Abstract
When treated with heat-killed bacterial cells, mosquito cells in culture respond by up-regulating several proteins. Among these is a 66-kDa protein (p66) that is secreted from cells derived from both Aedes aegypti and Aedes albopictus. p66 was degraded by proteolysis and gave a virtually identical pattern of peptide products for each mosquito species. The sequence of one peptide (31 amino acids) was determined and found to have similarity to insect transferrins. By using conserved regions of insect transferrin sequences, degenerate oligonucleotide PCR primers were designed and used to isolate a cDNA clone encoding an A. aegypti transferrin. The encoded protein contained a signal sequence that, when cleaved, would yield a mature protein of 68 kDa. It contained the 31-amino acid peptide, and the 3′ end exactly matched a cDNA encoding a polypeptide that is up-regulated when A. aegypti encapsulates filarial worms [Beerntsen, B. T., Severson, D. W. & Christensen, B. M. (1994) Exp. Parasitol. 79, 312–321]. This transferrin, like those of two other insect species, has conserved iron-binding residues in the N-terminal lobe but not in the C-terminal lobe, which also has large deletions in the polypeptide chain, compared with transferrins with functional C-terminal lobes. The hypothesis is developed that this transferrin plays a role similar to vertebrate lactoferrin in sequestering iron from invading organisms and that degradation of the structure of the C-terminal lobe might be a mechanism for evading pathogens that elaborate transferrin receptors to tap sequestered iron.
Keywords: cloning, sequence, cells, immunity
Transferrin in vertebrates is a major transport protein for iron in the blood. It is a glycoprotein of molecular weight about 80,000 with two iron-binding sites, each located within a separate and similar lobe of the protein (1, 2). It is believed that the two-lobed protein arose by a gene duplication in the course of evolution. Iron (FeIII) is bound by two tyrosine hydroxyls, an aspartate carboxyl, a histidine nitrogen, and (bi)carbonate that is ion-paired to an arginine guanidino group in each lobe (1). At physiological pH, the affinity for FeIII is very high (Kd ≈ 10−20 M).
Transferrin delivers iron to many cells by way of a membrane transferrin receptor, which has a high affinity for diferric transferrin, less affinity for the monoferric form, and low affinity for apotransferrin at physiological pH. When diferric transferrin bound to its membrane receptor is taken into an endocytotic vesicle that becomes an acidic endosome, iron dissociates from transferrin and is taken into the cell cytoplasm, whereas the transferrin receptor and apotransferrin are recycled to the plasma membrane, where the apotransferrin is released into the blood (3).
Vertebrate serum transferrin is classified as an acute-phase protein, because under conditions of stress or infection, its concentration can either rise or fall, depending upon the animal species (4). Human lactoferrin increases in plasma and other body fluids in response to infection and might play an antibiotic role by depriving invading organisms of iron needed for their proliferation (5). Similarly, other transferrin-like proteins (in eggs, milk, and tears, for example) also can serve an antibiotic role, sequestering iron from invading organisms.
Iron metabolism in insects and other invertebrates has been investigated relatively little, but enough is known to indicate that there are distinct and somewhat puzzling differences between insects and other organisms. For example, in most organisms the iron storage protein ferritin is contained in the cytoplasm. In contrast, in general, insect cytoplasm is deficient in ferritin, which is found instead in the export pathway of the cell and in the hemolymph (6). Ferritin itself also can be taken up by insect tissues and, thus, may serve as an iron transporter (7). Insect tissues also produce transferrins and secrete them into the hemolymph. In some species, insect transferrins are the same size as those of vertebrates and have identical iron-binding properties (8). However, in other insects the transferrins, although they have two lobes, have only one iron-binding site per molecule (9, 10). This raises questions about the function of transferrins in insect hemolymph.
Herein we will show that transferrin synthesis and secretion are increased on exposure of mosquito cells (from Aedes aegypti or Aedes albopictus) to bacteria, suggesting that mosquito transferrin participates as acute-phase protein that is up-regulated during the immune response. We also will describe the cloning and sequence of the A. aegypti transferrin and demonstrate that mosquito transferrin appears to be identical with a protein that is up-regulated by this mosquito during encapsulation of filarial worms (11). The significance of transferrin synthesis and its up-regulation during infection in insects will be discussed.
MATERIALS AND METHODS
Cells and Culture Conditions.
