Abstract
The adenovirus (Ad) E4 ORF3 protein is both necessary and sufficient to reorganize a nuclear subdomain, the PML nuclear body (PML-NB), from punctate structures into elongated nuclear tracks. PML-NB disruption is recapitulated by a variety of DNA viruses that encode proteins responsible for compromising PML-NB integrity through different mechanisms. PML-NB disruption has been correlated with the antagonism of both innate and intrinsic immune responses. The E4 ORF3 protein is required for adenoviral DNA replication in the interferon (IFN)-induced antiviral state. This may reflect the fact that PML itself, in addition to several other PML-NB proteins, is encoded by an interferon-stimulated gene. Here, we demonstrate that reorganization of the PML-NB by E4 ORF3 antagonizes an innate antiviral response mediated by both PML and Daxx. Reduction of either of these proteins is sufficient to restore the replicative capacity of virus with the E4 ORF3 protein deleted in the IFN-induced antiviral state. Further, we provide evidence that both the HSV1 ICP0 and HCMV IE1 proteins, which disrupt PML-NBs by mechanistically distinct strategies, behave in a manner functionally analogous to E4 ORF3 with respect to antagonizing the IFN-induced antiviral state. In addition, we assert that this innate antiviral strategy mediated by PML and Daxx does not involve transcriptional repression. While early gene transcription is modestly diminished in the absence of E4 ORF3 protein expression, this reduction does not affect early protein function. We propose that, in addition to its ability to repress gene expression, the PML-NB participates in additional innate immune activities.
The adenovirus (Ad) early region 4 (E4) encodes a variety of proteins responsible both for regulating the viral lytic program and for modulating various cellular processes. Among these proteins is a multifunctional gene product, E4 open reading frame 3 (ORF3) protein. The E4 ORF3 protein has been demonstrated to regulate Ad mRNA splicing, enhance the translation of late viral mRNAs, and promote cell cycle-independent virus growth (28, 53, 54, 61, 62). Furthermore, the E4 ORF3 protein performs functions critical for viral DNA (vDNA) replication.
In tissue culture, either the E4 ORF3 protein or the E4 ORF6 protein is required for efficient vDNA replication (6, 33). While these two proteins execute several complementary functions, expression of the E4 ORF3 protein is both necessary and sufficient to reorganize a nuclear subdomain, the promyelocytic leukemia protein (PML) nuclear body (PML-NB), alternatively referred to as POD and ND10, from punctate structures into elongated tracks throughout the nucleus (11, 16).
PML nucleates PML-NB formation, generating electron-dense nuclear punctae within the intrachromosomal regions of the nucleus (14). The integrity of these structures has been correlated with the regulation of cell proliferation, and PML functions as a tumor suppressor (60). In addition to these processes, PML-NBs have been implicated in multiple cellular responses, including apoptosis, DNA damage repair, cellular stress response, transcriptional regulation, antiviral defense, and posttranslational modification (3, 15, 21). This is likely a consequence of the exceedingly diverse population of proteins known to associate with the PML-NB. Among these proteins is the Mre11-Rad50-Nbs1 (MRN) DNA repair complex (42, 48). Unimpeded, the MRN complex promotes concatenation of Ad genomes, thereby inhibiting vDNA replication (5, 18, 66, 67, 73). Ad effectively counteracts this cellular response by two distinct measures. The E4 ORF6 protein targets the MRN complex components for degradation by the ubiquitin-mediated proteosome-dependent pathway (2, 41, 66). Alternatively, among the group C Ads, the E4 ORF3 protein is capable of sequestering both the nucleoplasmic and PML-NB-associated MRN complex proteins into the E4 ORF3 tracks with PML (18, 66, 67).
While this phenomenon is specific to a subset of Ads, the ability of the E4 ORF3 protein to rearrange both PML and a cellular transcription factor, TIF1α, is conserved among all serotypes investigated to date (76). Similarly conserved is the strict requirement for E4 ORF3 protein expression to facilitate vDNA replication during the interferon (IFN)-induced antiviral state (71). This association of PML-NB rearrangement and subversion of innate immunity is intuitive, as the PML protein is encoded by an IFN-stimulated gene (ISG), containing both an IFN-stimulated response element and a gamma-activated site responsive to type I IFNs and type II IFN, respectively, in its 5′ untranslated region (65). As such, upon treatment with either a type I IFN (e.g., IFN-α) or type II IFN (IFN-γ), both the size and number of PML bodies are dramatically augmented (29, 38). In addition to the PML protein itself, several ISGs have been reported to localize to PML-NBs (21). Among them are Sp100 and Daxx (29, 63), two proteins, in addition to PML, that are capable of functioning as constitutively expressed mediators of intrinsic immunity, termed restriction factors, against both herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV) in the absence of IFN induction (9, 10, 26, 27, 55, 58, 59, 68, 69, 74). PML and Sp100 also inhibit HSV-1 immediate-early gene expression in an IFN-inducible manner (12, 51). The Daxx protein facilitates inhibition of HCMV early gene expression via the recruitment of histone deacetylases (HDACs) to the major immediate-early promoter (MIEP) and the establishment of a repressive chromatin structure, thereby inhibiting the expression of critical immediate-early genes (59, 74). Similarly, PML and certain Sp100 isoforms have been demonstrated to repress the expression of HCMV and HSV-1 immediate-early genes (51, 68, 69).
Here, we demonstrate that the rearrangement of PML-NBs by the Ad E4 ORF3 protein antagonizes a PML-dependent, IFN-induced innate antiviral response. When expression of the PML protein was reduced, replication of an E4 ORF3 mutant virus during the IFN-induced antiviral state was rescued. Similarly, replication of an E4 ORF3 mutant virus was restored upon expression of either the HSV-1 ICP0 or HCMV IE1 protein during the IFN response. This rescue implies that there is functional conservation of viral disruption of PML-NBs in the IFN-induced antiviral state. Furthermore, a reduction in the level of the transcriptional corepressor, Daxx, restored replicative success to an E4 ORF3 mutant virus during the IFN response, thereby suggesting that PML at the PML-NB serves as a nucleation point from which Daxx may execute deleterious effects upon Ad vDNA replication. We demonstrate that this IFN-induced antiviral state exerted only a modest effect upon Ad early gene transcription that was not sufficient to inhibit early protein function. We propose that both Daxx and PML contribute to an additional antiviral defense different from that known to inhibit herpesvirus replication.
MATERIALS AND METHODS
Cells, IFN, viruses, and infections.
Vero and HT1080 cells were passaged in Dulbecco's modified Eagle medium supplemented with 10% bovine calf serum. The cells were pretreated for 24 h with IFN (1,000 U/ml IFN-α [Hoffman LaRoche] or 2,000 U/ml IFN-γ [R&D Systems]). Following IFN stimulation, the cells were infected with wild-type or mutant viruses. Viruses dl309 (phenotypically wild-type AD type 5 [Ad5]) and E4inORF3 (an E4 ORF3-minus mutant) were previously described (33, 36). Purified virus particles were obtained by CsCl equilibrium centrifugation (17). Cells were infected at 37°C using 200 virus particles/cell of dl309 or E4inORF3. In brief, to infect coverslips, medium was aspirated from the cells grown on coverslips and replaced with 0.5 ml inoculum. Alternatively, to infect 10-cm dishes, the medium was replaced with 1 ml inoculum. After 1 h, the viral suspension was aspirated and the cells were washed. Dulbecco's modified Eagle medium supplemented with 10% bovine calf serum was replaced, and IFN was added, as appropriate. The cells were then incubated at 37°C in 5% CO2 for the times indicated in the text.
