Abstract
Principal cells of the ventral cochlear nucleus (VCN) differ in the magnitudes of low-voltage-activated potassium (gKL) and hyperpolarization-activated (gh) conductances that determine the time course of signaling. Octopus cells have large gKL (500 nS) and gh (150 nS), bushy cells have smaller gKL (80 nS) and gh (30 nS), and T stellate cells have little gKL and a small gh (20 nS). gKL arises through potassium channels of which ~ 60% contain Kv1.1 subunits; gh arises through channels that include HCN1 subunits. The surfaces of cell bodies and dendrites of octopus cells in the dorsocaudal pole, and of similar cells along the ventrolateral edge of the PVCN, were brightly labeled by an antibody against HCN1 that was colocalized with labeling for Kv1.1. More anteriorly neurons with little surface labeling were intermingled among cell bodies and dendrites with surface labeling for both proteins, likely corresponding to T stellate and bushy cells. The membrane-associated labeling patterns for Kv1.1 and HCN1 were consistent with what is known about the distribution and the electrophysiological properties of the principal cells of the VCN. The cytoplasm of large cells and axonal paranodes contained immunoflurorescent labeling for only Kv1.1.
Keywords: low-voltage-activated potassium conductance, hyperpolarization-activated conductance, hearing, auditory system, brainstem auditory nuclei
INTRODUCTION
Extracting information about the location of sound sources and interpreting the meaning of those sounds begins with the delivery of acoustic information by auditory nerve fibers to the cochlear nuclei. On the basis of their histological staining, Osen identified nine cell types and showed that neurons in the cochlear nuclei are diverse but have a consistent organization (Osen, 1969). Over the last forty years, much has been learned about those cell types, their dendritic morphology, their projections through the brain stem, their responses to sounds, and their biophysical properties. The names of the cell types given to these neurons by Osen continue to be used, often together with designations based on dendritic and axonal labeling with which the Nissl staining corresponds: large spherical (bushy), small spherical (bushy), globular (bushy), multipolar (T and D stellate), octopus, pyramidal (fusiform), giant and granular. Again and again our group has found itself confirming distinctions between types and finding common features within cell types, attesting to the meticulousness of the observations that guided the original parcellation (Wu and Oertel, 1984; Oertel et al., 1990; Zhang and Oertel, 1993a; Zhang and Oertel, 1993b; Zhang and Oertel, 1994; Golding et al., 1995; Golding and Oertel, 1997; Cao et al., 2007). Recognition of differing cell types was the first step in recognizing that the processing of acoustic information occurs in parallel along multiple pathways, many tonotopically organized.
How neurons process information by transforming the firing of inputs depends on the morphology of the cell, on what inputs impinge on the cells and how they are arranged, on the properties of the synapses, and on how the synaptic currents are transformed to voltage changes by the electrical properties of postsynaptic neurons. Neurons in the cochlear nuclei differ in their electrical properties. Some cells in the VCN have low-voltage-activated potassium conductances (gKL) and some do not; the magnitude in those that do varies substantially between cells (Wu and Oertel, 1984; Manis and Marx, 1991). In octopus cells, gKL is large, in spherical and globular bushy cells the conductances are smaller and in the multipolar cells whose axons project through the trapezoid body (T stellate or planar stellate) cells they are tiny or undetectable (Manis and Marx, 1991; Doucet and Ryugo, 1997; Bal and Oertel, 2001; Ferragamo and Oertel, 2002; Rothman and Manis, 2003; Cao et al., 2007). These same cells also have hyperpolarization-activated conductances (gh) whose maximum amplitude, kinetics and half-activation voltage varies between cell types (Bal and Oertel, 2000; Cao et al., 2007; Rodrigues and Oertel, 2006; Fujino and Oertel, 2001). Inhibitory multipolar cells that project dorsalward through the intermediate acoustic stria (D stellate cells or radial stellate) have not been studied under voltage-clamp but express a rapid gh (Fujino and Oertel, 2001). At the resting potential both gKL and gh are partially activated, reducing the input resistance. The currents at the resting potential oppose one another. IKL has a reversal potential near EK at about −80 mV and is outward at rest whereas Ih reverses at about −40 mV and is inward at rest. Also, IKL is activated by depolarization whereas Ih is activated by hyperpolarization.
The presence of gKL and gh has two significant functional consequences. First, in reducing the input resistance they allow voltage changes to be rapid and to convey the timing of their peaks with temporal precision. Second, the rapid activation of gKL prevents slow depolarizations from evoking action potentials (Ferragamo and Oertel, 2002; McGinley and Oertel, 2006). As synaptic depolarizations are most rapid when excitation from convergent inputs is synchronous and the rising phases of EPSPs sum, the presence of gKL enhances the detection of coincident inputs and decreases temporal summation.
There is strong evidence that gKL is mediated by a heterogeneous population of tetrameric ion channels of the Kv1 family (also known as shaker or KCNA) (Bal and Oertel, 2001; Dodson et al., 2003; Lu et al., 2004; Rothman and Manis, 2003; Cao et al., 2007). Not all ion channels that mediate gKL contain Kv1.1 because only about 60% of gKL is sensitive to DTX-K, a blocker specific for Kv1.1-containing channels (Robertson et al., 1996; Owen et al., 1997; Wang et al., 1999). In mice that lack Kv1.1 subunits, IKL is reduced but not eliminated (Bal and Oertel, 2001; Brew et al., 2003; Cao et al., 2007).
