Abstract
Uropathogenic Escherichia coli (UPEC) cause bladder and kidney infections in human and mice. UPEC initiate many kidney infections by ascending out of infected bladders, but how this occurs is not well understood. To determine if the flagella were responsible for the ascension of UPEC to the kidneys, a fliC mutation in strain NU149 was created. The fliC mutant spread poorly on soft agar plates, and 12 hours post-inoculation of murine urinary tracts, ascension into the murine kidneys was compromised in this mutant strain compared to wild-type bacteria. Complementation of the mutation restored the ability to spread on soft agar plates and ascend into the murine kidneys. To confirm the fliC mutant results, an anti-flagella monoclonal antibody that has been previously described inhibited the spread of UPEC strain NU149 on soft agar plates. When the anti-flagella antibody was mixed with strain NU149 cells and the antibody-treated bacterial cells used to infect mice, significantly fewer mice had kidney infections than mice that were injected with strain NU149 cells mixed with normal mouse serum or anti-type 1 pili antibody. These results suggest E. coli flagella may be of importance in allowing the bacteria to ascend from the bladder and initiate kidney infections in humans, and the use of an antibody against the flagella could prevent the spread of UPEC into the kidneys.
Keywords: Escherichia coli, Flagella, Antibody, Urinary tract
Introduction
Urinary tract infections afflict millions of women worldwide each year. The costs associated have reached a staggering $2.4 billion a year with 4.5 to 6.8 million cases reported (Litwin et al., 2005; Foxman and Brown, 2003). Uropathogenic Escherichia coli (UPEC) are primarily responsible for these infections in humans (Hooton and Stamm, 1997). The route of infection is well established going from rectal to vaginal to urethral to bladder and then ascension up the ureters into the kidneys. Most of the infections occur in the bladder of the lower urinary tract (cystitis), but some women will also suffer from upper urinary tract infections involving the kidneys (pyelonephritis). UPEC pathogenicity is the result of the action of several virulence factors, including type 1 fimbriae, hemolysin, and a polysaccharide capsule (Johnson, 1991). Although motility caused by flagella may be needed to move the UPEC cells from the bladder into the kidneys (Mobley et al., 1994), no concrete linkage has been shown for this event.
Bacterial flagella are extracellular structures that allow directional movement in vitro and in vivo as a result of chemotaxis. The ability to move through the use of these extracellular flagella has been shown to be essential for the pathogenesis of other bacteria, including Proteus mirabilis (Burall et al., 2004; Mobley et al., 1996), Salmonella species (Schmitt et al., 2001), Helicobacter pylori (Terry et al., 2005), and even enteropathogenic E. coli (Giron et al., 2002). Recently, several studies have indicated that there is a role for motility and the presence of flagella in UPEC pathogenesis (Lane et al., 2005; Wright et al., 2005; Haugen et al., 2007). In these studies, there was a fitness advantage given to wild-type populations compared to flagella and chemotaxis mutants. However, the studies demonstrated that flagella were needed to ascend from the bladder into the kidneys of infected mice.
In this study, we have constructed a mutation in the fliC flagellin structural gene in the UPEC strain NU149 (Schaeffer et al., 1987) and have used a monoclonal antibody to flagellin (Schwan et al., 1990) in a blocking analysis to answer two questions: 1) are flagella needed for ascension of UPEC from the bladder up to the kidneys and 2) can an antibody to flagella added externally with the bacterial cell inoculum block kidney colonization by UPEC. We demonstrate through the use of a fliC mutant strain that flagella are needed by UPEC to ascend from the bladder to the kidneys of mice. This was confirmed by the blocking study with the anti-flagella monoclonal antibody. Complementation of the fliC mutation restored ascension to the kidneys back to wild type levels, fulfilling molecular Koch’s postulates (Falkow, 1988).
