Abstract
Although the aberrant actions of protein kinases have long been known to contribute to tumor promotion and carcinogenesis, roles for proteins phosphatases in the development of human cancer have only emerged in the last decade. In this review, we discuss the data obtained from studies examining the biological and pathological roles of a serine/threonine protein phosphate, PP5, which suggest that PP5 is a potentially important regulator of both hormone- and stress-induced networks that enable a cell to respond appropriately to genomic stress.
Keywords: phosphatase, PP5, cancer, oxidative stress
Introduction
Studies into the molecular mechanisms underlying tumor promotion and the unrestrained proliferation of cancer cells have revealed that many different protein kinases influence signaling networks that affect cancer cell growth [1–3]. Indeed, reversible phosphorylation has been shown to play an important role in the regulation of numerous signaling networks that control cell growth, differentiation, senescence and programmed cell death (apoptosis). Accordingly, there is great interest in the development of drugs to suppress the aberrant actions of “key” kinases that promote tumor formation and growth. More recently it has become clear that protein phosphatases are also dynamic and highly regulated enzymes [4–6], suggesting that the aberrant actions of certain phosphatases may also contribute to the development and progression of human cancer. Here we review the literature on a serine/threonine protein phosphatase designated as PP5 (human gene PPP5).
Overview of PP5 and Ser/Thr phosphatases
In eukaryotic organisms, serine/threonine protein phosphatases (PPases) have been grouped into two major families, designated as PPM (metal-dependent protein phosphatases) and PPP (phosphoprotein phosphatases). The phosphatases of the PPP family are among the most highly conserved proteins on earth, with homology across taxa greater than that of histones (2A and 2B) [7–11]. PP5 belongs to the PPP-family, which also contains PP1, PP2A, PP2B, PP4, PP6 and PP7 [8–17]. Most of the PPP-subfamilies contain isoforms, with mammals expressing two or more isoforms of PP1, PP2A, PP2B, and PP7 that share >80% identity. In contrast, throughout Eukaryota, there is a single form of PP5. PP5 also differs from most PPP-family phosphatases in that the principle substrate targeting, regulatory, and catalytic domains are contained in a single polypeptide chain. Whereas the catalytic, regulatory, and targeting subunits of PP1–PP4 are encoded by separate genes, with the holoenzyme comprised of two or more proteins that are held together weakly via non-covalent interactions. Unlike PP1 and PP2A, purified PP5 has low basal activity. Structural studies indicate that in the absence of other proteins the N-terminal domain folds to cover the catalytic site blocking acess to substrates [18, 19]. Thus, it is believed that the catalytic actions of PP5 occur predominately in protein complexes (Figure 1).
Figure 1. Structure of PP5.
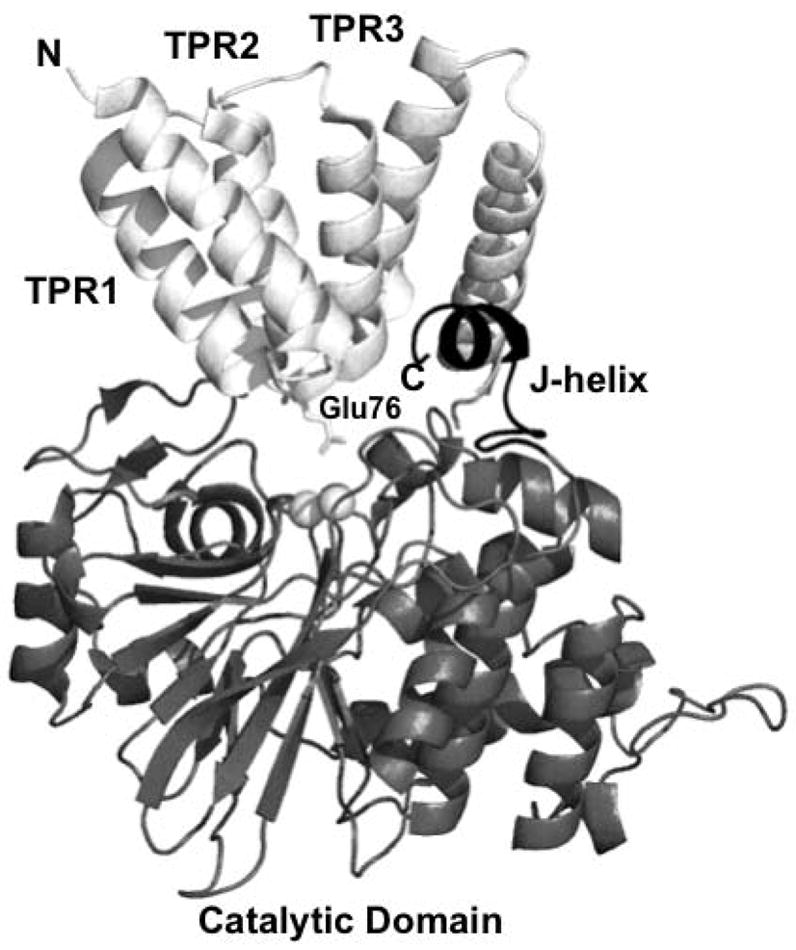
A ribbon representation of full-length PP5 (pdb code 1WAO). Also displayed are the active site metal ions (white spheres) and the side chain of glu76 (stick representation). PP5 has three principal regions, an N-terminal domain (white) containing three tetratricopeptide repeats (labeled TPR1, TPR2, and TPR3) a catalytic domain (gray), and a flexible linker that connects the N-terminal and catalytic domains. The 3 TPRs form 3 sets of antiparallel amphipathic alpha helices that are arranged into a partial superhelix and are involved in mediating protein-protein interactions. The catalytic domain has an overall fold that is strikingly similar to that of the catalaytic subuints of PP1, PP2A, and PP2B. Also present is a regulatory C-terminal subdomain (black) containing the J-helix that helps to maintain a closed, inactive conformation by stabilizing the position the N-terminal domain over the catalytic site, thereby blocking substrate access. The figure was produced using PYMOL (http://www.pymol.org).
Determining the biological roles of PP5 has proven challenging. The catalytic domain of PP5, originally designated as PP3, was first purified from a bovine brain extract [20]. Shortly thereafter the cDNA encoding PP5 was identified in cDNA libraries derived from rat adipocytes (designated as PPT), yeast (designated as PPT1), a human teratocarcinoma and mouse embryos [21–23]. The human gene encoding PP5 (designated PPP5c) was then identified on chromosome 19. It contains 13 exons with a transcript length of ~2 kb [24].
Although the fact is often ignored, PP5 is sensitive to inhibition by several natural toxins commonly employed to study the actions of PP1 and PP2A (i.e. okadaic acid, microcystins, nodularin, calyculin A, tautomycin and cantharidin). Therefore, although studies conducted with these PPase binding toxins are often interpreted as providing evidence that either PP1 or PP2A participates in a given event, in reality the involvement of PP5 should not have been excluded. The potential actions of toxin-sensitive PP4 and/or PP6 should not be ignored either.