A. albopictus C7-10 and A. aegypti Aag2 cells were maintained in Eagle’s medium containing glutamine, nonessential amino acids, and 5% fetal bovine serum, essentially as described (12, 13). For induction of immunity proteins, 3 × 106 cells were plated in 100-mm dishes, allowed to grow overnight, and then incubated for 6–24 h, in serum-free (E-O) medium with autoclaved Escherichia coli cells. For labeling of the proteins, the mosquito cells were incubated for an additional 5 h with Tran35S-label (ICN; ≈1,000 Ci/mmol, 10 μCi/ml; 1 Ci = 37 GBq). Cells were removed by centrifugation and the proteins were precipitated from the culture medium by addition of trichloroacetic acid (TCA) to a final concentration of 10%. The protein was recovered by centrifugation, and the pellet was washed with acetone/water, 9:1 (vol/vol) to remove residual TCA. Samples were dissolved in an appropriate buffer for SDS/PAGE as detailed (14). After electrophoresis, the gel was stained with Coomassie brilliant blue R-250, destained in 50% methanol/10% acetic acid for 30 min, washed in 25% methanol/5% acetic acid for 30 min, washed in water for 30 min, dried, and exposed at −75°C overnight.
Proteolysis, Peptide Separation, and Sequencing.
The band corresponding to the protein of interest was cut from stained SDS gels, and the gel slices were incubated for 2 h in distilled water, with two changes. The samples then were incubated in proteolysis buffer (125 mM Tris⋅HCl, pH 6.8/10% glycerol/0.1% SDS) for 1 h with gentle agitation. The equilibrated gel fragments were inserted into the sample wells of a SDS/15% polyacrylamide separation gel and overlaid with 15 μl of SV8 protease (830 units/mg, 1 mg/ml) in Tris⋅HCl (pH 6.8) containing 10% glycerol and with 10 μl proteolysis buffer without enzyme (15). The gel was electrophoresed at a constant current of 15 mA until the bromophenol blue dye front reached the separating gel. The current was turned off for 30 min to allow proteolytic digestion, and electrophoresis was resumed at 30 mA. For peptide sequencing, SV8 digestion products were electrotransferred to Immobilon-PSQ (Millipore) in CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) buffer (10 mM CAPS/10% methanol, pH 11.0), and the membrane was stained with 0.1% Coomassie brilliant blue R-250 in 50% methanol, destained in 50% methanol, rinsed in distilled water, and dried. Peptide sequencing was performed by the University of Minnesota Microchemical Facility.
Cloning and Sequence of a cDNA Encoding Mosquito Transferrin.
Total RNA was extracted from fourth-instar Rockefeller strain A. aegypti larvae, by using a powdered glass affinity matrix (RNaid matrix, Bio 101) as described by Noriega and Wells (16). Messenger RNA was purified by using the Micro-Fast Trac system (Invitrogen), and the cDNA library was constructed by using a ZAP-cDNA synthesis kit (Stratagene) according to the manufacturer’s instructions. Two oligonucleotide primers (5′- GAMCCYAAGGAYATGTAYGTRGC-3′ and 5′-YCWCKYTCWATIACITCYKTGTA-3′) were designed from the conserved regions of insect transferrin sequences (9, 10, 17). First-strand cDNA was synthesized from total RNA of fourth-instar larvae by using the T-primed first-strand kit (Pharmacia Biotech) and used for PCR. PCR conditions were 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min in 29 cycles with a final 10-min extension step at 72°C. An 870-bp PCR product was cloned into a pCR II vector by using TA cloning kit (Invitrogen) and sequenced.
The cloned PCR fragment was labeled with [α-32P]dCTP by using the random primers DNA labeling system (GIBCO/BRL), and the cDNA library was screened with this clone as a probe. Hybridization was carried out in 6× standard saline citrate (SSC)/0.1% SDS/0.025% nonfat dried milk at 68°C overnight and washed for two 5-min periods in 2× SSC/0.1% SDS and for two 30-min periods in 0.1× SSC/0.1% SDS. After the tertiary screening, phagemid was obtained by in vivo excision according to manufacturer’s instruction (Stratagene). DNA sequencing was performed by using the Sequenase Version 2.0 DNA sequencing kit according to manufacturer’s instructions (United States Biochemicals).
Expression in E. coli and Production of Antibody.
