase (37) when rumB′ is not disrupted by a cluster of antibiotic resistance genes as in SXTMO10 (Fig. 6). Similarly, the last genes that enter the recipient cell are xis, int, s002, and s003. Late transfer of int is remarkable as de novo expression of this gene has been found to be necessary in the recipient to promote the integration of the circular SXTMO10 molecule into the recipient's chromosome (28). Besides the requirement of int, little is known to date about the early steps leading to the establishment of SXT/R391 ICEs into the new host cell.
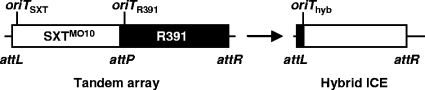
An important consequence of the new position of oriT in SXT/R391 ICEs is that previously published observations need to be reinterpreted. Hybrid ICEs, which appear to correspond to an assemblage of parts originating from different parental ICEs from the SXT/R391 family, can be detected in many clinical and environmental vibrios. ICEVchMex1 (9), ICEVchMoz3 (47), ICEPdaSpa1 (38), and ICEVchLao1 (48) are examples of what appear to be naturally occurring hybrids. A possible mechanism of hybrid ICE formation in which hybrid ICEs would form by transfer to the recipient of a DNA strand initiated at the oriT locus of the ICE located at the 5′ end and terminated at the oriT locus of the ICE located at the 3′ end of a tandem array was proposed (11) (Fig. 7). The region located between the oriTs of two ICEs integrated in a tandem fashion resembles and mimics the structure of the circular intermediate that serves as a substrate for transfer. Our observations indicate that such a mechanism would in fact be possible since we found that there is no barrier preventing the cross-recognition by the SXT/R391 ICEs of the oriT loci of other ICEs of the same family. However, the location of oriT so close to one of the borders of these ICEs likely limits the impact of such a mechanism of recombination on the diversity of the family and solely do not explain the variety of hybrid ICEs found in nature.
FIG. 7.
Schematic representation of an SXTMO10-R391 tandem array and possible corresponding hybrid formed by oriT recombination.
While prior characterizations of numerous oriTs of conjugative plasmids in both gram-positive and gram-negative bacteria have been made, oriTs of ICEs are not as well known or studied. To date, only two of them have been defined: the oriT locus of the conjugative transposon Tn916 from Enterococcus faecalis and the oriT locus of ICEBs1 from Bacillus subtilis (30, 31). Now that the oriT locus of the SXT/R391 family of ICEs has been identified, many aspects of the mechanism that initiate and terminate the strand transfer remain to be discovered, especially the role of mobI in these phenomena. Characterization of the interactions between the proteins MobI and TraI and the oriT sequence are ongoing.
Acknowledgments
We thank Matthew K. Waldor for the kind gift of many strains and critical reading of the manuscript. We are also grateful to Tony Pembroke for providing R997 and pMERPH. We acknowledge Joeli Marrero for helpful discussions.
This work was supported by a grant from the FQRNT new researcher start-up program. V.B. holds a Canada Research Chair in molecular biology (impact and evolution of bacterial mobile elements). D.C. holds a fellowship from the Cenci Bolognetti-Institut Pasteur Foundation, Italy.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Ahmed, A. M., S. Shinoda, and T. Shimamoto. 2005. A variant type of Vibrio cholerae SXT element in a multidrug-resistant strain of Vibrio fluvialis. FEMS Microbiol. Lett. 242241-247. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 3.Beaber, J. W., V. Burrus, B. Hochhut, and M. K. Waldor. 2002. Comparison of SXT and R391, two conjugative integrating elements: definition of a genetic backbone for the mobilization of resistance determinants. Cell. Mol. Life Sci. 592065-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaber, J. W., B. Hochhut, and M. K. Waldor. 2002. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J. Bacteriol. 1844259-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaber, J. W., B. Hochhut, and M. K. Waldor. 2004. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature 42772-74. [DOI] [PubMed] [Google Scholar]
- 6.Böltner, D., C. MacMahon, J. T. Pembroke, P. Strike, and A. M. Osborn. 2002. R391: a conjugative integrating mosaic comprised of phage, plasmid, and transposon elements. J. Bacteriol. 1845158-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrus, V., J. Marrero, and M. K. Waldor. 2006. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid 55173-183. [DOI] [PubMed] [Google Scholar]
