Abstract
Apolipoprotein E is associated with age-related risk for Alzheimer’s disease and plays critical roles in Aβ homeostasis. We report that ApoE plays a previously unappreciated role in facilitating the proteolytic clearance of soluble Aβ from the brain. The endolytic degradation of Aβ peptides within microglia by neprilysin and related enzymes is dramatically enhanced by ApoE. Similarly, Aβ degradation extracellularly by insulin degrading enzyme is facilitated by ApoE. The capacity of ApoE to promote Aβ degradation is dependent upon the ApoE isoform and its lipidation status. The enhanced expression of lipidated ApoE, through the activation of liver X receptors, stimulates Aβ degradation. Indeed, aged Tg2576 mice treated with the LXR agonist GW3965 exhibited a dramatic reduction in brain Aβ load. GW3965 treatment also reversed contextual memory deficits. These data demonstrate a novel mechanism through which ApoE facilitates the clearance of Aβ from the brain and suggest that LXR agonists may represent a novel therapy for AD.
Alzheimer’s disease (AD) is characterized by the accumulation and deposition of Aβ peptides within the brain, leading to the perturbation of synaptic function and neuronal loss that typifies the disease (Tanzi and Bertram, 2005). Genetic analysis of familial forms of AD has established the centrality of APP processing and Aβ production to disease pathogenesis. Aβ peptides are normally produced by neurons in the brain and cleared through efflux into the peripheral circulation (Zlokovic et al., 2005) and through their degradation by proteinases within the brain (Hardy and Selkoe, 2002).
An isoform of apolipoprotein E, ApoE4, has been shown to confer dramatically increased risk for late onset AD (LOAD) (Roses et al., 1995); however, the basis for this remains one of the major unanswered questions of disease pathogenesis. ApoE plays critical roles in regulating brain Aβ peptide levels, as well as their deposition and clearance (Holtzman, 2001; Zlokovic et al., 2005). Thus, processes that regulate ApoE expression and functional state could affect its ability to influence brain Aβ homeostasis. ApoE is the predominant apolipoprotein in the brain and is synthesized and secreted mainly by astrocytes (but also by microglia (Xu et al., 2006; Xu et al., 2000)) within unilamellar HDL-like particles (Fagan et al., 1999). ApoE is lipidated principally through the action of the ATP-binding cassette transporter ABCA1 (and related transporters) which acts in a variety of cell types to transfer both phospholipids and cholesterol to ApoE (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004) and in this way ApoE acts to traffic lipids throughout the brain. The lipidation status of ApoE is an important functional parameter, governing its conformation (Fisher and Ryan, 1999), intrinsic stability (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004), and interactions with membrane receptors (Dergunov et al., 2000; Ladu et al., 2006). Importantly, ApoE binds to Aβ and this, too, is influenced by its lipidation status (Tokuda et al., 2000). Studies with APP transgenic mice have demonstrated ApoE isoform-specific effects on the propensity of Aβ to be deposited in the brain (E4>E3>E2), the nature of the deposits and a gene dosage-related influence on the magnitude of these effects (Holtzman, 2004). ApoE lipidation status is also a significant determinant of whether its interaction with Aβ leads to efflux of the peptides from the brain, or alternatively to the formation of fibrils and their deposition into plaques (Bell et al., 2007; LaDu et al., 1995; Morikawa et al., 2005; Tokuda et al., 2000). Recently, three independent studies have reported that inactivation of the Abca1 gene in APP-expressing transgenic mice resulted in reduced levels of ApoE. Remarkably, these mice exhibited a seemingly paradoxical elevation of brain Aβ peptide levels and a doubling of Aβ plaque burden, without a significant effect on Aβ generation (Hirsch-Reinshagen et al., 2005; Koldamova et al., 2005a; Wahrle et al., 2005). The outcomes of these studies strongly suggested lipidated forms of ApoE act to enhance the clearance of Aβ peptides from the brain. The aim of the present study was to establish the mechanism by which ApoE and its lipidation affect Aβ homeostasis.
Liver X Receptors (LXRs) are ligand-activated transcription factors which induce the expression of Apoe, Abca1 and other genes of lipid metabolism (Beaven and Tontonoz, 2006). LXRs act physiologically as cellular cholesterol sensors and are activated by oxysterols. There are two LXR isoforms, LXRα and LXRβ, both of which are expressed in the brain (Wang et al., 2002) and their activation results in the rapid and robust increase in the levels of lipidated forms of ApoE (Jiang et al., 2003). Thus, regulation of LXR transcriptional activity provides a mechanism to regulate brain ApoE levels and its lipidation status.
The brain possesses robust intrinsic Aβ clearance mechanisms (Tanzi et al., 2004). Aβ peptides are proteolytically degraded within the brain principally by neprilysin (NEP) (Iwata et al., 2000) and insulin degrading enzyme (IDE, insulysin) (Kurochkin and Goto, 1994). Genetic inactivation of these genes (Farris et al., 2003; Iwata et al., 2001) or administration of inhibitors of these proteinases into the brain results in substantial elevation of Aβ levels in the brain and induction of plaque deposition (Dolev and Michaelson, 2004). Conversely, overexpression of IDE or neprilysin lowered brain Aβ levels and reduced plaque formation (Hemming et al., 2007; Leissring et al., 2003). It has been argued that the predominant mode of Aβ42 clearance from the brain is through its proteolytic degradation since this peptide is not efficiently exported through the vasculature (Deane et al., 2004). Microglia, the brain’s resident macrophages, play an essential role in Aβ clearance through their ability to take up and degrade soluble and fibrillar forms of Aβ (Rogers et al., 2002). Moreover, both microglia and astrocytes secrete proteinases, including IDE that mediate the degradation of Aβ peptides in the extracellular milieu (Qiu et al., 1998).
Despite considerable effort, the cellular mechanisms through which ApoE influences Aβ clearance remain unresolved. We report that ApoE acts to facilitate the proteolytic degradation of Aβ, a previously unappreciated action of this apolipoprotein. Moreover, the lipidation status of ApoE is a critical determinant of its ability to stimulate Aβ degradation and this finding provides a mechanistic explanation of the increased Aβ levels and deposition observed in APP expressing mice lacking the Abca1 gene. Importantly, we demonstrate that elevation of lipidated forms of ApoE, through activation of LXRs, results in reduced Aβ peptide and plaque levels in an animal model of AD and is associated with improved contextual memory. Therapeutic agents which increase the abundance of highly lipidated forms of ApoE, including LXR agonists, may attenuate disease pathogenesis and represent a promising strategy for the treatment of AD.
RESULTS
Microglia efficiently take up and degrade soluble Aβ
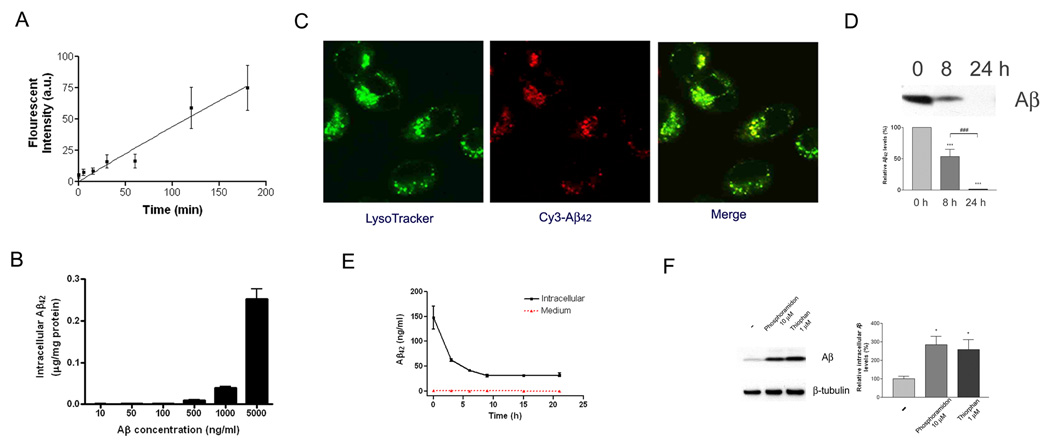
We first investigated the contribution of microglia to the clearance of soluble Aβ We found that these cells rapidly take up soluble forms of Aβ from the extracellular milieu (Figure 1A) through a non-saturable (Figure 1B) macropinocytotic uptake mechanism (Mandrekar et al., submitted for publication). The internalized Aβ is rapidly trafficked to a late endosomal/lysosomal compartment (Figure 1C). Indeed, incubation of BV-2 microglia with soluble Aβ42 resulted in the loss of Aβ from the medium, with complete clearance of the peptide within 24 hours (Figure 1D) that is reflective of both of its uptake into microglia as well as degradation by proteinases secreted by these cells into the extracellular milieu. Microglia efficiently degrade internalized soluble Aβ and we observed little or no resecretion of Aβ into the medium (Figure 1E). The intracellular degradation of Aβ by microglia is carried out principally by neprilysin and related proteases whose activity can be inhibited by phosphoramidon and thiorphan (Iwata et al., 2000; Tanzi et al., 2004), because in the presence of these protease inhibitors the degradation of internalized soluble Aβ was dramatically inhibited (Figure 1F).