A. aegypti transferrin cDNA was subcloned between BamHI and HindIII sites of a pET 32a vector (Novagen) by PCR that used Pfu polymerase (Stratagene). Oligonucleotide primers (5′-CGGGATCCCAACAGAAGGAAACGTTC-3′ and 5′-CCCAAGCTTCTACAAGCACATCAACCG-3′) were designed to remove the putative signal peptide and to contain BamHI and HindIII sites. The conditions for PCR were 95°C for 45 sec, 55°C for 45 sec, and 72°C for 2 min (29 cycles) with a final 10-min extension at 72°C. The PCR product and pET 32a vector were digested by BamHI and HindIII, ligated, and transformed into competent cells (INVα F′: Invitrogen). A colony containing the pET 32a with the target clone in the correct orientation was identified by restriction enzyme digestion, and the DNA sequence of the clone was confirmed by DNA sequencing. The plasmid containing the target clone was transformed into BL21 (DE3) cells. Overexpression of recombinant protein was carried out according to the manufacturer’s instructions (Novagen). BL21 (DE3) containing plasmid was inoculated into 500 ml of Luria–Bertani broth containing ampicillin (50 μg/ml) and incubated with vigorous shaking until the OD600 reached 0.4, then isopropyl β-d-thiogalactoside was added to a final concentration of 0.4 mM, and the culture was incubated for another 4 h. Cells were collected by centrifugation at 5,000 × g for 5 min, resuspended in 100 ml of 1 × binding buffer (5 mM imidazole/0.5 mM NaCl/20 mM Tris⋅HCl, pH 7.9), and broken by three 15-sec sonications at 50% maximum power (Branson Sonifier 250, Branson). The insoluble fraction containing recombinant transferrin was collected by centrifugation and resuspended in 100 ml of binding buffer by sonication. The insoluble fraction was collected by centrifugation and resuspended by sonication in 20 ml of binding buffer containing 6 M urea. The sample was kept on ice for 1 h and centrifuged at 39,000 × g for 20 min. The supernatant was passed through a 0.45-μm (pore size) filter and was subjected to affinity column chromatography using the His⋅Tag system (Novagen) according to manufacturer’s instruction. Purified recombinant protein was mixed with 2× SDS sample buffer (20% glycerol/10% 2-mercaptoethanol/6% SDS/0.1% bromophenol blue/125 mM Tris⋅HCl, pH 6.8), boiled for 3 min, and subjected to a SDS/9% polyacrylamide gel. After electrophoresis the gel was stained in 0.2 M CuCl2 solution (18) and the recombinant transferrin band was excised from the gel, the gel slice was washed for three 10-min periods in 0.25 M EDTA/0.25 M Tris⋅HCl, pH 9.0. Protein in the gel slice was eluted by using model 422 Electro-Eluter (Bio-Rad).
About 300 μg of recombinant protein was emulsified with TiterMax adjuvant (CytRx, Norcross, GA) and injected into each hind quadriceps of a female New Zealand White rabbit. Three weeks after the first injection, a 100-μg booster was injected. Two weeks after boosting, blood was collected and used for serum preparation.
Northern Blot Analysis.
Total RNA was separated on 1% agarose gels under denaturing conditions (6.6% formaldehyde/1× Mops). After electrophoresis, the gel was rinsed with 20× SSC. The RNA was transferred onto a Zetabind (Cuno) in 20× SSC overnight. The membrane was pretreated according to manufacturer’s instruction before incubation in prehybridization solution [5× Denhardt’s reagent/5× SSC/herring sperm DNA (200 μg/ml)/43% formamide/0.1% SDS] at 42°C overnight. The probe was labeled using Multiprime DNA labeling system (Amersham), heat-denatured, and added to the prehybridization solution, and the membrane was incubated at 42°C overnight. After hybridization, the membrane was washed for two 20-min periods in 2× SSC/0.1% SDS at 42°C, for two 30-min periods in 0.1× SSC/0.1% SDS at 65°C. The membrane was exposed to x-ray film at −80°C overnight.
Western Blot Analysis.
TCA-precipitated protein sample was sonicated in 62.5 mM Tris⋅HCl containing 0.1% SDS, 2% DTT, 10% glycerol, and 0.1% bromophenol blue and electrophoresed on a SDS/10% polyacrylamide gel. After electrophoresis the gel was rinsed in CAPS buffer (10 mM CAPS/10% methanol, pH 11.0). The proteins were transferred onto a Protran membrane (Schleicher & Schuell) by using a Mini Trans-Blot Cell (Bio-Rad). The transferrin on the membrane was detected with antiserum (1:5,000 dilution) against recombinant transferrin as primary antibody and alkaline phosphatase-conjugated goat anti-rabbit IgG for the secondary antibody as described in the Immuno-blot system (Bio-Rad).