Materials and methods
Bacterial strains, plasmids, and growth conditions
The uropathogenic NU149 strain of E. coli (Schaeffer et al., 1987) was grown in Luria broth as previously described (Hultgren et al., 1986) to allow for optimal expression of both type 1 pili and flagella. Previously, this strain has been shown to be capable of ascending from bladders into murine kidneys (Schaeffer et al., 1987; Schwan et al., 2002), causing pyelonephritis in some mice (Schaeffer et al., 1987). Strain DH5α MCR was used as a recipient for transformations. Luria agar (LA) was used during the transformations with the addition of the following antibiotics: kanamycin, 40 μg/ml or ampicillin, 100 μg/ml (Sigma Chemical Company, St. Louis, MO). The λ Red recombinase system with plasmids pKD4, pKD46, and pCP20 was used as previously described (Datsenko and Wanner, 2000).
Creation of a fliC mutation in uropathogenic E. coli strain NU149
To create a mutation in the fliC gene that encodes for the flagellin monomers, the Red recombinase system described by Datsenko and Wanner (2000), was used. Briefly, the primer pair FliC3 (5′ CAATACGTAATCAACGACTTGCAATATAGGATAACGAATCTGTGTAGGCTGG AGCTGCTTCG 3′) and FliC4 (5′ TTTGGCGTTGCCGTCAGACTCAGTTAATCAGGTTACAACGACATATGAATATC CTCCTTAG 3′) was used to create a PCR product, using pKD4 plasmid DNA as a template. The PCR conditions that were used were an initial denaturation at 95°C for 5 min followed by 35 cycles of 95°C, 1 min; 55°C, 1 min, and 72°C, 2 min. The resulting PCR product was concentrated and separated on a 0.8% agarose gel, cut out, and the DNA extracted from the agarose gel. With this purified PCR product, an electroporation was performed on strain NU149/pKD46 cells as previously described (Datsenko and Wanner, 2000), selecting for transformants on LA with kanamycin. One transformant, NU149 fliC 2 was chosen for further analysis. To remove the kanamycin resistance gene, strain NU149 fliC 2 had the pCP20 plasmid electroporated into it and processed as previously noted (Datsenko and Wanner, 2000). An enzyme immunoassay was used to confirm the loss of the flagellin in the fliC mutant strain as previously described (Schwan et al., 2002), using the anti-flagellin monoclonal antibody as the primary antibody in the assay.
The fliC mutation was complemented by PCR amplifying the fliC gene with the primer pair of FliC9 (5′ CGTTGCTGACAAATTGCGCT 3′) and FliC10 (5′ ATGGTGAGTTTACTGTCGCT 3′). Amplification was carried out under the following conditions: initial denaturation 95°C for 5 min then 33 cycles of 95°C, 1 min; 55°C, 1 min, and 72°C, 3 min. The 2316-bp product was ligated to pGEM3Z DNA digested with SmaI, and the ligation mix was added to DH5α MCR cells in a transformation reaction. Transformants were selected on LA containing ampicillin. One transformant, DH5α MCR/pWS15, was selected for further use. The pWS15 plasmid was purified using a commercial kit (Qiagen) and then electroporated into strain NU149 fliC 2 with selection on LA with ampicillin.
Soft agar assay for bacterial motility
A soft agar motility test was performed as previously described (Craven and Montie, 1981) for the wild type versus fliC mutant and complemented mutant analysis and with the following modifications when antibodies were used. A 1% (v/v) concentration set at 10 μg/ml of either the 2DE1B7 (anti-flagella) or C3C (anti-type 1 pili) monoclonal antibody (Schwan et al., 1990) was added to the agar. Strain NU149 was inoculated into the center of the agar plate and the amount of bacterial spread measured after 6 h post-inoculation at 37°C.
Murine urinary tract infection model
A murine urinary tract infection model was used to assess the role flagella may play in E. coli cells infecting murine urinary tract kidneys (Schaeffer et al., 1987). Six hundred microliters of mid-logarithmic phase grown bacteria set at 108 CFU/ml in phosphate-buffered saline (PBS, pH 7.4) were used as the inocula. A total of 8 mice per E. coli strain were transurethrally inoculated (50 μl per mouse) with the fliC mutant (strain NU149 fliC 2), the complemented fliC mutant (strain NU149 fliC 2/pWS15) or wild-type strain NU149 cells. After 12 h post-inoculation, each organ from each mouse was processed by adding one ml PBS, the organs were homogenized with a tissue grinder, aliquots were 10-fold serially diluted in PBS, and 100-μl aliquots were plated onto Luria agar to ascertain viable counts.