Figure 1. Microglia efficiently take up and degrade soluble Aβ.
(A) Kinetics of soluble Aβ uptake by microglia. BV2 microglia were incubated with exogenous Alexa488-labeled soluble Aβ42 for the indicated periods. The uptake of fluorescently-labeled soluble Aβ was measured using flow cytometry. (B) Soluble Aβ is dose-dependently taken up by microglia. BV2 microglia were incubated for 3 hours with indicated concentrations of unmodified soluble Aβ42. The intracellular Aβ42 levels were measured using ELISA. The results were normalized to total cellular protein. (C) Soluble Aβ is rapidly trafficked to lysosomes for degradation. Confocal imaging of live BV-2 microglia 15 min after addition of 2 µg/ml soluble Cy3-Aβ42 demonstrated localization of Aβ (red) within lysosomes. Lysosomes were stained using LysoTracker (green). (D) Exogenous soluble A β was efficiently cleared from the medium by microglia. BV2 microglia were incubated with 2 µg/ml Aβ42 for the indicated time. The media were collected and immunoblotted for Aβ(***P<0.001; ###P<0.001). (E) Soluble Aβ can be degraded by microglia. BV2 microglia were incubated for 3 hours with 2 µg/ml unmodified soluble Aβ42. After washout of the remaining Aβ42 in the media, the intracellular Aβ42 levels were measured using ELISA. The media were also monitored for resecreted Aβ. (F) Neprilysin and related proteinases mediate intracellular degradation of soluble Aβ by microglia. Primary microglia from wild type mice were pretreated with vehicle, 10 µM phosphoramidon or 1 µM thiorphan for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 in the presence of vehicle or drug for an additional 24 hours. The data represent the outcome of 3 independent experiments (*P<0.05).
HDL apolipoproteins enhance the cellular degradation of soluble Aβ by microglia
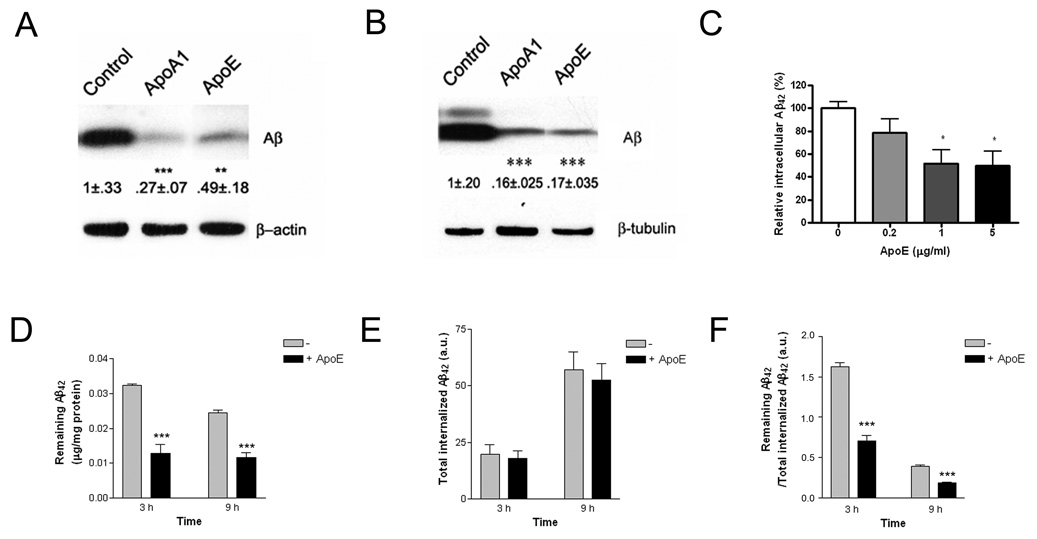
ApoE has been postulated to facilitate the clearance of Aβ peptides from the brain and we tested whether ApoE and related apolipoproteins influenced the ability of microglia to degrade soluble Aβ. ApoA-I is another major HDL-associated apolipoprotein both in the periphery and in the brain (Koch et al., 2001). Importantly, it functions and is trafficked in a manner similar to ApoE (Smith et al., 2004; Wang et al., 2001). ApoA-I is also lipidated by ABCA1 (Denis et al., 2004) and avidly binds Aβ peptides (Harr et al., 1996; Koldamova et al., 2001); thus, providing an independent measure of HDL apolipoprotein function. BV-2 microglia (Figure 2A) or primary microglia (Figure 2B) were incubated with soluble Aβ42 in the presence of exogenously supplied ApoE or ApoA-I. Coincubation with purified human ApoE or ApoA-I resulted in a dramatic stimulation of Aβ clearance from the microglia. ApoE and ApoA-I elicited a similar reduction in intracellular Aβ levels, demonstrating that the observed effects are reflective of the common actions of these apolipoproteins. The effect of ApoE on microglial Aβ clearance is dose-dependent (Figure 2C). We measured the efficiency with which ApoE stimulated the clearance of Aβ from microglia by monitoring in parallel both the cumulative uptake of Aβ using fluorescently labeled Aβ42 and the intracellular levels of the intact Aβ42 peptide by ELISA (Figure 2D–F). Fluorescently labeled Aβ is taken up by microglia and degraded, however, the fluorophore is retained within the cells and the total cellular fluorescence is an independent measurement of total Aβ uptake (Mandrekar et al, submitted for publication). We found that the ApoE-stimulated reduction in intracellular Aβ levels was a consequence of enhanced degradation and was observed at both 3 and 9 hours of incubation. These data demonstrate that the degradation of soluble Aβ within microglia is significantly enhanced by HDL apolipoproteins.
Figure 2. HDL Apolipoproteins increase soluble Aβ degradation in microglia.
BV2 microglia (A) or primary microglia from wild type mice (B) were incubated with 2 µg/ml Aβ42 in the presence or absence of 20 µg/ml ApoA-I or 5 µg/ml ApoE for 3 hours or 24 hours respectively. The intracellular Aβ42 levels were quantified from three independent experiments and data normalized to loading controls. Values are expressed as percentage relative to the value of controls (**P<0.01; ***P<0.001). (C) ApoE dose-dependently increased Aβ degradation in microglia. BV2 microglia were incubated with 2 µg/ml Aβ42 in the presence of increasing concentrations of purified human ApoE (0, 0.2, 1 or 5 µg/ml) for 3 hours, intracellular Aβ42 levels were quantified using ELISA and data normalized to total protein (*P<0.05). (D–F) ApoE enhanced soluble Aβ degradation. BV2 microglia were incubated with 2 µg/ml Aβ42 or 2 µg/ml Alexa488-labeled Aβ42 in the presence or absence of 1 µg/ml purified human ApoE for indicated time. (D) The remaining intracellular Aβ42 levels were quantified using ELISA and data normalized to total protein (***P<0.001). (E) The total internalized Aβ42 over the same intervals was monitored using flow cytometry. (F) The efficiency of Aβ degradation was expressed as a ratio of remaining intact Aβ42 over total internalized Aβ42 (***P<0.001).