RESULTS
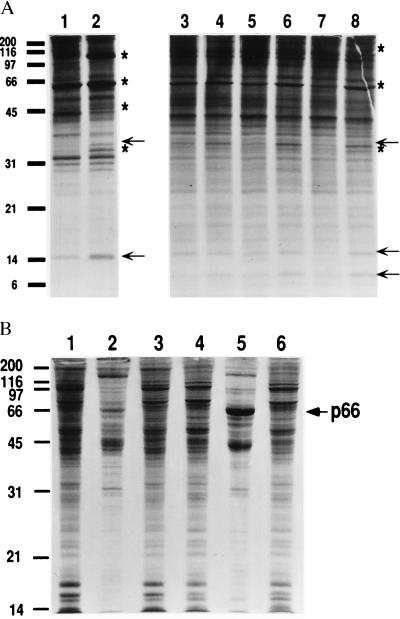
We earlier have described the induction of antibacterial substances when A. albopictus cells were treated with heat-killed bacteria (14). In addition to a cecropin-like peptide, four larger polypeptides of 111, 66, 53 and 32 kDa were synthesized and secreted. These polypeptides were designated by the letter p followed by the mass in kilodaltons (e.g., p111). Two additional bands, p35 and p16, also were observed in the present analysis (Fig. 1A, lanes 1 and 2). Similar results were obtained with A. aegypti cells (Fig. 1A, lanes 3–8), except that an additional small polypeptide was secreted by these cells. Of particular interest was the protein p66, because it is similar in size to a potential immunity protein described in filarial-worm-infected blackflies and mosquitoes (19, 20).
Figure 1.
(A) Induction of secreted immunity proteins by cultured mosquito cells. A. albopictus C7-10 (lanes 1 and 2) and A. aegypti Aag2 cells (lanes 3–8) were incubated without (control) or with 103 heat-killed bacteria per cell in E-O medium for 14 h (lanes 1, 2, 5, and 6), 6 h (lanes 3 and 4), or 24 h (lanes 7 and 8) and then labeled with Tran35S-label for an additional 5 h. The autoradiogram shows TCA-precipitable radiolabeled protein from the culture medium (45,000 cpm per lane) analyzed on a SDS/12.5% polyacrylamide gel. Values at left indicate molecular mass in kDa. Stars in lanes 2 and 8 identify proteins as described (14); arrows indicate new proteins. (B) Intracellular and extracellular distribution of p66. Control (lanes 1–3) or bacteria-treated (16 h with 103 heat-killed bacteria per cell; lanes 4–6) cells were labeled with Tran35S-label. Cells from two plates were collected separately by centrifugation and 2.5 × 105 cpm were analyzed in lanes 1, 3, 4, and 6. Protein from combined supernatants from plates 1 and 3 and from plates 4 and 6 were precipitated with TCA, and 2.5 × 105 cpm were analyzed in lanes 2 and 5, respectively. The arrow at the right indicates the position of p66.
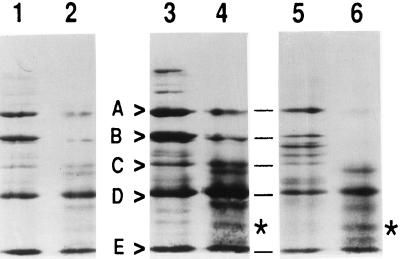
It was shown that p66 was secreted from the cells on induction with heat-killed bacteria (Fig. 1B). Growth of the cells with [3H]mannose resulted in several labeled proteins, but p66 was not labeled. Furthermore, treatment with N-glucosidase F (New England Biolabs) had no effect on the mobility of p66 on SDS gels (data not shown). Thus, p66 did not seem to be a glycoprotein. Digestion of p66 from both A. aegypti and A. albopictus cells with SV8 protease gave comparable peptide patterns on SDS/PAGE (Fig. 2), suggesting that the sequences of p66 from either A. aegypti or A. albopictus cells were very similar or possibly identical.
Figure 2.
Analysis of p66 by SV8 protease. Aag2 and C7-10 cells were treated (lanes 3–6) with 103 heat-killed bacteria per cell for 16 h, labeled with Tran35S-label for an additional 5 h, and 106 cpm per lane were electrophoresed on an SDS/8% polyacrylamide gel. Control cells (lanes 1 and 2) were not induced with bacteria before labeling. Material corresponding to p66 was cut from the gel, subjected to SV8 protease digestion, and reanalyzed on a SDS/15% polyacrylamide gel. Lanes 1, 3, and 5 represent products from an initial SV8 treatment; lanes 2, 4, and 6 represent products from a second preparation of protease. Starred band indicates a peptide that is prominent with the second protease treatment. Lanes 1–4 contain p66 from A. albopictus cells, and lanes 5 and 6 contain p66 from A. aegypti cells.