It is likely that gh is mediated through channels of the HCN (for hyperpolarization-activated and cyclic nucleotide-gated channels) family. These channels are tetrameric and are composed of subunits HCN1, HCN2, HCN3 and HCN4 (Santoro et al., 2000; Robinson and Siegelbaum, 2003). Homomeric channels differ in their rates of activation and sensitivity to cyclic nucleotides. Homomeric HCN1 channels activate most rapidly and are least modulated by cAMP (Santoro et al. 1998). HCN4 channels activate most slowly and both HCN2 and HCN4 channels are strongly modulated by cAMP (Seifert et al., 1999; Ludwig et al., 1999). Heteromeric channels composed of HCN1 and HCN2 subunits are intermediate in their kinetics and sensitivity to cAMP (Chen et al., 2001; Ulens and Tytgat, 2001). HCN1, HCN2, and HCN4 are expressed in the VCN (Santoro et al., 2000; Notomi and Shigemoto, 2004; Koch et al., 2004).
Here we relate measurements of currents from octopus, bushy and one type of multipolar cell (T stellate cells) to immunohistochemical labeling for the ion channels that mediate voltage-gated currents. An antibody against Kv1.1 was used to label a part of gKL and an antibody against HCN1 was used to label what is perhaps only part of gh.
EXPERIMENTAL PROCEDURES
Mice
Mice of the outbred ICR strain and obtained from Sprague-Dawley (Madison, WI) were used in these studies. All animal protocols were approved by the Institutional Animal Care and Use Committee of the School of Medicine and Public Health at the University of Wisconsin, Madison.
Electrophysiology
Recordings were made from coronal slices from mice between 18 and 21 days old as described previously (Bal and Oertel, 2000; Cao and Oertel, 2005; Cao et al., 2007). Slices were cut 200 µm thick in a physiological saline that contained (in mM): 130 NaCl, 3 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 20 NaHCO3, 3 HEPES, 10 glucose, saturated with 95 % O2/5% CO2, pH 7.3–7.4, at between 24°C and 27°C. Control recordings were made in a slightly altered saline that contained (in mM): 138 NaCl; 4.2 KCl, 2.4 CaCl2, 1.3 MgCl2, 0.25 CdCl2, 10 HEPES, 10 glucose, 0.001 tetrodotoxin (TTX), 0.040 6,7-dinitroquinoxaline-2,3-dione (DNQX) (Tocris Cookson, UK), 0.001 strychnine, pH 7.4 and saturated with 100% O2 at 33°C. In some experiments, 50 µM ZD7288, 10 mM TEA, or 50 nM α-DTX were added to isolate the IKL, IKH, and Ih. All reported voltages were compensated for a −12 mV junction potential. Slices were viewed through a Zeiss Axioskop at 63X. Patch-clamp pipettes had resistances between 4–6 MΩ and were filled with a solution consisting of (in mM): 108 potassium gluconate, 9 HEPES, 9 EGTA, 4.5 MgCl2, 14 phosphocreatinine (tris salt), 4 ATP (Na salt) and 0.3 GTP (tris salt), pH 7.4. Recordings were made with an Axopatch 200A amplifier (Axon Instruments). Stimulation, recording, and analysis was done with pClamp 8 software (Axon Instruments). All chemicals were from Sigma unless specified otherwise.
Immunohistochemistry
Mice between 19 and 29 days old were deeply anaesthetized with intraperitoneal injections of between 0.6 and 0.8 ml avertin (Sigma). Animals were perfused through the heart with normal physiological saline to which 0.02% heparin had been added. After 2 min it was followed by 30 min perfusion with 4% paraformaldehyde in 0.1 M NaH2PO4, 0.1 M Na2HPO4, pH 7.4 (PBS). After perfusion, the cochlear nuclei were dissected and postfixed further for 10 min. The tissue was washed with graded series of cryoprotective solutions (10% sucrose in PBS for 1 hr, 15% sucrose solution for 2 hr, and 20% sucrose solution overnight) at 4° C. On the following day, 30 µm sections were cut on a cryostat. The sections were left to dry overnight at room temperature. Sections were stored at −20°C.
Immunolabeling was with commercially available antibodies. The primary antibody used to label Kv1.1 was obtained from Sigma (Clone K20/78). It is a monoclonal antibody against a synthetic peptide of residues 458–476 (EEDMNNSIAHYRQANIRTG) of the C-terminus of the Kv1.1 α subunit from rats that has been extensively characterized. Its specificity for Kv1.1 subunits was shown by labeling only COS-1 cells that had been transfected with Kv1.1, by immunoprecipitating the Kv1.1 polypeptide, preabsorbtion with the Kv1.1 peptide blocked immunoreactivity, and immunocytochemical staining patterns were identical to those obtained with a rabbit polyclonal antibody (Bekele-Arcuri et al., 1996; Rasband et al., 1998; Vabnick et al, 1999). The primary antibody was detected with a TRITC-conjugated goat anti-mouse antibody, also from Sigma. No labeling was detected when the primary antibody was omitted. The primary antibody used to label HCN1 was obtained from Alomone Labs (Lot AN-09). It was a polyclonal antibody against a peptide corresponding to the intracellular residues 6–24 at the N-terminal of HCN1 of rats. The antibody was affinity purified and shown by the manufacturer to lose immunoreactivity when preincubated with the control peptide antigen. The lyophilized antibody was reconstituted in deionized water. This primary antibody was detected with a FITC-conjugated, goat anti-rabbit secondary antibody from Molecular Probes. In the absence of the primary antibody, no labeling was detected.