Expression vectors, plasmid transfection, and shRNA-expressing constructs.
All transfections using protein and short hairpin RNA (shRNA) expression vectors were performed upon subconfluent monolayers of cells using Lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's protocol. The pICP0 construct (1.6 μg/ml; KOS strain; from Sandra Weller, University of Connecticut Health Center [57]) or a pCGN-IE1 construct (from Thomas Shenk, Princeton University [52]) was used in the transfection assays. These studies were performed in Vero cells. All experiments using shRNAs were performed in HT1080 cells. shRNA sequences were cloned into a modified pSuper vector (OligoEngine), which contained a blasticidin resistance expression cassette (from Dafna Bar-Sagi, New York University). PML shRNA targets were previously described by Xu et al. (75) and Everett et al. (27). Likewise, Michaelson and Leder published the Daxx shRNA target sequence (47). Sp100 shRNA targets were designed by Open Biosystems and corresponded to target sequences common to all Sp100 isoforms (CTGTGAAACAGAACAGATA and GCTGCTCTATGACATTGTA). The shRNA expression constructs (1.6 μg/ml) were utilized to transfect HT1080 cells. Twenty-four hours posttransfection, cells expressing the shRNAs were selected with 10 μg/ml blasticidin. The selection conditions were maintained for 48 h in the cases of PML and Daxx shRNA or 72 h in the case of Sp100 shRNA.
Immunofluorescence.
Vero and HT1080 cells were seeded onto glass coverslips and infected as described above. At 24 h postinfection, the cells were washed with phosphate-buffered saline (PBS), fixed, and permeabilized with −20°C methanol and then washed with PBS. Cells were blocked in PBS containing 10% goat serum for 1 h at room temperature. Antibodies were diluted in PBS containing 10% goat serum and incubated with the fixed cells for an hour at room temperature. The antibodies and dilutions used were as follows: 1:50 anti-DNA binding protein (DBP) mouse monoclonal antibody B6-8 (from Arnold Levine, Cancer Institute of New Jersey), 1:300 anti-PML rabbit polyclonal antibody (H-238; Santa Cruz Biotechnology), 1:300 anti-hemagglutinin rabbit polyclonal antibody (Y-11; Santa Cruz Biotechnology), 1:150 anti-HSV-1 ICP0 mouse monoclonal antibody (11060; Santa Cruz Biotechnology), 1:2,500 anti-DBP rabbit polyclonal antibody (from Peter van der Vleit, University Medical Centre, Utrecht, The Netherlands), 1:300 anti-PML mouse monoclonal antibody (PG-M3; Santa Cruz Biotechnology), 1:300 anti-Daxx rabbit polyclonal antibody (M-112; Santa Cruz Biotechnology), 1:5,000 anti-Sp100 rabbit polyclonal antibody (1380; Chemicon), and 1:600 anti-Mre11 rabbit polyclonal antibody (100-142; Novus). The cells were then washed with PBS and incubated with 1:300 dilutions of Alexa 350-conjugated goat anti-mouse immunoglobulin G (IgG) (Molecular Probes), Alexa350-conjugated goat anti-rabbit IgG (Molecular Probes), fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Zymed), fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Zymed), Texas red-isothiocyanate-conjugated goat anti-rabbit IgG (Zymed), or Texas red-isothiocyanate-conjugated goat anti-mouse IgG (Zymed) for 45 min at room temperature in the dark. Subsequently, the cells were washed with PBS and the coverslips were mounted onto slides using Immu-Mount (Thermo Electron). Microscopy was conducted on a Zeiss Axiovert 200 M digital deconvolution microscope. Images were captured and analyzed with Axiovision 4.5 software. Ad vDNA replication was assessed by determination of DBP localization. The results presented represent the analysis of 100 cells in three independent experiments for each data point.
Real-time PCR analysis.
Total RNA was isolated using an RNeasy kit and Qiashredder (Qiagen) according to the manufacturer's protocol. Five micrograms of RNA was reverse transcribed using oligo(dT) and a first-strand synthesis kit (Roche), following the manufacturer's instructions. Primers were designed using Primer3 software to contain roughly 50% GC content (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and to amplify specific viral genes as follows: E1A forward (TCCGGTCCTTCTAACA) and E1B reverse (TGCGCCTTTTACTGCTGCTG); E2B forward (CGCGCGTCGAAGTAGTCTAT) and E2B reverse (CGGTGGAAGATGCTACCCTA); DBP forward (CCGTAGTGGCATCAAAAGCT) and DBP reverse (GTCTCGCAAGGCCAAGAT); E4 ORF6 forward (TCCCGCGTTAGAACCATATC) and E4 ORF6 reverse (CCATTTGGCATGACACTACG); GAPDH forward (GTCAGTGGTGGACCTGACCT) and GAPDH reverse (TGACCAAGTGGTCGTTGAGG). Quantitative PCR (Q-PCR)analysis was performed on a LightCycler (Roche) utilizing the cDNA templates and different primer pairs. The reaction mixture consisted of FastStart DNA Master Sybr green 1 (Roche), 3 mM MgCl2, and 10 μM of each primer. In brief, the PCR program consisted of 95°C for 5 min, proceeded to 38 amplification cycles of 95°C for 5 seconds, 58 to 60°C, depending upon the primer set, for 5 seconds, followed by 72°C for 10 seconds and a final incubation at 85°C to eliminate primer dimers prior to quantification. The identities of the products obtained were confirmed by melting curve analysis, and the results were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels for each sample. The final results represent the averages of three independent experiments.
RESULTS
The E4 ORF3 protein antagonizes a PML-dependent antiviral effect.
We previously demonstrated that the E4 ORF3 protein is required for efficient Ad vDNA replication during the IFN-induced antiviral state in Vero monkey cells (71). This viral protein is both necessary and sufficient to rearrange PML bodies from punctate nuclear subdomains into nuclear track structures (11, 16). To test whether PML is implicated, either directly or indirectly, in the IFN-induced antiviral response antagonized by the E4 ORF3 protein, we used shRNAs to reduce the expression of PML in HT1080 cells, a human fibrosarcoma cell line responsive to IFN. HT1080 cells were used due to the description of small interfering RNAs and shRNAs to reduce the expression of specific human proteins. To confirm the efficacy of the shRNA constructs, cells grown on coverslips were transfected with shRNA expression vectors to target PML. After 24 h, the cells were placed under blasticidin selection to select for transfected cells. After an additional 24 h, the cells were fixed and immunostained with an antibody against PML, revealing the absence of PML bodies in those nuclei successfully transfected with the shRNA expression vectors (Fig. 1A). Transfection of control vector alone did not affect PML-NB integrity (Fig. 1C).
FIG. 1.
Reduction in PML-NB-associated PML, Daxx, and Sp100 using shRNAs. HT1080 cells grown on coverslips were transfected with plasmids expressing shRNAs against PML (A and B), Daxx (D and E), or Sp100 (G and H) or with control vector alone (C, F, and I). Subsequently, the cells were fixed and immunostained with an antibody against PML (A and C), Daxx (D and F), and Sp100 (G and I). The images represent deconvolved, compressed Z stacks. Differential interference contrast images are shown in panels B, E, and H.