PP5 protein complexes
To date PP5 has been identified in complexes containing many proteins know to participate in signaling networks that initiate or regulate a variety of cellular events, including the glucocorticoid receptor (GR)- heat shock protein 90 (Hsp-90)-heterocomplex [13, 25, 26], the CDC16/CDC27 subunits of the anaphase-promoting complex [27], cryptochrome 2 [28], Hsp90-dependent heme-regulated eIF2α kinase [29], apoptosis signal-regulating kinase 1 (ASK1) [30], DNA-PKcs (DNA-dependent Ser/Thr protein kinase) [31], ATM (ataxia-telangiectasia mutated kinase) [32], ATR (ATM and Rad 3 related kinase) [33], the A-regulatory subunit of protein phosphatase type 2A [34], the G12-α/G13-α subunits of heterotrimeric G proteins [35], Rac [36] and Raf1 [37]. However, unlike protein kinases where a binding partner is often a good indication of a substrate, PP5 has less defined binding interactions with its substrates (i.e. PP5 has no apparent consensus substrate binding sequence). Therefore, in many cases binding may not indicate an actual substrate, but rather a partner in a complex of proteins that contains a substrate(s).
PP5 and the glucocorticoid receptor
One of the first and most studied protein complexes containing PP5 is the glucocorticoid receptor (GR) heat shock protein 90 (Hsp-90) complex [25]. In this complex PP5 binds directly to Hsp-90. Mutational studies revealed that PP5 binds via its N-terminal tetratricopeptide repeat (TPR) domain directly to the C-terminal region of Hsp-90 [13]. Binding also disrupts the auto-inhibitory conformation maintained by the interaction of the three TPRs in the N-terminal domain with the C-terminal J helix and catalytic domain [18]. Thus, the association of PP5 with Hsp-90 “activates” PP5 by allowing substrate access to the catalytic site [19].
The role played by PP5 in GR-signaling is complex. Hsp-90 is an essential molecular chaperone that is responsible for the activation or maturation of several proteins in key signal transduction pathways, including steroid hormone receptors, helix-loop-helix transcription factors, and protein kinases (e.g. Raf-1 and Src [reviewed in 38, 40]). Steroid receptors, such as GR, bind hormone after assembling into “mature” complexes containing several proteins. Upon hormone binding, the GRs are released from the Hsp-90 heterocomplex, translocate into the nucleus, dimerize and bind DNA. There, steroid receptors interact with co-transcriptional regulatory proteins to modulate the transcription of genes.
Initially it was proposed that PP5 is required for optimal GR signaling. This hypothesis was derived from studies using a “dominate negative” truncation mutant of PP5, in which a dramatic inhibition of transcriptional activation by glucocorticoids was observed following the over expression of the N-terminal TPR domain of PP5 [25]. In contrast, studies using PP5 antisense oligonucleotides revealed a marked enhancement of dexamethasone-induced transcriptional activation suggesting the opposite, that PP5 antagonized GR-signaling [41]. Using antisense oligonucleotides targeting PP5, a modest increase in receptor binding to DNA was observed even without the addition of agonist. In addition, in the presence of dexamethasone (a potent GR-agonist) the suppression of PP5 expression produced a marked increase in GR-DNA binding, which was observed concomitantly with increased nuclear accumulation of the receptor [42]. Additional experiments revealed that the binding of PP5 to the GR-Hsp-90 complex occurs in the cytoplasm in a competitive manner with two immunophilin-proteins, FKBP51 and FKBP52, which also contain TPR-domains [13, 25, 26]. The binding of FKBP52 augments glucocorticoid signaling [26, 43], where as FKBP51 suppresses GR-signaling [44]. The binding of hormone to the GR was then shown to induce the substitution of one immunophilin (FKBP51) for another (FKBP52), with GR-Hsp90-FKBP52 heterocomplex binding with dynein and then moving from the cytoplasm to nucleus [45]. In contrast, the binding of GR-Hsp90 with FKBP51 favors cytoplasmic retention of the receptor complex and exhibits an inhibitory role in GR-function. Subsequently it was shown that PP5 exerts a hierarchical effect on this aspect of GR-function (FKBP52 > PP5 > FKBP51) [26] (Figure 2). This finding appears to reconcile the conflicting data produced with the TPR “dominant-negative” mutant and the antisense oligonucleotides. That is, the over expression of the TRP-domain of PP5 prevents the binding of FKBP52, favoring the cytoplasmic retention of the GR-complex in a state with low affinity for hormone. The suppression of PP5 expression has the opposite effect, facilitating FKBP52-GR interactions, which favors hormone binding, increased nuclear translocation and enhanced transcriptional activity. However, the process is likely to be more complex, for recently PP5 has been implicated in the dephosphorylation of GRs at sites shown to differentially affect GR target gene expression [46]. Thus, in addition to affecting nuclear translocation of GR via its association with Hsp-90, PP5’s direct actions on the GR may influence the ability of the hormone-activated receptor to interact with transcriptional coregulators.
Figure 2. Model of FKBP51, FKBP52 and PP5 regulation of GR function.