Sections were stained over two days. Sections were rinsed in PBS for 30 min and placed in a blocking solution for 1.5 hr that contained 400 µl normal goat serum, 0.3 % Triton-x-100, 200 mg bovine gamma globulin. Primary antibodies were diluted 1:100 with blocking solution and applied to the sections overnight at 4°C. On the following day, sections were rinsed three times for 10 min with PBS followed by incubation in a secondary antibody. The sections were washed with PBS and were mounted with vectashield mounting medium (Vectors Laboratories). Confocal images of the slides were acquired using a confocal system (Olympus Fluoview FV1000). Images in figures were scaled and some were cropped; otherwise images were not manipulated in any way.
RESULTS
Electrophysiology
Whole-cell patch-clamp recordings in the voltage-clamp mode reveal the properties of the ion channels that mediate IKL and Ih. Examples of such recordings are illustrated in Figure 1. In all recordings some currents were blocked pharmacologically: 0.25 mM Cd2+ blocked voltage-gated Ca2+ currents, 1 µM TTX blocked voltage-gated Na+ currents, 40 µM DNQX blocked spontaneous EPSCs through AMPA receptors, and 1 µM strychnine blocked spontaneous IPSCs through glycine receptors. The upper panels illustrate measurements of IKL when Ih was blocked with 50 µM ZD7288. The lower panels illustrate measurements of Ih when IKL was blocked with 50nM α–DTX. The magnitudes of the conductances that underly those currents reflect the surface expression of the ion channels that mediate them. Descriptions of those conductances have been reported: gKL in octopus cells was described by (Bal and Oertel, 2001; Cao and Oertel, 2005), gh in octopus cells was described by (Bal and Oertel, 2000; Cao and Oertel, 2005), gKL and gh in bushy cells by (Cao et al., 2007), and gh in T stellate cells by (Rodrigues and Oertel, 2006). Figure 1 illustrates the large differences in the magnitudes of currents in octopus, bushy and T stellate cells.
Figure 1.

Examples of measurements of gKL (upper traces) and gh (lower traces) in each of the three major types of principal cells are illustrated. Families of voltage pulses were imposed through patch-electrodes in the whole-cell configuration. Because these conductances have overlapping voltage-sensitivity, they were measured in the presence of blockers of other conductances. In all these recordings voltage-sensitive Na+ currents were blocked by 1 µM tetrodotoxin, Ca2+ currents by 0.25 mM Cd2+, and glycinergic and glutamatergic synaptic currents were blocked by 1 µM strychnine and 40 µM DNQX, respectively. In addition gKL was measured in the presence of 50 µM ZD7288, a blocker of gh and gh was measured in the presence of 50 nM α-DTX, a blocker of Kv1 channels.
Measurements of the magnitudes of currents are summarized in Table 1. The gKLmax is more than 6 times greater in octopus than in bushy cells and is undetectable in T stellate cells. The maximum values of gh are about 5 times greater in octopus than in bushy cells and almost 8 times greater than in T stellate cells.
Table 1.
Summary of properties of conductances measured in cells of the VCN. For the three groups of principal cells, the maximal conductances, half-maximal activation voltage, and DTX-K-sensitivity are shown for gKL; the maximal gh and its half-maximal activation voltage are also shown.
Immunohistochemistry
An overview of immunohistochemical labeling is given in Figure 2. Labeling in the DCN was brightest in the outermost layers for both Kv1.1 (Fig. 2A,C) and HCN1 (Fig. 2B,C). At higher magnification it is evident that the ependymal cell layer was brightly labeled for Kv1.1 but not for HCN1 (Fig. 2C1). The molecular layer (Fig. 2, ml) was labeled for both antigens. Labeling for Kv1.1 was mainly diffuse but an occasional brightly labeled red spot was evident (eg. Fig. 2C1, arrow). Labeling for HCN1 looks diffuse at low magnification but at higher magnification its punctate nature is evident; labeled processes were not observed (Fig. 2C1). In the deep layer (Fig. 2, dl) an occasional neuron and its dendrites were outlined in both red and green but most labeling was diffuse and dim and could not be associated with particular cell types.
Figure 2.