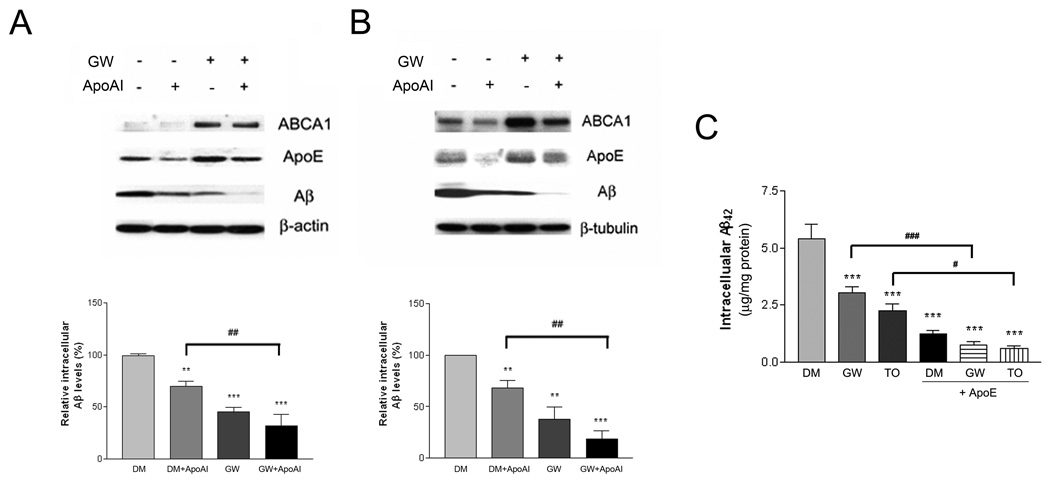
Activation of LXR increased the expression of ApoE and ABCA1 (Burns et al., 2006; Koldamova et al., 2003; Sun et al., 2003), resulting in elevated levels of lipidated ApoE in astrocytes (Supplemental Figure 1A and B). Microglia express both LXR isoforms and respond to LXR agonists (Zelcer et al., 2007). These cells also express and secrete lipidated forms of ApoE (Xu et al., 2000). We tested whether elevation of lipidated ApoE levels would result in enhanced Aβ degradation by microglia by treating these cells with an agonist of LXRs. BV-2 microglia (Figure 3A) or primary microglia (Figure 3B) were pretreated with LXR-specific agonist GW3965 for 18 hours and then incubated with exogenous soluble Aβ42 for an additional 3 or 24 hours, respectively. The GW3965-treated microglia exhibited significantly lower levels of intracellular Aβ42 due to the enhanced degradation of Aβ in the drug-treated cells compared to vehicle-treated controls. Similar effects were observed using another LXR agonist T0901317 (Figure 3C). LXR activation was verified by the observation of increased expression of its target gene products, ABCA1 and ApoE (Figure 3A and B). Significantly, the combined treatment with both the LXR agonist and provision of exogenous HDL apolipoproteins resulted in a further reduction of intracellular Aβ levels, evaluated either by Western analysis (Figure 3A, B) or by ELISA (Figure 3C). Control experiments established that the uptake of soluble Aβ was not affected by the presence of either exogenous ApoA-I or LXR activation (Supplemental Figure 2A). LXR activation did not affect the expression of neprilysin in microglia (Supplemental Figure 3). These data suggest that LXR activation facilitates the degradation of Aβ peptides and this is associated with induction of LXR target genes including ABCA1 and ApoE and elevation of lipidated ApoE levels.
Figure 3. LXR activation enhances intracellular Aβ degradation in microglia.
BV2 microglia (A) or primary microglia from wild type mice (B) were pretreated with DMSO or 1 µM GW3965 for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 in the presence or absence of 20 µg/ml ApoA-I for 3 hours or 24 hours, respectively. Cellular lysates were subjected to SDS-PAGE and Western blotted for ABCA1, ApoE, Aβ and β-actin or β-tubulin. The levels of Aβ were normalized to β-actin or β-tubulin as loading controls. Relative intracellular Aβ levels were quantified from three independent experiments (**P<0.01; ***P<0.001; ##P<0.01). (C) Primary microglia from wild type mice were pretreated with DMSO, 1 µM GW3965 or 1 µM T0901317 for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 in the presence or absence of 5 µg/ml purified human plasma ApoE for 24 hours. Intracellular Aβ levels were quantified using ELISA for Aβ42 and normalized to total protein. The data represent the outcome of 4 independent experiments (***P<0.001; #P<0.05; ###P<0.001).
ApoE is essential for efficient intracellular degradation of soluble Aβ by microglia
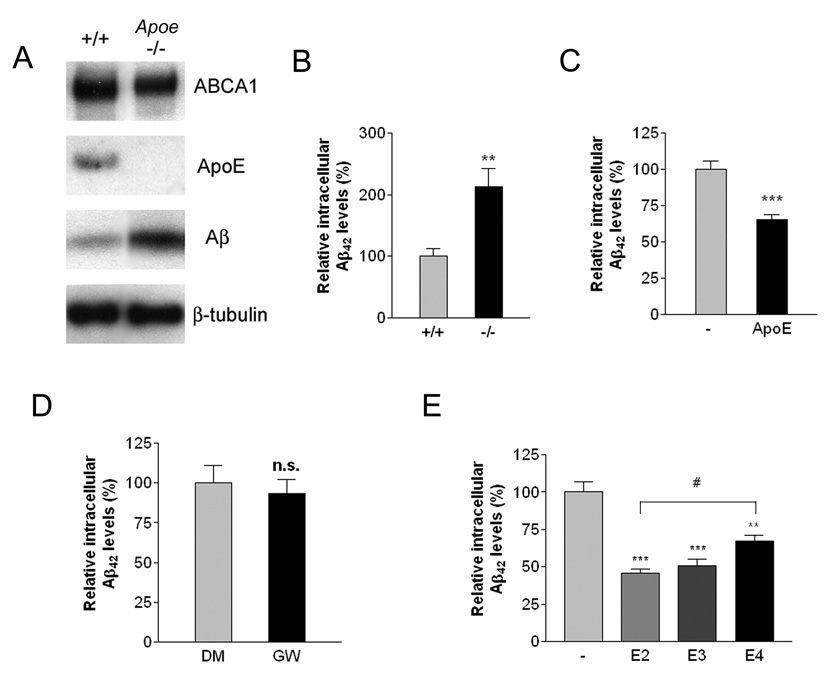
To directly assess whether ApoE was essential for efficient intracellular Aβ degradation, wild type or Apoe−/− microglia were isolated and incubated with soluble Aβ42. Loss of ApoE does not impair soluble Aβ uptake by microglia (Supplemental Figure 2C). Wild type microglia efficiently degrade Aβ, resulting in lower cellular Aβ levels when examined 24 hours later. However, the degradation of soluble Aβ by Apoe-deficient microglia was significantly impaired (Figure 4A, B). When the Apoe null microglia were provided with exogenous ApoE (Figure 4C), it restored their ability to efficiently degrade soluble Aβ, verifying that intracellular Aβ degradation is facilitated by ApoE. Treatment of Apoe-deficient microglia with GW3965 was without effect, demonstrating the effect of LXR agonists on Aβ degradation is dependent on expression of ApoE (Figure 4D).
Figure 4. ApoE is essential for efficient degradation of Aβ.
(A) Loss of ApoE resulted in intracellular accumulation of Aβ. Primary microglia from wild type or Apoe −/− mice were treated with 2 µg/ml Aβ42 for 24 hours. The lysates were subjected to SDS-PAGE and Western blotted for ABCA1, ApoE, Aβ and β-tubulin as a loading control. Blots shown are representative of three independent experiments. (B) Intracellular Aβ levels were quantified using ELISA for Aβ42 and normalized to total protein. The data represent the outcome of 5 independent experiments (**P<0.01). (C) Exogenous ApoE rescued the Aβ degrading deficiency in Apoe −/− microglia. Primary microglia from Apoe −/− mice were incubated with 2 µg/ml Aβ42 in the absence or presence of 1 µg/ml ApoE for an additional 24 hours. Intracellular Aβ42 levels were quantified using ELISA and the data normalized to total protein (***P<0.001). (D) ApoE is required for LXR-mediated effect on Aβ degradation. Primary microglia from Apoe −/− mice were pretreated with DMSO or 1 µM GW3965 for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 for an additional 24 hours. Intracellular Aβ42 levels were quantified using ELISA and data normalized to total protein. (E) Native ApoE particles enhanced Aβ degradation in an isoform-dependent manner. Primary microglia from ApoE −/− mice were treated with 2 µg/ml Aβ42 in the absence or the presence of 200 ng/ml purified ApoE-containing native HDL particles isolated from immortalized astrocytes expressing the human ApoE isoforms for 24 hours. The levels of remaining intracellular Aβ were quantified using ELISA for Aβ42. The data represent the outcome of 4 independent experiments (**P<0.01; ***P<0.001; #P<0.05).