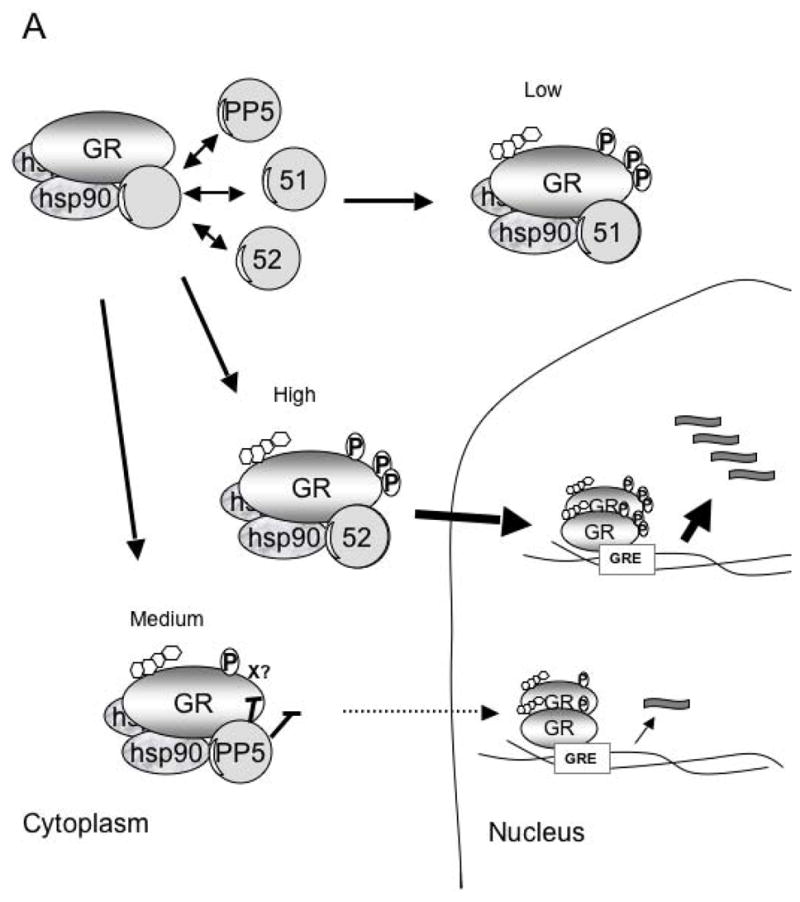
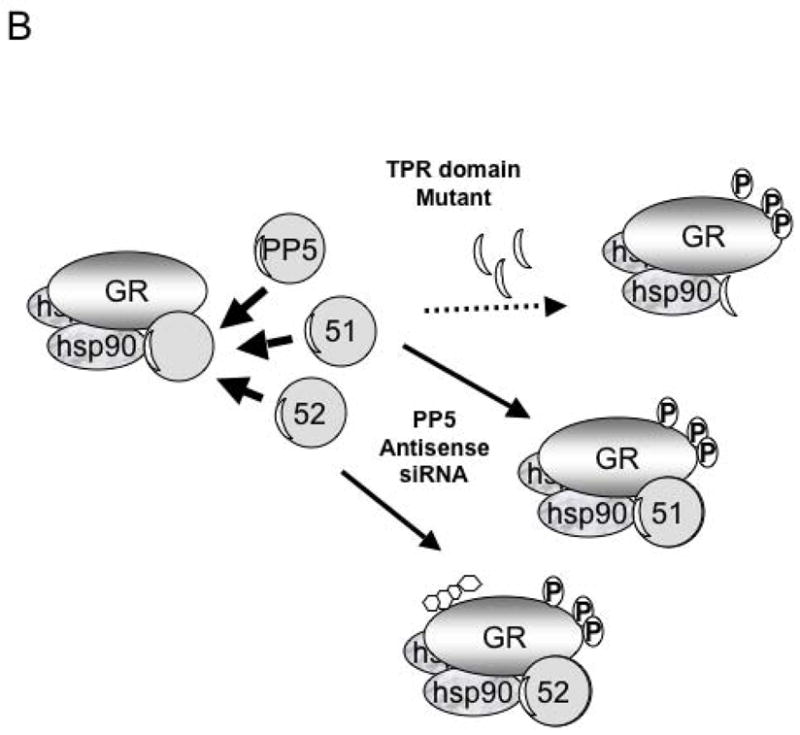
A) The GR-heterocomplex can contain FKBP51, FKBP52 or PP5. In the basal state the inactive GR-heterocomplex contains FKBP51 as the predominant TPR protein. In this state GR has low hormone binding activity. The acquisition of PP5 by the GR-complex results in a GR-with intermediate hormone-binding and transcriptional activities. When the GR acquires FKBP52, it has the highest transcription activity due to both enhanced hormone binding affinity and enhanced nuclear translocation. B) The model above enlightens previous conflicting experiments such that the over expression of the TPR-domain (thought to be a dominant-negative mutant) of PP5 prevents the binding of FKBP52, favoring the cytoplasmic retention of the GR-complex in a state with low affinity for hormone. The suppression of PP5 expression has the opposite effect, facilitating FKBP52-GR interactions, which favors hormone binding, increased nuclear translocation and enhanced transcriptional activity.
PP5 and other Hsp-90 associated proteins
PP5 has also been observed in Hsp-90 heterocomplexes containing other proteins, including heme-regulated eIF2α kinase [29] and heat shock factor 1 (Hsf-1) [47]. PP5 appears to negatively modulate the maturation of the Hsp-90 dependent heme-regulated kinase [29] and to function as a negative modulator of Hsf-1 [47]. The over expression of the PP5 TPR domain facilitates the dissociation of peroxisome proliferator activated receptors (PPARα and PPARβ) from Hsp-90, which is associated with increased transcriptional activity measured with a PPAR-reporter plasmid assay [48]. The over expression of the TPR domain has also been found to result in the down regulation of aryl hydrocarbon receptor levels, suggesting that PP5 may also play a role in the stabilization of the aryl hydrocarbon receptor [49]. These observations suggest PP5 may play a broad role in the molecular chaperone activity of Hsp-90. However, at this time the physiological roles played by PP5 when associated with these protein complexes are not clear.
PP5 as a negative regulator of p53-tumor suppressor protein
The suppression of PP5 expression with ISIS 15534, a potent antisense oligonucleotide targeting human PP5, suppressed the growth of some, but not all, cancer cells in culture [50]. Later it was observed that the growth suppression achieved with ISIS 15534 occurred only in cells that contain functional p53 and undergo G1-growth arrest when treated with dexamethasone [41]. The ablation of PP5 has little effect on the binding of hormone to GR. However, treatment with ISIS 15534 enhanced both dexamethasone-induced phosphorylation of p53 at Ser15 and the expression of the G1-cyclin dependent kinase inhibitor, p21Waf1/cip1 [51].
p53 is a well known tumor suppressor protein that functions as a stress-induced transcription factor. Phosphorylation at Ser15 affects p53 in several ways: 1) it decreases the binding affinity between Mdm2 (p53-murine double minute 2, an Ub-E3 ligase) and p53, disrupting a negative feedback loop leading to the proteolytic degradation of p53; 2) it increases the transcriptional efficiency of certain p53-responsive genes; 3) by masking a nuclear export signal contained near the amino terminus, phosphorylation at Ser15 allows the nuclear accumulation of p53 [52, 53, 54]. Thus, by suppressing the GR-dependent phosphorylation of Ser15, PP5 appears to function as a negative regulator of p53 [41, 50, 51]. However, even the marked over expression of PP5 does not prevent the UV-induced increase in p53 protein levels or phosphorylation, suggesting that the main role of PP5 in p53 signaling is to help keep the basal activity of p53 low in cells that have not encountered genomic stress. In addition, although PP5 can dephosphorylate p53 in vitro, in vivo PP5 is likely acting upstream, possibly by augmenting actions of a GR-induced kinase, such as serum-glucocorticoid inducible kinase-1 (SGK-1), which is up-regulated in cells treated with ISIS 15534 [41].