Because initial sequencing efforts suggested that the N-terminal end of p66 was blocked, an SV8 protease-derived peptide from A. albopictus p66 was subjected to Edman degradation. The 31-amino acid sequence FRTLEEPKAEFRYEGIILVRKSDNFRSLAXL showed strong similarity to insect transferrins, so we proceeded to clone and sequence the transferrin from an A. aegypti cDNA library.
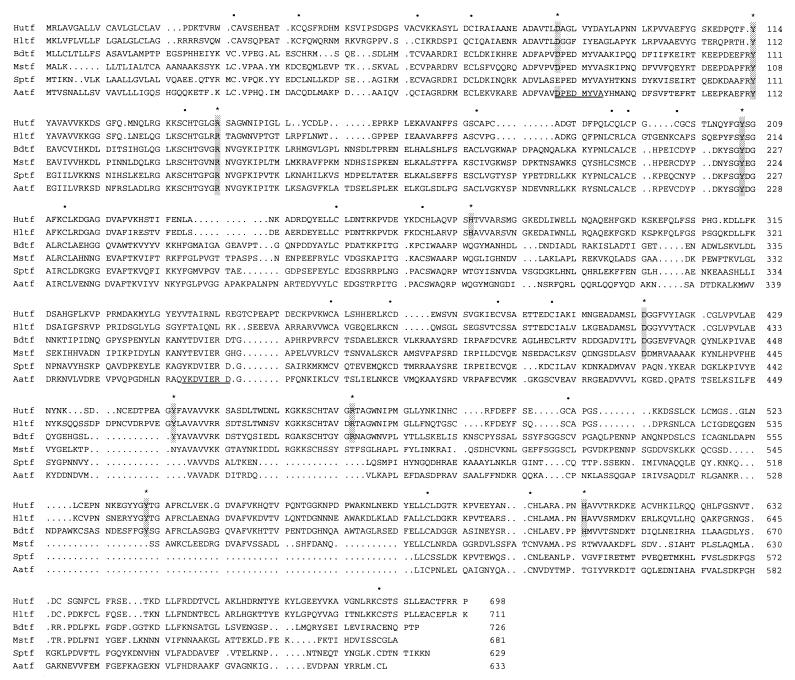
From an examination of three available insect transferrin sequences, we were able to design two degenerate oligonucleotides for use in a PCR. A PCR product of the appropriate size (870 bp) was obtained, cloned, and sequenced. It showed a high degree of identity with a transferrin from the fleshfly (10). The PCR product was labeled and used as a probe to screen a mosquito cDNA library. About 106 plaques were screened and 10 positive plaques were obtained. Two clones were found to be full length (2 kb) and the nucleotide sequence of one of them was determined (Fig. 3). The deduced amino acid sequence consists of 633 amino acids. A typical signal sequence is seen at the N-terminal end, and cleavage after residue 22 (Fig. 3, arrow) would be predicted by the rules developed by von Heijne (21). The glutamine at position 23 would then likely be converted to a pyroglutamate, resulting in N-terminal blockage. The calculated molecular weight of the mature polypeptide is 68,401, which is slightly larger than the value predicted from SDS/PAGE. In the N-terminal lobe, we found the sequence of the peptide isolated from the protease digest (Fig. 3, double underlined).
Figure 3.
Nucleotide sequence of mosquito transferrin cDNA and its deduced amino acid sequence. Putative signal peptide cleavage site is indicated by an arrow. Possible polyadenylation signals are in boldface type. Sequence of peptide from Edman degradation is double underlined. Partial cDNA sequence reported by Beerntsen et al. (11) is underlined.
According to crystallographic studies of vertebrate transferrins, five amino acid residues are necessary for iron binding in each lobe (22, 23). Four out of five iron-binding residues are conserved in the N-terminal lobe of mosquito transferrin, but none of them were conserved in the C-terminal lobe (Fig. 4). Cysteine residues were also highly conserved in the N-terminal lobe but not in the C-terminal lobe (Fig. 4).
Figure 4.
