Abstract
Human immunodeficiency virus type 1 (HIV-1), introduced into the brain by HIV-1-infected monocytes which migrate across the blood-brain barrier (BBB), infects resident macrophages and microglia and initiates a process that causes HIV-1-associated neurocognitive disorders. The mechanism by which HIV-1 infection circumvents the BBB-restricted passage of systemic leukocytes into the brain and disrupts the integrity of the BBB is not known. Circulating lipopolysaccharide (LPS), which can compromise the integrity of the BBB, is significantly increased in HIV-1-infected individuals. We hypothesized that HIV-1 infection increases monocyte capacity to migrate across the BBB, which is further facilitated by a compromise of BBB integrity mediated by the increased systemic LPS levels present in HIV-1-infected individuals. To investigate this possibility, we examined the in vivo BBB migration of monocytes derived from our novel mouse model, JR-CSF/EYFP mice, which are transgenic for both a long terminal repeat-regulated full-length infectious HIV-1 provirus and ROSA-26-regulated enhanced yellow fluorescent protein. We demonstrated that JR-CSF/EYFP mouse monocytes displayed an increased capacity to enter the brain by crossing either an intact BBB or a BBB whose integrity was partially compromised by systemic LPS. We also demonstrated that the JR-CSF mouse BBB was more susceptible to disruption by systemic LPS than the control wild-type mouse BBB. These results demonstrated that HIV-1 infection increased the ability of monocytes to enter the brain and increased the sensitivity of the BBB to disruption by systemic LPS, which is elevated in HIV-1-infected individuals. These mice represent a new in vivo system for studying the mechanism by which HIV-1-infected monocytes migrate into the brain.
Human immunodeficiency virus type 1 (HIV-1) enters the brain during the establishment and systemic dissemination of HIV-1 infection and either directly or indirectly causes a broad range of HIV-1-associated neurocognitive disorders, including asymptomatic neurocognitive impairment, HIV-1-associated mild neurocognitive disorder, and HIV-1-associated dementia (HAD) (6). HAD, a devastating neurological disease that is a frequent consequence of HIV-1 infection (42, 43), is associated with the pathological findings of HIV-1 encephalitis, characterized by multinucleated giant-cell formation, microglial nodules, astrogliosis, myelin pallor, and neocortical atrophy (48). The major route by which HIV-1 enters the brain is transmigration of HIV-1-infected monocytes across the blood-brain barrier (BBB) early in the course of infection (23, 27, 36, 44). After entry into the brain, these peripheral HIV-1-infected monocytes produce HIV-1 that subsequently infects resident brain microglia and macrophages (3, 7, 37, 38, 66). HAD is not caused by direct HIV-1 infection of neurons but rather is a consequence of the cumulative neurotoxic effects of multiple factors produced by HIV-1-infected or HIV-1-exposed cells in the brain, such as microglia and macrophages (33, 48, 67). Circulating monocytes are normally precluded from migrating into the brain by the BBB, an anatomical barrier that restricts the exchange of cells and soluble factors from the blood into the brain parenchyma (47, 57). The BBB is composed of several components that include specialized endothelial cells which form tight junctions at cellular contact points, the end feet of astrocytes that surround the blood vessels, the capillary basement membrane, and pericytes which are embedded in the basement membrane (31). The BBB prevents migration of inflammatory cells from the periphery into the brain, with the exception of a small number of leukocytes engaged in immune surveillance that can pass across the BBB without altering BBB integrity. The restricted passage of leukocytes from the systemic circulation into the brain across the BBB may be disrupted during the course of HIV-1 infection (53, 54). Compromise of the integrity of the BBB allows circulating HIV-1-infected monocytes to enter the brain, which further disrupts BBB integrity and permits the influx into the brain of more HIV-1-infected monocytes; this further disrupts BBB integrity and allows the entry of increased numbers of HIV-1-infected monocytes, which subsequently infect resident brain monocytes and microglia with HIV-1 (23, 25). The precise mechanism by which HIV-1-infected monocytes cross the intact BBB and subsequently disrupt the integrity of the BBB is not known.