PP5, Ras, Rac and Raf-1
Another developing area of research involves PP5 as an effector of GTPase signaling networks, which control events as diverse as metastasis and neuronal development. Yeast two-hybrid screening identified interactions between PP5 and both Gα12 and Gα13 [35]. These G-proteins are known to regulate the activity of a small GTPase, Rho, through Rho guanine nucleotide exchange factor (RhoGEF) [36]. The site of interaction is the TPR-domain of PP5, and binding is associated with the activation of PP5 catalytic activity. Although the physiological importance of this interactions is not yet clear, recently PP5 has been shown to modulate thyroid hormone signaling via Rho and another member of Ras-related family of monomeric GTPases, Rac. Rac and Rho mediate opposing signaling effects in response to thyroid hormone on KCNH2 potassium channels [55], with Rho inhibiting and Rac stimulating, KCNH2 activity [36]. Current research shows that inhibition of PP5 with okadaic acid blocked channel stimulation by hormone and Rac, while the expression of a toxin insensitive mutant of PP5 (Y451A) restored signaling [36]. Expression of the PP5 TPR domain blocked channel stimulation by hormone. Furthermore, mutation of the TPR domain of the insensitive PP5 mutant at two predicted sites of interaction with Rac blocked the ability of the Y451A substitution to rescue KCNH2 activity in the presence of okadaic acid. [36]. These studies implicate PP5 as a modulator of Rac/Rho signaling, and future studies designed to examine the details of this binding interaction and other Rac versus Rho signaling pathways are warranted.
PP5 was also shown to play a role in the inactivation of Raf-1. Raf-1 is a serine/threonine kinase that functions as a downstream effector of Ras-GTPases, playing a key role in Ras-Raf-MEK/ERK pathways transmitting mitogenic, differentiative and oncogenic signals to down stream kinases [56]. Raf-1 activation involves the dephosphorylation of an inhibitory site (Ser259) located in its regulatory domain by PP2A [56], the recruitment of Raf-1 to the plasma membrane, and its association with Ras. This is followed by phosphorylation of an activating residue (Ser338), resulting in the stabilization of Raf-1 in an activate conformation. Using a proteomic approach, PP5 was identified as a protein that interacts with Raf-1 [35]. PP5/Raf-1 association occurs in response to growth factor stimulation (EGF) and results in the selective dephosphorylation of Ser338, removing phosphate from a critical site that helps maintain Raf-1 activation. Thus, PP5 inactives Raf-1, suppressing the down stream activation of MEK. Since another PPP-family phosphatase (PP2A) serves to dephosphorylate/activate Raf-1 (Ser259), the coordinated efforts of PP2A and PP5 appear to be important for Raf-1 signaling. Interestingly, PP5 has been shown to bind the A-subunit of the PP2A holoenzyme, which functions to tether the catalytic (PP2Ac) and substrate targeting B-subunits of PP2A [34]. Therefore, it will be interesting to determine if PP5 regulates the incorporation of a particular B-subunit into the PP2Ac/A/B holoenzyme, enabling the formation of PP2Ac/A/B trimer that recognizes phospho-Ser259 on Raf-1. In addition, the stability of Raf-1 protein is influenced by its association with Hsp-90. Thus, again the involvement of PP5 may be further up-stream and more complex than suggested by the current literature.
PP5 and cell growth
Other studies using ISIS 15534 revealed that the growth of MCF-7 breast cancer cells that were not sensitive to growth arrest by treatment with dexamethasone were growth suppressed when PP5 expression was ablated [51,57]. Subsequent studies revealed that PP5 protein levels are decreased by the removal of estrogen from the culture media and that the PP5 promoter possesses a functional estrogen response element [57]. In culture, the constitutive over expression of PP5 relieved the estrogen dependency of MCF-7 cells, allowing rapid proliferation in estrogen-depleted media [57]. PP5 over expression has also been reported to induce the binding of PP5 to ERs, resulting in the suppression of ER-dependent transcription [58]. This may implicate PP5 in a feedback control mechanism. In a MCF-7 mouse xenograph model of tumor development, the constitutive over expression of PP5 was associated with accelerated tumor growth in a high estrogen environment [59]. However, PP5 over expression alone failed to produce spontaneous tumors in a low estrogen environment. Therefore, although the over expression of PP5 appears to provide a growth advantage to estrogen responsive tumors, at this time the physiological role of estrogen-induced PP5 expression is not yet clear.
PP5 and cellular responses to stress
Several studies suggest that many of the growth regulating actions of PP5 are responsive to cellular stress, with PP5 acting in the regulation of signaling cascades induced by oxidative stress, DNA-damage and hypoxia (Figure 3). An increase in PP5 protein levels is also observed following prolonged hypoxia or treatment with reagents that induce oxidative stress [30, 60]. This increase in expression under low oxygen conditions is mediated by the activation and stabilization of a transcription factor, hypoxia inducible factor-1 (HIF-1), which binds to a HIF-1 response element in the PP5 promoter [60]. Both, hypoxia and acute oxidative stress also induce the association of PP5 with apoptosis signal regulating kinase (ASK1) [30, 60]. ASK1 is a member of the MAPKKK family of kinases that activates both p38 and MKK4/JNK pathways. Most reports indicate that ASK1 initiates a signaling cascade that favors apoptosis, but the activation of ASK1 has also been reported to aid differentiation and survival [61, 62]. Current research indicates that after exposure to oxidative stress (e.g. treatment with H202) ASK1 is transiently activated by autophosphorylation at Thr845. In vitro, PP5 can dephosphorylate ASK1 at Thr845, suggesting that PP5 can inactivate ASK1 [30]. However, the ablation of PP5 expression using siRNA or antisense oligonucleotides results only in the prolonged activation of the ASK1/MKK4/JNK arm of ASK-signaling, without affecting the phosphorylation of p38 [60]. Thus, the association of ASK1 with PP5 may suppress the ability of ASK1 to phosphorylate/activate MKK4, possibly implicating MKK4 or an ASK1/MKK4 scaffolding protein as substrate [30, 60, 62]. Other studies have shown that a decrease in PP5 activity following rapamycin treatment subsequently leads to an increase in ASK1-mediated apoptosis [63]. However, the role for PP5 in rapamycin mediated activation of ASK1 and apoptosis was only observed in p53−/− cells. This supports studies suggesting p53 acts to suppress rapamycin-induced activation of ASK1 [63] and may further link the actions of p53, PP5 and ASK1.
Figure 3. Roles of PP5 in the regulation of stress-induced signaling cascades.