For the blocking studies, the bacteria prepared as described above were mixed with normal mouse serum collected from four- to six-week-old female BALB/c mice or 10 μg of 2DE1B7 mouse anti-flagella monoclonal antibody or 10 μg of 3C3 mouse anti-type 1 pili monoclonal antibody that have been characterized previously (Schwan et al., 1990). For each arm of the study, 50 μl per mouse of the normal serum, anti-flagella antibody-coated UPEC cells, or anti-type 1 pili antibody-coated UPEC cells were then transurethrally injected into the bladder of 8 to 10 four- to six-week-old female BALB/c mice. After one day post-inoculation, the mice were euthanized and the bladders and kidneys collected and processed as noted above. This procedure was repeated once more to give a total of eighteen mice per arm of the study with the same processing described above. As a control, 20 BALB/c mice were transurethrally inoculated with 50 μl per mouse of NU149 cells suspended in PBS. The mice were euthanized and bladders and kidneys collected after 30 min post-inoculation.
Aggregate formation
Since the anti-flagellin monoclonal antibody could form bacterial aggregates that would influence the colonization results, both macroscopic and microscopic examinations of the reaction mixes were performed. Bacterial suspensions mixed with the same concentrations of monoclonal antibody or normal serum noted above were examined visually for gross aggregate formation. Next, aliquots were placed on glass slides and examined for microscopic bacterial aggregates on a Nikon microscope using phase contrast at 1000× magnification.
Statistics
A Fisher’s t test was used to calculate statistical variation. P ≤ 0.05 was considered significant.
Results
Inhibition of motility in vitro using anti-flagella antibody or through a fliC mutation
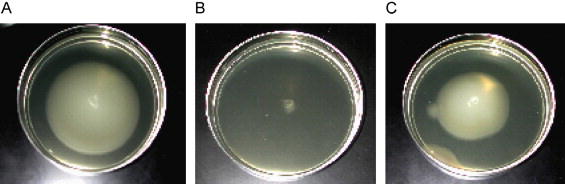
A fliC mutant strain was constructed and tested in the motility assay compared to wild-type bacteria. After 6 h, the fliC mutant strain had barely moved across the agar surface (5 mm), whereas the wild-type bacteria moved a significant distance (49 mm; Fig. 1A). When the fliC mutant strain was complemented with a full-length fliC gene on a multicopy plasmid, the swarming motility was restored (47 mm), demonstrating that the fliC mutation affected swarming motility. To ensure the mutation affected flagellin expression, the fliC mutant was tested in an enzyme immunoassay with anti-flagella antibody. No reactivity was observed against the monoclonal antibody (data not shown). A monoclonal antibody to E. coli strain NU149 flagella has been previously described (Schwan et al., 1990). The anti-flagella antibody was added to agar and used in an in vitro motility assay to determine if it could inhibit the spread of strain NU149 bacteria on a soft agar plate. After 6 h, strain NU149 spread out an average of 49 mm on a soft agar plate with PBS added (Figs. 1B and 2A). However, when anti-flagella antibody 2DE1B7 was added to the soft agar, the motility of the bacteria was completely inhibited (Figs. 1B and 2B; 0 mm; P < 0.0002). On the other hand, an anti-type 1 pilus monoclonal antibody (3C3) showed a small but significant difference in bacterial spread across the plate (Fig. 1B and 2C; 31.7 mm; P< 0.03) compared to the control. These results indicated that the anti-flagella antibody was able to inhibit the spread of the UPEC cells in vitro, confirming the results shown for the fliC mutant strain.
Fig. 1.