The plasma of HIV-1-infected individuals contains markedly elevated levels of lipopolysaccharide (LPS) due to the increased microbial translocation across a gut mucosa barrier which is compromised by HIV-1-mediated depletion of mucosal CD4 T lymphocytes (11, 22). The mean LPS plasma level (75 pg/ml) in HIV-1 progressors is markedly higher than the plasma level of LPS (14 pg/ml) shown to activate the systemic immune system and induce inflammatory cytokine production in LPS-injected HIV-1-naive human volunteers (22) and was associated with in vivo activation of the innate and adaptive immune systems of HIV-1 progressors (11, 22). The in vivo integrity of the BBB can be compromised by exposure to elevated levels of LPS in the circulation (70). We hypothesized that the increased levels of systemic LPS in HIV-1-infected individuals disrupt BBB integrity and permit the influx of increasing numbers of HIV-1-infected monocytes, which further compromise BBB integrity, and permit the entry of increasing numbers of HIV-1-infected monocytes, which ultimately initiates the development of HAD. We also postulated that HIV-1 infection of monocytes may increase their capacity to migrate across the intact and partially compromised BBB. An in vitro model of the BBB that consists of brain microvascular endothelial cells and astrocytes cultured on opposite sides of a semipermeable coated membrane has permitted investigation of the mechanism by which HIV-1-infected monocytes cross and damage the BBB (52, 68). Although this model has been very useful for studying some aspects of BBB function, it does not fully recapitulate the in vivo function of the BBB. As an in vivo model to investigate whether HIV-1-infection alters the capacity of monocytes to cross the intact BBB and whether HIV-1 infection increases the sensitivity of the BBB to disruption by systemic LPS, we used our well-characterized system consisting of JR-CSF mice, which are transgenic for an infectious HIV-1 provirus (13, 46, 64).
Our JR-CSF transgenic mouse line circumvents the block of HIV-1 entry into mouse cells by carrying as a transgene a full-length infectious HIV-1 provirus derived from the primary R5-tropic clinical isolate HIV-1JR-CSF, and these mice display plasma viremia at levels comparable to that observed in HIV-1-infected patients (46, 64). Furthermore, infectious HIV-1 is produced by monocytes and microglia from the JR-CSF mice, and LPS-stimulated JR-CSF mouse monocytes and microglia produce higher levels of MCP-1 than monocytes and microglia from LPS-stimulated control mice (65). Because JR-CSF mouse monocytes and microglia carry an HIV-1 provirus regulated by the HIV-1 long terminal repeat, we used this model to investigate whether HIV-1 infection of monocytes increases their in vivo capacity to cross the intact and LPS-compromised BBB and migrate into the brain. In the current study, we demonstrated that monocytes from JR-CSF mice displayed an increased capacity to cross both the intact and compromised BBB into the brain parenchyma and that the BBB of JR-CSF mice is more susceptible to disruption by inflammatory signals, such as LPS, than that of control wild-type (WT) mice.
MATERIALS AND METHODS
Construction of mice transgenic for HIV-1JR-CSF and EYFP genes.
The JR-CSF transgenic mouse line was constructed using a plasmid containing an infectious molecular clone of HIV-1JR-CSF (PYK-JR-CSF, obtained from the NIH AIDS Research and Reference Reagent Program), that was cloned from the lymphocytes of an HIV-1-infected patient soon after the initiation of culture (13). This construct contains the full-length genomic sequence of HIV-1JR-CSF, as well as 0.5 kb of 3′ and 2.2 kb of 5′ flanking sequences, and produces infectious virions after transfection into cells (15, 45). The PYK-JR-CSF plasmid was linearized with EcoRI and microinjected into the pronuclei of fertilized embryos derived from F1 FVB × C57/B6 mouse crosses as described previously (12). R26-EYFP mice, which ubiquitously express enhanced yellow fluorescent protein (EYFP), were obtained from Frank Costantini (60) and crossed with JR-CSF mice to generate transgenic mice which carry both the JR-CSF and EYFP transgenes (JR-CSF/EYFP mice). Offspring were screened by PCR analysis of genomic DNA extracted from tail DNA using primer pairs specific for amplification of HIV-1 Gag DNA (13) and EYFP genes (16). All of the procedures used in this study were approved by the Albert Einstein College of Medicine Animal Institute Committee.
Isolation of CD11b+ monocytes from bone marrow of R26-EYFP or JR-CSF/EYFP transgenic mice.
Bone marrow cells flushed from mouse femurs by lavage with phosphate-buffered saline (PBS) were dispersed with vigorous pipetting, washed, and resuspended in PBS. Mononuclear cells were isolated from the bone marrow cells by Ficoll-Hypaque density centrifugation, washed twice with PBS, incubated with MACS MicroBeads coupled to antibody directed to the monocyte-specific marker CD11b (39), and passed through a positive selection autoMACS separation column using the AutoMACS automated bench-top magnetic cell sorter (Miltenyi Biotec Inc., Auburn, CA) as described previously (61). The purity of the sorted cells was determined by flow cytometry, and greater than 90% of the positively selected cells expressed CD11b.
