Abstract
In order to further test whether or not psychostimulant drugs activate CART peptide-containing cells in the nucleus accumbens, we examined the fraction of CART positive cells that co-immunostained for c-Fos after administration of saline or cocaine (10 and 25 mg/kg i.p.). There was about a 45% increase in the fraction of cells that stained for both CART and c-Fos after administration of cocaine, but there was no change in the fraction after administration of saline. Moreover, the increase was not found 24 hours after injection and is therefore reversible. These results support the notion that psychostimulant drugs activate CART cells in the nucleus accumbens, even under conditions where it is difficult to show a change in CART levels.
Introduction
CART mRNA was discovered as a transcript whose levels increased after acute administration of cocaine or amphetamine (Douglass et al., 1995). Evidence for translation of the mRNA was that part of its coding sequence corresponded to the amino acid sequence of a previously identified peptide from ovine hypothalamus (Speiss et al., 1981). The increase in the level of the transcript suggested that the product of this gene was a substance related to the action of psychostimulant drugs, and the increased level further suggested an increased need or release of the gene product. Since that discovery, many publications have shown that the product of the gene is at least a pair of peptides (rat CART 62–102, CART 55–102) that function as peptide neurotransmitters. In the ventral striatum, CART peptides are co transmitters with GABA in some of the medium spiny neurons. In the nervous system, the peptides are localized in widespread but discrete groups of neurons that suggest many functions of the peptides. The general importance of CART peptides in a variety of physiologic functions, including food intake, stress, drug reward, endocrine control, and other processes has been demonstrated (Dominguez et al., 2004; Murphy 2005; Larsen et al., 2002; Hurd et al., 1999; Hunter and Kuhar 2003; Thim et al., 1998; Kuhar et al., 2005; also see the August issue of Peptides for reviews).
Several studies have not confirmed the up regulation of CART mRNA under the original conditions of a single, acute injection of drug (Vrang et al., 2002; Marie-Claire et al., 2003; Hunter et al., 2005; Jones and Kuhar 2006). Rather, binge or repeated dosing has more reliably resulted in increases of CART mRNA and peptide in the ventral striatum (Fagergren and Hurd, 1999; Brenz Verca et al., 2001; Hunter et al., 2005), and corticosterone may play a role in this increase (Hunter et al., 2005; Hunter et al., 2007). Nevertheless, these disparate results have lead to controversy over whether or not administration of psychostimulant drugs activate CART-containing neurons in the nucleus accumbens. An appropriate issue has been that CART neurons could be activated without a significant change in levels of CART peptide or mRNA, and that searching for changes in levels is not a reliable approach for determining neuronal activity.
In this study, in order to additionally test if psychostimulants activate CART-containing neurons, we measured c-Fos levels, another possible indicator of activation, in accumbal CART neurons after acute administration of cocaine. This approach would not require changes in CART levels to suggest changes in the activity of CART neurons. It is known that cocaine administration can increase Fos levels in brain (Marie-Claire et al., 2003; Graybiel et al., 1990; Gutstein et al 1998; Erdtmann-Vourliotis et al., 1999), but the colocalization of CART and Fos in the nucleus accumbens has not yet been reported. In this study we test if cocaine administration, in physiologically active doses, results in an increase in c-Fos in CART-containing neurons in the rat nucleus accumbens.
Materials and Methods
Animals
Thirty six adult, male, Sprague-Dawley rats (Charles River, Wilmington MA) were injected with either cocaine (10 or 25mg/kg, i.p.) or saline (vehicle) and anesthetized with chloral hydrate either thirty minutes, one hour or 24 hours after injection. Within minutes of loss of tail pinch reflex, all rats were transcardially perfused with 100 ml of cold, oxygenated Ringer’s solution. This was followed by perfusion with 500 ml of fixative containing 4.0% paraformaldehyde and 0.1% glutaraldehyde, dissolved in phosphate buffer (PB, 0.1M, pH 7.4). Brains were removed from the skull and stored in 30% sucrose solution in PB at 4°C until brains were saturated. The brains were then cut into 60μm-thick coronal sections on a freezing microtome. Sections were put in a 1.0% sodium borohydride solution dissolved in phosphate buffered saline (PBS) for 20 min and rinsed with PBS before being processed for immunocytochemistry. The anesthesia and euthanasia procedures were carried out according to the National Institutes of Health Guidelines and a protocol describing these experiments has been approved by the Institutional Animal Care and Use Committee of Emory University.