To ascertain whether the E4 ORF3 protein antagonizes a PML-dependent antiviral phenomenon, HT1080 cells grown on coverslips were transfected with PML shRNA expression vectors or empty vector alone. Twenty four hours after transfection, the cells were placed under selection and stimulated with either IFN-α or IFN-γ. After an additional 24 h, the cells were infected with either dl309 (phenotypically wild-type Ad5) or E4inORF3 (an E4 ORF3 mutant virus). After 24 h, the cells were fixed and immunostained with an antibody against the viral DBP. The presence of circular DBP-positive viral replication centers in the nuclei of infected cells is indicative of robust Ad vDNA replication, whereas diffuse nucleoplasmic localization of the protein signifies a lack of efficient replication (71, 72). Following transfection of the control vector, vDNA replication was evident in the case of untreated cells infected with both dl309 and mutant E4inORF3 (Fig. 2D and G). As previously reported, dl309 exhibited active replication in cells pretreated with either IFN-α or IFN-γ (Fig. 2E and F), whereas the E4 ORF3 mutant predominantly displayed diffuse DBP staining indicative of an inhibition of vDNA replication (71). Quantification of these results is presented in Fig. 3A.
FIG. 2.
The E4 ORF3 protein antagonizes an IFN-induced, PML-dependent antiviral effect. HT1080 cells grown on coverslips were transfected with vectors expressing PML shRNAs (J to O) or empty vector alone (D to I). Twenty-four hours after transfection, the cells were untreated (A, D, G, J, and M), or stimulated with IFN-α (B, E, H, K, and N) or IFN-γ (C, F, I, L, and O). After an additional 24 h, the cells were infected with dl309 (D to F and J to L) or E4inORF3 (G to I and M to O) or were uninfected (A to C). Twenty-four hours after infection, the cells were fixed and immunostained with an antibody against DBP (D to O) or PML (A to C). The images represent deconvolved, compressed Z stacks.
FIG. 3.
Viral DNA replication in HT1080 cells following IFN treatment with or without PML reduction. (A) The percentages of cells exhibiting viral replication centers are presented in cells, with or without IFN pretreatment, infected with dl309 or mutant E4inORF3. Replication was quantified by counting cells that exhibited viral replication centers in comparison to cells that displayed diffuse nuclear DBP staining. (B) Quantification of vDNA replication following PML reduction using shRNAs. The percentages of cells exhibiting viral replication centers are presented in cells infected with dl309 or E4inORF4 and demonstrating PML reduction, with or without IFN pretreatment. Replication was quantified by counting cells that exhibited viral replication centers in comparison to cells that displayed diffuse nuclear DBP staining. The results presented in both panels represent the analysis of 100 cells for each data point in three independent experiments. The error bars represent standard deviations.
When PML-NBs were disrupted using shRNAs, mutant E4inORF3 exhibited vDNA replication in cells pretreated with either IFN-α or IFN-γ (Fig. 2N and O) and comparable to cells infected with dl309 (Fig. 2K and L). The percentages of cells supporting active vDNA replication were quantified in three independent experiments. Infected cells exhibiting substantial PML-NB disruption were scored for the presence of vDNA replication centers or diffuse DBP expression. This analysis revealed that PML-NB disruption effectively restored replication competence to the E4 ORF3 mutant virus in the antiviral state induced by either IFN-α or IFN-γ to an extent comparable to that observed in unstimulated cells (Fig. 3B). This is in contrast to the significant reduction in vDNA replication with this mutant in IFN-treated cells in the absence of PML-NB disruption (Fig. 3A). These results clearly implicate PML as a participant in an IFN-induced innate antiviral defense antagonized by the E4 ORF3 protein.
The disruption of PML-NBs by HSV-1 ICP0 and HCMV IE1 is functionally equivalent to disruption by E4 ORF3 during the IFN-induced antiviral state.
The Ad E4 ORF3 protein is not unique in its ability to disrupt PML bodies (21). Among several virus-encoded gene products, the HCMV IE1 protein prompts the dispersal of PML from the PML-NB (1, 35, 37), while the HSV-1 ICP0 protein promotes the proteosome-dependent degradation of PML and, consequently, PML-NB disruption (23, 44). While these proteins execute these processes using mechanisms distinct from that of the Ad E4 ORF3 protein, all three viral proteins accomplish the same objective: to disrupt PML-NBs and to alter the subnuclear localization of a wide variety of proteins known to associate with these structures. For this reason, we opted to investigate if the HSV-1 ICP0 and HCMV IE1 proteins were capable of substituting for the E4 ORF3 protein to promote Ad vDNA replication during the IFN-induced antiviral state.
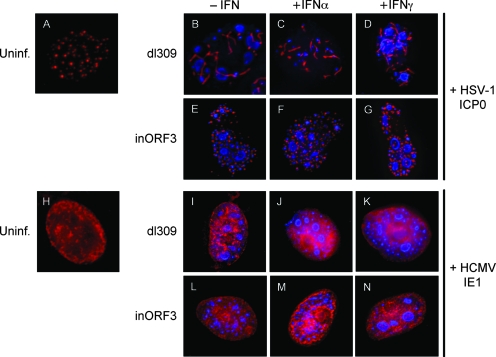
Vero cells grown on coverslips were transfected with either pICP0 or pIE1 expression vectors. Six hours posttransfection, the cells were stimulated with either IFN-α or IFN-γ. After an additional 24 h, the cells remained uninfected or were subjected to infection by either dl309 or E4inORF3. Vero cells were used, since they exhibit a robust antiviral effect with the E4 ORF3 mutant virus following IFN stimulation (71). The ICP0 protein assumed a punctate staining pattern within the nuclei of cells (Fig. 4A), as previously described (23, 44, 45). The ICP0 protein has been established to associate with PML bodies and to promote proteosome-dependent degradation of PML, thereby causing dispersal of PML body-associated proteins throughout the nucleoplasm (4, 20, 22). When ICP0-expressing cells were infected with dl309, the virus demonstrated replication competence under both uninduced and IFN-induced conditions (Fig. 4B to D). Of interest, ICP0 was relocalized from punctate dots into E4 ORF3-induced nuclear tracks following infection with dl309 (Fig. 4B to D). When ICP0-expressing cells were infected with E4inORF3, a significant percentage of cells exhibited active vDNA replication (Fig. 4E to G).
FIG. 4.
HSV-1 ICPO and HCMV IE1 support replication of an E4 ORF3 mutant virus in the IFN-induced antiviral state. Vero cells were transfected with expression vectors for HSV1 ICP0 (A to G) or HCMV IE1 (H to N). Six hours after transfection, the cells remained untreated (A, B, E, H, I, and L) or were stimulated with either IFN-α (C, F, J, and M) or IFN-γ (D, G, K, and N) for 24 h. The cells were then infected with dl309 (B to D and I to K) or E4inORF3 (E to G and L to N) or remained uninfected (Uninf.) (A and H). Twenty-four hours after infection, the cells were fixed and immunostained with antibodies against ICP0 (A to G), hemagglutinin (H to N), and DBP (B to G and I to N). The images represent deconvolved, compressed Z stacks. Texas red-conjugated secondary antibodies were used to detect ICP0 and IE1 (red), and an Alexa 350-conjugated antibody was used to detect DBP (blue).