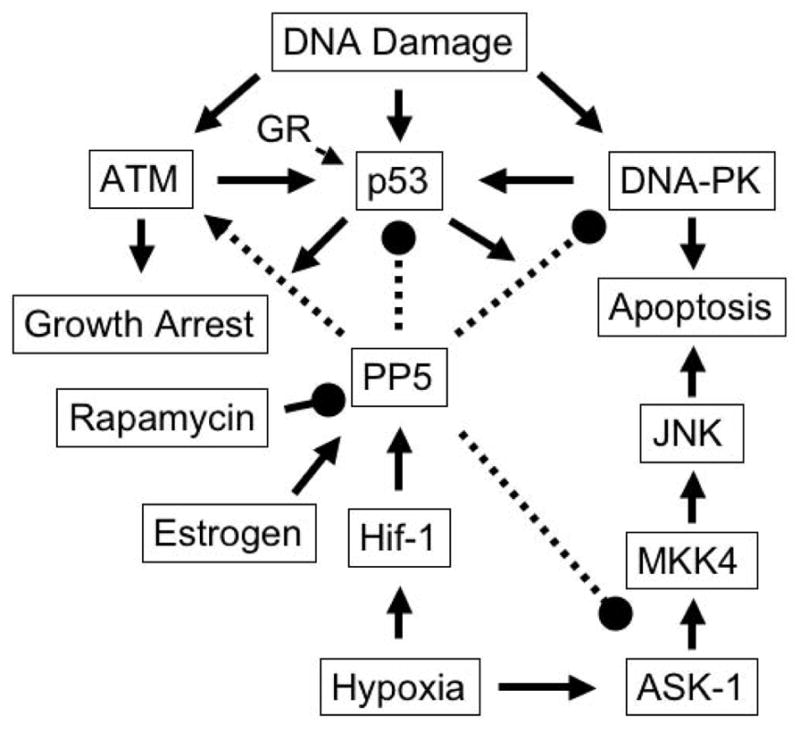
In response to DNA damage, ATM, p53 and DNA-PK are activated, triggering and/or propagating signaling cascades leading to growth arrest or apoptosis. Both hypoxia and 17-β estradiol induce PP5 transcription, leading to the suppression of p53-, DNA-PK- and ASK-1-mediated responses that result in growth arrest or apoptosis. In p53 −/− cells rapamycin produces a decrease in PP5 activity and a concomitant increase in ASK-1 mediated apoptosis suggesting that the suppression of PP5 activity contributes to the antitumor activity of rapamycin. PP5 has also been reported to play a positive role in the propagation of an ATM-mediated response leading to growth arrest. Arrows indicate stimulatory actions. Lines with filled circles at the end indicate inhibitory actions.
In response to DNA-damage, PP5 has been reported to associate with ATM (ataxia telangiectasia mutated kinase) [32], ATR (ATM and Rad3 related kinase) [33] and a DNA-damage activated protein kinase, DNA-PKcs [31]. DNA breaks can have serious consequences if they are not repaired, leading to cell death, genomic instability and tumorigenesis. Therefore, humans have evolved elaborate mechanisms to repair or eliminate cells that have encountered genomic damage. All three of the proteins PP5 associates with in response to DNA-damage are serine/threonine kinases that are key regulators of cellular responses to genomic stress. DNA-PKcs plays a key role in the repair of double-strand breaks via non-homologous end-joining repair [64]. Studies suppressing the expression of PP5 with antisense oligonucleotides or siRNA suggest that the interaction of PP5 with DNA-PKcs is associated with the dephosphorylation of a functional site (Thr2609) on DNA-PKcs, suggesting PP5 acts as a negative regulator of DNA-PKcs [31].
In contrast to its inhibitory role in DNA-PKcs activation, PP5 appears to function as positive regulator in ATM and ATR signaling. ATM and ATR are two related protein kinases that are key regulators of DNA-damage cell cycle checkpoint controls, coordinating many processes that induce cell cycle arrest, G2/M-, and S-phase checkpoint control mechanisms [65]. In ATM signaling the suppression of PP5 expression prevents ATM-mediated G-1 growth arrest [33]. After exposure to ionizing radiation, cells with reduced levels of PP5 also fail to arrest at the normal G2/M checkpoint and have decreased ATM activity [66]. Similarly, in ATR-signaling PP5 has been reported to be necessary for ATR-mediated checkpoint control (31), again with the suppression of PP5 resulting in impaired ATR-mediated phosphorylation of known down stream substrates that mediate intra S-phase checkpoint responses.
PP5 and cancer
To date, there have only been a few studies that directly implicate PP5 in the development of cancer. The first observation linking PP5 with cell growth comes from studies in yeast, where the homologue of PP5 (PPT1) is expressed at elevated levels in proliferating cells [67]. In rats PP5 mRNA levels were reported as markedly elevated in highly malignant ascites hepatomas [68]. Antibody microarray expression studies of mantle-cell lymphomas also indicate PP5 is overexpressed ≥ two-fold compared to controls [69]. However, the number of patients in this study was limited (data from 6 patients). Immunostaining of human tumor tissue microarrays (>250 samples) also revealed a positive correlation between elevated levels of PP5 and human breast cancer, and the PP5 gene has been linked to a region of chromosome amplification in osteosarcoma samples from human patients [70, 71]. A survey of PP5 (locus at 19q13.3) on the NCI CGAP web site [72] comparing Mitelman Breakpoint data section reveals a large number of alterations (1212 cases) at this chromosomal site for several types of cancers. However, the data is correlative, and a direct demonstration that aberrant PP5 expression contributes to tumorigenesis has not been confirmed experimentally. Nonetheless, when considered with the above mentioned observations linking the expression of PP5 to both HIF-1 and estrogen (which have both been linked to the progression of human cancer) and the participation of PP5 in the regulation of GR- and stress-induced signaling networks that suppress cell cycle progression or induce apoptosis, there is certainly circumstantial evidence suggesting that aberrant expression of PP5 may aid the development or progression of human cancer. On theo ther hand, recent studies implicating PP5 as a negative regulator of Raf1 and reports suggesting that PP5 is needed for ATM- and ATR-signaling may argue for the contrary.
Structural considerations for PP5 drug design
For studies to determine the biological roles of PP5, and possibly for development of novel antitumor drugs, a specific inhibitor of PP5 is desired. In the absence of enzymes, the uncatalyzed hydrolysis of simple phosphate monoester dianions is very slow. The half-time for the uncatalyzed hydrolysis of alkyl phosphate dianions at 25 °C is over 1 trillion years; knon = ~2 × 10−20 s−1 [73]. In comparison, typical substrate turnover rates (kcat) for PPP family phosphatases range from 1 to 100 s−1. Therefore, PPases enhance the rate of hydrolysis by a factor of ~1021, placing them among the most powerful known catalysts on earth (catalytic proficiencies ([kcat/kM]/knon) of ~1025–1026 M−1 [73].
The high resolution (1.6 Å) structure of PP5 indicates that phosphomonoester hydrolysis occurs through in-line nucleophilic attack requiring the activation of a bound water molecule to the more nucleophilic hydroxide, its precise alignment with the electrophilic phosphorus atom of the substrate phosphoryl group, and profound stabilization of the altered substrate in the transition state [18]. The structure of the PP5 catalytic domain shows that the essential catalytic motif contains 6 conserved features: Asp274, His304, Asp271, the backbone carbonyl of His427, the two active site metal ions, and W1 (water/hydroxide coordinated to the two active site metal ions). Sequence alignments and comparisons to other crystal structures reveal that this motif is common to all members of the PPP family phosphatases, as is the hydrogen bond network that ensures this catalytic activity (Figure 4). The necessary alignment of substrate and nucleophile is facilitated by substrate contacts with M1, M2, Arg275, Asn303, His304, and Arg400 and interactions of W1 with M1, M2, and His427. Thus, there are many sights for inhibitor binding that should disrupt catalytic activity.