- 8.Burrus, V., G. Pavlovic, B. Decaris, and G. Guedon. 2002. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46601-610. [DOI] [PubMed] [Google Scholar]
- 9.Burrus, V., R. Quezada-Calvillo, J. Marrero, and M. K. Waldor. 2006. SXT-related integrating conjugative element in New World Vibrio cholerae. Appl. Environ. Microbiol. 723054-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrus, V., and M. K. Waldor. 2003. Control of SXT integration and excision. J. Bacteriol. 1855045-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrus, V., and M. K. Waldor. 2004. Formation of SXT tandem arrays and SXT-R391 hybrids. J. Bacteriol. 1862636-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrus, V., and M. K. Waldor. 2004. Shaping bacterial genomes with integrative and conjugative elements. Res. Microbiol. 155376-386. [DOI] [PubMed] [Google Scholar]
- 13.Cabezon, E., J. I. Sastre, and F. de la Cruz. 1997. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol. Gen. Genet. 254400-406. [DOI] [PubMed] [Google Scholar]
- 14.César, C. E., and M. Llosa. 2007. TrwC-mediated site-specific recombination is controlled by host factors altering local DNA topology. J. Bacteriol. 1899037-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, I., P. J. Christie, and D. Dubnau. 2005. The ins and outs of DNA transfer in bacteria. Science 3101456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coetzee, J. N., N. Datta, and R. W. Hedges. 1972. R factors from Proteus rettgeri. J. Gen. Microbiol. 72543-552. [DOI] [PubMed] [Google Scholar]
- 17.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 166127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost, L. S., K. Ippen-Ihler, and R. A. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Mol. Biol. Rev. 58162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost, L. S., R. Leplae, A. O. Summers, and A. Toussaint. 2005. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 3722-732. [DOI] [PubMed] [Google Scholar]
- 21.Furuya, N., and T. Komano. 2000. Initiation and Termination of DNA transfer during conjugation of IncI1 plasmid R64: roles of two sets of inverted repeat sequences within oriT in termination of R64 transfer. J. Bacteriol. 1823191-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandoso, G., P. Avila, A. Cayon, M. A. Hernando, M. Llosa, and F. de la Cruz. 2000. Two active-site tyrosyl residues of protein TrwC act sequentially at the origin of transfer during plasmid R388 conjugation. J. Mol. Biol. 2951163-1172. [DOI] [PubMed] [Google Scholar]
- 23.Grohmann, E., G. Muth, and M. Espinosa. 2003. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67277-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guasch, A., M. Lucas, G. Moncalian, M. Cabezas, R. Perez-Luque, F. X. Gomis-Ruth, F. de la Cruz, and M. Coll. 2003. Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. Nat. Struct. Biol. 101002-1010. [DOI] [PubMed] [Google Scholar]
- 25.Hinerfeld, D., and G. Churchward. 2001. Specific binding of integrase to the origin of transfer (oriT) of the conjugative transposon Tn916. J. Bacteriol. 1832947-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochhut, B., J. W. Beaber, R. Woodgate, and M. K. Waldor. 2001. Formation of chromosomal tandem arrays of the SXT element and R391, two conjugative chromosomally integrating elements that share an attachment site. J. Bacteriol. 1831124-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochhut, B., J. Marrero, and M. K. Waldor. 2000. Mobilization of plasmids and chromosomal DNA mediated by the SXT element, a constin found in Vibrio cholerae O139. J. Bacteriol. 1822043-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochhut, B., and M. K. Waldor. 1999. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol. Microbiol. 3299-110. [DOI] [PubMed] [Google Scholar]
- 29.Iwanaga, M., C. Toma, T. Miyazato, S. Insisiengmay, N. Nakasone, and M. Ehara. 2004. Antibiotic resistance conferred by a class I integron and SXT constin in Vibrio cholerae O1 strains isolated in Laos. Antimicrob. Agents Chemother. 482364-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaworski, D. D., and D. B. Clewell. 1995. A functional origin of transfer (oriT) on the conjugative transposon Tn916. J. Bacteriol. 1776644-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, C. A., and A. D. Grossman. 2007. Identification of the origin of transfer (oriT) and DNA relaxase required for conjugation of the integrative and conjugative element ICEBs1 of Bacillus subtilis. J. Bacteriol. 1897254-7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llosa, M., and F. de la Cruz. 2005. Bacterial conjugation: a potential tool for genomic engineering. Res. Microbiol. 1561-6. [DOI] [PubMed] [Google Scholar]