Comparison of transferrin sequences. Putative iron-binding residues are shadowed and indicated by the stars. Conserved cysteine residues are indicated by the dots. Hutf, human transferrin (GenBank/EMBL accession number M12530); Hltf, human lactoferrin (X53961); Sptf, flesh fly (Sarcophaga peregrina) transferrin (D28940); Aatf, mosquito (Aedes aegypti) transferrin (AF019117); Bdtf, cockroach (Blaberus discoidalis) transferrin (L05340); Mstf, moth (Manduca sexta) transferrin (M36296). The conserved regions in insect transferrins used for designing of PCR primers are underlined.
The deduced amino acid sequence for mosquito transferrin is compared with those of other insects and human in Fig. 4. Compared with the other sequences shown by using gap program (oldpep.cmp matrix, GCG), the mosquito transferrin has the following percentage of amino acid identities: fleshfly, 45%; cockroach, 42%; moth, 39%; human serum transferrin, 27%; human lactoferrin, 25%.
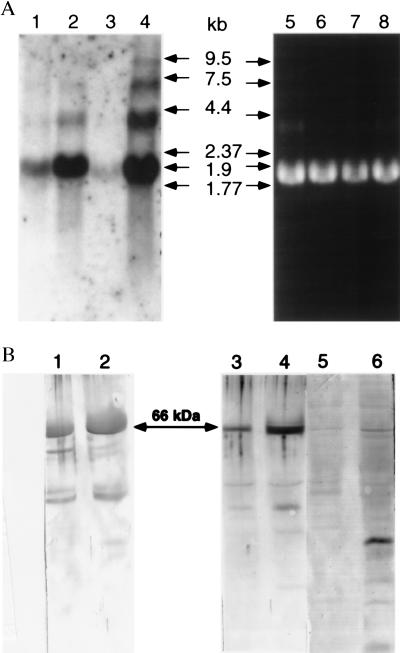
Northern and Western blot analyses were performed on A. aegypti cells and culture supernatants with or without stimulation with heat-killed bacteria (Fig. 5). Although minimal increase in transferrin mRNA was detected after 6 h (data not shown), a 16-h treatment with heat-killed bacteria resulted in a substantial increase in a major band of 1.9 kb in both A. albopictus and A. aegypti cells (Fig. 5A, lanes 2 and 4). Larger bands in the induced lanes may correspond to precursor molecules or to cross-hybridizing mRNA encoding a related protein(s).
Figure 5.
Detection of transferrin and its mRNA from cell culture. (A) Northern blotting. Total RNA was isolated from C7-10 cells (lanes 1, 2, 5, and 6) and Aag2 cells (lanes 3, 4, 7, and 8) by using Qiagen RNeasy mini kit (Qiagen, Chatsworth, CA), and 10-μg samples were electrophoresed on a 1% agarose gel containing formaldehyde. Lanes 1–4 show hybridization signal with A. aegypti transferrin cDNA labeled with [α-32P]dATP to a specific activity of 1010 cpm/μg. Lanes: 1 and 3, control cells; 2 and 4, induced cells (16 h in E-O medium); 5–8, ethidium bromide-stained gel. (B) Western blotting. Antiserum against recombinant transferrin was used as the primary antibody to detect the mosquito transferrin in cell culture supernatants from 1 × 107 C7-10 cells (lanes 1 and 2) and 3 × 106 Aag2 cells (lanes 3–6). Lanes 1, 3, and 5, supernatant from control cells; lanes 2, 4, and 6, supernatant from treated cells used for the RNA samples shown in A. Lanes 1–4 were treated with a 1:5,000 dilution of immune serum; minor bands of lower molecular mass may represent degradation products. In lanes 5 and 6, preimmune serum was used in place of primary antibody.
Polyclonal antibodies raised to the recombinant protein produced in E. coli gave a single band in Western blots of mosquito homogenates; preimmune serum gave no reaction (data not shown). By Western blot analysis, a predominant band measuring 66 kDa was enriched in treated C7-10 (Fig. 5B, lane 2) and Aag2 cells (Fig. 5B, lane 4), relative to untreated controls (Fig. 5B, lanes 1 and 3).
DISCUSSION
In response to infection, insects produce an array of peptides and proteins that have antibiotic activities (24, 25). From the results presented herein, it now seems that insect transferrins should be added to the list of such agents. Transferrins impede the growth of bacteria and other invading organisms in some vertebrates by sequestering iron; the insect proteins may play an identical role.
In the 3′ end of the nucleotide sequence of mosquito transferrin (Fig. 3), we found a perfect match for the cDNA fragment reported by Beerntsen et al. (11) for an 84-kDa protein that is up-regulated in A. aegypti infected with filarial worms. This group also reported that the protein was not glycosylated and was blocked at the N terminus, which agrees with our results. Although the protein has a higher apparent mass than p66, the estimation of the molecular mass was by SDS/PAGE in different gel systems, which could account for the difference. Judging from these results, there seems to be little doubt that the protein is mosquito transferrin.