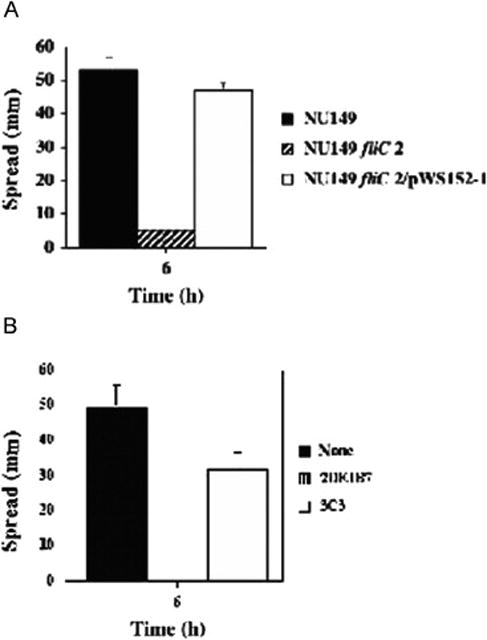
Soft agar motility assays of E. coli strain NU149. (A) Comparison of E. coli strain NU149 (black) to a fliC mutant strain (NU149 fliC 2; striped), or a complemented fliC mutant strain (NU149 fliC 2/pWS15; white). (B) Comparison of E. coli strain NU149 and the addition of PBS (black), monoclonal antibody 2DE1B7 (anti-flagella; striped), or monoclonal antibody C3C (anti-type 1 pili; white). Spread of the bacteria was measured in mm after 6 h incubation at 37°C. The results are the mean ± standard deviation of three separate runs.
Fig. 2.
Soft agar motility assay using E. coli strain NU149 and the addition of (A) PBS, (B) monoclonal antibody 2DE1B7 (anti-flagella), and (C) monoclonal antibody C3C (anti-type 1 pili).
Determining anti-flagella antibody aggregation of the E. coli cells
Since intact antibody can sometimes cause aggregation of the bacteria that would diminish bacterial motility, the mixtures of bacteria with normal serum or monoclonal antibody were examined for both macroscopic and microscopic aggregation. After the monoclonal antibody was mixed with the strain NU149 cells, the tubes were first observed for macroscopic aggregates. Seeing no visible aggregates, aliquots were next examined under a phase-contrast microscope for microscopic aggregates. No aggregates were observed when using either the anti-flagella or the anti-type 1 pili monoclonal antibody (data not shown), indicating that the reduction in motility was not caused by the formation of bacterial aggregates, rather the reduction was due to binding to the flagella.
A mutation in the fliC gene prevents UPEC ascension into the kidneys
Upper urinary tract infections of the kidney (pyelonephritis) are thought to be the result of UPEC cells ascending from the bladder, up through the ureters, and ultimately reaching the kidneys. Many believe that E. coli flagella may be responsible for the ascension into the kidneys. To verify that the bacterial flagella were involved in the ascension, the fliC mutant strain was used in an ascending urinary tract infection model. Mice infected with strain NU149 fliC 2 displayed no bacterial counts in the kidneys after 12 h, which was significantly different from both the wild-type strain NU149 (median = 6.15 × 103, P < 0.03) as well as the complemented fliC mutant strain (NU149 fliC 2/pWS15; median = 5.55 × 103, P < 0.02; Fig. 3). Thus, the role of flagella in ascension into the murine kidneys was established using a fliC mutant strain.
Fig. 3.
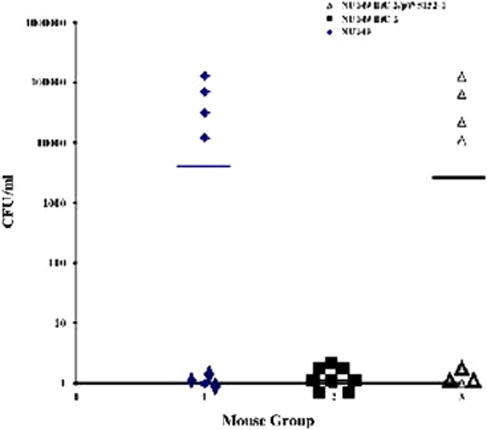
Independent challenges of mice with E. coli strain NU149, a fliC mutant, and a complemented fliC mutant strain. The E. coli strain NU149 (group 1, closed diamond), NU149 fliC 2 mutant strain (group 2, closed square), or strain NU149 fliC 2/pWS15 complemented with the pWS15 plasmid (group 3, open triangle) were individually inoculated into the bladders of female BALB/c mice. After 12 h, the kidneys were harvested to determine bacterial concentrations. Each data point represents the log10 CFU/ml per one mouse. Horizontal bars represent the median values of the populations.