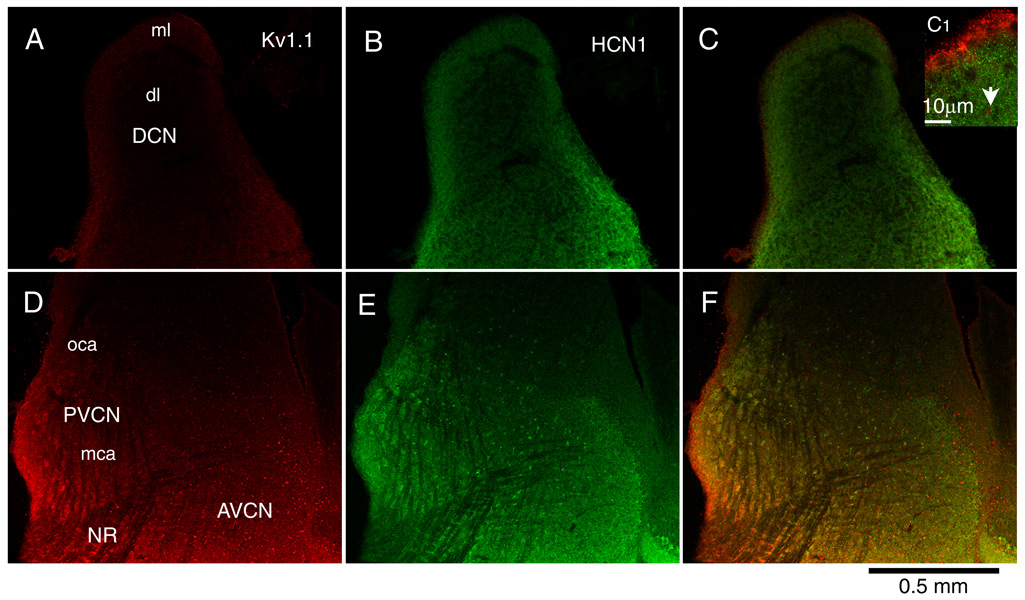
Labeling for Kv1.1 (left, red) and for HCN1 (middle, green) and the merged image that reveals colocalization in yellow (right) in parasagittal sections of the cochlear nuclear complex show differing patterns. A. The molecular layer (ml) of the DCN revealed brighter staining for Kv1.1 than the deep layer (dl). The labeling was especially bright at the ependymal layer but that is not obvious at the low magnification that is shown. B. The molecular layer was also more brightly stained for HCN1 than the deep layer whereas the granule cell lamina that separates the DCN and VCN shows little staining. C. The difference in labeling between the molecular layer and deep layers is evident in the merged image and is particularly clear at higher magnification in the inset (C1). C1. The ependymal layer is brightly labeled for Kv1.1 whereas the molecular layer shows evenly distributed punctate staining for HCN1. An example of a spot that resembles a paranode is indicated by the arrow. D. The brightest labeling for Kv1.1 was associated with octopus cells in the caudal, posterior VCN. In more medial sections, octopus cells lay in a triangular region in the most caudal and dorsal part of the nucleus but in this relatively lateral section, brightly labeled cells extended ventrally along the posterior edge of the nucleus almost to the nerve root. Labeling for Kv1.1 increased toward the anterior VCN; brightly labeled spots are evident. E. Octopus cells and scattered neurons in the vicinity of the nerve root were brightly labeled for HCN1 among cells that were only weakly labeled. Labeling for HCN1 was bright anteriorly revealing the border between the labeled magnocellular and unlabeled parvocellular regions. The dim labeling of the bundles of auditory nerve fibers contrasts with the brighter staining of the VCN cells. F. The merged image reveals the same patterns as D and E.
Both antibodies labeled the VCN (Fig. 2D–F). Labeling for Kv1.1 was brightest posteriorly near the octopus cell area of the PVCN (Fig. 2D, F oca) and anteriorly (Fig. 2D, F AVCN) and somewhat dimmer around the nerve root (Fig. 2D, F NR). Labeling for HCN1 showed the same overall pattern as Kv1.1, being brightest posteriorly in the vicinity of the octopus cell area (Fig. 2E, F). In more medial sections, these brightly lit neurons lay within the triangular octopus cell area but in more lateral sections, like that illustrated in Figure 2, the brightly labeled cells extended along the edge of the PVCN almost to the nerve root. The ventral and most anterior parts of the AVCN were also strongly labeled. Some neurons scattered throughout the nucleus were particularly brightly labeled for HCN1. Punctate labeling outlined the magnocellular region of the AVCN, defining the border between the large principal cells and granule cell domains that were not strongly labeled. Bundles of auditory nerve fibers were dimly labeled for Kv1.1 but unlabeled for HCN1.
Labeling for Kv1.1 and for HCN1 in the VCN differed (Figure 3). Labeling for HCN1 tended to outline cell bodies and processes and to be low in the cytoplasm, as was observed by others (Koch et al., 2004). The antibody against Kv1.1 labeled not only the surfaces of some cells but also the cytoplasm of almost all of the larger cell bodies. The brightness of cytoplasmic labeling varied between cells and was not always even.
Figure 3.

Labeling differs between populations of cells. A–C. Within the octopus cell area many cell bodies and processes were brightly labeled for both Kv1.1 and HCN1. Much of the label for Kv1.1 was colocalized with label for HCN1 but some was not. Elongated bright spots (arrows) resemble those associated with myelinated nerve fibers and likely labeled paranodes. D–F. Along the posterior and ventral border of the VCN some cells and processes were brightly labeled for both antigens. The cells and their processes resembled those in the octopus cell area. They were intermingled with cells that were only dimly labeled (<) that occupy the multipolar cell area. G–I. In the nerve root, antibodies against Kv1.1 labeled numerous elongated spots (arrows). The regions near the arrows are shown at higher magnification in Figure 4. Among the bundle of fibers was a single cell in which label for HCN1 was colocalized with label for Kv1.1. J–L. In the AVCN many cell bodies were surrounded with bright label for both antigens but few processes were distinct. Presumably many of these cells were bushy cells whose fine labeled processes could not be resolved. In the AVCN, too, labeling for Kv1.1 revealed bright spots that were presumably labeled paranodes (arrows). All images were from the same section.