Antisera
Commercially available antibodies for c-Fos raised in mouse (Calbiochem, San Diego CA), and custom antibodies for CART peptide raised in rabbit ( C4 in Koylu et al., 1997) were used. Secondary antibodies raised in donkey coupled to either CY3 or FITC (Jackson Laboratories, West Grove, PA) were used to visualize the localization of the proteins of interest.
Immunofluorescent Localization of CART and c-Fos
The tissue sections were preincubated in a solution containing 5% normal donkey serum (NDS), 1.0% bovine serum albumin (BSA), and 0.3% Triton X-100 in PBS for one hour. They were then incubated overnight with primary antisera for CART and/or c-Fos diluted to 0.5–1.0 μg/ml in a solution containing 1.0% NDS, 1.0% BSA, 0.3% Triton X-100 in PBS. Next, the sections were rinsed in PBS and transferred for one hour to a secondary antibody solution containing FITC- (for CART) and CY3-(for c-Fos) conjugated secondary antibodies diluted 1:100 in the primary antibody diluent solution. The tissue sections were then washed in PBS and incubated in cupric sulfate solution for 30 minutes to enhance visualization. Sections were then mounted on gelatin-coated slides, dried, and a coverslip was applied with Vectashield mounting agent (Vector, Burlingame, CA) and sealed using clear fingernail polish. Specimens were observed throughout the rostrocaudal length (2.7 to 0.7 mm according to Paxinos and Watson,1986) of the nucleus accumbens using a Zeiss LSM 410 confocal microscope. Accumbal neurons that displayed CART-IR were scanned on the confocal microscope for the presence of double labeling. The total number of CART positive cells examined under each condition are given in Table 1. Due to the limitations of darkfield, oil-immersion confocal microscopy, where it is difficult to precisely define the border between core and shell, no distinction made between the accumbens core and shell.
Table 1.
Total number of CART-positive cells examined under each condition. The number of Fos-positive CART cells is divided by the total number of CART-positive cells to determine the proportion of cells activated by cocaine, shown graphically in Figure 2.
| Saline | Cocaine, 10mg/kg | Cocaine, 25mg/kg | |
|---|---|---|---|
| 30 min | 232 | 254 | 273 |
| 1hr | 189 | 222 | 239 |
| 24hrs | 252 | 218 | 246 |
Results
Animals were prepared and tissues processed as described in Methods. CART positive cells and cFos positive cells were examined in multiple sections from each animal as described in Methods. The total number of Immunofluorescent cells that stained for CART are given in Table 1 for each experimental condition. CART cells were rated as either co-staining or not co-staining with c-Fos. Examples of such accumbal cells are shown in Figure 1.
Figure 1.
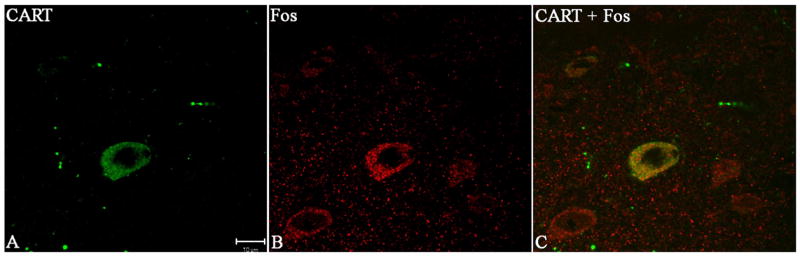
Examples of immunostaining for c-Fos and CART peptide in neurons of the nucleus accumbens. In A, a CART positive cell is stained green and a c-Fos positive cell is stained red in B. In C, the neuron co-staining for both c-Fos and CART is yellow which is expected. All three frames show the same tissue field.