Intravenous injection of EYFP or JR-CSF/EYFP monocytes and intraperitoneal injection of LPS.
Highly purified monocytes (5 × 106 cells) that were isolated from the bone marrow of R26-EYFP or JR-CSF/EYFP transgenic mice as described above were intravenously injected into the tail veins of 1- to 2-month-old mice after they were anesthetized with pentobarbital (40 to 80 mg/kg of body weight). Some mice were intraperitoneally injected with LPS (3 mg/kg) from Escherichia coli 0111:B4 (Sigma, St. Louis, MO) 3 hours before intravenous injection with the mouse monocytes (5 × 106 cells). After 4 days, the mice were anesthetized, exsanguinated, and intracardially injected with 10 ml of PBS to flush the residual blood from the circulation, and their brains and spleens were harvested and analyzed.
Detection by PCR of R26-EYFP or JR-CSF/EYFP mouse monocytes in peripheral blood, spleens, and brains of injected mice.
DNA was extracted from the mouse brains, spleens, or peripheral blood (500 μl) using the Easy-DNA kit (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer's instructions and resuspended in 150 μl Tris-EDTA buffer. The concentration of DNA samples was determined using a Smartspec 3000 spectrophotometer (Bio-Rad), and equivalent DNA concentrations for each sample were analyzed by PCR. The presence of R26-EYFP monocytes and JR-CSF/EYFP monocytes in the tissues of the injected mice was detected by PCR amplification using 10 μl genomic DNA as the template for the first round (40 cycles at 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min). In some studies, we increased the sensitivity for detection of EYFP DNA by using 5 μl from the first round of PCR amplification as a template for a second PCR amplification of 40 cycles using the same amplification protocol. Taq platinum polymerase (Invitrogen Corporation, Carlsbad, CA) and EYFP-specific primers (5′-TACGGCAAGCTGACCCTGAAGTTC-3′ and 5′-CGTCCTTGAAGATGGTGCG-3′) were added to the PCR mix, and the amplimers were detected by gel electrophoresis as described previously (30). No PCR products were detected after either the first- or second-round PCR amplifications of control mouse DNA with these primer pairs, which confirmed the specificity of the PCR. To provide a reference standard for semiquantitative measurement of the EYFP DNA concentration, a region in exon 2 of the mouse exonuclease 1 gene was amplified (30 cycles at 94°C for 30 s, 56°C for 1 min, and 72°C for 1 min) from 5 μl DNA samples from the indicated tissues or blood using exonuclease gene-specific primers (5′-GGGATTCAAGGGTTACTTCAGTTC-3′ and 5′-TTTCAGCACAAGCAATAGCCC-3′). Semiquantitative measurement of the number of migrated cells in the brain was performed by comparing the densities of the EYFP amplimer bands to the densities of the exonuclease amplimer bands using the Kodak Image Station 440 analyzer system and Kodak 1D 3.5 image software (Eastman Kodak, Rochester, NY).
Visualization by fluorescence microscopy of EYFP-expressing cells in brains of mice injected with JR-CSF/EYFP mouse monocytes.
Four days after injection of LPS-treated JR-CSF mice with JR-CSF/EYFP mouse monocytes, the mice were anesthetized and perfused by intracardiac injection with 0.9% NaCl followed by 4% paraformaldehyde. The brains were incubated for 24 h in 30% sucrose in PBS and snap-frozen in optimal-cutting-temperature compound as described previously (18). Coronal cryostatic sections (10-μm thickness) from the mid-coronal region of the brain were cut onto slides and coverslipped in one drop of mounting medium with 4′,6′-diamidino-2-phenylindole (Vector Laboratories, Inc., Burlingame, CA). Fluorescent JR-CSF/EYFP monocytes in the brain were detected and visualized by confocal fluorescence microscopy, and representative images were captured using a Nikon DN100 digital camera.
Evaluation of ZO-1 and occludin expression in brain vasculature.
After mice were perfused by cardiac injection with PBS, their brains were removed, fixed in 10% buffered formalin, bisected coronally, and embedded in paraffin. Sections (5 μm thick) were cut onto slides, deparaffinized in xylene, and rehydrated in serial ethanol solutions. After treatment with pepsin (ZYMED Laboratories) at 37°C for 10 min, the slides were incubated with sheep serum and bovine serum albumin blocking solution to eliminate nonspecific reactivity and then incubated with rabbit anti-zona occludens-1 (ZO-1) (ZYMED Laboratories) and goat antioccludin (Santa Cruz Biotechnology) at optimized concentrations. The slides were washed, incubated with sheep anti-rabbit immunoglobulin G Cy3-conjugated antibody (Sigma) and donkey anti-goat immunoglobulin G Cy5-conjugated antibody (Jackson ImmunoResearch Laboratories), washed again, and mounted with the Prolong Gold antifade reagent containing DAPI (4′6-diamidino-2-phenylindole) (Molecular Probes). The sections were analyzed for cerebral blood vessel occludin and ZO-1-specific fluorescence with a Leica AOBS laser scanning confocal microscope system, and representative images were captured using a Nikon DN100 digital camera.