The differences in the labeling patterns over different regions of the VCN are best appreciated at high magnification (Figure 3). Labeling was bright in the octopus cell area for both antigens (Fig. 3A–C, Fig.4 D–F). Cell bodies and large processes were outlined by red and green labels. In some cases the large processes emanated from the cell body indicating that they were dendrites. The close proximity of Kv1.1 and HCN1 proteins near the membrane is evident in the yellow color in the merged images (Fig. 3C, Fig. 4F). It is likely that labeling is associated with the membrane and that the membrane labeling reflects the presence of ion channels that contain these subunits. The merged images show that within the octopus cell area some punctate labeling for Kv1.1 was not associated with labeling for HCN1. Two of these are marked with arrows in Figures 3A and C and likely reflect labeling of paranodes of axons (Fig. 3A, C).
Figure 4.

Spots labeled for Kv1.1 in the nerve root have structure. Two regions, in the vicinity of each of the arrows in Figure 3G, are shown at higher magnification. Labeled spots are often elongated and seem to comprise pairs of parallel labeled strips.
An unexpected finding was that brightly labeled cells with thick dendrites were located not only in the tear-drop-shaped octopus cell area but extend ventrally toward the nerve root along the edge of the posterior VCN. Some of these cells are illustrated in Figure 3D–F and in Figure 5 A–C, G–I. A comparison between labeled cells within the octopus cell area (Fig 3. A–C, Fig. 5D–F) with the labeled cells near the caudoventral border of the VCN (Fig. 3 D–F, Fig. 5 A–C, G–I) shows similar bright surface labeling of cell bodies and processes for both Kv1.1 and HCN1. In these more ventral regions, brightly labeled cell bodies and dendritic processes intermingle with more dimly labeled cell bodies and processes (Fig. 3 D–F, <). The most parsimonious interpretation of this result is that the OCA occupies not only the region at the most dorsal and caudal VCN but that it also extends laterally to more ventral regions.
Figure 5.

Comparison of octopus cells within the octopus cell area with brightly labeled cells along the ventral and caudal border of the VCN in different animals. The size of neurons and the diameters of labeled processes were similar, consistent with all being octopus cells. A–C. Brightly labeled neurons near the caudoventral border of the VCN; edge is outside the picture at the left. D–F. Octopus cells in the midst of the octopus cell area. G–H. Brightly labeled neurons near the caudoventral border. As elsewhere in the VCN, elongated spots that are likely to be perinodes of myelinated nerve fibers were labeled for Kv1.1 but not for HCN1 (rightward arrowheads).
Dimly labeled cells were most common in the multipolar cell area. The distinction between cells was especially clear where they lie adjacent to octopus cells (Figure 3D–F <)). The cytoplasm of most multipolar cell bodies was lightly stained for Kv1.1 but little labeling was evident near the surfaces of cell bodies or processes. It is likely that many of the dimly labeled cells were T stellate cells as these are the most numerous cells in the multipolar cell area. An occasional cell with long, straight dendrites was outlined with the label for HCN1. The shape of dendrites and the similarity of the distribution of such cells with those labeled for glycine indicates that these may have been D stellate cells (Oertel et al., 1990; Wickesberg et al., 1994; Doucet and Ryugo, 1997).
The nerve root contains bundles of auditory nerve axons as well as a few cell bodies. Occasional cell bodies were labeled for HCN1 but bundles of nerve fibers showed no labeling (Fig. 3H, I). The nerve root contains numerous elongated Kv1.1-positive, HCN1-negative spots (Fig. 3G–I). Two groups of the spots, above the arrows in Figure 3G, are illustrated at higher magnification in Figure 4. Labeled spots were often elongated and appeared as doublets. These labeled, elongated, parallel doublets bear a striking resemblance to the paranodal distribution of Kv1.1 in myelinated nerve nerve fibers (Wang et al., 1993; Mi et al., 1995; Rasband et al, 1998; Zhou et al., 1998b; Vabnick et al., 1999; Adamson et al., 2002, Caminos et al., 2005).
The anterior VCN was also brightly labeled but staining was variable and processes were difficult to resolve (Fig. 3J–L, Fig. 6). Many cell bodies were diffusely labeled for Kv1.1 and surface staining was not obvious (Fig. 3J) whereas HCN1 labeled the surfaces but not the cytoplasm (Fig. 3K). The merged images show that much of the labeling was colocalized, suggesting that Kv1.1, like HCN1, also labeled surfaces (Fig. 3L). If HCN1 generally labels membranes, the colocalization of both antibodies indicates that Kv1.1, too, is localized in membranes. Colocalization in indistinct structures between cells could reflect labeling of the membranes of the small, intertwined dendritic processes of bushy cells. Figure 6 shows that in the AVCN, cells with surfaces that were strongly labeled for HCN1 lay among neurons with weakly labeled surfaces (Fig. 6B o). Cells labeled strongly for Kv1.1 lay adjacent to cells that were weakly labeled for HCN1 and Kv1.1 (Fig. 6C). The magnitude of gKL and gh varies considerably between bushy cells (Cao et al., 2007); whether the variability in staining of neurons in the anterior VCN reflects variability in the expression of these subunits in bushy cells or differences in cell type is unknown. As elsewhere in the VCN, spots labeled brightly for Kv1.1 but not HCN1 are evident.