Human ApoE isoforms confer different risk for LOAD (E4>E3>E2) (Roses et al., 1995). We investigated whether the individual ApoE isoforms differ in their ability to facilitate the proteolytic degradation of soluble Aβ by microglia. We isolated ApoE-containing HDL particles produced by immortalized astrocytes derived from mice in which human Apoe2, e3 or e4 alleles were knocked into endogenous mouse Apoe locus (Morikawa et al., 2005). Primary microglia from Apoe−/− animals were treated with Aβ42 in the absence or the presence of the same amount of purified human ApoE-containing HDL particles. While all isoforms of human ApoE increased clearance of soluble Aβ, ApoE2 exhibited the strongest effect whereas ApoE4 was significantly less efficient in promoting the degradation of soluble Aβ (Figure 4E). Addition of ApoE particles did not significantly influence the uptake of soluble Aβ by Apoe−/− microglia. These data demonstrate an ApoE isoform-specific promotion of the proteolytic degradation of soluble Aβ that is consistent with the linkage of these isoforms to disease risk.
The lipidation status of ApoE influences its ability to promote the intracellular degradation of soluble Aβ
The degree of ApoE lipidation and HDL particle size is governed primarily by the expression levels of ABCA1 (Supplemental Figure 1A, D, F and G) which, in turn, is directly regulated by LXRs (Supplemental Figure 1A) (Lee and Parks, 2005). We investigated whether ABCA1 affects the ability of ApoE to facilitate the proteolytic degradation of soluble Aβ by microglia. We isolated primary microglia from Abca1−/−, +/− and wild type mice and compared their ability to degrade exogenously supplied soluble Aβ. The Abca1 genotype did not significantly influence the uptake of soluble Aβ by microglia (Supplemental Figure 2C). Microglia expressing Abca1 were able to efficiently degrade soluble Aβ. However, Abca1 null microglia, which cannot efficiently lipidate ApoE, exhibited significantly higher intracellular Aβ levels, reflecting their impaired ability to degrade the soluble Aβ peptides (Figure 5A). When we induced the expression of ABCA1 and ApoE through the activation of LXRs we observed a significant enhancement of Aβ clearance in wild type cells, however, this effect was substantially diminished in microglia from Abca1 null animals (Figure 5B, C). LXR agonist treatment resulted in induction of cellular ABCA1 and ApoE levels in a genotype related manner, providing an internal control for LXR action. These data demonstrate that ABCA1 acts to facilitate soluble Aβ clearance through mechanisms that are reliant upon apolipoprotein function and are subject to regulation by LXR agonists, supporting the conclusion that lipidated forms of ApoE act to facilitate intracellular Aβ degradation by microglia.
Figure 5. ABCA1 influences the intracellular degradation of Aβ by microglia.
(A) Loss of Abca1 impairs Aβ degradation in primary microglia. Primary microglia from Abca1 +/+, +/− and −/− mice were treated with 2 µg/ml Aβ42 for 24 hours. The cellular levels of Aβ42 were measured in lysates by ELISA. The data were normalized to total protein and represent the outcome of 5 independent experiments. (B) Primary microglia from Abca1 +/+ and −/− mice were pretreated with DMSO or 1 µM GW3965 for 18 hours. The cells were then incubated with 2 µg/ml Aβ42 in the presence or absence of 1 µM GW3965 for 24 hours. Cellular lysates were subjected to SDS-PAGE and Western blotted for ABCA1, ApoE, Aβ and β-actin. Representative blots from 3 independent experiments are shown. (C) Primary microglia from Abca1 +/+ and −/− mice were treated as above. Intracellular Aβ levels were quantified using ELISA for Aβ42 and normalized to total protein. The data represent the outcome of 6 independent experiments (*P<0.05; ***P<0.001).
ApoE lipidation status influences the extracellular degradation of Aβ by IDE
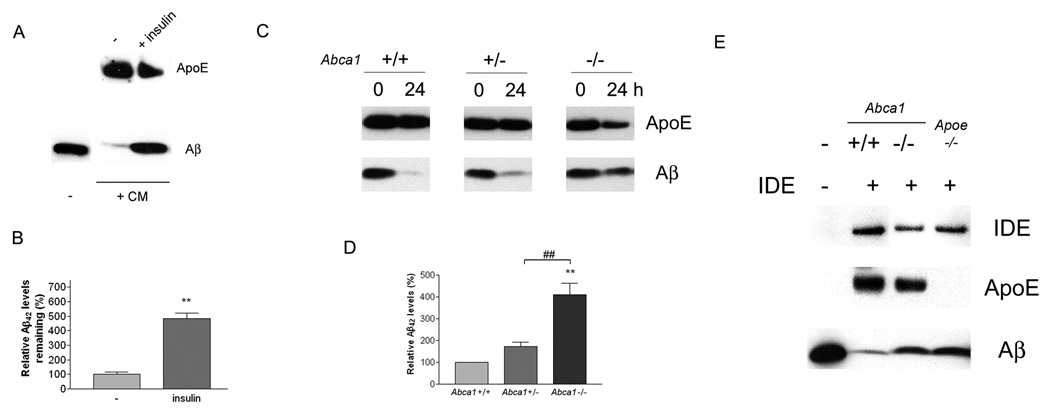
The proteolytic degradation of Aβ occurs both within cells and in the extracellular milieu of the brain. Aβ peptides are degraded in the extracellular space and interstitial fluid of the brain principally by insulin degrading enzyme (IDE) with minor contributions by other secreted proteinases (Qiu and Folstein, 2006). IDE has been reported to be secreted by microglia and to degrade soluble Aβ (Qiu et al., 1998), but it is unknown whether astrocytes act similarly. We first verified that active IDE is synthesized and secreted by astrocytes (Supplemental Figure 4) and upon addition of soluble Aβ to the astrocyte-conditioned medium the Aβ peptide is efficiently degraded (Figure 6A,B). The degradation of Aβ was inhibited in the presence insulin, a competitive inhibitor of IDE, supporting the notion that IDE (and/or related proteinases whose activity is also inhibited by insulin) was responsible for Aβ proteolysis in the conditioned media.
Figure 6. Extracellular degradation of soluble Aβ is dependent on IDE and related proteinases, and is influenced by the lipidation status of ApoE.
(A) Astrocyte-conditioned medium degrades soluble Aβ by IDE and related proteinases. Astrocyte-conditioned medium from wild type mice was incubated with Aβ42 in the presence or absence of 10 µM insulin for 24 hours. The samples were then subjected to SDS-PAGE and Western blotted for ApoE and Aβ. (B) After 24 hours, the samples were analyzed for Aβ42 using ELISA. The data represent the outcome of 3 independent experiments (**P<0.01). (C) Lipidation status of ApoE regulates Aβ degradation in astrocyte-conditioned medium. Conditioned media from astrocytes derived from Abca1 +/+, +/−, and −/− mice were incubated with 1 µg/ml Aβ42 for 0 or 24 hours and the reaction mixtures were subjected to SDS-PAGE and Western blotted for ApoE and Aβ. Representative blots from three independent experiments are shown. (D) The amount of Aβ42 remaining in the medium after 24 hours was quantified using ELISA. The data represent the outcome of 3 independent experiments (**P<0.01; ##P<0.01). (E) ApoE-containing HDL particles promote Aβ degradation by recombinant IDE. ApoE-containing HDL particles were collected from astrocyte-conditioned medium derived from wild type, Abca1−/− and Apoe−/− mice by immunoprecipitation and the complexes were incubated with 500 ng/ml recombinant IDE and 2 µg/ml Aβ42 for 1 hour. The reaction mixtures were then resolved by SDS-PAGE and Western blotted for IDE, ApoE and Aβ.
Next, we tested if ApoE promoted the degradation of soluble Aβ extracellularly by IDE in the same fashion as we observed within microglia and to ascertain if the ABCA1-dependent lipidation was important for this process. Astrocytes are the predominant source of ApoE in the brain and secrete it into the extracellular space, thus we employed conditioned medium from these cells containing both ApoE and IDE and evaluated Aβ degradation. Soluble Aβ42 was added to astrocyte-conditioned medium and Aβ levels were measured 24 hours later. We found conditioned medium from wild type astrocytes efficiently degraded Aβ (Figure 6C). However, conditioned medium obtained from Abca1 −/− astrocytes that contain poorly lipidated forms of ApoE (Supplemental Figure 1) exhibited significantly higher (four fold) levels of remaining Aβ measured either by Western analysis (Figure 6C) or by ELISA (Figure 6D). The reduced levels of ApoE in Abca1 −/− conditioned media after 24 hours are consistent with previous reports that suggested the stability of ApoE is regulated by its lipidation status (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004). The reduced degradation of Aβ is not due to differences in the levels of IDE or overall enzymatic activity in the conditioned medium derived from Abca1 −/− compared to wild type astrocytes (Supplemental Figure 4). These data demonstrate that the lipidation status of ApoE regulates its stability and influences extracellular soluble Aβ proteolysis by IDE (and/or related proteinases).