RESULTS
JR-CSF/EYFP mouse monocytes but not control mouse monocytes cross the intact BBB into the brain.
We previously demonstrated that bone marrow-derived monocytes from JR-CSF mice support HIV-1 replication, produce HIV-1 that infects activated human peripheral blood mononuclear cells in vitro (13), and introduce disseminated HIV-1 infection in vivo into SCID mice implanted with pieces of human fetal thymus and liver (thy/liv-SCID-hu mice) (64). We postulated that JR-CSF mouse monocytes that carry an integrated long terminal repeat-regulated HIV-1 provirus could be used to recapitulate the behavior of HIV-1-infected monocytes and enable us to study the process of in vivo migration of HIV-1-infected monocytes across the BBB into the brain. To establish a system where these migrant cells could be detected in the brains of recipient mice by PCR and fluorescence microscopy, we crossed the JR-CSF mice with R26-EYFP mice, which carry an EYFP gene under the control of the ubiquitously expressed ROSA26 promoter, to generate JR-CSF/EYFP mice which express both transgenes. To determine whether monocytes expressing HIV-1 proteins displayed an increased capacity to cross the intact BBB, JR-CSF/EYFP or R26-EYFP mouse monocytes (5 × 106 cells) were intravenously injected into BALB/c SCID mice. Four days after injection of the monocytes, circulating leukocytes were flushed from the blood vessels of the mice by intracardiac injection of PBS, their brains were harvested, the meninges were removed, and their brain DNA was extracted. Carriage of the EYFP transgene permitted us to detect the presence of transferred JR-CSF/EYFP and R26-EYFP mouse monocytes in the brains of the recipient mice by PCR amplification with EYFP-specific primers of DNA extracted from the mouse brains. No EYFP DNA was detected in the brains of any of the injected mice after a single round of PCR. After a second round of PCR amplification, while no EYFP DNA was detected by PCR in any of the 10 BALB/c SCID mice injected with R26-EYFP mouse monocytes, EYFP DNA was detected by PCR in 3 of 10 BALB/c SCID mice injected with JR-CSF/EYFP mouse monocytes (Fig. 1). Analysis of the brain DNA with PCR amplification using exonuclease-specific primers demonstrated that comparable levels of DNA were present in the brain samples analyzed. Variability in detection of EYFP DNA in the peripheral blood likely reflects the differential rate of in vivo clearance of the injected cells. These results indicated that HIV-1-infected monocytes display an increased capacity to cross an intact BBB and enter the brain.
FIG. 1.
JR-CSF/EYFP monocytes migrate into the intact brain. DNA was extracted from brains, spleens, and blood of SCID mice 4 days after intravenous injection with either the EYFP monocytes or JR-CSF/EYFP monocytes. DNA extracted from an EYFP mouse or a WT mouse provide the positive or negative control, respectively. PCR amplification products from two rounds of PCR using primers specific for the EYFP gene or one round of PCR using mouse exonuclease gene-specific primers were resolved on an agarose gel containing ethidium bromide. Quantitative analysis of the EYFP-specific bands was done to determine the relative number of migrated cells in the brain. The number shown is the density of EYFP-specific bands normalized to the density of the exonuclease-specific bands in the indicated mouse brain. (A) BALB/c mice injected with EYFP mouse monocytes. (B) BALB/c mice injected with JR-CSF/EYFP mouse monocytes.
JR-CSF/EYFP mouse monocytes and not control R26-EYFP mouse monocytes cross the BBB of LPS-treated BALB/c mice.