Figure 6.

Labeling in the AVCN was variable. Cells whose surfaces were strongly labeled lay adjacent to cells whose surfaces were not labeled (example marked with an o). Much of the label for Kv1.1 and for HCN1 was colocalized but as elsewhere in the VCN nodes were labeled brightly with only Kv1.1 (rightward arrowheads).
DISCUSSION
There was a strong correlation between electrophysiological measurements of voltage-sensitive conductances and surface labeling for subunits of ion channels that mediate those conductances. The octopus cells that have exceptionally strong gKL and gh have surfaces that are the most brightly labeled for HCN1 that is colocalized with Kv1.1 at the surfaces of cell bodies and dendrites. An unexpected finding was that cells whose labeling for these antigens resembles that of octopus cells were found not only within the octopus cell area but also along the lateral and caudal surface of the VCN almost to the nerve root where their brightly labeled dendrites were intermingled among dimly labeled neurons. In the AVCN, where most bushy cells are located, many cell bodies were outlined by colocalized labels for Kv1.1 and HCN1. There was also diffuse, colocalized labeling for both antigens between cells that could reflect labeling on dendrites of bushy cells. Labeling was dimmest in the multipolar cell area where most T stellate cells are located. The cytoplasm of cell bodies was diffusely labeled for Kv1.1 but little surface labeling for Kv1.1 or HCN1 was detected in cells of this region. Among cells with little or no superficial labeling, an occasional cell and its dendrites were brightly labeled for HCN1. These may have been D stellate cells. While gh has not been measured in D stellate cells under voltage-clamp, current clamp recordings indicate that D stellate cells have a strong and rapid gh (Fujino and Oertel, 2001).
In the present study the surface labeling by antibodies to HCN1 is not only interesting in its own right but it also serves as a marker of the surface to which the localization of Kv1.1 can be related so that Kv1.1 in the cytoplasm can be distinguished from surface labeling within the resolution of light microscopy (~0.2 µm). Our results show that at the surfaces of many cell bodies and dendrites, including those of octopus and presumed bushy cells, HCN1 and Kv1.1 are colocalized. Taken together, the findings that HCN1 labels cellular surfaces, that Kv1.1 and HCN1 are colocalized, and that the cells that are most strongly labeled for HCN1 and Kv1.1 also have the largest gKL and gh support the interpretation that fluorescent labels associated with the surface reveal ion channels in the plasma membranes. The cytoplasm of most neurons in the VCN was labled diffusely by antibodies to Kv1 but protein in the cytoplasm cannot contribute to the electrophysiologically measured conductances.
Labeling patterns for HCN1 and Kv1.1 in the present study are consistent with earlier studies in the VCN. Koch and colleagues revealed the exceptionally strong surface labeling of octopus cells by antibodies to HCN1 and also showed that in the AVCN, where many bushy and some T stellate cells are located, weakly labeled cells were interspersed among strongly labeled ones. In contrast to the expression of HCN1, that labeled the surfaces of neurons, label for HCN2 was also found in the cytoplasm (Koch et al., 2004). Kv1.1 protein has been detected in the AVCN (Dodson et al., 2003; Caminos et al., 2005). Furthermore, the labeling patterns for Kv1.1 and HCN1 proteins in the cochlear nuclei are consistent with expression of mRNA. High levels of mRNA for Kv1.1 were detected by in situ hybridization where high levels of the protein have been detected with immunohistochemical methods in the cochlear nuclei (Wang et al., 1994; Caminos et al., 2005). Levels were reported to be particularly high where octopus and bushy cells are located (Grigg et al., 2000). In rats the levels of expression of Kv1.1 mRNA are relatively low at birth but increase rapidly to attain adult levels at P15 (Bortone et al., 2006). Caminos et al. (2005) showed that in the AVCN of rats somatic labeling decreases and axonal labeling increases over the first month or two after birth, raising the possibility that our immunohistochemical and electrophysiological measurements may reflect some immaturity.
In addition to labeling the surfaces of some neurons, antibodies to Kv1.1 also labeled paranodes in fibers of the auditory nerve and possibly in axons of other neurons. Kv1.1 has been localized to paranodes of axons (Wang et al., 1993; Mi et al., 1995; Rasband et al., 1998; Zhou et al., 1998a; Reid et al., 1999; Vabnick et al., 1999; Caminos et al., 2005); in peripheral axons Kv1.1 has been shown to prevent aberrant firing. In the cochlear nuclei punctate Kv1.1 labeling was localized to paranodes with multiple approaches, including colabeling with markers of dendrites and electron microscopy (Caminos et al., 2005). It is perhaps not surprising that some expression of Kv1.1 was detected in all large neurons in the VCN because even the T stellate cells in which little gKL was detected in recordings from the cell body have myelinated axons but it was not possible to examined axons of T stellate cells specifically. In other neurons, too, Kv1 channels have been detected at terminals but not at the cell body (Southan and Robertson, 2000). The granule cells that separate the VCN from the DCN have unmyelinated axons and are unlabeled for Kv1.1. Mice survive even when Kv1.1 is absent and gKL is reduced, indicating either that their action is not essential or that the increased excitability is reduced in some other way (Smart et al., 1998; Zhou et al., 1998; Brew et al., 2003).