Mixing anti-flagella antibody with bacteria inhibits infection of murine kidneys
Since the fliC mutant strain failed to ascend into murine kidneys, an anti-flagella antibody that blocked motility of the UPEC bacteria cells in vitro was next used to test whether it could block ascension of the UPEC cells from the bladder into the kidneys of mice, confirming the fliC mutant results. Female BALB/c mice were inoculated transurethrally with UPEC strain NU149 cells mixed with normal mouse serum, anti-type 1 pili antibody 3C3, or anti-flagella antibody 2DE1B7. Strain NU149 has been shown to ascend into murine kidneys in the murine urinary tract infection model and cause pyelonephritis in some of the mice (Schaeffer et al., 1987). To ensure that the inoculations deposited bacteria only in the bladder, not the kidneys, the kidneys of 20 mice were checked after 30 min post-inoculation. The kidneys from only one mouse of the 20 inoculated showed viable UPEC cells (data not shown). This showed that the infection model was truly an ascending model.
After one day post-inoculation, the kidneys and bladders from the infected mice were collected and processed to determine viable counts. Comparing both the normal serum-and anti-flagellar antibody-treated populations; the bladders were uniformly colonized at a high level (median of 6.6 × 104 CFU/ml), indicating no effect by the anti-flagellar antibody on bladder infections in mice (data not shown). On the other hand, the anti-type 1 pili antibody significantly reduced bladder colonization of the mice (median of 1.8 × 102 CFU/ml). For the normal serum-treated arm, 9 of 18 (50%) mice had kidneys colonized with strain NU149 bacteria, indicating that ascension into the kidneys had occurred in half of the animals. In those kidneys that were infected, the mean viable count was 3.3 × 104 CFU/ml. The anti-type 1 pili antibody arm had 5 of 18 (28%) mice with infected kidneys with a mean viable count of 7.8 × 103 CFU/ml (P < 0.102). On the other hand, only one of eighteen mice (5%) had a kidney infection in the anti-flagella antibody-treated arm, demonstrating a significant difference from the normal serum arm (P < 0.02). Moreover, the viable count of the one infected kidney was 4.5 × 102 CFU/ml (Fig. 4). These results show that the anti-flagella antibody appeared to block the UPEC bacteria from ascending from the murine bladders into the murine kidneys but the antitype 1 pili antibody did not significantly affect bacterial ascension to the kidneys, confirming that UPEC flagella are needed for bacteria to move into the kidneys.
Fig. 4.
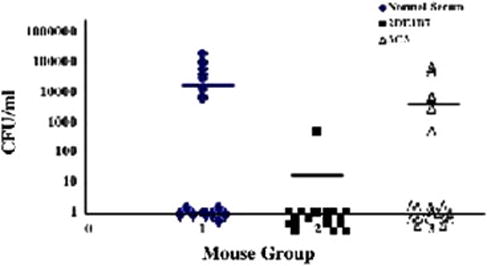
Independent challenges of mice with E. coli strain NU149 mixed with antibody. The E. coli bacteria were mixed with normal mouse serum (group 1, closed diamond), 2DE1B7 anti-flagella antibody (group 2, closed square), or anti-type 1 pili antibody (group 3, open triangle) and then individually inoculated transurethrally into the bladders of female BALB/c mice. After 24 h, the kidneys were harvested to determine bacterial concentrations. Each data point represents the log10 CFU/ml per one mouse. Horizontal bars represent the median values of the populations.
Discussion
Pyelonephritis (a severe kidney infection) occurs after a series of events bringing bacteria from the exterior of the human body, up to the bladder, and eventually settling into the kidneys. It has been assumed that flagella-based motility may allow UPEC bacteria to ascend from the bladder up to the kidneys. Although there has been speculation that flagella may contribute to this ascension process, no solid data has been shown to support this idea. Previously, Bahrani et al. (2002) implicated flagella as something needed by UPEC bacteria to cause murine urinary tract infection. More recently, three studies have demonstrated that flagella contribute in some way to UPEC pathogenesis in the murine, and presumably, the human urinary tract (Haugen et al., 2007; Lane et al, 2005; Wright et al., 2005). All three papers used molecular Koch’s postulates (Falkow, 1988) as a basis for their respective studies. By using a co-challenge model of infection with both wild-type and fliC mutant strains, Lane et al. (2005) demonstrated some role for flagella in the early stages of a urinary tract infection, but individual strain analysis was not done at the very early time point that was done in the present study. In general, the previous studies examined differences after several days or more post-inoculation of the bacteria into the urinary tract, so early events (i.e. after a day or less) would have been missed.