Transferrins now have been cloned from four insect species (deduced amino acid sequences are shown in Fig. 4). Cockroach transferrin bears a strong resemblance to vertebrate transferrins in its size and iron-binding and spectral properties (8), in spite of an only 45% amino acid identity with human serum transferrin (17). The other three transferrins show significant differences from the vertebrate proteins. That of the moth Manduca sexta is about the same size as the human protein but has only 28% amino acid identity and has an iron-binding site only in the N-terminal lobe (9). The Dipteran transferrins have 45% amino acid identity with each other but only 27% with human serum transferrin. Furthermore, they are considerably smaller than the vertebrate transferrins, and this is because of large deletions in the C-terminal lobe, which also lacks iron-binding ligands. Thus the latter three insect transferrins have only one iron-binding site, which resides in the N-terminal lobe.
Given these differences in the structure and iron-binding capacity of the Lepidopteran and Dipteran proteins, one must question whether these insect members of the transferrin superfamily serve the same function in insects that their homologues do in vertebrates. In the case of the fleshfly, for example, Kurama et al. (10) have suggested that transferrin is a vitellogenic protein that is taken up by developing oocytes. The fact that these proteins are present constitutively in insect hemolymph suggests that they are involved in iron transport between tissues. Vertebrate transferrin receptors, which function to move iron into some cells, show highest affinity for diferric transferrin, and less for monoferric or apotransferrin. Therefore, if insects use the transferrin receptor pathway to carry iron into cells, the insect receptor must have sufficient affinity for a monoferric form to operate effectively.
The fact that the same protein that is present constitutively can be up-regulated on infection might suggest that in mosquitoes the protein is playing both the role of vertebrate lactoferrin and that of serum transferrin. Lactoferrin functions only as an antibiotic iron-sequestering agent and not as an iron transport protein (5). If mosquito transferrin acts primarily as an antibiotic agent such as lactoferrin, then how is iron transport in the hemolymph accomplished? The answer may lay in the unusual properties of the mosquito ferritin subunit, which is encoded by a gene that endows it with a signal peptide so that it can be exported into the hemolymph (26). Thus apoferritin could be produced by insect cells and secreted into the hemolymph to be transported to a loading site for iron, e.g., the midgut. Ferritin loaded with iron has been shown to be taken up by insect cells (7), and this would provide a route for delivery of iron that is separate from a transferrin-mediated route.
Up-regulation of an antibiotic transferrin after infection with bacteria or filarial worms would be an appropriate response. A vertebrate type of transferrin with two iron-binding sites would seem to be a more effective antibiotic agent than mosquito transferrin with its single iron-binding site. However, some bacteria that are pathogenic to vertebrates have acquired transferrin receptors that allow them to acquire iron from transferrins (27), and the process has been termed “iron piracy” (28). These receptors interact principally with the C-terminal lobe, rather than the N-terminal lobe of the vertebrate transferrins (29, 30). It may be that the insect transferrins have evolved to modify the C-terminal lobe in such a way that it no longer binds to the receptors elaborated by the pathogens. The insect in this way would choose to sacrifice half of the iron binding capacity of the transferrin molecule to defeat the “piracy” action of the pathogen.
Acknowledgments
We thank Dr. Joy Winzerling (Department of Nutrition and the Center for Insect Science, University of Arizona) and Dr. Rolf Ziegler (Institute of Zoology, University of Halle) for critical proofreading and Mr. Dianzheng Zhang for technical assistance. This work was supported by Grants AI-36258 (to A.M.F.) and GM-29238 (to J.H.L.) from the National Institutes of Health, and by the University of Minnesota Agricultural Experiment Station (contribution 22,589), St. Paul, Minnesota.
ABBREVIATIONS
- TCA
trichloroacetic acid
- CAPS
3-[cyclohexylamino]-1-propanesulfonic acid
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF019117).