When quantified, ≥70% of dl309-infected cells were observed to replicate vDNA, irrespective of IFN treatment (Fig. 5A). In the absence of IFN treatment, roughly 60% of cells infected with mutant E4inORF3 supported vDNA replication (data not shown). When cells were stimulated with IFN-α and subsequently subjected to infection by E4inORF3, 31% of infected cells exhibited robust vDNA replication, as indicated by multiple viral replication centers. Ninety-one percent of these cells were found to express the ICP0 protein to a level detectable by immunofluorescence microscopy (Fig. 5B). Of the cells infected with E4inORF3 and stimulated with IFN-α that did not support vDNA replication and exhibited diffuse DBP staining, 82% did not express the ICP0 protein (Fig. 5B). Similar to what was observed in the case of cells induced with IFN-α, those cells subjected to IFN-γ treatment prior to infection by mutant E4inORF3 supported vDNA replication in 25% of infected cells. Of this population, 89% of the cells demonstrating vDNA replication expressed the ICP0 protein to a level detectable by immunofluorescence microscopy (Fig. 5B). Those cells incapable of permitting vDNA replication accounted for 75% of the population infected with E4inORF3. Of this group, 95% of the cells did not express the ICP0 protein. These data strongly indicate that the HSV-1 ICP0 protein is capable of complementing the absence of the E4 ORF3 protein to promote Ad vDNA replication during the antiviral states induced by IFN-α and IFN-γ.
FIG. 5.
Quantification of the rescue of mutant E4inORF by HSV-1 ICP0 and HCMV IE1 in the IFN-induced antiviral state. One hundred cells infected with mutant E4inORF3 were counted in three independent experiments and scored based upon the appearance of viral replication centers or a diffuse DBP expression pattern. The percentages of cells exhibiting viral replication centers are indicated as replic. +; the percentages of cells exhibiting diffuse DBP staining are indicated as replic. −. Those cells exhibiting viral replication centers were then scored for the presence or absence of HSV-1 ICP0 expression (A) or HCMV IE1 expression (B). Cells exhibiting a diffuse DBP expression pattern were analyzed in the same manner. The proportion of HSV-1 ICP0- or HCMV IE1-expressing cells to nonexpressing cells within each population was then determined. The gray bars represent cells that expressed detectable HSV-1 ICP0 or HCMV IE1; the black bars represent cells that did not express detectable HSV-1 ICP or HCMV EI1. The cells were pretreated with either IFN-α (+IFN-α) or IFN-γ (+IFN-γ).
Upon its expression, the HCMV IE1 protein assumed a diffuse nucleoplasmic localization (Fig. 4H), as previously described (1, 35, 37). When IE1-expressing cells were infected with dl309, vDNA replication centers were observed regardless of whether the cells had been pretreated with either IFN-α or IFN-γ (Fig. 4I to K). In contrast, mutant E4inORF3 did not replicate during the antiviral state induced by either IFN-α or IFN-γ (data not shown and reference 71). In contrast, when cells expressing the IE1 protein were infected with E4inORF3, vDNA replication centers were observed under both uninduced and IFN-induced conditions (Fig. 4L to M). When quantified, 75% of dl309-infected cells exhibited vDNA replication under both unstimulated and IFN-stimulated conditions (data not shown). Similarly, roughly 60% of unstimulated cells infected with mutant E4inORF3 supported robust vDNA replication (data not shown). When subjected to IFN-α pretreatment, 33% of E4inORF3-infected cells demonstrated vDNA replication centers. Of this population, 87% expressed the HCMV IE1 protein (Fig. 5B). Eighty-nine percent of the infected cells that were deficient for inORF3 replication did not show detectable IE1 expression (Fig. 5B). Consistent with these observations, in cells pretreated with IFN-γ and infected with E4inORF3, 24% of infected nuclei displayed vDNA replication centers, and 87% of these cells expressed the IE1 protein (Fig. 5B). Those cells incapable of permitting vDNA replication accounted for 76% of the population infected with E4inORF3, and of this group, 85% did not express the IE1 protein (Fig. 5B).
Taken together, these data show that the Ad E4 ORF3, HSV-1 ICP0, and HCMV IE1 proteins are capable of promoting efficient Ad vDNA replication in the antiviral state induced by either IFN-α or IFN-γ. While these viral proteins employ distinct mechanisms to promote PML-NB disruption, the data suggest functional conservation in their abilities to alter PML-NB-associated, IFN-induced antiviral activities.
Roles of PML and Daxx in the IFN-induced antiviral state during Ad infection.
Although the above-mentioned data implicated PML in the antiviral response induced by IFNs, it remained unclear whether PML functions as the direct antiviral effector or whether PML indirectly mediates the function of another protein. A variety of proteins localize to PML-NBs via protein-protein interactions (3, 50). Several of these proteins, including Daxx and Sp100, exhibit antiviral activities against human herpesviruses (9, 10, 26, 51, 55, 58, 59, 68, 69, 74). Therefore, we opted to examine the potential involvement of Daxx and Sp100 in the IFN-induced antiviral state that inhibits the replication of an Ad E4 ORF3 mutant virus.
To address this question, we reduced Daxx expression in HT1080 cells by shRNA expression, using the approach described above for PML. Immunofluorescence microscopy revealed the expected punctate nuclear Daxx localization in those cells transfected with vector alone (Fig. 1F). In contrast, the cells subjected to Daxx shRNA expression exhibited a marked absence of Daxx nuclear foci (Fig. 1D). Daxx knockdown did not abrogate PML body formation in HT1080 cells (data not shown). As anticipated, in the absence of shRNA expression, Daxx localized to punctate nuclear bodies, known to colocalize with PML (Fig. 6A). When cells were stimulated with either IFN-α or IFN-γ, augmentation of the number of Daxx punctae was observed (Fig. 6B and C), consistent with the fact that Daxx is an ISG (63).
FIG. 6.
Daxx, but not Sp100, reduction restores vDNA replication to an E4 ORF3 mutant virus in the IFN-induced antiviral state. HT1080 cells grown on coverslips were transfected with a vector for the expression of a Daxx shRNA (D to I) or with vectors for the expression of Sp100 shRNAs (M to R) or were not transfected (A to C and J to L). Six hours after transfection, the cells were stimulated with IFN-α (B, E, H, K, N, and Q) or IFN-γ (C, F, I, L, O, and R) for 24 h or left untreated (A, D, G, J, M, and P). The cells were then infected with dl309 (D to F and M to O) or E4inORF3 (G to I and P to R) or were left uninfected (Uninf.) (A to C and J to L). Twenty-four hours after infection, the cells were fixed and immunostained for Daxx (A to C), Sp100 (J to L), and DBP (D to I and M to R). The images represent deconvolved, compressed Z-stacks.