Our study demonstrates that an E. coli-specific anti-flagella monoclonal antibody completely blocked soft agar motility of the UPEC strain compared to a normal serum control. Flagella are need by UPEC cells to move across the agar surface because of a chemotactic gradient that is established. Several flagellar and chemotactic genes contribute to the ultimate functioning of the flagella (Silverman and Simon, 1977). The fliC gene encoding the structural subunits of flagella is transcriptionally activated in vitro under conditions that promote motility (Wright et al., 2005). An anti-type 1 pili monoclonal antibody partially blocked motility of the UPEC bacteria, presumably because of steric hindrance as the result of the antibody attaching to the type 1 pili and impeding the action of the bacterial flagella. Alternatively, recent data suggests that type 1 pili may provide a supporting function in swarming motility (Inoue et al., 2007).
Even more striking than the motility assay analysis were the in vivo animal results. This ascending model of infection first described by Hagberg et al. (1983) that we later refined (Schaeffer et al., 1987) is truly an ascending model as demonstrated by the very low number of kidneys displaying viable counts moments after inoculation of the urinary tract. Flagella were shown to be necessary for ascension into the kidneys by using a fliC mutant strain in the murine urinary tract infection model system, comparing the results to the wild-type bacteria and a fliC mutant strain complemented with the fliC gene. Fifty percent of the murine kidneys were infected when wild-type bacteria were used, but no mice infected with the fliC mutant strain displayed kidney colonization. Moreover, complementation of the fliC mutation allowed the bacteria to colonize the murine kidneys at levels and percentages comparable to the wild-type strain. A limitation of our study is we only examined the ascension event and strain NU149 is not a prototypic pyelonephritis-causing strain of E. coli, although it is capable of producing pyelonephritis under certain circumstances (Schaeffer et al., 1987). Certainly, testing a more typical pyelonephritis-associated strain with a fliC mutation would substantiate the role of flagella in the ability of UPEC to initiate pyelonephritis in humans.
We verified that flagella are important for kidney ascension through the antibody blocking study. In the normal serum arm of the study, the UPEC bacteria infected half of the kidneys, whereas 28% of the kidneys were infected in the anti-type 1 pili antibody arm. However, the kidneys of only one mouse were infected in the anti-flagella antibody arm. Thus, the anti-flagella monoclonal antibody significantly prevented colonization of murine kidneys by UPEC bacteria transurethrally inoculated into the mice. This suggests that the bacterial flagella were needed by the bacteria to ascend into the kidneys of the mice.
The prior studies that have been performed have indicated that non-motile or non-chemotactic mutants have a subtle survival advantage compared to the wild-type bacteria (Lane et al., 2005; Wright et al., 2005). In these past studies, it is certainly possible that during inoculation the kidneys were bathed in the non-motile bacterial inoculum. More likely is the scenario where urine reflux from the bladder into the kidneys allowed the murine kidneys to become infected several days after inoculation (Loeb and Quimby, 1989). A shorter time course would have minimal effects caused by the reflux.
Once the heavily flagellated UPEC cells reach the kidneys, their benefit in the infection process may wane. The transcriptome study performed by Snyder et al. (2004) indicated that genes involved in flagella biosynthesis and chemotaxis were down-regulated over 10 days in UPEC bacteria colonizing murine urinary tracts. Several environmental cues found in the murine urinary tract may act directly on down-regulating these genes. Some preliminary work we have done suggests that certain acid tolerance gene proteins and the OmpR protein may be acting in unison to repress fliC expression (W. Schwan, unpublished data). For the bacteria, the long-term presence of flagella on their cell surface will target those cells for elimination by the immune system. Phagocytic cells will more likely phagocytize flagellated bacteria (Sharon et al., 1981) and a vigorous anti-flagella antibody response will be mounted (Bahrani et al., 1991; Ebersole and Molinari, 1977).