Treatment of mice with a low dose of LPS (3 mg/kg) partially compromises the integrity of the BBB without disrupting the capillary structure (9, 69), and this approach has been used to investigate the in vivo capacity of factors to modulate BBB permeability (1, 63). We postulated that LPS-mediated compromise of the BBB is relevant to HIV-1 infection because the BBB in HIV-1-infected individuals may be partially compromised in a similar manner by their increased levels of LPS in plasma (11). Therefore, we examined whether the JR-CSF/EYFP mouse monocytes that support HIV-1 replication displayed an increased capacity to migrate across a BBB whose integrity was partially compromised by LPS and enter the brain. Three hours after BALB/c mice were intraperitoneally injected with LPS (3 mg/kg), the mice were intravenously injected with either R26-EYFP or JR-CSF/EYFP mouse monocytes (5 × 106 cells). Four days later, the mouse brains were harvested and the DNA was extracted and analyzed with a single round of PCR with EYFP-specific primers (Fig. 2). We did not detect passage of R26-EYFP mouse monocytes into the brains of any of the 15 BALB/c mice injected with R26-EYFP mouse monocytes. In contrast, we detected migration of JR-CSF/EYFP mouse monocytes into the brains of 4 of 15 BALB/c mice injected with JR-CSF/EYFP mouse monocytes. These results indicated that monocytes carrying the JR-CSF provirus displayed an increased capacity to cross a BBB whose integrity was partially compromised by LPS treatment.
FIG. 2.
Increased migration of JR-CSF/EYFP mouse monocytes into the brains of LPS-injected BALB/c mice. DNA was extracted from brains of LPS-treated BALB/c mice 4 days after intravenous injection with either EYFP monocytes (n = 15 mice) or JR-CSF/EYFP monocytes (n = 15 mice), and the presence of EYFP DNA was detected by a single round of PCR amplification using primers specific for the EYFP gene. DNA extracted from an EYFP mouse or a WT mouse provided the positive or negative control, respectively. The percentage of BALB/c mice in which EYFP DNA was detected after injection with either EYFP mouse monocytes or JR-CSF/EYFP mouse monocytes is shown.
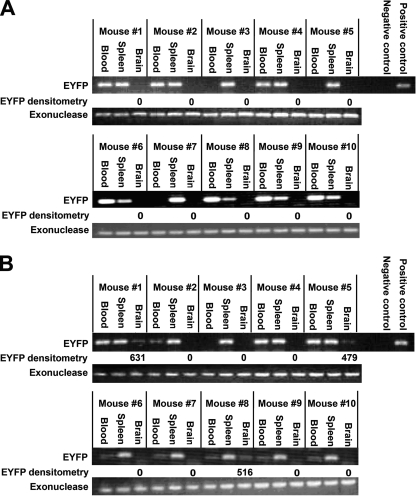
The BBB in JR-CSF mice is more sensitive to disruption by systemic LPS.
We previously demonstrated that HIV-1 replication occurs in the brains of JR-CSF mice (65). We used these mice to examine whether systemic viremia and local production of HIV-1 proteins was associated with increased sensitivity of the BBB to disruption by LPS treatment, as indicated by increased migration of monocytes from the peripheral circulation into the brain. JR-CSF mice or BALB/c mice were intraperitoneally injected with LPS (3 mg/kg), and 3 h later the mice were injected with monocytes (5 × 106 cells) isolated from JR-CSF/EYFP mice. After 4 days, the injected mice were sacrificed and their peripheral blood, spleens, and brains were analyzed for the presence of transferred monocytes by EYFP-specific PCR. PCR analysis of the brains of the LPS-treated BALB/c mice injected with JR-CSF/EYFP mouse monocytes demonstrated passage of JR-CSF/EYFP mouse monocytes into the brains of 3 out of 10 injected BALB/c mice (Fig. 3A). In contrast, we detected passage of JR-CSF/EYFP mouse monocytes into the brains of 7 out of 10 injected JR-CSF mice (Fig. 3B). The relative number of JR-CSF/EYFP mouse monocytes in the brains of the injected BALB/c or JR-CSF mice was determined by comparing the intensity of the EYFP-PCR-amplified product detected in mouse brains normalized to the intensity of the exonuclease-specific PCR products of the brain DNA by densitometric analysis. Densitometric analysis of the PCR products detected in the mouse brains where JR-CSF mouse monocytes entered indicated that threefold-higher numbers of JR-CSF/EYFP mouse monocytes penetrated the BBB of JR-CSF mice (mean = 1,178) than the BBB of BALB/c mice (mean = 373).
FIG. 3.
Increased migration of JR-CSF/EYFP mouse monocytes into the brains of LPS-injected JR-CSF mice. JR-CSF mice and BALB/c mice were treated with LPS (3 mg/kg) and then intravenously injected with JR-CSF/EYFP monocytes (5 × 106 cells). Four days later, DNA was extracted from the mouse brains, spleens, and peripheral blood leukocytes. PCR products amplified by a single round using primers specific for the EYFP gene or mouse exonuclease gene were resolved on an agarose gel containing ethidium bromide. Densitometric analysis of the EYFP-specific bands normalized to the exonuclease-specific bands was performed to determine the relative number of migrated monocytes. (A) PCR analysis of JR-CSF/EYFP mouse monocytes injected into BALB/c mice. (B) PCR analysis of JR-CSF/EYFP mouse monocytes injected into JR-CSF mice. (C) Detection by immunofluorescence microscopy of JR-CSF/EYFP mouse monocytes in the brains of injected JR-CSF mice (n = 3 mice). A representative image from a section cut through the anterior cingulate gyrus of each mouse brain is shown. EYF, enhanced yellow fluorescence; DAPI, 4′,6-diamidino-2-phenylindole.