Tonotopic gradients in the expression of gKL have been described in mammals and in birds raising the question whether such gradients exist in the mammalian cochlear nuclei. Labeling for both Kv1.1 and HCN1 is not uniform across the cochlear nuclei, being strongest most rostrally and most caudally. In the LSO of very young rats gKL is most prominent in the lateral limb that ultimately encodes low frequency sounds (Barnes-Davies et al., 2004). In the medial nucleus of the trapezoid body it is most prominent medially where high frequencies are encoded (Brew and Forsythe, 2005). In nucleus magnocellularis and nucleus laminaris of birds, too, gKL and gh follow the tonotopic gradient (Fukui and Ohmori, 2004; Kuba et al., 2005). In the mammalian cochlear nucleus it is impossible to resolve tonotopic differences within a population of similar cells because the distribution of cell types is itself not uniform.
In summary, the present study leads to the following conclusions. 1) Surface labeling for Kv1.1 and HCN1 are correlated with the magnitude of maximal gKL and gh measured in neurons in those areas. Surface labeling is strong for octopus cells, and for a population of cells that is sparsely distributed over the VCN that might correspond to D stellate cells, less strong for bushy cells, and least for T stellate cells. 2) In octopus cells, and probably also bushy cells, gKL and gh are likely to be generated both at the cell body and along dendrites. 3) Neurons whose bright surface labeling for Kv1.1 and HCN1 resembles that of octopus cells are found not only in the octopus cell area but also along the ventral and lateral surface of the PVCN to near the nerve root. 4) Punctate label for Kv1.1 along axons is likely to be at paranodes.
ACKNOWLEDGEMENTS
We are most grateful to Ed Chapman for giving us the opportunity to use the confocal microscope and to Camin Dean and Felix Yeh who answered our questions patiently and graciously. Two anonymous reviewers pointed out several significant shortcomings and omissions in a very nice way; thank you! We also thank Samantha Wright for her valuable comments. We also thank Ravi Kochhar and the departmental office staff for their continuing support. This work was supported by a grant from NIH DC 00176.
COMPREHENSIVE LIST OF ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- D stellate
multipolar cells whose axons exit dorsalward
- DTX
dendrotoxin
- EPSP
excitatory postsynaptic potential
- gKL
low-voltage-activated potassium conductance
- gh
hyperpolarization-activated conductance
- HCN
hyperpolarization and cyclic nucleotide gated
- Kv1
potassium channels in the shaker or KCNA family
- T stellate
multipolar cells whose axons exit through the trapezoid body
- TTX
tetrodotoxin
- VCN
ventral cochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Bal R, Oertel D. Hyperpolarization-activated, mixed-cation current (Ih) in octopus cells of the mammalian cochlear nucleus. J Neurophysiol. 2000;84:806–817. doi: 10.1152/jn.2000.84.2.806. [DOI] [PubMed] [Google Scholar]
- Bal R, Oertel D. Potassium currents in octopus cells of the mammalian cochlear nuclei. J Neurophysiol. 2001;86:2299–2311. doi: 10.1152/jn.2001.86.5.2299. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. doi: 10.1111/j.0953-816x.2003.03133.x. [DOI] [PubMed] [Google Scholar]
- Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel alpha- and beta-subunit polypeptides. Neuropharm. 1996;35:851–865. doi: 10.1016/0028-3908(96)00128-1. [DOI] [PubMed] [Google Scholar]
- Bortone DS, Mitchell K, Manis PB. Developmental time course of potassium channel expression in the rat cochlear nucleus. Hear Res. 2006;211:114–125. doi: 10.1016/j.heares.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Systematic variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hear Res. 2005;206:116–132. doi: 10.1016/j.heares.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol (Lond) 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminos E, Vale C, Lujan R, Martinez-Galan JR, Juiz JM. Developmental regulation and adult maintenance of potassium channel proteins (Kv 1.1 and Kv 1.2) in the cochlear nucleus of the rat. Brain Res. 2005;1056:118–131. doi: 10.1016/j.brainres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Cao X, Oertel D. Temperature affects voltage-sensitive conductances differentially in octopus cells of the mammalian cochlear nucleus. J Neurophysiol. 2005;94:821–832. doi: 10.1152/jn.01049.2004. [DOI] [PubMed] [Google Scholar]
- Cao XJ, Shatadal S, Oertel D. Voltage-sensitive conductances of bushy cells of the mammalian ventral cochlear nucleus. Journal of Neurophysiology. 2007;97:3961–3975. doi: 10.1152/jn.00052.2007. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J Physiol. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Projections from the ventral cochlear nucleus to the dorsal cochlear nucleus in rats. J Comp Neurol. 1997;385:245–264. [PubMed] [Google Scholar]