We then employed an in vitro assay to verify that the lipidation status of ApoE regulated the degradation of Aβ by IDE. We immunoprecipitated ApoE-containing HDL particles from the conditioned medium of wild type and Abca1 −/− astrocytes and then incubated the particles with Aβ42 in the presence of recombinant IDE for 1 hour. Parallel samples from Apoe−/− astrocytes provide a negative control for this experiment. We found that Aβ, in the presence of nascent highly lipidated forms of ApoE derived from wild type animals, was more efficiently degraded by IDE. In contrast, poorly-lipidated forms of ApoE derived from Abca1 −/− mice were much less effective in facilitating soluble Aβ degradation (Figure 6 E). These data demonstrate that the capacity of IDE to degrade Aβ is governed by the lipidation status of ApoE.
The LXR agonist GW3965 reduces plaque pathology and improves memory in Tg2576 mice
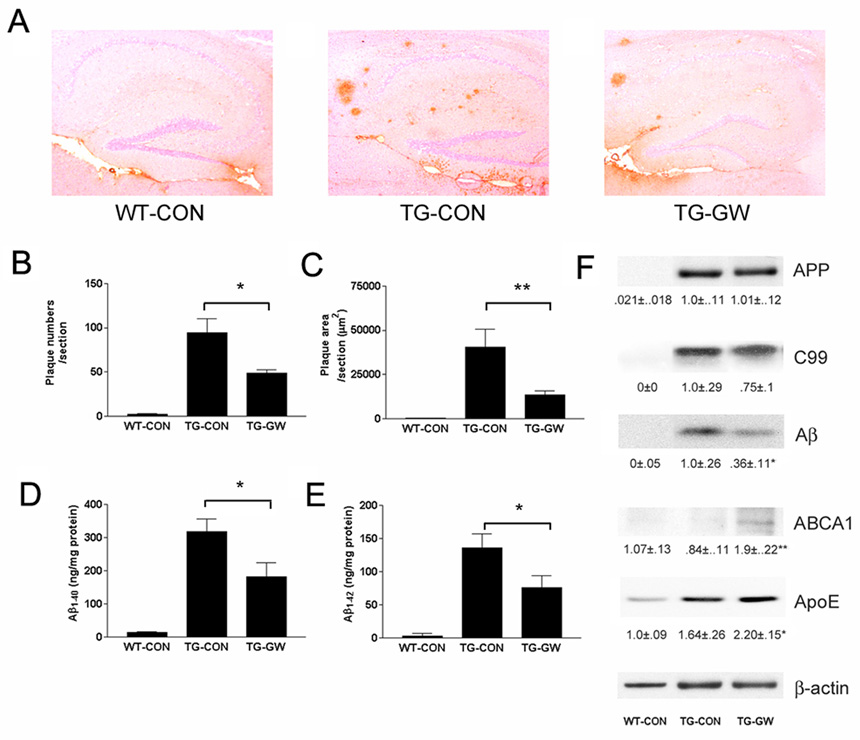
We investigated the effects of LXR agonist treatment on AD-related pathology in aged Tg2576 mice that overexpress hAPPswe (Hsiao et al., 1995) and exhibit extensive deposits of Aβ by one year of age (Kawarabayashi et al., 2001). One year old Tg2576 mice were treated orally with the LXR agonist GW3965 for 4 months. We found that GW3965-treated animals exhibited many fewer plaques (Figure 7 A), with an approximate 50% reduction in overall plaque number (Figure 7B) and 67% reduction in plaque load (Figure 7 C) in the hippocampus compared with Tg2576 mice fed a control diet. We found a corresponding approximate 50% reduction in the levels of total Aβ40 (Figure 7D) and Aβ42 (Figure 7E) peptides in the brains of the drug-treated animals. Importantly, LXR agonist treatment did not affect APP expression levels, or alter the levels of C-terminal APP fragments (Figure 7F). Thus, drug treatment did not significantly affect APP metabolism or Aβ generation in vivo. GW3965 administration resulted in the elevation in brain levels of ApoE and ABCA1, demonstrating the ability of this drug to act within the brain upon LXR target genes (Figure 7F). LXRs have been demonstrated to exhibit robust anti-inflammatory actions in the brain (Zelcer et al., 2007), and we observed that the LXR-mediated reduction in Aβ deposition was accompanied by a corresponding decrease in the number of activated, plaque-associated microglia and interleukin-6 mRNA levels (Supplemental Figure 5A–D). These data demonstrate that LXR activation results in reduced Aβ deposition in an animal model of AD that is postulated to occur through facilitating Aβ clearance from the brain.
Figure 7. LXR agonist treatment reduces Aβ levels and ameliorates plaque burden in Tg2576 mice.
(A) Aged Tg2576 mice (12 month-old) or genetically similar controls were treated for 4 months with normal chow or chow containing GW3965 (120 mg/kg, 33 mg/kg/day). Aβ plaque burden was monitored by 6E10 staining in the hippocampus. Plaque number and plaque area were quantified in (B) and (C) respectively (n=5, *P<0.05; **P<0.01). The levels of Aβ40 (D) and Aβ42 (E) were quantified using ELISA (*P<0.05). (F) The levels of full-length APP, C99 C-terminal fragment, total Aβ, ABCA1 and ApoE were monitored by Western analysis. The results were normalized to β-actin (n=5, *P<0.05; **P<0.01). Quantification of the data is shown under the Western blots.
Finally, as pre-plaque Tg2576 mice have been shown to display an Aβ-dependent deficit in contextual memory (Jacobsen et al., 2006), we examined the effects of GW3965 on memory processes in these animals. Twenty week-old Tg2576 mice were treated orally for 6 days with GW3965 or vehicle and trained and tested for contextual memory following the contextual fear conditioning procedure. Treatment of Tg2576 mice with GW3965 resulted in significantly improved contextual memory scores that were not significantly different from those observed in the wild-type animals (Figure 8). No effect of GW3965 treatment was observed in wild-type animals. Together, these results demonstrated that LXR activation not only ameliorates amyloid plaque pathology and neuroinflammation, but also improves the memory in an animal model of AD.
Figure 8. GW3965 significantly improves contextual memory in Tg2576 mice.
Tg2576 or wild type mice (20 week-old) were orally treated with vehicle or 50 mg/kg/day of GW3965 for 6 days and subjected to contextual memory assessment as outlined in the methods. Treatment of the LXR agonist improved hippocampal-dependent contextual memory in the heterozygous Tg2576 mice (n = 11, *P<0.05). No significant effect of treatment was observed in contextual memory in the wild-type littermate control mice. No significant effect of genotype was observed in hippocampal-independent cue conditioning (data not shown).
DISCUSSION
Sporadic, late onset AD is the most common form of the disease and the only established genetic risk factor is possession of one or more ApoE4 alleles (Roses, 1996; Tanzi and Bertram, 2005). It remains unclear how ApoE participates in disease pathogenesis. ApoE is implicated as a critical regulator of the propensity of Aβ to be deposited within the brain. Examination of APP expressing mice lacking the murine Apoe gene failed to develop compact amyloid plaques, but exhibited elevated levels of Aβ peptides within the brain (Bales et al., 1997). Strikingly, when human ApoE isoforms were expressed in these animals there was a delay in the onset of plaque deposition and a significant reduction in plaque burden that was isoform-specific (E2>E3>E4) and gene dose-dependent (DeMattos et al., 2004). These data suggested that ApoE influenced Aβ deposition and promoted Aβ clearance, with the ApoE4 isoform being less effective than other isoforms in facilitating removal of these peptides from the brain.