Migration of the injected JR-CSF/EYFP monocytes across the BBB into the brain parenchyma was visualized by examining 20 coronal slices from the brains of control uninjected JR-CSF mice and the brains of LPS-treated JR-CSF mice 4 days after intravenous injection with JR-CSF/EYFP monocytes. The expression of EYFP permitted highly sensitive and specific visualization and localization of the fluorescent JR-CSF/EYFP mouse monocytes in the parenchyma of the brains. Fluorescent cells were not detected in the coronal sections from the brains of uninjected mice, ruling out the presence of cells displaying nonspecific fluorescence. In contrast, several EYFP-expressing cells were observed in the parenchyma of JR-CSF mice injected with JR-CSF/EYFP monocytes. Representative photomicrographs of sections from three JR-CSF mice evaluated by fluorescence microscopy are shown in Fig. 3C. Taken together, these results indicate that HIV-1-infected monocytes migrated across the BBB into the brain parenchyma, where they could potentially introduce HIV-1 infection and initiate neurotoxic processes.
Cerebral blood vessels in LPS-treated JR-CSF mice display increased disruption of BBB-specific cerebral vascular tight junctions.
We investigated whether the functional evidence for increased compromise of BBB integrity indicated by the passage of monocytes into the brains of JR-CSF mice correlated with anatomical evidence of increased disruption of the BBB. The BBB is composed of tight junctions between epithelial cells formed by the interaction of continuous intramembranous strands of several proteins, including ZO-1 and occludin (20). Anatomical compromise of the BBB due to HIV-1 infection could be detected by visualizing breaks in the intramembranous strands by immunohistological analysis of ZO-1 and occludin in cerebral blood vessels (20). All of the cerebral blood vessels in untreated WT and JR-CSF mice displayed strong and continuous interendothelial patterns of equivalent intensity of occludin and ZO-1, indicating the presence of an intact BBB (Fig. 4). To determine if systemic treatment of mice with LPS more severely compromised the functional activity of the BBB in JR-CSF mice, we compared the effect of peripheral LPS injection on the integrity of ZO-1 and occludins in the cerebral vessels of JR-CSF mice to that for control BALB/c mice. In the brains of LPS-treated BALB/c mice, some cerebral vessels displayed intermittent breaks in occludin and ZO-1 continuity. In contrast, a markedly greater disruption of the anatomical BBB of the cerebral blood vessels in the LPS-treated JR-CSF mice was observed, as indicated by the weak and fragmented expression of occludin and ZO-1 by the majority of cortical blood vessels (Fig. 4A). Semiquantitative measurement of the extent of BBB integrity compromise in the brains of LPS-treated mice was performed as described previously (20) and demonstrated that significantly more vessels (P < 0.02) displayed BBB disruption in JR-CSF mouse brains than in BALB/c mouse brains (Fig. 4B). Taken together, these results indicated that the BBB of JR-CSF mice was more susceptible to disruption by LPS than the BBB of WT mice.
FIG. 4.
Evaluation of BBB barrier integrity in WT or JR-CSF mouse brains by immunostaining for ZO-1 and occludin. Four days after JR-CSF or BALB/c mice (n = 4 mice/group) were treated by intraperitoneal injection with LPS, coronal sections of the brain were analyzed by immunofluorescence. (A) Images of representative mouse brain sections stained for detection of the cell nucleus, ZO-1, and occludin and a merged view are shown in the indicated quadrants. (B) Semiquantitative measurement of the number of cortical blood vessels with weak and fragmented expression of occludin and ZO-1 in the perihippocampal cortex region of the mouse brains (n = 4 mice/group) was performed by analyzing four sections from each mouse brain, and the results are expressed as the average number (± standard errors of the means) of blood vessels with fragmented staining/low-power field (LPF)/mouse brain/group. The fields evaluated were located in similar regions of the cortex for each section.