To test the consequences of Daxx reduction in the IFN-induced antiviral state, HT1080 cells were transfected with the Daxx shRNA expression vector and subsequently placed under blasticidin selection, followed by treatment with either IFN-α or IFN-γ. After 24 h, the cells were infected with either dl309 or mutant E4inORF3. When an additional 24 h had elapsed, the cells were fixed and immunostained with antibodies against both Daxx and DBP. Upon Daxx reduction, cells infected with dl309 displayed replication competence under both unstimulated and IFN-stimulated conditions (Fig. 6D to F). Furthermore, replication centers were observed when cells exhibiting Daxx reduction were infected with E4inORF3 in the absence of IFN treatment (Fig. 6G). Importantly, in those cells both demonstrating Daxx reduction and induced with either IFN-α or IFN-γ, replication of mutant E4inORF3 vDNA was rescued, as indicated by the presence of active vDNA replication centers (Fig. 6H and I). When quantified, this analysis confirmed that a reduction in PML-NB-associated Daxx restored replication competence to the E4 ORF3 mutant virus during the antiviral state induced by either IFN-α or IFN-γ to an extent comparable to that observed in unstimulated cells (Fig. 7A). This dramatic rescue suggests that the Daxx protein plays a critical role in inhibiting mutant E4inORF3 replication in the IFN-induced antiviral state, thereby suggesting that the E4 ORF3 protein functions to antagonize the antiviral effects of Daxx. By extension, PML rearrangement by E4 ORF3 may serve to promote Daxx sequestration into the E4 ORF3 tracks.
FIG. 7.
Viral DNA replication in HT1080 cells following IFN treatment with or without Daxx or Sp100 reduction. (A) Quantification of vDNA replication following Daxx reduction using shRNAs. The percentages of cells exhibiting viral replication centers are presented in cells infected with dl309 or E4inORF4 and demonstrating Daxx reduction, with or without IFN pretreatment. (B) Quantification of vDNA replication following Sp100 reduction using shRNAs. The percentages of cells exhibiting viral replication centers are presented in cells infected with dl309 or E4inORF4 and demonstrating Sp100 reduction, with or without IFN pretreatment. The data analysis was performed as described for Fig. 3. The error bars represent standard deviations.
Sp100 does not contribute to the antiviral effect antagonized by the E4 ORF3 protein.
To determine whether the PML-NB-mediated antiviral response was specific to PML and Daxx or whether it reflected a global ability of proteins that localize to the PML-NB to inhibit Ad vDNA replication, we reduced expression of the PML-NB component Sp100, using shRNAs. HT1080 cells grown on coverslips were transfected with vectors expressing shRNAs directed against Sp100 following the approach described for PML and Daxx. Transfected cells were selected with blasticidin, and after 48 h, the coverslips were fixed and immunostained with an antibody against Sp100. Immunofluorescence microscopy revealed both a nucleoplasmic population of Sp100 and Sp100 localized to nuclear foci in those cells transfected with vector alone (Fig. 1I). In contrast, those cells transfected with the shRNA expression vector demonstrated a marked absence of Sp100 immunostaining at PML-NBs (Fig. 1G). As anticipated, in the absence of shRNA expression, Daxx localized to punctate nuclear bodies, known to colocalize with PML (Fig. 6J). When cells were stimulated with either IFN-α or IFN-γ, augmentation of the number of Sp100 punctae was observed (Fig. 6K and L). This is consistent with the fact that Sp100 is an ISG (29).
To ascertain the effects of Sp100 reduction in the IFN-induced antiviral state, HT1080 cells were transfected with Sp100 shRNA expression vectors. Following blasticidin selection for 48 h, the cells were stimulated with either IFN-α or IFN-γ for 24 h. The cells were infected with either dl309 or mutant E4inORF3. Twenty four hours postinfection, the cells were fixed and immunostained with antibodies against both DBP and Sp100. In those cells both deficient for Sp100 expression and infected with dl309, vDNA replication centers were observed irrespective of whether the cells had been stimulated with either IFN-α or IFN-γ (Fig. 6 M to O). Unlike the instances in which either PML or Daxx expression was reduced and replication of mutant E4inORF3 was rescued during an IFN response, replication of mutant E4inORF3 was observed only in untreated cells, not in cells reduced for Sp100 localization to PML-NBs and pretreated with either IFN-α or IFN-γ (Fig. 6P to R). Quantification of these results is presented in Fig. 7B. These data demonstrate that Sp100, while implicated in intrinsic and IFN-induced responses against HSV-1 (26, 51), does not appear to participate in the anti-Ad response induced by IFNs and antagonized by the E4 ORF3 protein. Furthermore, these data indicate that the observed results may be attributed to specific functions of both PML and Daxx.
IFN stimulation induces a modest decrease in Ad early mRNA expression, although it does not impede early protein function.
Daxx is a transcriptional corepressor established to recruit both HDAC1 and HDAC2 (32), and Daxx facilitates inhibition of HCMV immediate-early gene expression via the establishment of a repressive chromatin structure at the MIEP (59, 74). Since the data described above implicate Daxx in an IFN-induced antiviral defense mediated by the E4 ORF3 protein, we examined if the absence of the E4 ORF3 protein during the IFN-induced antiviral state resulted in the repression of Ad early gene expression.
In the absence of the E4 ORF3 protein, Ad vDNA replication is dependent upon the expression of six early gene products: E1A, E1B 55,000-molecular-weight protein (55K), terminal protein, Ad DNA polymerase, E4 ORF6 protein, and DBP. We analyzed the levels of mRNA transcripts that correspond to these gene products in Vero cells infected with dl309 or E4inORF3, with or without pretreatment with either IFN-α or IFN-γ. Twenty four hours postinfection, total RNA was harvested and reversed transcribed. The resulting cDNAs were subjected to Q-PCR analysis to assess the expression levels of these six critical early genes (Fig. 8). Lack of DNA template in the RNA samples was confirmed by Q-PCR (data not shown).
FIG. 8.
IFN treatment induces a modest decrease in Ad early transcript levels. Vero cells were untreated or stimulated with either IFN-α or IFN-γ for 24 h. The cells were then infected with dl309 or mutant E4inORF3. Twenty-four hours after infection, total RNA was harvested and cDNAs were generated. cDNA levels were quantified by Q-PCR analysis using primers specific for E1A, E1B, E2B, DBP, and E4 ORF6 protein. The data were normalized to GAPDH levels. The results represent the averages of three independent experiments. The error bars represent standard deviations.
In those cells infected with dl309, treatment with either IFN-α or IFN-γ promoted a negligible to modest (two- to threefold) decrease in transcript levels for all six early genes. Because this virus is known to exhibit replication competence during the IFN-induced antiviral state (71), as described above, we conclude that a reduction in early transcript expression of this magnitude exerts a negligible effect upon vDNA replication. Similarly, modest reductions in the expression of these early genes were evident in IFN-treated cells infected with E4inORF3, although the effects were slightly more pronounced following IFN-γ treatment. These data suggest that the inhibition of vDNA replication by mutant E4inORF3 following IFN treatment is not attributable to repression of early gene expression.