Age-related impairment of Aβ homeostatic mechanisms has been postulated to be a critical determinant of disease risk. Aβ peptides are normally generated at high levels in the brain (approximately 8%/hour) and are cleared at an equivalent rate in both humans and mice (Bateman et al., 2006). Thus, even modest reductions in clearance of soluble Aβ could result in elevated levels of Aβ peptides and ultimately their deposition within the brain. The clearance of Aβ peptides from the brain is accomplished by their proteolytic degradation within the brain and by their efflux through the blood-brain barrier (Tanzi et al., 2004). ApoE has been postulated to facilitate Aβ clearance across the blood—brain barrier, owing to its ability to form complexes with Aβ and to be exported to the peripheral circulation via LRP1 in vascular endothelial cells (Deane et al., 2004). However, a recent quantitative analysis has revealed that ApoE:Aβ complexes are inefficiently cleared through this mechanism and lipidated forms of ApoE are not significantly trafficked by this process (Bell et al., 2007). These finding suggest that ApoE promotes Aβ clearance from the brain principally through intrinsic proteolytic mechanisms. The physiological importance of intrinsic Aβ proteolysis has been demonstrated in vivo. Mice in which Nep or Ide have been genetically inactivated exhibit higher Aβ levels and enhanced plaque deposition in the brain (Farris et al., 2003; Farris et al., 2007; Iwata et al., 2001).
The present study documents a novel mechanism through which ApoE stimulates the degradation of soluble Aβ peptides within the brain. We show that ApoE facilitates the proteolytic degradation of soluble Aβ, both within microglia and in the extracellular milieu, through the action of two distinct classes of proteinases. Significantly, the proteolysis of Aβ is dramatically enhanced in the presence of lipidated apolipoproteins (Figure 9). The transfer of lipids to ApoE is accomplished principally by ABCA1 and several recent studies have provided direct evidence for the functional importance of this process in Aβ homeostasis. Three independent studies in different hAPP mice lacking the Abca1 gene, have demonstrated that the absence of Abca1 results in low levels of poorly-lipidated ApoE in the brain. Importantly, Abca1 inactivation did not affect APP processing or Aβ production. However, these animals exhibited much greater Aβ plaque loads and higher levels of insoluble forms of ApoE, reflective of the codeposition of these molecules (Hirsch-Reinshagen et al., 2005; Koldamova et al., 2005a; Wahrle et al., 2005). Thus, the dominant effect of Abca1 inactivation in vivo is to impair Aβ clearance, without a significant impact on Aβ synthesis.
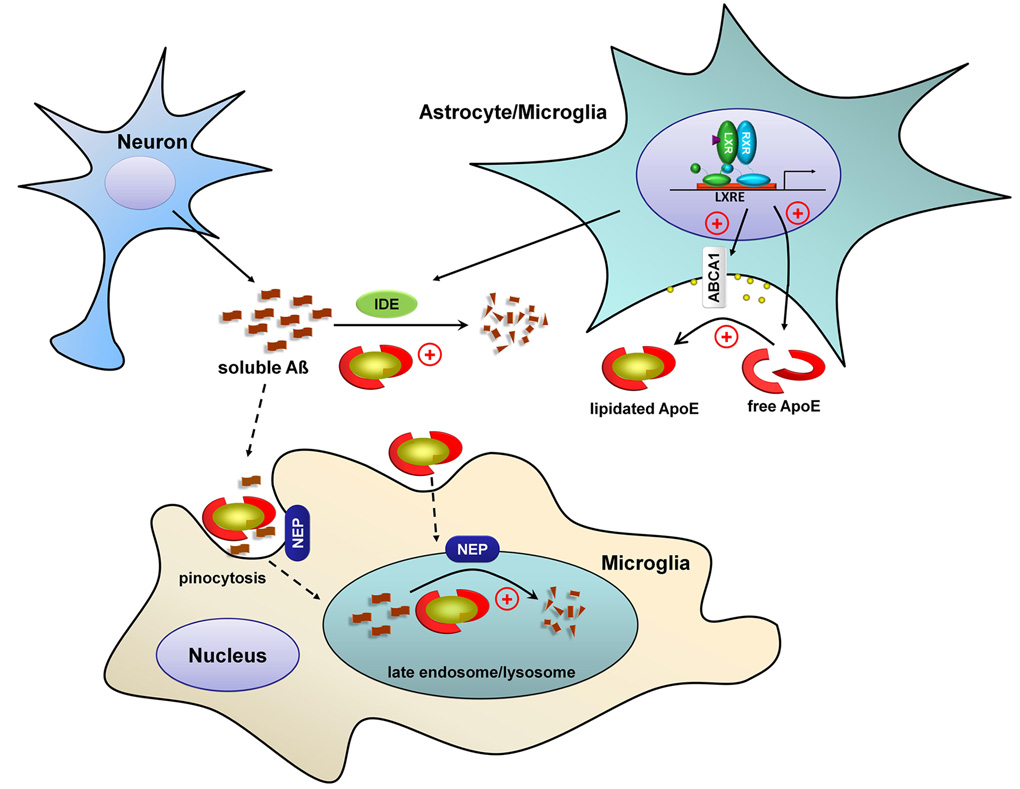
Figure 9. ApoE and its lipidation status regulates the proteolytic degradation of soluble Aβ.
The level of soluble Aβ is homeostatically controlled by its production by neurons and its subsequent clearance. Soluble Aβ can be cleared by proteolytic enzymes including NEP and IDE acting both intracellularly and extracellularly. ApoE is principally synthesized and secreted by glia. The lipid transporter ABCA1 mediates the lipidation of ApoE. Liver X receptors regulate the expression of both ABCA1 and ApoE and their activation results in increased levels of lipidated ApoE. The degradation of soluble Aβ both intracellularly and extracellularly is enhanced by increasing ApoE and its lipidation.
We demonstrate that the proteolytic degradation of Aβ is stimulated by LXR agonist treatment and is reliant upon the LXR target genes, Apoe and Abca1. ApoE secreted from wild type glia is fully lipidated (Hirsch-Reinshagen et al., 2004; Wahrle et al., 2004) and enables soluble Aβ to be efficiently degraded by proteinases. Our findings are consistent with the previous report that Aβ present within lipidated ApoE:Aβ complexes isolated from human brains was more susceptible to proteolytic degradation than with purified ApoE, which is poorly lipidated (Russo et al., 1998). We favor a model in which ApoE interacts with Aβ acting to chaperone its proteolysis. However, we cannot exclude the possibility that enhanced intracellular proteolytic degradation of Aβ might arise from HDL-mediated changes in cellular membrane lipid composition, as Abca1 null microglia retain a partial response to the LXR agonist GW3965. Indeed, LXR has been demonstrated to induce the expression of other cholesterol transporters and apolipoproteins involved in HDL metabolism such as Abcg1 and Apod, respectively (Zelcer et al., 2007). The ApoE-dependent soluble Aβ clearance mechanisms reported here appear to be distinct from those that occur in adult astrocytes which involve the ApoE receptor LRP1 and deposited forms of Aβ (Koistinaho et al., 2004).
Much of the initial interest in LXR action centered on the roles of these receptors and ABCA1 on neuronal APP processing and Aβ generation. LXR activation in cell culture models yielded conflicting data and was shown to either increase (Fukumoto et al., 2002) or suppress Aβ production (Burns et al., 2006; Koldamova et al., 2003; Sun et al., 2003). Subsequent studies in mice have uniformly found that LXR agonist treatment of wild type (Burns et al., 2006) or young APP-expressing mice (Koldamova et al., 2005b; Lefterov et al., 2007; Riddell et al., 2007) resulted in a significant decrease in brain Aβ levels, and we report similar results in aged Tg2576 mice treated with GW3965. Moreover, we have recently reported that hAPP mice in which either Lxrα or Lxrβ have been knocked out exhibit a significant increase in Aβ plaque pathology (Zelcer et al., 2007), consistent with the view that LXR activation facilitates Aβ clearance from the brain. These recent studies also found that LXRs inhibited the microglia-mediated inflammatory response and inflammatory gene expression, owing to their ability to functionally inactivate the promoters of proinflammatory genes (Zelcer and Tontonoz, 2006). We have reproduced this finding in aged Tg2576 mice with existing plaque pathology. Thus, LXRs can act to ameliorate AD pathogenesis through their action on both Aβ clearance and suppression of the plaque-related inflammatory response.
The behavioral impairment associated with overexpression of hAPP has been postulated to be due to elevated levels of soluble forms of Aβ within the brain (Comery et al., 2005; Riddell et al., 2007; Walsh and Selkoe, 2004). We found that treatment of Tg2576 mice with the LXR agonist GW3965 resulted in a dramatic improvement in contextual memory and this finding, too, is consistent with the LXR-stimulated Aβ clearance.