Thus, in the short term, heavily flagellated UPEC cells likely allow for ascension into the kidneys of the urinary tract. Upon reaching the kidneys, the flagella are no longer needed and will in fact be detrimental to the survival of the bacteria. By down-regulating expression of these surface antigens, the bacteria can survive for a longer period of time in either the murine or human upper urinary tract.
Acknowledgments
This study was funded by an NIH grant 1R15AI47801A2 to W.R. Schwan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahrani FK, Johnson DE, Robbins D, Mobley HL. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991;59:3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Burall LS, Harro JM, Li X, Lockatell CV, Himpsl SD, Hebel JR, Johnson DE, Mobley HL. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect Immun. 2004;72:2922–2938. doi: 10.1128/IAI.72.5.2922-2938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RC, Montie TC. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can J Microbiol. 1981;27:458–460. doi: 10.1139/m81-070. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JL, Molinari JA. Gastrointestinal antibody responses in axenic mice to topically administered Escherichia coli. Infect Immun. 1977;16:938–946. doi: 10.1128/iai.16.3.938-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;(10 Suppl 2):S274–S276. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Foxman B, Brown P. Epidemiology or urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin N Am. 2003;17:353–360. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- Giron JA, Torres AG, Freer E, Kaper JB. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol. 2002;44:361–379. doi: 10.1046/j.1365-2958.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen BJ, Pellett S, Redford P, Hamilton HL, Roesch PL, Welch RA. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect Immun. 2007;75:278–289. doi: 10.1128/IAI.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin N Am. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- Hultgren SJ, Schwan WR, Schaeffer AJ, Duncan JL. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect Immun. 1986;54:613–620. doi: 10.1128/iai.54.3.613-620.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Shingaki R, Hirose S, Waki K, Mori H, Fukui K. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J Bacteriol. 2007;189:950–957. doi: 10.1128/JB.01294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MC, Lockatell CV, Monterosso G, Lamphier D, Weinert J, Hebel JR, Johnson DE, Mobley HLT. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect Immun. 2005;73:7644–7656. doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin MS, Saigal CS, Yano EM, Avila C, Geschwind SA, Hanley JM, Joyce GF, Madison R, Pace J, Polich SM, Wang M. Urologic diseases in America Project: analytical methods and principal findings. J Urol. 2005;173:933–937. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- Loeb WF, Quimby FW. The clinical chemistry of laboratory animals. Pergamon Press; New York, NY: 1989. [Google Scholar]
- Mobley HL, Island MD, Massad G. Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis. Kidney Int Suppl. 1994;47:S129–S136. [PubMed] [Google Scholar]
- Mobley HL, Belas R, Lockatell V, Chippendale G, Trifillis AL, Johnson SE, Warren JW. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer AJ, Schwan WR, Hultgren SJ, Duncan JL. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect Immun. 1987;55:373–380. doi: 10.1128/iai.55.2.373-380.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O’Brien AD. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect Immun. 2001;69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan WR, Waltenbaugh C, Duncan JL. Bacteria as solid phase in a concentration fluorescence immunoassay analysis of antibodies to surface antigens. J Immunol Methods. 1990;126:247–252. doi: 10.1016/0022-1759(90)90157-q. [DOI] [PubMed] [Google Scholar]
- Schwan WR, Lee JL, Lenard FA, Matthews BT, Beck MT. Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun. 2002;70:1391–1402. doi: 10.1128/IAI.70.3.1391-1402.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N, Eshdat Y, Silverblatt FJ, Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Silverman M, Simon MI. Bacterial flagella. Annu Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]
- Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun. 2004;72:6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry K, Williams SM, Connolly L, Ottemann KM. Chemotaxis plays multiple roles during Helicobacter pylori animal infection. Infect Immun. 2005;73:803–811. doi: 10.1128/IAI.73.2.803-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun. 2005;73:7657–7668. doi: 10.1128/IAI.73.11.7657-7668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