- 33.Lukashin, A., and M. Borodovsky. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 261107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matson, S., and B. Morton. 1991. Escherichia coli DNA helicase I catalyzes a site- and strand-specific nicking reaction at the F plasmid oriT. J. Biol. Chem. 26616232-16237. [PubMed] [Google Scholar]
- 35.Mazel, D., and J. Davies. 1999. Antibiotic resistance in microbes. Cell. Mol. Life Sci. 56742-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath, B. M., J. A. O'Halloran, and J. T. Pembroke. 2005. Pre-exposure to UV irradiation increases the transfer frequency of the IncJ conjugative transposon-like elements R391, R392, R705, R706, R997 and pMERPH and is recA+ dependent. FEMS Microbiol. Lett. 243461-465. [DOI] [PubMed] [Google Scholar]
- 37.Mead, S., A. Vaisman, M. Valjavec-Gratian, K. Karata, D. Vandewiele, and R. Woodgate. 2007. Characterization of polVR391: a Y-family polymerase encoded by rumA′B from the IncJ conjugative transposon, R391. Mol. Microbiol. 63797-810. [DOI] [PubMed] [Google Scholar]
- 38.Osorio, C. R., J. Marrero, R. A. F. Wozniak, M. L. Lemos, V. Burrus, and M. K. Waldor. 2008. Genomic and functional analysis of ICEPdaSpa1, a fish-pathogen-derived SXT-related integrating conjugative element that can mobilize a virulence plasmid. J. Bacteriol. 1903353-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker, C., E. Becker, X. Zhang, S. Jandle, and R. Meyer. 2005. Elements in the co-evolution of relaxases and their origins of transfer. Plasmid 53113-118. [DOI] [PubMed] [Google Scholar]
- 40.Pembroke, J. T., and A. V. Piterina. 2006. A novel ICE in the genome of Shewanella putrefaciens W3-18-1: comparison with the SXT/R391 ICE-like elements. FEMS Microbiol. Lett. 26480-88. [DOI] [PubMed] [Google Scholar]
- 41.Peters, S. E., J. L. Hobman, P. Strike, and D. A. Ritchie. 1991. Novel mercury resistance determinants carried by IncJ plasmids pMERPH and R391. Mol. Gen. Genet. 228294-299. [DOI] [PubMed] [Google Scholar]
- 42.Ragonese, H., D. Haisch, E. Villareal, J.-H. Choi, and S. W. Matson. 2007. The F plasmid-encoded TraM protein stimulates relaxosome-mediated cleavage at oriT through an interaction with TraI. Mol. Microbiol. 631173-1184. [DOI] [PubMed] [Google Scholar]
- 43.Reygers, U., R. Wessel, H. Muller, and H. Hoffmann-Berling. 1991. Endonuclease activity of Escherichia coli DNA helicase I directed against the transfer origin of the F factor. EMBO J. 102689-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice, L. B. 1998. Tn916 family conjugative transposons and dissemination of antimicrobial resistance determinants. Antimicrob. Agents Chemother. 421871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocco, J. M., and G. Churchward. 2006. The integrase of the conjugative transposon Tn916 directs strand- and sequence-specific cleavage of the origin of conjugal transfer, oriT, by the endonuclease Orf20. J. Bacteriol. 1882207-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 531-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taviani, E., D. Ceccarelli, N. Lazaro, S. Bani, P. Cappuccinelli, R. R. Colwell, and M. M. Colombo. 2008. Environmental Vibrio spp., isolated from Mozambique, contain a polymorphic group of integrative conjugative elements and class 1 integrons. FEMS Microbiol. Ecol. 6445-54. [DOI] [PubMed] [Google Scholar]
- 48.Toma, C., N. Nakasone, T. Song, and M. Iwanaga. 2005. Vibrio cholerae SXT element, Laos. Emerg. Infect. Dis. 11346-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldor, M. K., H. Tschape, and J. J. Mekalanos. 1996. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J. Bacteriol. 1784157-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittle, G., N. Shoemaker, and A. Salyers. 2002. The role of Bacteroides conjugative transposons in the dissemination of antibiotic resistance genes. Cell. Mol. Life Sci. 592044-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams, S. L., and J. F. Schildbach. 2006. Examination of an inverted repeat within the F factor origin of transfer: context dependence of F TraI relaxase DNA specificity. Nucleic Acids Res. 34426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]