DISCUSSION
Although HAD does not occur until several years after infection, several lines of evidence have indicated that the entry of HIV-1 into the brain occurs early in the course of infection (33). Antibodies directed to HIV-1 envelope protein were detected in the cerebrospinal fluid of neurologically asymptomatic HIV-1-positive subjects (5, 29), HIV-1 was isolated from the cerebrospinal fluid of asymptomatic HIV-1-positive individuals (17), and HIV-1 viral particles (21) and HIV-1 DNA (2) were detected in the brains of asymptomatic HIV-1-positive subjects. Several observations support the critical role of migration of HIV-1-infected monocytes across the BBB into the brain as the major mechanism by which HIV-1 infection is introduced into the brain (19, 33, 36, 66). First, the phenotype of the majority of HIV-1 isolated from the brain is monocyte tropic (28). Second, HIV-1-infected cells detected in the brains of HIV-1-infected individuals are predominantly from the macrophage lineage (19, 35, 66). Third, primate studies have indicated that the presence of monocyte-tropic strains in the central nervous system (CNS) is required for the development of simian immunodeficiency virus (SIV) encephalitis (4, 34, 59). Fourth, the capacity of brain vessel endothelium to bind monocytes is greatly increased by SIV infection (58). Finally, the perivascular presence of SIV-infected macrophages/microglia is detected in the brains of primates soon after infection with SIV (41). Studies using an in vitro BBB model have demonstrated that greater numbers of HIV-1-infected monocytes than uninfected monocytes passed across a membrane coated with brain microvascular endothelial cells on one side and astrocytes on the other side (24, 52). However, investigation of the mechanism by which HIV-1-infected monocytes migrate across the BBB from the peripheral circulation into the brain has been limited by the absence of an in vivo model of HIV-1 infection.
Although there are differences between the biological behaviors of murine and human mononuclear phagocytes such as microglia and macrophages, mouse models provide informative in vivo systems for studying BBB function and the deleterious effect of HIV-1 on the CNS. Knockout mouse models (55), transgenic mouse models using tissue-specific promoters (14), and experimental mouse models of CNS inflammation (26) have been used to delineate the mechanisms by which cells traffic across the BBB from the periphery and into the brain. Mouse models were developed to study HIV-1-induced neuropathology using SCID mice stereotactically injected with HIV-1-infected monocytes or microglia in their basal ganglia/cortex, and these mice developed neurodegeneration and associated cognitive impairment related to macrophage/microglia activation (48, 50, 51). This mouse model has also been used to evaluate the efficacy of therapies to block HIV-1-induced neurological damage (49). In another mouse model, used to study the pathogenesis of neuro-AIDS, transgenic mice expressing HIV-1 gp120 under the control of the GFAP promoter displayed neuronal and glial changes that resembled the abnormalities observed in the brains of HIV-1-infected humans (62). To investigate the in vivo impact of HIV-1 infection on BBB compromise and the migration of HIV-1-infected monocytes across the BBB, we developed a novel in vivo mouse model consisting of mice transgenic both for the HIV-1 JR-CSF provirus populated with monocytes that produce infectious HIV-1 (13) and for the R26-EYFP transgene whose monocytes express EYFP. These double-transgenic mice provided a source of HIV-1-infected monocytes that can be readily detected by PCR and fluorescence microscopy in the brains of recipient mice after adoptive transfer. To determine whether HIV-1 infection increases the capacity of monocytes to migrate into the brain, we investigated whether JR-CSF mouse monocytes that support HIV-1 replication displayed an increased capacity to migrate across the intact BBB from the systemic circulation into the brain. While there was no detectable migration of monocytes from control R26-EYFP mice across the intact BBB, intravenously injected JR-CSF/EYFP monocytes that express HIV-1 proteins migrated across the intact BBB into some mouse brains. Nevertheless, few JR-CSF/EYFP monocytes initially crossed the intact BBB, as indicated by the requirement to perform two rounds of PCR to detect JR-CSF/EYFP monocytes carrying the HIV-1 provirus in the mouse brains. Production of HIV-1 proteins in infected monocytes may increase their capacity to cross the intact BBB by inducing upregulated expression of cellular adhesion molecules, which increases their adherence to microvascular endothelial cells. Another mechanism by which HIV-1 infection enables monocytes to cross the intact BBB may be related to the expression of surface gp120, which has been shown by in vitro studies to function as a weak lectin that facilitates adsorptive endocytosis (8) and to alter expression of tight junction proteins in brain endothelial cells (32).