In summary, we provide data documenting a previously unappreciated action of ApoE in facilitating the proteolytic clearance of Aβ peptides from the brain. We postulate that ApoE acts both within microglia and in the extracellular space to affect the clearance of Aβ through promoting its proteolysis by at least two distinct classes of proteinases. Importantly, the ApoE4 isoform, which is associated with increased risk for AD, exhibits an impaired ability to promote Aβ proteolysis compared to the ApoE2 and ApoE3 isoforms. There is a remarkable correspondence in the effects of LXR activation and Abca1 inactivation (or overexpression) in animal models of AD that support an essential role for the lipidation of ApoE in removing soluble Aβ from the brain. The present study provides a mechanistic explanation of both the effects of Abca1 inactivation (or overexpression (Wahrle et al., 2008)) and the Lxrα and Lxrβ knockouts (Zelcer et al., 2007) on Aβ plaque pathology in animal models of AD. Our data suggests that therapeutic agents which increase the levels of lipidated forms of ApoE, including LXR agonists, represent a new and potentially efficacious therapy for AD.
Experimental Procedures
Animals
Tg2576 mice or wild type littermates (5 animals/group), 12 months of age, were fed the AIN-76A standard rodent diet alone or containing GW3965 (120 mg/kg; 33 mg/kg/day) ad libitum for 4 months. The animals were sacrificed, and the right hemispheres were fixed and processed for immunohistochemical analysis. The left hemispheres were snap-frozen on dry ice and subject to serial extraction of total RNA, DNA and protein. Wild type mice (C57BL/6), Apoe null mice (B6129P2-Apoetm1Unc/J) and Abca1 heterozygote mice (DBA/1-Abca1tm1Jdm/J) were obtained from Jackson Laboratory (Bar Harbor, ME). Abca1+/+, +/− and −/− mice were bred from Abca1 heterozygote mice (DBA/1-Abca1tm1Jdm/J). Tg2576 mice were maintained by crossing to B6SJLF1/J animals.
Cell culture
BV2 microglia were maintained in DMEM containing 2% FBS. Primary microglia and astrocytes were prepared from P0–P3 mice and purified microglia and astrocyte cultures were obtained as previously described (Koenigsknecht and Landreth, 2004; Zander et al., 2002). Immortalized astrocytes derived from human ApoE knockin mice (Morikawa et al., 2005) were maintained in DMEM containing 10% FBS, 500 µg/ml G418 and 1 mM sodium pyruvate.
Preparation of Aβ peptides
Aβ42 or Aβ40 lyophilized powder (American Peptide Company, Sunnyvale, CA) was dissolved to a final concentration of 1 mg/mL in DMSO and stored at −80°C until use. Fluorescently labeled peptides were prepared by first dissolving the peptides to a concentration of 2 mmol/L in sterile H2O. The Aβ42 was then labeled with Cy3 (Amersham Biosciences, Pittsburgh, PA) or Alexa488 fluorophores (Invitrogen, Carlsbad, CA) using manufacturer’s protocol. The Aβ42 reaction mixture was allowed to fibrillize at 37°C overnight after which unincorporated dyes were removed by ultracentrifugation at 4°C. The pellet was then resuspended in DMSO, sonicated and ultracentrifuged. This was subsequently repeated until the fAβ was solubilized in DMSO. The supernatant contains the operationally defined “soluble Aβ” which exhibits an electrophoretic mobility corresponding to 4 kDa on SDS-PAGE and is predicted to consist of primarily monomeric and small oligomeric species (Shen and Murphy, 1995).
Western Blot analysis
Protein concentrations of cell lysates or brain extracts were measured using the BCA method (Pierce, Rockford, IL). For Western blot analysis of Aβ, 10–20% Tricine gels or 4–15% Bis-Tris gels (Invitrogen, Carlsbad, CA) were used. The following primary antibodies were used: anti-human Aβ, 6E10 (Covance, Dedham, MA); anti-ApoE (Calbiochem, San Diego, CA); anti-β-actin; anti-β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA); anti-ABCA1 (Novus Biologicals, Littleton, CO); anti-APP CTF (Millipore, Billerica, MA).
Immunohistochemistry and image analysis
Post-fixed hemispheres were sectioned sagittally into 10 µm sections using a cryostat. Sections were mounted, air-dried, and then stored at 4°C until use. For Aβ immunohistochemistry, sections (3 per mouse, about 1.2 mm–1.5mm from the midline, spaced 0.1 mm apart from each other) were incubated in 70% formic acid for 3 min and the endogenous tissue peroxidase activity was quenched by incubation with 1% peroxide in methanol for 10 min. Sections were then microwaved in distilled water for 3 min, and then incubated with blocking solution (5% normal goat serum and 0.1% Triton X-100 in PBS) for 1 hour. Sections were incubated with primary antibody in the blocking solution overnight at 4°C. The antigens were detected by secondary antibodies using standard ABC-DAB methods. Sections were counterstained with hematoxylin. The 6E10 antibody against human Aβ was used to stain Aβ plaques. Images were analyzed using Image Pro-Plus software (Media Cybernetics, Silver Spring, MD).
Aβ ELISAs
Aβ40 and Aβ42 ELISAs were performed using commercial kits (Invitrogen, Carlsbad, California) following the manufacturer’s instructions. Alternatively, Aβ1-x, Aβ40 or Aβ42 ELISAs were performed using 6E10 as the capture antibody and monoclonal 4G8, anti-Aβ40 or anti-Aβ42 HRP-conjugated antibodies (Covance, Dedham, MA) as the detection antibodies. Synthetic Aβ40 or Aβ42 were used to generate a standard curve for each experiment. The plates were developed using TMB substrate kit (Pierce, Rockford, IL) and the reaction was stopped by addition of equal volume of 1 M HCl. The results were read using a Spectramax colorimetric plate reader (Molecular Devices, Sunnyvale, CA).
Conditioned media harvesting
Confluent primary astrocytes were washed twice with PBS (pH7.4) and incubated with fresh serum-free F12-DMEM media for 24 hours. Immortalized astrocytes were washed twice with PBS (pH7.4) and incubated with fresh serum-free DMEM supplemented with 1 mM sodium pyruvate. Conditioned media were collected and sterilized by filtering through 0.22 µm filters.
Flow cytometry
BV2 or primary microglia were incubated with 2 µg/ml Alexa488 labeled Aβ42 in serum-free DMEM or F12 DMEM, respectively, in the presence of 10 µg/ml BSA for 3 hours, washed extensively with PBS and fixed with 4% paraformaldehyde for 5 min. Cells were washed again with PBS and collected for flow cytometry using Beckman-Coulter XL flow cytometer. The total amount of Aβ internalized was determined by fluorescent intensity of Alexa488 as measured by flow cytometry.
Live cell imaging
For live cell imaging, microglial BV-2 cells were plated overnight in DMEM containing 2% FBS on Delta T tissue culture plates. Cells were incubated with 2 µg/ml Cy3-Aβ and 100 nmol/L Lysotracker Green DND-26 (Invitrogen, Carlsbad, CA) in serum-free DMEM containing 10 µg/ml BSA and imaged using a Zeiss LSM 510 confocal microscope.
Intracellular Aβ clearance
BV2 or primary mouse microglia cells were incubated with DMSO or 1 µmol/L GW3965 for 18 hours at 37°C. Cells were then treated with 2 µg/ml soluble Aβ42 in serum-free medium containing 10 µg/ml BSA for 3 hours or 24 hours respectively in the presence or absence of drug. For experiments using apolipoproteins, purified human HDL ApoA-I (Sigma, Saint Louis, MO), purified human plasma ApoE (rPeptide, Athens, GA), native ApoE2, ApoE3, or ApoE4 particles were applied at the same time as soluble Aβ42. Aβ42 levels in the cell lysates were determined by immunoblotting with the anti-Aβ antibody 6E10. Briefly, cells were extensively washed with PBS to ensure removing Aβ which is attached to the cell surface. Cells were then lysed in ice-cold RIPA buffer (Upstate Biotechnology, Lake Placid, NY), sonicated briefly, then collected by centrifugation at 13,000 rpm at 4 °C for 15 min. The samples were resolved by 4–15% Bis-Tris SDS-PAGE. ABCA1, ApoE (for positive controls) and β-actin or β-tubulin levels (for loading controls) in the cell lysates were measured by Western blot. For ELISA measurements, cells were treated similarly, washed with PBS and lysed in 1% SDS. Aβ42 levels were measured using ELISA and normalized to total protein.