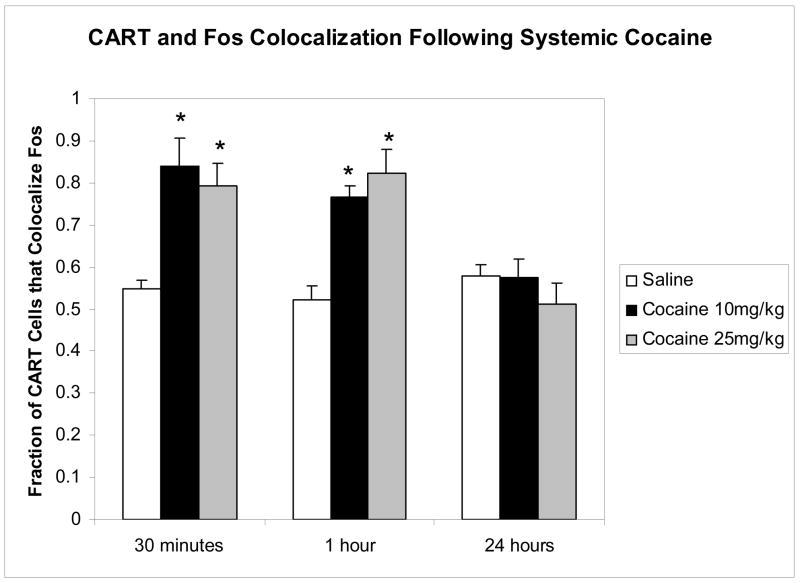
Figure 2 shows the quantitative analysis of the data. A two way analysis of variance revealed a significant interaction, a significant effect of time and a significant effect of dose on the fraction of CART cells staining for c-Fos, all at the P<0.0001 level. Subsequent analysis of the data at each time point with a one way ANOVA revealed significant differences at 30 min [F(2,6)= 122.8, P<0.0001] and 1 hr [F(2,6)=169.3, P<0.0001], but not at 24 hours [F(2,6)=0.0586, P=0.94]. Tukey’s post hoc analysis revealed that, at 30 min, the fractions at both cocaine doses were significantly greater than that at saline (P<0.001), but were not significantly different from each other. The same results were found for fractions measured at 1 hour after cocaine. However, 24 hours after cocaine, there were no significant differences among the fractions for the different doses of cocaine (Figure 2).
Figure 2.
Effect of cocaine administration on the fraction of CART positive cells staining for c-Fos in the nucleus accumbens. Tissues were prepared and examined as described in Methods. Administration of either 10 or 25 mg/kg of cocaine i.p., resulted in a significant increase in the fraction of CART positive cells that stained for cFos in the nucleus accumbens (2 way ANOVA, with a P<0.0001 effect in interaction, drug, and time). Asterisks indicate a significant difference (P<0.05) between values from cocaine treated animals and saline treated animals at the same time point. At 24 hours after cocaine injection, there was no difference among the groups. N = 3 animals for each bar. Data are mean +/− SEM for each bar.
Discussion
Several studies support the notion that psychostimulants, which increase extracellular dopamine levels, can activate CART-containing neurons in the nucleus accumbens through dopaminergic mechanisms. One is that CART neurons in the accumbens receive nerve terminals that contain tyrosine hydroxylase (Smith et al., 1999). Another study showed that accumbal CART neurons express protein or mRNAs for dopamine receptors (Hubert and Kuhar 2005; Beaudry et al., 2004), and that the neurons are affected by some dopamine agonists and antagonists (Hunter et al., 2006; Beaudry et al., 2004). Moreover, various publications indicate a CART-dopamine interaction in the nucleus accumbens (Dominguez et al., 2004; Philpot and Smith, 2006; Jaworski and Jones,2006). However, it has not been clear that administration of psychostimulants such as cocaine results in an activation of CART neurons in the nucleus accumbens. Part of the problem is that most studies used the levels of CART mRNA or peptide in the ventral striatum as an indicator of changed activity. However, the activity of the neurons could change without a measurable change in CART levels, provided the changes in activity were small or if the rate of synthesis of the peptide could keep up with demand. Therefore, we measured the effect of cocaine administration on the fraction of CART neurons that contain c-Fos, a marker for increased activity and possibly neuroplasticity, in the nucleus accumbens.
It has long been known that measurement of neurotransmitter levels alone are not adequate for an accurate determination of neuronal activity, but measurement of turnover rates of neurotransmitters is more sensitive (Iversen and Glowinski, 1966; Brodie et al., 1966). Turnover measurements reflect release and/or synthesis rates that have been found to change and reflect activity even when levels do not. Similarly, protein levels may not change while changes in turnover rates are significant (for example, Kimmel et al., 2003). However, methods for measuring turnover rates of most peptides have not been developed. Therefore, other approaches are useful, and we utilize induction of c-Fos levels as a rough indicator of activity.