Figure 4. Structural comparison of PP5 to other members of the PPP.
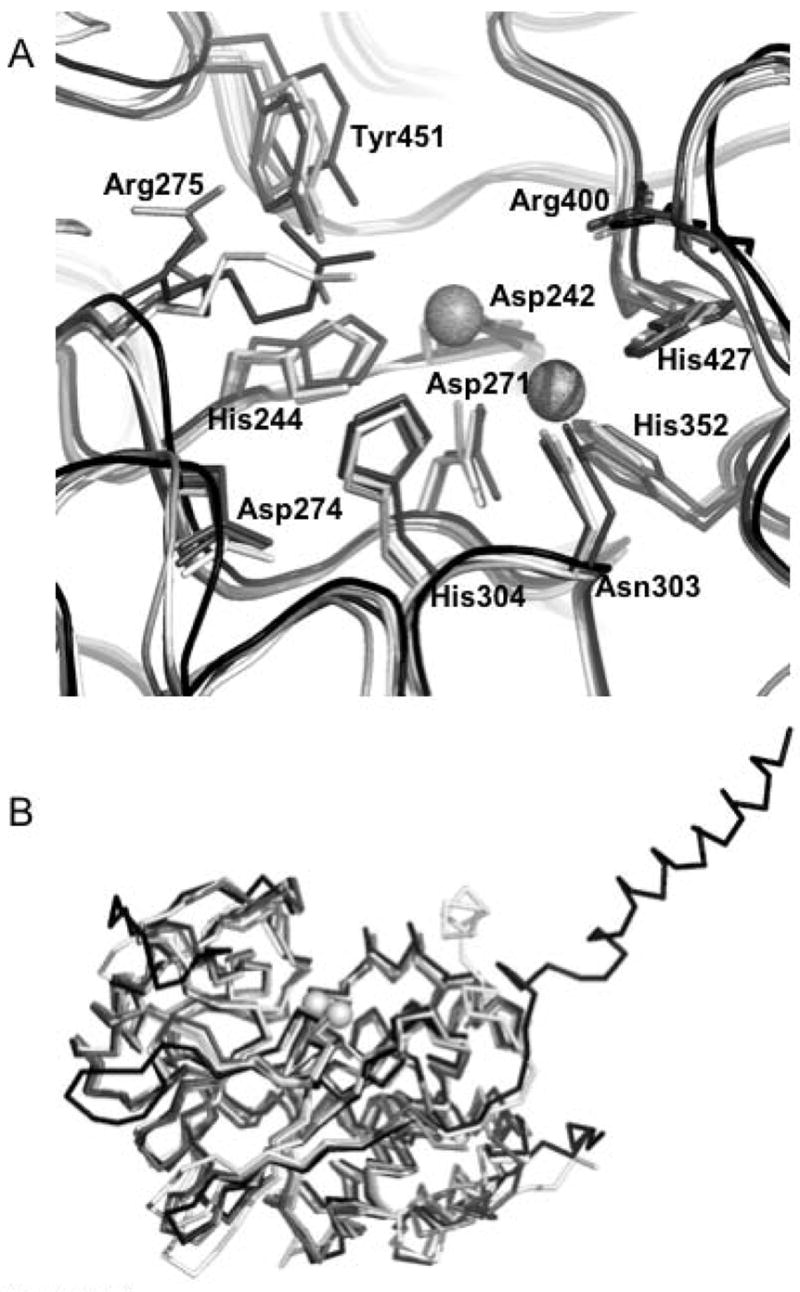
family. A. Superposition of the active sites of (1S95:white), PP2A (2IE4:light gray), PP1 (1JK7:dark gray), and PP2B (1TCO:black) showing the positions and conformations of 11 absolutely conserved residues. The active site metal positions are derived from 1JK7. PP5 numbering of residues is observed. B. Superposition (shown as Cα traces) of the catalytic domains of PP5 (1S95:white), PP2A (2IE4:light gray), PP1 (1JK7:dark gray), and PP2B (1TCO:black). The active site metal positions are derived from 1JK7. These structural alignments show the remarkable similarity of the overall fold in these four representatives of the PPP family. This similarity is particularly evident in and around the catalytic center where the conserved residues are found in essentially identical conformations (an exception being Arg275 which takes on different conformations depending upon which ligand is bound in the active site). Structural alignments were done with STRAP (http://www.charite.de/bioinf/strap/) and the figure was prepared with PyMOL (http://www.pymol.org).
Still, the development of a specific inhibitor may be challenging, for the catalytic core of PP5 is highly homologous with other eukaryotic PPP phosphatases. In addition both structural and mutational studies indicate that PP1, PP2A, PP2B and PP5 share a common catalytic mechanism that is likely common to the PPP-family. Superposition of the structure of PP5c onto the structures of other eukaryotic PPP phosphatases such as PP1, PP2A and PP2B gives root mean square deviations of <2.0 Å within the highly homologous ~270-residue region used in the calculations. Nonetheless, comparisons of the loop regions of PP5, PP1, PP2A and PP2B reveal differences that may be relevant for drug discovery efforts, such as sequence/conformational differences in the β12/β13 loop that plays an important role in microcystin-LR and okadaic acid-mediated inhibition of catalytic activity. Such structural differences closely apposed to the conserved catalytic site suggest the feasibility of developing type-specific inhibitors.
Conclusions
Although to date, there is no direct experimental proof that PP5 over expression aids tumor promotion or carcinogenesis, both the correlative and experimental studies discussed above provide substantial data demonstrating that PP5 potentially plays a key role in the regulation of both hormone- and stress-induced signaling networks that allow a cell to respond appropriately to genomic stress. Therefore, it seems likely that aberrant alterations of PP5 function may indeed contribute to neoplastic transformation and cancer progression. In addition, because many of the pathways that PP5 has been shown to influence share common components and are themselves targets for cancer drug development (i.e. p53, ASK-1, GR-Hsp90, ATR, ATM, DNA-PKcs), understanding the biological actions of PP5 should not only provide insight into cell behavior, they should also aid the development of new methods for the medical management of human cancers.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NCI grant CA-60750 to REH), the National Center for Research Resources (NCRR P2PRR016478 to TG).