To validate this conclusion, we examined the levels of Mre11, with and without IFN treatment, in cells infected with dl309 or E4inORF3. The E1B 55K and E4 ORF6 proteins act in concert to recruit an E3 ubiquitin ligase complex to promote the proteosome-dependent degradation of a variety of cellular proteins, including Mre11 and p53 (30, 56, 66). To assay E1B 55K and E4 ORF6 protein functions, Vero cells were stimulated with either IFN-α or IFN-γ or remained untreated. Twenty-four hours later, the cells were infected with either dl309 or E4inORF3. Mre11 proteins levels were examined using immunofluorescence microscopy 24 h after infection; this assay was utilized, since only ∼20 to 30% of Vero cells are infected in these experiments, which precluded direct protein quantification by Western blot analysis. In uninfected cells, comparable levels of Mre11 were evident with or without IFN treatment (Fig. 9A to C). In cells infected with dl309 or E4inORF3, a similar level of reduction in Mre11 levels was evident, irrespective of IFN-α or IFN-γ treatment (Fig. 9D, F, H, J, L, and N). When the expression patterns of Ad DBP were analyzed, viral replication centers were readily evident in cells infected with dl309, irrespective of IFN treatment (Fig. 9E, G, and I), and in cells infected with E4inORF3 without IFN treatment (Fig. 9K), whereas only diffuse DBP expression was observed in cells infected with E4inORF3 following treatment with IFN-α or IFN-γ (Fig. 9M and O), as expected. Taken together, these data suggest that while stimulation by either IFN-α or IFN-γ induces a modest decrease in Ad early mRNA transcript levels, this reduction does not reduce Ad protein expression to an extent capable of compromising early protein function. Therefore, while Daxx and PML produce inhibitory effects upon Ad vDNA replication in the IFN-induced antiviral state, the mechanism of this antiviral strategy is not based solely upon transcriptional repression.
FIG. 9.
IFN treatment does not affect the ability of mutant E4inORF3 to target Mre11 for degradation. Vero cells were untreated (A, D, E, J, and K) or stimulated with either IFN-α (B, F, G, L, and M) or IFN-γ (C, H, I, N, and O) for 24 h. The cells were then infected with dl309 (D to I) or mutant E4inORF3 (J to O). Twenty-four hours after infection, the cells were fixed and immunostained for Mre11 (A, B, C, D, F, H, J, L, and N) or DBP (E, G, I, K, M, and O). The images represent deconvolved, compressed Z stacks.
DISCUSSION
The PML-NB has been implicated in both the intrinsic and innate responses to viral infection and is a target for inactivation during viral infection (21). Upon treatment of cells with either type I or type II IFNs, PML-NBs undergo a dramatic augmentation in both size and number (29, 38). This is likely a consequence of the fact that PML gene expression and protein levels are induced by IFNs (29, 38). Furthermore, a variety of other proteins that localize to these nuclear structures are also ISGs, implying a function for the PML-NB as a depot for IFN-inducible antiviral effectors (21). It is a common theme of DNA viruses both to localize to and to replicate their genomes in proximity to PML-NBs (43). It has also been reported that de novo PML-NB assembly occurs at the sites of viral genome deposition in the nucleus (24). While the genomes successful in vDNA replication are those associated with PML-NBs early after infection (64), indicating that these structures may enhance vDNA replication, many viral proteins mediate PML-NB disruption, suggesting antiviral activity or activities of these domains. In fact, failure to disrupt PML-NBs during infection with wild-type HSV-1 or ICP0 mutant viruses inhibits vDNA replication, in either the absence or presence of IFN treatment (7, 13, 49, 70). The PML protein has been documented to exhibit antiviral effects against HCMV, as well as an HSV-1 ICP0 mutant, in an intrinsic capacity (12, 26, 27, 68, 69). An Ad E4 ORF3 mutant in an otherwise wild-type virus background was capable of sustaining near-wild-type levels of vDNA synthesis under standard tissue culture conditions (6, 33), suggesting that if PML, or any other PML-NB-associated protein(s), executes an intrinsic defense against Ad infection, it is overcome by a mechanism that does not involve the E4 ORF3 protein. We previously demonstrated that the E4 ORF3 protein is necessary for efficient Ad vDNA replication in the IFN-induced antiviral state (71). Thus, the essential function of E4 ORF3 is only revealed following IFN stimulation of cells.
We provide several lines of evidence that the PML protein, and by inference PML-NBs, mediates an IFN-induced innate defense against Ad infection in the absence of the E4 ORF3 protein. First, the replication of an E4 ORF3 mutant virus, otherwise deficient during the IFN-mediated antiviral response, was restored to levels observed in the absence of IFN stimulation when PML-NBs were eliminated using shRNAs directed against PML (Fig. 2 and 3B). Second, an E4 ORF3 mutant virus replicated efficiently during an IFN response in cells expressing either the HSV-1 ICP0 or HCMV IE1 protein (Fig. 4 and 5). These data suggest that the E4 ORF3, HCMV IE1, and HSV-1 ICP0 proteins behave in functionally analogous manners with respect to antagonizing the IFN-induced antiviral state. While all three viral proteins mediate PML-NB disruption by employing mechanistically distinct strategies, they share the ability to disrupt PML-NB integrity. This observation parallels those obtained with an HSV-1 ICP0 mutant that was found to be sensitive to the antiviral activities of IFN in a PML-dependent capacity (12, 49). Multiple PML isoforms exist due to alternative splicing of 3′ exons (3). Future experiments will be required to determine if a specific PML isoform is involved in the IFN response.
It remained unclear whether IFN-induced antiviral defense against Ad is mediated directly by the PML protein or through the activity of the PML-NB. For this reason, we examined the consequences of depletion of two other IFN-inducible PML-NB components, Daxx and Sp100 (29, 63). Upon depletion of PML-NB-associated Daxx by shRNA, replication of an E4 ORF3 mutant virus during an IFN-induced antiviral state was rescued to levels comparable to those observed in unstimulated cells (Fig. 6 and 7A). These results demonstrate a role for Daxx in the IFN-induced antiviral response antagonized by the E4 ORF3 protein. PML mediates the association of Daxx with the PML-NB via the association of a SUMO interaction motif in Daxx with sumoylated PML (3). It is logical to assume that HCMV IE1 protein-mediated desumoylation of PML would effectively antagonize this interaction. In fact, we have noted that expression of either the HCMV IE1 or HSV1 ICP0 protein alters Daxx localization (data not shown). In contrast, depletion of Sp100 from PML-NBs using shRNAs did not rescue vDNA replication of an E4 ORF3 mutant virus during an IFN response (Fig. 6 and 7B). It was recently shown that Sp100 inhibits early gene expression of an HSV-1 ICP0 mutant and that a reduction of Sp100 protein levels restored early transcription of this mutant virus, demonstrating that Sp100 can negatively regulate HSV-1 transcription (26). Similarly, Daxx is a component of the intrinsic antiviral response directed against HCMV and has been reported to interact with the HCMV MIEP to repress HCMV early gene transcription (59, 74). Our results distinguish the roles of Daxx and Sp100 in the intrinsic response that inhibits HCMV and HSV-1 early gene expression from the IFN-induced innate response that inhibits Ad vDNA replication in the absence of E4 ORF3, since Daxx, but not Sp100, is required to inhibit Ad replication. Multiple Sp100 isoforms exist due to alternative splicing patterns (51). Our studies show that a reduction of PML-NB-associated Sp100 does not block the inhibitory effect of IFNs on an Ad E4 ORF3 mutant virus, but they do not determine whether a specific Sp100 isoform may inhibit Ad replication outside the context of the PML-NB.