The results of this study were straightforward. Injection of cocaine, at 10 or 25 mg/kg i.p., for 30 min or 1 hr caused an increase in the fraction of CART cells that stained for c-Fos, strongly suggesting that cocaine increases the activity of accumbal CART cells. C-Fos is also associated with plasticity (McClung et al., 2004). Twenty-four hrs after cocaine injection, there were no significant differences in the fractions indicating that the increase in c-Fos in CART cells was reversible. In several other studies, CART levels have been measured in the accumbens after cocaine, and no changes in levels after acute cocaine were found (Vrang et al., 2002; Marie-Claire et al., 2003; Hunter et al., 2005; Jones and Kuhar 2006). This makes it unlikely that the overall number of CART positive cells changed in the accumbens after drug administration.
The doses of cocaine and the time points were selected because of the data showing increases in c-Fos under roughly these conditions (Marie-Claire et al., 2003; Graybiel et al., 1990; Gutstein et al 1998; Erdtmann-Vourliotis et al., 1999). Now we show that the increase in c-Fos occurs at least partly in CART positive cells. Also, it is known that cocaine can increase locomotor activity under these conditions (For example, see Jaworski et al., 2003a: 2007). This strongly supports that idea the cocaine activates CART cells in the nucleus accumbens and implicates CART in drug abuse, and other studies with CART implicate CART in drug abuse as well (Jaworski and Jones, 2006; Jaworski et al 2006; Hurd et al., 1999; Freeman et al., 2007; Dayas et al., 2007; Moffett et al., 2006; Salinas et al., 2006; Couceyro et al., 2005; Kim et al., 2003; Kim et al., 2007; Yoon et al., 2007).
The fraction of cells staining with both CART and cFos after either 10 or 25 mg/kg were the same at 30 min and 1 hour. This suggests that the effects of cocaine on CART were maximal at these times and doses. A major hypothesis is that CART peptides in the accumbens act in a corrective or homeostatic mechanism such that the effects of high levels of dopamine, including those caused by psychostimulants, are opposed by CART peptide in accumbal neurons (Jaworski et al 2003a,b, 2006, 2007). Evidence for this has been that injection of CART into the nucleus accumbens has no effect on locomotor activity, but coinjection of CART with cocaine or dopamine results in a decrease in the cocaine- or dopamine-induced locomotor activity (Jaworski et al 2003a,b, 2006, 2007). Other neurotransmitters could also be involved in such a mechanism that tends to limit and control accumbal output.
Acknowledgments
The authors acknowledge the support of NIH grants RR00165, DA10732, DA015162, DA00418, and DA21056, and the support of the Georgia Research Alliance. We also acknowledge Dr Doug Jones for his helpful suggestions with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–240. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- Brenz Verca MS, Widmer DA, Wagner GC, Dreyer J. Cocaine induced expression of the tetraspanin CD81 and its relation to hypothalamic function. Mol Cell Neurosci. 2001;17:303–316. doi: 10.1006/mcne.2000.0942. [DOI] [PubMed] [Google Scholar]
- Brodie BB, Costa E, Dlabac A, Neff NH, Smookler HH. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. JPET. 1966;154:493–8. [PubMed] [Google Scholar]
- Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005 Dec;315(3):1091–100. doi: 10.1124/jpet.105.091678. Epub 2005 Aug 11. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli Linked to Ethanol Availability Activate Hypothalamic CART and Orexin Neurons in a Reinstatement Model of Relapse. Biol Psychiatry. 2007 Jun 12; doi: 10.1016/j.biopsych.2007.02.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Vicentic A, Del Giudice EM, Jaworski J, Hunter RG, Kuhar MJ. CART peptides: modulators of mesolimbic dopamine, feeding, and stress. Ann N Y Acad Sci. 2004;1025:363–9. doi: 10.1196/annals.1316.044. Review. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Riechert U, Hollt V. Acute injection of drugs with low addictive potential (delta(9)-tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine) Brain Res Mol Brain Res. 1999;71:313–324. doi: 10.1016/s0169-328x(99)00207-7. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–3452. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent Alterations in Mesolimbic Gene Expression with Abstinence from Cocaine Self-Administration. Neuropsychopharmacology. 2007 Sep 12; doi: 10.1038/sj.npp.1301577. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein HB, Thome JL, Fine JL, Watson SJ, Akil H. Pattern of c-fos mRNA induction in rat brain by acute morphine. Can J Physiol Pharmacol. 1998;76:294–303. [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006 Dec;40(6):409–15. doi: 10.1016/j.npep.2006.09.001. Epub 2006 Oct 24. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–864. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Targets CNS Neurol Disord. 2003;2(3):201–5. doi: 10.2174/1568007033482896. Review. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Bellani R, Bloss E, Costa A, Romeo RD, McEwen BS. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res. 2007 Jun 4;1152:234–40. doi: 10.1016/j.brainres.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Svensson P, Pontén M. The role of dopamine, dynorphin, and CART systems in the ventral striatum and amygdala in cocaine abuse. Ann N Y Acad Sci. 1999;877:499–506. doi: 10.1111/j.1749-6632.1999.tb09285.x. Review. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Glowinski J. Regional studies of catecholamines in the rat brain. II. Rate of turnover of catecholamines in various brain regions. J Neurochem. 1966;13:671–82. doi: 10.1111/j.1471-4159.1966.tb09874.x. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal Injection of CART (Cocaine-Amphetamine Regulated Transcript) Peptide Reduces Cocaine-Induced Locomotor Activity. J Pharmacol Exp Ther. 2003a;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Vicentic A, Hunter RG, Kimmel HL, Kuhar MJ. CART Peptides are Modulators of Mesolimbic Dopamine and Psychostimulants. Life Sci. 2003b;73:741–747. doi: 10.1016/s0024-3205(03)00394-1. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Intra-accumbal Injection of Cocaine- and Amphetamine-Regulated Transcript Peptide Alters Cocaine Self-administration. Program No. 195.9. 2006 Neuroscience Meeting Planner; Society for Neuroscience, Atlanta, GA. 2006. Online. [Google Scholar]
- Jaworski NJ, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55–102 Reduces the Locomotor Effect of Systemic Cocaine in Rats: An Isobolographic Analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Kuhar MJ. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system. J Pharmacol Exp Ther. 2006 Apr;317(1):454–61. doi: 10.1124/jpet.105.096123. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003 Dec;37(6):369–73. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kim S, Yoon HS, Kim JH. CART peptide 55–102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul Pept. 2007 Dec 1;144(1–3):6–9. doi: 10.1016/j.regpep.2007.07.003. Epub 2007 Jul 17. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ. Withdrawal from repeated cocaine alters dopamine transporter protein turnover in the rat striatum. JPET. 2003;304:15–21. doi: 10.1124/jpet.102.038018. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical Localization of Novel CART Peptides in Rat Hypothalamus, Pituitary and Adrenal Gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. AAPS J. 2005;7(1):E259–65. doi: 10.1208/aapsj070125. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Vrang N, Tang-Christensen M, Jensen PB, Hay-Schmidt A, Rømer J, Bjerre-Knudsen L, Kristensen P. Ups and downs for neuropeptides in body weight homeostasis: pharmacological potential of cocaine amphetamine regulated transcript and pre-proglucagon-derived peptides. Eur J Pharmacol. 2002;440(2–3):159–72. doi: 10.1016/s0014-2999(02)01426-7. Review. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Laurendeau I, Canestrelli C, Courtin C, Vidaud M, Roques B, Noble F. Fos but not CART (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real-time quantitative polymerase chain reaction in rat brain. Neurosci Lett. 2003;345:77–80. doi: 10.1016/s0304-3940(03)00307-0. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004 2004 Dec 20;132(2):146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, Kuhar M. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006 Aug;27(8):2037–45. doi: 10.1016/j.peptides.2006.03.035. Epub 2006 Jun 8. Review. [DOI] [PubMed] [Google Scholar]
- Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic. 2005;4(2):95–111. doi: 10.1093/bfgp/4.2.95. Review. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. Academic Press; NY, NY: 1986. [Google Scholar]
- Salinas A, Wilde JD, Maldve RE. Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem. 2006 Apr;97(2):408–15. doi: 10.1111/j.1471-4159.2006.03745.x. Epub 2006 Mar 15. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide – immunoreactive neurons in the nucleus accumbens in monkeys; ultrastructural analysis, colocalization studies and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Speiss J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine Hypothalamus. Biochem. 1981;20:1394–7. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Thim L, Kristensen P, Larsen PJ, Wulff BS. CART, a new anorectic peptide. Int J Biochem Cell Biol. 1998;30(12):1281–4. doi: 10.1016/s1357-2725(98)00110-1. Review. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–1218. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007 Aug;53(2):344–51. doi: 10.1016/j.neuropharm.2007.05.014. Epub 2007 May 29. [DOI] [PubMed] [Google Scholar]