References
- 1.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 2.Milano A, De Rosa V, Iaffaioli RV, Caponigro F. Downstream intracellular effectors of epidermal growth factor receptor as targets for anticancer therapy. Expert Opinion on Therapeutic Targets. 2007;11:771–782. doi: 10.1517/14728222.11.6.771. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. J Zhejiang Univ Sci B. 2007;8(6):377–397. doi: 10.1631/jzus.2007.B0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenolikar S, Nairn AC. Protein phosphatases: Recent Progress. Adv Sec Mess and Phosphopro Res. 1991;23:1–119. [PubMed] [Google Scholar]
- 5.Klumpp S, Krieglstein J. Serine/threonine protein phosphatases in apoptosis. Curr Opin Pharmacol. 2002;2(4):458–462. doi: 10.1016/s1471-4892(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 6.Cohen PT. Protein phosphatase 1-targeted in many directions. J Cell Sci. 2002;115(Pt 2):241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 8.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22(7):245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 9.Cohen PTW, Brewis ND, Hughes V, Mann DJ. Serine/threonine protein phosphatases: an expanding family. FEBS Lett. 1990;268:355–358. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- 10.Cohen PTW. Cloning of protein-serine/threonine phosphatases. Methods in Enzymology. 1991;201:398–408. doi: 10.1016/0076-6879(91)01036-2. [DOI] [PubMed] [Google Scholar]
- 11.Arndt KT, Styles CA, Fink GR. A suppressor of HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989;56:527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Z, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21WAF1/Cip1 and mediate growth arrest. J Biol Chem. 1998;273(20):12250–12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor. hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272(26):16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- 14.Skinner J, Sinclair C, Romeo C, Armstrong D, Charbonneau H, Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J Biol Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Cheng A, Honkanen RE. Genomic organization of the human PP4 gene encoding a serine/threonine protein phosphatase (PP4) suggest a common ancestry with PP2A. Genomics. 1997;44:336–343. doi: 10.1006/geno.1997.4891. [DOI] [PubMed] [Google Scholar]
- 16.Bastians H, Ponstingl H. The novel human protein serine/threonine phosphatase 6 is a functional homologue of budding yeast Sit4p and fission yeast ppe1, which are involved in cell cycle regulation. J Cell Sci. 1996;109(Pt 12):2865–2874. doi: 10.1242/jcs.109.12.2865. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Honkanen RE. Molecular cloning, expression and characterization of a novel human serine/threonine protein phosphatase, PP7, that is homologous to Drosophila retinal degeneration C gene product (rdgC) J Biol Chem. 1998;273(3):1462–1468. doi: 10.1074/jbc.273.3.1462. [DOI] [PubMed] [Google Scholar]
- 18.Swingle MR, Honkanen RE, Ciszak EM. Structural basis for the catalytic activity of human serine/threonine protein phosphatase-5. JBC. 2004;279(32):33992–33999. doi: 10.1074/jbc.M402855200. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Roe SM, Cliff MJ, Williams MA, Ladbury JE, Cohen PTW, et al. Molecular basis for TPR domain-mediated regulation of protein phosphatase 5. The EMBO Journal. 2005;24:1–10. doi: 10.1038/sj.emboj.7600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honkanen RE, Zwiller J, Daily SL, Khatra BS, Dukelow M, Boynton AL. Identification, purification, and characterization of a novel serine/threonine protein phosphatase from bovine brain. J Biol Chem. 1991;266(10):6614–6619. [PubMed] [Google Scholar]
- 21.Becker W, Kentrup H, Klumpp S, Schultz JE, Joost HG. Molecular cloning of a protein serinethreonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. JBC. 1994;269:22586–22592. [PubMed] [Google Scholar]
- 22.Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PTW. A novel human serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO. 1994;13(18):4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci USA. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Lagercrantz J, Zickert P, Bajalica-Lagercrantz S, Zetterberg A. Chromosomal localization and 5′ sequence of the human protein serine/threonine phosphatase 5 gene. Biochem and Biophys Res Comm. 1996;218:514–517. doi: 10.1006/bbrc.1996.0092. [DOI] [PubMed] [Google Scholar]
- 25.Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- 26.Davies TH, Ning YM, Sanchez ER. Differential control of glucocorticoid receptor hormone-binding function by tetratricopeptide repeat (TPR) proteins and the immunosuppressive ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- 27.Ollendorff V, Donoghue DJ. The serine/threonine phosphatase PP5 interacts with CDC16 and CDC27, two tetratricopeptide repeat-containing subunits of the anaphase-promoting complex. J Biol Chem. 1997;272(51):32011–32018. doi: 10.1074/jbc.272.51.32011. [DOI] [PubMed] [Google Scholar]
- 28.Zhao S, Sancar A. Human blue-light photoreceptor hCRY2 specifically interacts with protein serine/threonine phosphatase 5 and modulates its activity. Photochem Photobiol. 1997;66:727–731. doi: 10.1111/j.1751-1097.1997.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 29.Shao J, Hartson SD, Matts RL. Evidence that protein phosphatase 5 functions to negatively modulate the maturation of the Hsp90-dependent heme-regulated eIF2alpha kinase. Biochemistry. 2002;41:6770–6779. doi: 10.1021/bi025737a. [DOI] [PubMed] [Google Scholar]
- 30.Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, et al. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wechsler T, Chen BP, Harper R, Morotomi-Yano K, Huang BC, Meek K, et al. DNA-PKcs function regulated specifically by protein phosphatase 5. Proc Natl Acad Sci USA. 2004;101:1247–1252. doi: 10.1073/pnas.0307765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali A, Zhang J, Bao S, Liu I, Otterness D, Dean NM, et al. Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev. 2004;18:249–254. doi: 10.1101/gad.1176004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Bao S, Furumai R, Kucera KS, Ali A, Dean NM, et al. Protein Phosphatase 5 is required for ATR-mediated checkpoint activation. Mol Cell Biol. 2005;25(22):9910–9919. doi: 10.1128/MCB.25.22.9910-9919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubert EJ, Hong Y, Sarge KD. Interaction between protein phosphatase 5 and the A subunit of protein phosphatase 2A: evidence for a heterotrimeric form of protein phosphatase 5. J Biol Chem. 2001;276(42):38582–38587. doi: 10.1074/jbc.M106906200. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi Y, Katoh H, Mori K, Negishi M. Galpha(12) and Galpha(13) interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol. 2002;12:1353. doi: 10.1016/s0960-9822(02)01034-5. [DOI] [PubMed] [Google Scholar]
- 36.Gentile S, Darden T, Erxleben C, Romeo C, Russo A, Martin N, et al. Rac GTPase signaling through the PP5 protein phosphatase. PNAS. 2006;103:5202–5206. doi: 10.1073/pnas.0600080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Kriegsheim A, Pitt A, Grindlay GJ, Kolch W, Dhillon AS. Regulation of the Raf-MEK- ERK pathway by protein phosphatase 5. Nat Cell Biol. 2006;8(9):1011–1016. doi: 10.1038/ncb1465. [DOI] [PubMed] [Google Scholar]