References
- 1.Aisen P. In: Iron Metabolism in Health and Disease. Brock J H, Halliday J W, Pippard M J, Ward R J, editors. London: Saunders; 1994. pp. 1–30. [Google Scholar]
- 2.Winzerling J J, Law J H. Annu Rev Nutr. 1997;17:501–526. doi: 10.1146/annurev.nutr.17.1.501. [DOI] [PubMed] [Google Scholar]
- 3.Thorstensen K, Romslo I. Biochem J. 1990;271:1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powanda M C, Moyer E D. In: Infection: The Physiological and Metabolic Responses of the Host. Powanda M C, Canonico P G, editors. Amsterdam: Elsevier/North Holland; 1981. pp. 271–296. [Google Scholar]
- 5.Kontoghiorghes G J, Weinberg E D. Blood Rev. 1995;9:33–45. doi: 10.1016/0268-960x(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 6.Locke M, Nichol H. Annu Rev Entomol. 1992;37:195–215. [Google Scholar]
- 7.Huebers H A, Huebers E, Finch C A, Webb B A, Truman J W, Riddiford L M, Martin A W, Massover W H. J Comp Physiol B. 1988;158:291–300. doi: 10.1007/BF00695327. [DOI] [PubMed] [Google Scholar]
- 8.Gasdaska J R, Law J H, Bender C J, Aisen P. J Inorg Biochem. 1996;64:247–258. doi: 10.1016/s0162-0134(96)00052-9. [DOI] [PubMed] [Google Scholar]
- 9.Bartfeld N S, Law J H. J Biol Chem. 1990;265:21684–21691. [PubMed] [Google Scholar]
- 10.Kurama T, Kurata S, Natori S. Eur J Biochem. 1995;228:229–235. [PubMed] [Google Scholar]
- 11.Beerntsen B T, Severson D W, Christensen B M. Exp Parasitol. 1994;79:312–321. doi: 10.1006/expr.1994.1094. [DOI] [PubMed] [Google Scholar]
- 12.Fallon A M. J Tissue Cult Methods. 1989;12:1–6. [Google Scholar]
- 13.Lan Q, Fallon A M. Am J Trop Med Hyg. 1990;43:669–676. doi: 10.4269/ajtmh.1990.43.669. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez V P, Gerenday A, Fallon A M. Am J Trop Med Hyg. 1994;50:440–447. doi: 10.4269/ajtmh.1994.50.440. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland D W, Fischer S G, Kirschner M W, Laemmli U K. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 16.Noriega F G, Wells M A. Insect Mol Biol. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 17.Jamroz R C, Gasdaska J R, Bradfield J Y, Law J H. Proc Natl Acad Sci USA. 1993;90:1320–1324. doi: 10.1073/pnas.90.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Levin A, Branton D. Anal Biochem. 1987;166:308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- 19.Townson H, Chaithong U. Ann Trop Med Parasitol. 1991;85:149–163. doi: 10.1080/00034983.1991.11812541. [DOI] [PubMed] [Google Scholar]
- 20.Ham P J, Albuquerque C, Baxter A J, Chalk R, Hagen H E. Trans R Soc Trop Med Hyg. 1994;88:132–135. doi: 10.1016/0035-9203(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 21.von Heijne G. Eur J Biochem. 1983;133:17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- 22.Bailey S, Evans R W, Garratt R C, Gorinsky B, Hasnain S, Horsburgh C, Jhoti H, Lindley P F, Mydin A, Sarra R, Watson J L. Biochemistry. 1988;27:5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- 23.Anderson B F, Baker H M, Norris G E, Rice D W, Baker E N. J Mol Biol. 1989;209:711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann J A, Reichhart J-M, Hetru C. Curr Opin Immunol. 1996;8:8–13. doi: 10.1016/s0952-7915(96)80098-7. [DOI] [PubMed] [Google Scholar]
- 25.Kanost M R, Kawooya J K, Law J H, Ryan R O, Van Huedsen M C, Ziegler R. Adv Insect Physiol. 1990;22:330–396. [Google Scholar]
- 26.Dunkov B C, Zhang D, Choumarov K, Winzerling J J, Law J H. Arch Insect Biochem Physiol. 1995;29:293–307. doi: 10.1002/arch.940290307. [DOI] [PubMed] [Google Scholar]
- 27.Schryvers A B, Gonzalez G C. Can J Microbiol. 1990;36:145–147. doi: 10.1139/m90-026. [DOI] [PubMed] [Google Scholar]
- 28.Cornelissen C N, Sparling P F. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 29.Alcantara J, Yu R-H, Schryvers A B. Mol Microbiol. 1993;8:1135–1143. doi: 10.1111/j.1365-2958.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 30.Retzer M D, Kabani A, Button L L, Yu R-H, Schryvers A B. J Biol Chem. 1996;271:1166–1173. doi: 10.1074/jbc.271.2.1166. [DOI] [PubMed] [Google Scholar]