Inhibition of HSV1 infection by IFN, PML, and Sp100 is associated with the downregulation of early gene expression, as are the intrinsic effects of Daxx against HCMV infection (12, 19, 49, 51, 58, 59, 68-70). Daxx is a transcriptional corepressor known to associate with HDAC1 and HDAC2 (32, 39, 40). In light of these results, we analyzed whether the IFN response inhibits Ad early transcription. When the expression levels of five critical early genes, E1A, E1B, E2B, DPB, and E4 ORF6, were assayed by quantitative-PCR analysis, only modest decreases in early gene expression were observed during the IFN response (Fig. 8). This situation was true for both wild-type Ad5 and an E4 ORF3 mutant, although early gene transcription appeared slightly more susceptible to the effects of IFN in the case of the mutant. These results are consistent with previous Western blot analyses that indicated only minor changes in the levels of the E1A and DBP proteins following IFN stimulation and infection with either wild-type Ad5 or an E4 ORF3 mutant (71). While these effects resulted in no greater than a two- to threefold reduction in early transcription, it was imperative to determine whether the effects resulted in functional inhibition of early protein activity. To address this question, the functions of the E1B 55K and E4 ORF6 proteins were measured by determining the extent of Mre11 degradation in the absence or presence of IFN treatment and infection with wild-type Ad5 or the E4 ORF3 mutant virus. The E1B 55K/E4 ORF6 complex recruits an E3 ubiquitin ligase complex to target cellular proteins, such as Mre11 and p53, for ubiquitin-mediated, proteosome-dependent degradation (30, 56, 66). When Mre11 degradation was examined, it was evident that both wild-type Ad5 and the E4 ORF3 mutant virus were capable of directing efficient Mre11 degradation irrespective of IFN treatment (Fig. 9). These data demonstrate that early protein function was not compromised as a result of the modestly diminished early gene transcription observed in the antiviral state induced by either IFN-α or IFN-γ.
We propose that, in addition to their participation in an intrinsic response to human herpesviruses (9, 26, 27, 31, 55, 58, 59, 68, 69, 74), both PML and Daxx, perhaps as a function of the PML-NB, actively contribute to an IFN-mediated innate antiviral response that is not based solely on transcriptional repression. While Daxx may execute transcriptional repression during the intrinsic response to certain viral infections, Ad appears to be resistant to any significant transcriptional repression during the IFN-induced antiviral state. Our results are consistent with two models of PML and Daxx function during the IFN-induced antiviral state. First, the role of PML may be to recruit Daxx to PML-NBs, where Daxx functions as the direct IFN-induced antiviral effector. Alternatively, PML and Daxx may coordinately function to inhibit Ad vDNA replication during the IFN response. In either case, a reduction in or inhibition of either cellular protein would be sufficient to block their antiviral effect. The IFN-induced antiviral response to Ad infection may reflect a regulation of the subnuclear localization Ad genomes to sites that inhibit vDNA replication, an idea consistent with analyses of herpesviruses and viral genome deposition near PML-NBs early after infection (8, 24-26, 34, 46, 68, 69), or these cellular proteins may inhibit Ad vDNA replication by acting on viral replication proteins and/or the viral origin of replication. Regardless of the precise mechanism, what remains clear is that the PML-NB participates in a critical IFN-induced innate response to viral infection. PML-NB integrity is essential to facilitating both the effects of intrinsic restriction factors and induced innate effectors, underscoring its complex function as a site of host antiviral defense that is targeted for inactivation during viral infection.
Acknowledgments
We thank several colleagues for the generous gifts of antibodies, including Thomas Dobner for antibody against E4 ORF3 and Arnold Levine and Peter van der Vleit for antibodies against DBP. We acknowledge the excellent technical assistance of Mary Anderson, Ilana Shoshani, and Sarah van Scoy. We thank members of our laboratory for informed discussions.
This work was supported by NIH grant CA122677. A.J.U. was supported by NIH Training Grant CA009176.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 714599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo, F. D., T. H. Stracker, C. T. Carson, D. V. Lee, and M. D. Weitzman. 2005. Adenovirus type 5 E4orf3 protein targets the Mre11 complex to cytoplasmic aggresomes. J. Virol. 7911382-11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardi, R., and P. P. Pandolfi. 2007. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 81006-1016. [DOI] [PubMed] [Google Scholar]
- 4.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer, J., K. Rohleder, and G. Ketner. 1999. Adenovirus E4 34k and E4 11k inhibit double strand break repair and are physically associated with the cellular DNA-dependent protein kinase. Virology 263307-312. [DOI] [PubMed] [Google Scholar]
- 6.Bridge, E., and G. Ketner. 1989. Redundant control of adenovirus late gene expression by early region 4. J. Virol. 63631-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkham, J., D. M. Coen, C. B. Hwang, and S. K. Weller. 2001. Interactions of herpes simplex virus type 1 with ND10 and recruitment of PML to replication compartments. J. Virol. 752353-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkham, J., D. M. Coen, and S. K. Weller. 1998. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 7210100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantrell, S. R., and W. A. Bresnahan. 2006. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J. Virol. 806188-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantrell, S. R., and W. A. Bresnahan. 2005. Interaction between the human cytomegalovirus UL82 gene product (pp71) and hDaxx regulates immediate-early gene expression and viral replication. J. Virol. 797792-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho, T., J. S. Seeler, K. Ohman, P. Jordan, U. Pettersson, G. Akusjarvi, M. Carmo-Fonseca, and A. Dejean. 1995. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J. Cell Biol. 13145-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 777101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelbi-Alix, M. K., F. Quignon, L. Pelicano, M. H. Koken, and H. de The. 1998. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J. Virol. 721043-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ching, R. W., G. Dellaire, C. H. Eskiw, and D. P. Bazett-Jones. 2005. PML bodies: a meeting place for genomic loci? J. Cell Sci. 118847-854. [DOI] [PubMed] [Google Scholar]
- 15.Dellaire, G., and D. P. Bazett-Jones. 2004. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26963-977. [DOI] [PubMed] [Google Scholar]
- 16.Doucas, V., A. M. Ishov, A. Romo, H. Juguilon, M. D. Weitzman, R. M. Evans, and G. G. Maul. 1996. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10196-207. [DOI] [PubMed] [Google Scholar]
- 17.Evans, J. D., and P. Hearing. 2003. Distinct roles of the adenovirus E4 ORF3 protein in viral DNA replication and inhibition of genome concatenation. J. Virol. 775295-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, J. D., and P. Hearing. 2005. Relocalization of the Mre11-Rad50-Nbs1 complex by the adenovirus E4 ORF3 protein is required for viral replication. J. Virol. 796207-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 207266-7273. [DOI] [PubMed] [Google Scholar]
- 20.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 749994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett, R. D., and M. K. Chelbi-Alix. 2007. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89819-830. [DOI] [PubMed] [Google Scholar]
- 22.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 726581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 135062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett, R. D., and J. Murray. 2005. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 795078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everett, R. D., J. Murray, A. Orr, and C. M. Preston. 2007. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 8110991-11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, R. D., C. Parada, P. Gripon, H. Sirma, and A. Orr. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 822661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 807995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrum, F. D., and D. A. Ornelles. 1999. Roles for the E4 orf6, orf3, and E1B 55-kilodalton proteins in cell cycle-independent adenovirus replication. J. Virol. 737474-7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grotzinger, T., T. Sternsdorf, K. Jensen, and H. Will. 1996. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML). Eur. J. Biochem. 238554-560. [DOI] [PubMed] [Google Scholar]