- 38.Richter K, Buchner J. Hsp90: Chaperoning signal transduction. J Cell Physiol. 2001;188(3):281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 39.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 40.Frydman J, Höhfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- 41.Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon IV, et al. Ser/thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38(28):8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]
- 42.Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, et al. Serine/threonine phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2(6):1471–2121. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, et al. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107– 4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 45.Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- 47.Conde R, Xavier J, McLoughlin C, Chinkers M, Ovsenek N. Protein Phosphatase 5 is a negative modulator of Heat Shock Factor 1. J Biol Chem. 2005;280:28989–28996. doi: 10.1074/jbc.M503594200. [DOI] [PubMed] [Google Scholar]
- 48.Sumanasekera WK, Tien ES, Davis JW, II, Turpey R, Perdew GH, Vanden Heuvel JP. Heat Shock Protein-90 (Hsp90) acts as a repressor of Peroxisome Proliferator-Activated Receptor alpha (PPARalpha) and PPARbeta Activity. Biochemistry. 2003;42(36):10726–10735. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 49.Meyer BK, Petrulis JR, Perdew JH. Aryl hydrocarbon (Ah) receptor levels are selectively modulated by hsp90-associated immunophilin homolog XAP2. Cell Stress Chaperones. 2000;5(3):243–254. doi: 10.1379/1466-1268(2000)005<0243:aharla>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuo Z, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21WAF1/Cip1 and mediate growth arrest. J Biol Chem. 1998;273:12250– 12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- 51.Urban G, Golden T, Aragon IV, Cowsert L, Cooper SR, Dean NM, et al. Identification of a functional link for the p53-tumor suppressor protein in dexamethasone induced growth suppression. J Biol Chem. 2003;278(11):9747–9753. doi: 10.1074/jbc.M210993200. [DOI] [PubMed] [Google Scholar]
- 52.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Xiong Y. A p53 Amino-terminal nuclear export signal inhibited by DNA damage- induced phosphorylation. Science. 2001;292:1910–1915. doi: 10.1126/science.1058637. [DOI] [PubMed] [Google Scholar]
- 54.Bean LJ, Stark GR. Phosphorylation of serines 15 and 37 is necessary for efficient accumulation of p53 following irradiation with UV. Oncogene. 2001;20:1076–1084. doi: 10.1038/sj.onc.1204204. [DOI] [PubMed] [Google Scholar]
- 55.Storey NM, O’Bryan JP, Armstrong DL. Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr Biol. 2002;12:27–33. doi: 10.1016/s0960-9822(01)00625-x. [DOI] [PubMed] [Google Scholar]
- 56.Dougherty M, Müller J, Ritt D, Zhou M, Zhou X, Copeland T, et al. Regulation of Raf-1 by direct feedback phosphorylation. Molecular Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 57.Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an Estrogen-inducible Phosphatase (PP5) that converts MCF-7 Human Breast Carcinoma Cells into an Estrogen-Independent phenotype when expressed constitutively. J Biol Chem. 2001;276(29):27638–27646. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda k, Ogawa S, Tsukui T, Horie-Inoue K, Ouchi Y, Kato S, et al. Protein Phosphatase 5 Is a Negative regulator of Estrogen Receptor-Mediated transcription. Molecular Endocrinology. 2004;18:1131–1143. doi: 10.1210/me.2003-0308. [DOI] [PubMed] [Google Scholar]
- 59.Golden T, Aragon IV, Zhou G, Cooper SR, Dean NM, Honkanen RE. Constitutive over expression of serine/threonine protein phosphatase 5 (PP5) augments estrogen-dependent tumor growth in mice. Cancer Letters. 2004;215:95–100. doi: 10.1016/j.canlet.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Zhou G, Golden T, Aragon IV, Honkanen RE. Ser/thr protein phosphatase 5 (PP5) inactivates hypoxia-induced activation of an ASK-1/MKK-4/JNK-signaling cascade. J Biol Chem. 2004;279:46595–46605. doi: 10.1074/jbc.M408320200. [DOI] [PubMed] [Google Scholar]
- 61.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 62.Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 63.Huang S, Shu L, Easton J, Harwood FC, Germain GS, Ichijo H, et al. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–36496. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 64.DiBiase SJ, Zeng ZC, Chen R, Hyslop T, Curran WJ, Jr, Iliakis G. DNA-dependent Protein Kinase stimulates an independently active, Nonhomologous, End-Joining Apparatus. Can Res. 2000;60:1245–1253. [PubMed] [Google Scholar]
- 65.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 66.Yong W, Bao S, Chen H, Dapei L, Sanchez ER, Shou W. Mice lacking protein phosphatase5 are defective in ataxia telangiectasia mutated (ATM)-mediated cell cycle arrest. J Biol Chem. 2007;282:14690–14694. doi: 10.1074/jbc.C700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong J-Y, Johns J, Sinclair C, Park J-M, Rossie S. Characterization of Saccharomyces cerevisiae protein Ser/Thr phosphatase T1 and comparison to its mammalian homolog PP5. BMC Cell Biology. 2003;4:3. doi: 10.1186/1471-2121-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shirato H, Shima H, Nakagama H, Fukuda H, Watanabe Y, Ogawa K, et al. Expression in hepatomas and chromosomal localization of rat protein phosphatase 5 gene. Int J of Oncol. 2000;17:909–912. doi: 10.3892/ijo.17.5.909. [DOI] [PubMed] [Google Scholar]
- 69.Ghobrial IM, McCormick DJ, Kaufmann SH, Leontovich AA, Loegering DA, Dai NT, et al. Proteomic analysis of mantle-cell lymphoma by protein microarray. Blood. 2005;105:3722–3730. doi: 10.1182/blood-2004-10-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honkanen, R.E. and Golden T.G., Unpublished observation.
- 71.Atiye J, Wolf M, Kaur S, Monni O, Böhling T, Kivioja A, et al. Gene amplifications in osteosarcoma-CGH microarray analysis. Genes Chromosomes Cancer. 2005;42(2):158–163. doi: 10.1002/gcc.20120. [DOI] [PubMed] [Google Scholar]
- 72.http://cgap.nci.nih.gov/Genes/GeneFinder
- 73.Lad C, Williams NH, Wolfenden R. The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases. Proc Natl Acad Sci U S A. 2003;100:5607–5610. doi: 10.1073/pnas.0631607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maynes JT, Bateman KS, Cherney MM, Das AK, Luu HA, Holmes CF, James MN. Crystal structure of the tumor-promoter okadaic acid bound to protein phosphatase-1. J Biol Chem. 2001;276:44078–44082. doi: 10.1074/jbc.M107656200. [DOI] [PubMed] [Google Scholar]
- 75.Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, Fleming MA, Caron PR, Hsiao K, Navia MA. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 76.Xing Y, Xu Y, Chen Y, Jeffrey PD, Chao Y, Lin Z, Li Z, Strack S, Stock JB, Shi Y. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell. 2006;127:341–53. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]