Aβ degradation in astrocyte-conditioned medium
Conditioned media from Abca1+/+, +/− and −/− astrocyte cultures were incubated with 1 µg/ml Aβ42 for 0 or 24 hours. Aβ42 in the media was assessed using Western analysis or ELISA.
In vitro IDE assay
ApoE-containing HDL particles were immunprecipitated from the conditioned medium using the anti-ApoE antibody. Recombinant (500 ng/ml) IDE (R&D Systems, Minneapolis, MN) was incubated with 2 µg/ml Aβ42 for 1 hour in the presence of ApoE containing HDL particles. The samples were resolved by 4–15% Bis-Tris SDS-PAGE. The levels of IDE, ApoE and Aβ were monitored using Western analysis.
Contextual Fear Conditioning Studies
Following six days of treatment by oral gavage with 50 mg/kg/day GW3965 or vehicle, 20 week old Tg2576 mice (n=11/genotype/treatment) were trained and tested on two consecutive days as described previously (Comery et al., 2005).
Statistical analysis
To compare differences between the experimental groups, two-tailed t-test or one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test was performed using GraphPad Prizm software (GraphPad Software, San Diego, CA). Contextual memory was analyzed using a two-way ANOVA and post hoc pairwise comparison made using SAS Statistical Software (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgements
We would like to thank Iliya Lefterov and Radosveta Koldamova for their comments on the manuscript. This work was supported by a grant from the Blanchette Hooker Rockefeller Foundation (GL), and grants from the NIH AG-13956 (DMH), AG-020202 (GL) and HL30568 and HL66088 (PT). PT is an investigator of the Howard Hughes Medical Institute. NZ was supported by a fellowship from the Human Frontier Science Program Foundation. BW is supported by a Ruth L. Kirschstein National Research Service Award (F32 AG24031).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV. Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MP, Vardanian L, Pajoohesh-Ganji A, Wang L, Cooper M, Harris DC, Duff K, Rebeck GW. The effects of ABCA1 on cholesterol efflux and Abeta levels in vitro and in vivo. J Neurochem. 2006;98:792–800. doi: 10.1111/j.1471-4159.2006.03925.x. [DOI] [PubMed] [Google Scholar]
- Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, Zhou H, Kreft AF, Pangalos MN, Sonnenberg-Reines J, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2005;25:8898–8902. doi: 10.1523/JNEUROSCI.2693-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O'Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr. Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–7394. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- Dergunov AD, Smirnova EA, Merched A, Visvikis S, Siest G, Yakushkin VV, Tsibulsky V. Conformation of apolipoprotein E both in free and in lipid-bound form may determine the avidity of triglyceride-rich lipoproteins to the LDL receptor: structural and kinetic study. Biochim Biophys Acta. 2000;1484:14–28. doi: 10.1016/s1388-1981(99)00196-1. [DOI] [PubMed] [Google Scholar]
- Dolev I, Michaelson DM. A nontransgenic mouse model shows inducible amyloid-beta (Abeta) peptide deposition and elucidates the role of apolipoprotein E in the amyloid cascade. Proc Natl Acad Sci U S A. 2004;101:13909–13914. doi: 10.1073/pnas.0404458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/ −), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris W, Schutz SG, Cirrito JR, Shankar GM, Sun X, George A, Leissring MA, Walsh DM, Qiu WQ, Holtzman DM, Selkoe DJ. Loss of neprilysin function promotes amyloid plaque formation and causes cerebral amyloid angiopathy. Am J Pathol. 2007;171:241–251. doi: 10.2353/ajpath.2007.070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CA, Ryan RO. Lipid binding-induced conformational changes in the N-terminal domain of human apolipoprotein E. J Lipid Res. 1999;40:93–99. [PubMed] [Google Scholar]
- Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW. Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Abeta levels. J Biol Chem. 2002;277:48508–48513. doi: 10.1074/jbc.M209085200. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A-I in Alzheimer's disease. J Neurochem. 1996;66:2429–2435. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. 2007;4:e262. doi: 10.1371/journal.pmed.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Maia LF, Burgess BL, Blain JF, Naus KE, McIsaac SA, Parkinson PF, Chan JY, Tansley GH, Hayden MR, et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J Biol Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer's disease and cerebral amyloid angiopathy. J Mol Neurosci. 2001;17:147–155. doi: 10.1385/JMN:17:2:147. [DOI] [PubMed] [Google Scholar]
- Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci. 2004;23:247–254. doi: 10.1385/JMN:23:3:247. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XC, Beyer TP, Li Z, Liu J, Quan W, Schmidt RJ, Zhang Y, Bensch WR, Eacho PI, Cao G. Enlargement of high density lipoprotein in mice via liver X receptor activation requires apolipoprotein E and is abolished by cholesteryl ester transfer protein expression. J Biol Chem. 2003;278:49072–49078. doi: 10.1074/jbc.M304274200. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–9846. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005a;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Ikonomovic MD, Skoko J, Lefterov PI, Isanski BA, DeKosky ST, Lazo JS. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278:13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein A-I directly interacts with amyloid precursor protein and inhibits A beta aggregation and toxicity. Biochemistry. 2001;40:3553–3560. doi: 10.1021/bi002186k. [DOI] [PubMed] [Google Scholar]
- Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, Walter M, Roth MG, Lazo JS. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J Biol Chem. 2005b;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- Kurochkin IV, Goto S. Alzheimer's beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995;270:9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- Ladu MJ, Stine WB, Jr., Narita M, Getz GS, Reardon CA, Bu G. Self-Assembly of HEK Cell-Secreted ApoE Particles Resembles ApoE Enrichment of Lipoproteins as a Ligand for the LDL Receptor-Related Protein. Biochemistry. 2006;45:381–390. doi: 10.1021/bi051765s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Lefterov I, Bookout A, Wang Z, Staufenbiel M, Mangelsdorf D, Koldamova R. Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Mol Neurodegener. 2007;2:20. doi: 10.1186/1750-1326-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, O'Dell MA, Fagan AM, Lashuel HA, Walz T, et al. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Roses AD, Saunders AM, Corder EH, Pericak-Vance MA, Han SH, Einstein G, Hulette C, Schmechel DE, Holsti M, Huang D, et al. Influence of the susceptibility genes apolipoprotein E-epsilon 4 and apolipoprotein E-epsilon 2 on the rate of disease expressivity of late-onset Alzheimer's disease. Arzneimittelforschung. 1995;45:413–417. [PubMed] [Google Scholar]
- Russo C, Angelini G, Dapino D, Piccini A, Piombo G, Schettini G, Chen S, Teller JK, Zaccheo D, Gambetti P, Tabaton M. Opposite roles of apolipoprotein E in normal brains and in Alzheimer's disease. Proc Natl Acad Sci U S A. 1998;95:15598–15602. doi: 10.1073/pnas.95.26.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CL, Murphy RM. Solvent effects on self-assembly of beta-amyloid peptide. Biophys J. 1995;69:640–651. doi: 10.1016/S0006-3495(95)79940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Le Goff W, Settle M, Brubaker G, Waelde C, Horwitz A, Oda MN. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid Res. 2004;45:635–644. doi: 10.1194/jlr.M300336-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yao J, Kim TW, Tall AR. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J Biol Chem. 2003;278:27688–27694. doi: 10.1074/jbc.M300760200. [DOI] [PubMed] [Google Scholar]
- Tanzi R, Moir R, Wagner S. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer's amyloid beta peptides. Biochem J. 2000;348(Pt 2):359–365. [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Hartman RE, Bales KR, Paul SM, Holtzman DM. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem. 2005;280:43236–43242. doi: 10.1074/jbc.M508780200. [DOI] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Kim J, Li A, Knoten A, Jain S, Hirsch-Reinshagen V, Wellington CL, Bales KR, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008 doi: 10.1172/JCI33622. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci U S A. 2002;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–23747. doi: 10.1074/jbc.M102348200. [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li Y, Cyras C, Sanan DA, Cordell B. Isolation and characterization of apolipoproteins from murine microglia. Identification of a low density lipoprotein-like apolipoprotein J-rich but E-poor spherical particle. J Biol Chem. 2000;275:31770–31777. doi: 10.1074/jbc.M002796200. [DOI] [PubMed] [Google Scholar]
- Zander T, Kraus JA, Grommes C, Schlegel U, Feinstein D, Klockgether T, Landreth G, Koenigsknecht J, Heneka MT. Induction of apoptosis in human and rat glioma by agonists of the nuclear receptor PPARgamma. J Neurochem. 2002;81:1052–1060. doi: 10.1046/j.1471-4159.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.