- 30.Harada, J. N., A. Shevchenko, A. Shevchenko, D. C. Pallas, and A. J. Berk. 2002. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 769194-9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann, H., H. Sindre, and T. Stamminger. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 765769-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 1153319-3330. [DOI] [PubMed] [Google Scholar]
- 33.Huang, M. M., and P. Hearing. 1989. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J. Virol. 632605-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishov, A. M., and G. G. Maul. 1996. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J. Cell Biol. 134815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 1385-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones, N., and T. Shenk. 1979. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell 17683-689. [DOI] [PubMed] [Google Scholar]
- 37.Korioth, F., G. G. Maul, B. Plachter, T. Stamminger, and J. Frey. 1996. The nuclear domain 10 (ND10) is disrupted by the human cytomegalovirus gene product IE1. Exp. Cell Res. 229155-158. [DOI] [PubMed] [Google Scholar]
- 38.Lavau, C., A. Marchio, M. Fagioli, J. Jansen, B. Falini, P. Lebon, F. Grosveld, P. P. Pandolfi, P. G. Pelicci, and A. Dejean. 1995. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene 11871-876. [PubMed] [Google Scholar]
- 39.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 201784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19745-753. [DOI] [PubMed] [Google Scholar]
- 41.Liu, Y., A. Shevchenko, A. Shevchenko, and A. J. Berk. 2005. Adenovirus exploits the cellular aggresome response to accelerate inactivation of the MRN complex. J. Virol. 7914004-14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lombard, D. B., and L. Guarente. 2000. Nijmegen breakage syndrome disease protein and MRE11 at PML nuclear bodies and meiotic telomeres. Cancer Res. 602331-2334. [PubMed] [Google Scholar]
- 43.Maul, G. G. 1998. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20660-667. [DOI] [PubMed] [Google Scholar]
- 44.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 751223-1233. [DOI] [PubMed] [Google Scholar]
- 45.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 742679-2690. [DOI] [PubMed] [Google Scholar]
- 46.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 21767-75. [DOI] [PubMed] [Google Scholar]
- 47.Michaelson, J. S., and P. Leder. 2003. RNAi reveals anti-apoptotic and transcriptionally repressive activities of DAXX. J. Cell Sci. 116345-352. [DOI] [PubMed] [Google Scholar]
- 48.Mirzoeva, O. K., and J. H. Petrini. 2001. DNA damage-dependent nuclear dynamics of the Mre11 complex. Mol. Cell. Biol. 21281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 742052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negorev, D., and G. G. Maul. 2001. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 207234-7242. [DOI] [PubMed] [Google Scholar]
- 51.Negorev, D. G., O. V. Vladimirova, A. Ivanov, F. Rauscher III, and G. G. Maul. 2006. Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. J. Virol. 808019-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nevels, M., C. Paulus, and T. Shenk. 2004. Human cytomegalovirus immediate-early 1 protein facilitates viral replication by antagonizing histone deacetylation. Proc. Natl. Acad. Sci. USA 10117234-17239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nordqvist, K., K. Ohman, and G. Akusjarvi. 1994. Human adenovirus encodes two proteins which have opposite effects on accumulation of alternatively spliced mRNAs. Mol. Cell. Biol. 14437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohman, K., K. Nordqvist, and G. Akusjarvi. 1993. Two adenovirus proteins with redundant activities in virus growth facilitate tripartite leader mRNA accumulation. Virology 19450-58. [DOI] [PubMed] [Google Scholar]
- 55.Preston, C. M., and M. J. Nicholl. 2006. Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 871113-1121. [DOI] [PubMed] [Google Scholar]
- 56.Querido, E., P. Blanchette, Q. Yan, T. Kamura, M. Morrison, D. Boivin, W. G. Kaelin, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2001. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 153104-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saffert, R. T., and R. F. Kalejta. 2007. Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J. Virol. 819109-9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 803863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salomoni, P., and P. P. Pandolfi. 2002. The role of PML in tumor suppression. Cell 108165-170. [DOI] [PubMed] [Google Scholar]
- 61.Shepard, R. N., and D. A. Ornelles. 2004. Diverse roles for E4orf3 at late times of infection revealed in an E1B 55-kilodalton protein mutant background. J. Virol. 789924-9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shepard, R. N., and D. A. Ornelles. 2003. E4orf3 is necessary for enhanced S-phase replication of cell cycle-restricted subgroup C adenoviruses. J. Virol. 778593-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimoda, K., K. Kamesaki, A. Numata, K. Aoki, T. Matsuda, K. Oritani, S. Tamiya, K. Kato, K. Takase, R. Imamura, T. Yamamoto, T. Miyamoto, K. Nagafuji, H. Gondo, S. Nagafuchi, K. Nakayama, and M. Harada. 2002. Cutting edge: tyk2 is required for the induction and nuclear translocation of Daxx which regulates IFN-alpha-induced suppression of B lymphocyte formation. J. Immunol. 1694707-4711. [DOI] [PubMed] [Google Scholar]
- 64.Sourvinos, G., and R. D. Everett. 2002. Visualization of parental HSV-1 genomes and replication compartments in association with ND10 in live infected cells. EMBO J. 214989-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stadler, M., M. K. Chelbi-Alix, M. H. Koken, L. Venturini, C. Lee, A. Saib, F. Quignon, L. Pelicano, M. C. Guillemin, C. Schindler, et al. 1995. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene 112565-2573. [PubMed] [Google Scholar]
- 66.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418348-352. [DOI] [PubMed] [Google Scholar]
- 67.Stracker, T. H., D. V. Lee, C. T. Carson, F. D. Araujo, D. A. Ornelles, and M. D. Weitzman. 2005. Serotype-specific reorganization of the Mre11 complex by adenoviral E4orf3 proteins. J. Virol. 796664-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tavalai, N., P. Papior, S. Rechter, M. Leis, and T. Stamminger. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 808006-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tavalai, N., P. Papior, S. Rechter, and T. Stamminger. 2008. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 82126-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor, J. L., D. Unverrich, W. J. O'Brien, and K. W. Wilcox. 2000. Interferon coordinately inhibits the disruption of PML-positive ND10 and immediate-early gene expression by herpes simplex virus. J. Interferon Cytokine Res. 20805-815. [DOI] [PubMed] [Google Scholar]
- 71.Ullman, A. J., N. C. Reich, and P. Hearing. 2007. Adenovirus E4 ORF3 protein inhibits the interferon-mediated antiviral response. J. Virol. 814744-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voelkerding, K., and D. F. Klessig. 1986. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J. Virol. 60353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weiden, M. D., and H. S. Ginsberg. 1994. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc. Natl. Acad. Sci. USA 91153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodhall, D. L., I. J. Groves, M. B. Reeves, G. Wilkinson, and J. H. Sinclair. 2006. Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J. Biol. Chem. 28137652-37660. [DOI] [PubMed] [Google Scholar]
- 75.Xu, Z. X., A. Timanova-Atanasova, R. X. Zhao, and K. S. Chang. 2003. PML colocalizes with and stabilizes the DNA damage response protein TopBP1. Mol. Cell. Biol. 234247-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yondola, M. A., and P. Hearing. 2007. The adenovirus E4 ORF3 protein binds and reorganizes the TRIM family member transcriptional intermediary factor 1 alpha. J. Virol. 814264-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]