- Ferragamo MJ, Oertel D. Octopus cells of the mammalian ventral cochlear nucleus sense the rate of depolarization. J Neurophysiol. 2002;87:2262–2270. doi: 10.1152/jn.00587.2001. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci. 2001;21:7372–7383. doi: 10.1523/JNEUROSCI.21-18-07372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I, Ohmori H. Tonotopic gradients of membrane and synaptic properties for neurons of the chicken nucleus magnocellularis. J Neurosci. 2004;24:7514–7523. doi: 10.1523/JNEUROSCI.0566-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78:248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Golding NL, Robertson D, Oertel D. Recordings from slices indicate that octopus cells of the cochlear nucleus detect coincident firing of auditory nerve fibers with temporal precision. J Neurosci. 1995;15:3138–3153. doi: 10.1523/JNEUROSCI.15-04-03138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JJ, Brew HM, Tempel BL. Differential expression of voltage-gated potassium channel genes in auditory nuclei of the mouse brainstem. Hear Res. 2000;140:77–90. doi: 10.1016/s0378-5955(99)00187-2. [DOI] [PubMed] [Google Scholar]
- Koch U, Braun M, Kapfer C, Grothe B. Distribution of HCN1 and HCN2 in rat auditory brainstem nuclei. Eur J Neurosci. 2004;20:79–91. doi: 10.1111/j.0953-816X.2004.03456.x. [DOI] [PubMed] [Google Scholar]
- Kuba H, Yamada R, Fukui I, Ohmori H. Tonotopic specialization of auditory coincidence detection in nucleus laminaris of the chick. J Neurosci. 2005;25:1924–1934. doi: 10.1523/JNEUROSCI.4428-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Monsivais P, Tempel BL, Rubel EW. Activity-dependent regulation of the potassium channel subunits Kv1.1 and Kv3.1. J Comp Neurol. 2004;470:93–106. doi: 10.1002/cne.11037. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Marx SO. Outward currents in isolated ventral cochlear nucleus neurons. J Neurosci. 1991;11:2865–2880. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley MJ, Oertel D. Rate thresholds determine the precision of temporal integration in principal cells of the ventral cochlear nucleus. Hear Res. 2006;216–217:52–63. doi: 10.1016/j.heares.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Mi H, Deerinck TJ, Ellisman MH, Schwarz TL. Differential distribution of closely related potassium channels in rat Schwann cells. J Neurosci. 1995;15:3761–3774. doi: 10.1523/JNEUROSCI.15-05-03761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wu SH, Garb MW, Dizack C. Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J Comp Neurol. 1990;295:136–154. doi: 10.1002/cne.902950112. [DOI] [PubMed] [Google Scholar]
- Osen KK. Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol. 1969;136:453–484. doi: 10.1002/cne.901360407. [DOI] [PubMed] [Google Scholar]
- Owen DG, Hall A, Stephens G, Stow J, Robertson B. The relative potencies of dendrotoxins as blockers of the cloned voltage-gated K+ channel, mKv1.1 (MK-1), when stably expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;120:1029–1034. doi: 10.1038/sj.bjp.0701004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Scholz A, Bostock H, Vogel W. Human axons contain at least five types of voltage-dependent potassium channel. J Physiol (Lond) 1999;518:681–696. doi: 10.1111/j.1469-7793.1999.0681p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Owen D, Stow J, Butler C, Newland C. Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Let. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: From molecules to physiological function. Ann Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Rodrigues ARA, Oertel D. Hyperpolarization-activated currents regulate excitability in stellate cells of the mammalian ventral cochlear nucleus. J Neurophysiol. 2006;95:76–87. doi: 10.1152/jn.00624.2005. [DOI] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. Differential expression of three distinct potassium currents in the ventral cochlear nucleus. J Neurophysiol. 2003;89:3070–3082. doi: 10.1152/jn.00125.2002. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp UB. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc Nat Acad Sci USA. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K(+) currents in cerebellar basket and purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Gi- and Gs-coupled receptors up-regulate the cAMP cascade to modulate HCN2, but not HCN1 pacemaker channels. Pflugers Arch. 2001;442:928–942. doi: 10.1007/s004240100617. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FC, Bell N, Reid P, Smith LA, McIntosh P, Robertson B, Dolly JO. Identification of residues in dendrotoxin K responsible for its discrimination between neuronal K+ channels containing Kv1.1 and 1.2 alpha subunits. Eur J Biochem. 1999;263:222–229. doi: 10.1046/j.1432-1327.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickesberg RE, Whitlon DS, Oertel D. In vitro modulation of somatic glycine-like immunoreactivity. J Comp Neurol. 1994;339:311–327. doi: 10.1002/cne.903390302. [DOI] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci. 1984;4:1577–1588. doi: 10.1523/JNEUROSCI.04-06-01577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993a;69:1384–1397. doi: 10.1152/jn.1993.69.5.1384. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Giant cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993b;69:1398–1408. doi: 10.1152/jn.1993.69.5.1398. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Neuronal circuits associated with the output of the dorsal cochlear nucleus through fusiform cells. J Neurophysiol. 1994;71:914–930. doi: 10.1152/jn.1994.71.3.914. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang CL, Messing A, Chiu SY. Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: role of potassium channels under the myelin sheath in young nerves. J Neurosci. 1998a;18:7200–7215. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang CL, Messing A, Chiu SY. Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: role of potassium channels under the myelin sheath in young nerves. J Neurosci. 1998b;18:7200–7215. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