The capacity of HIV-1-infected monocytes to enter the brain was increased when the integrity of the BBB was partially compromised by the injection of LPS, particularly in the brains of JR-CSF mice, where the BBB is composed of cells carrying the HIV-1 provirus. The BBB in the JR-CSF mice was more sensitive to disruption by LPS than the BBB of control mice, indicating that HIV-1 infection may increase the sensitivity of the components that form the BBB to becoming compromised by systemic LPS. It is possible that increased susceptibility of the BBB in the JR-CSF mice that support HIV-1 replication to LPS treatment may be related to the capacity of HIV-1-infected monocytes to alter the brain microvascular proteome (56). Our analysis of BBB function carried out by measuring the migration of monocytes from the systemic circulation into the brain was complemented by examination of the integrity of BBB proteins in the cerebral blood vessels. We demonstrated that LPS treatment more potently disrupted the tight junctions constituted by ZO-1 and occludin in the cerebral blood vessels of JR-CSF mice than did LPS treatment of control mice. In concert with increased compromise of the BBB, systemic LPS may stimulate monocyte migration into the brain by stimulating HIV-1-infected resident macrophages and microglia in the brain to secrete MCP-1, the chemokine that most potently induces migration of peripheral monocytes into the brain parenchyma (10). We previously reported that LPS induced more MCP-1 production in vivo in JR-CSF mouse brains and in vitro in JR-CSF mouse microglia than was the case with LPS-stimulated brains and microglia from control BALB/c mice (65). Studies using an in vitro tissue culture BBB model demonstrated that HIV-1-infected monocytes are more responsive to MCP-1-mediated migration than uninfected monocytes (24). Our demonstration that the intravenously injected JR-CSF/EYFP monocytes were visualized in the parenchyma of the brain complemented our functional analysis of monocyte migration by PCR to confirm that HIV-1-infected monocytes transmigrated across the BBB and entered the parenchyma of the brain. The combination of compromising the BBB and inducing chemokine production that recruits monocytes into the brain may contribute to the increased migration of monocytes into the brains of LPS-injected JR-CSF mice compared to results with the brains of LPS-injected control mice. These complementary mechanisms may also be functioning in HIV-1-infected individuals to facilitate the migration of HIV-1-infected monocytes into their brains. Treatment with highly active antiretroviral therapy (HAART) has markedly reduced the incidence of neurological manifestations of HIV-1 infection (40). Treatment with HAART also reduces the elevated systemic levels of LPS present in HIV-1-infected individuals (11). An intriguing possibility is that HAART may lower the incidence of HAD not only by reducing HIV-1 replication but also by reducing systemic levels of LPS and thereby reducing compromise of the BBB and migration of HIV-1-infected monocytes from the systemic circulation into the brain.
The results of this study demonstrate that carriage of the HIV-1 provirus by monocytes increased their ability to enter the brain, particularly after partial compromise of the BBB in response to LPS treatment, and indicate the applicability of these mice as an in vivo system to study the mechanism by which HIV-1-infected monocytes migrate into the brain and how these migrated cells subsequently cause CNS damage. A major advantage of our transgenic system is that it lends itself to application of the powerful tool of manipulating mouse genetics for the study of neuro-AIDS by crossing the JR-CSF mice with mice that either carry gene deletions or are transgenic for factors associated with passage of monocytes across the BBB, enabling us to identify genes whose expression plays a role in HIV-1-mediated disruption of the BBB. Furthermore, we plan to extend these studies to another transgenic mouse line we have generated, JR-CSF/hu-cycT1 mice, which display increased HIV-1 replication due to their capacity to support HIV-1 Tat function because of their transgenic expression of human cyclin T1 (61). This in vivo mouse model would also permit examination of the capacities of various therapies to protect the BBB from compromise by determining the effects of candidate treatments on preventing or decreasing the migration of HIV-1-infected monocytes across the BBB. These future studies should contribute to our understanding of the role of the immune system and other in vivo factors in controlling traffic of HIV-1-infected monocytes across the BBB and their role in compromising subsequent BBB function.
Acknowledgments
This work was supported by the National Institutes of Health (National Institute of Neurological Disorders and Stroke, grant NS39201, National Institute of Allergy and Infectious Diseases, grant AI067136, and the Center for AIDS Research, grant AI51519).
Footnotes
Published ahead of print on 28 May 2008.
REFERENCES
- 1.Ahishali, B., M. Kaya, R. Kalayci, H. Uzun, B. Bilgic, N. Arican, I. Elmas, S. Aydin, and M. Kucuk. 2005. Effects of lipopolysaccharide on the blood-brain barrier permeability in prolonged nitric oxide blockade-induced hypertensive rats. Int. J. Neurosci. 115151-168. [DOI] [PubMed] [Google Scholar]
- 2.An, S. F., B. Giometto, and F. Scaravilli. 1996. HIV-1 DNA in brains in AIDS and pre-AIDS: correlation with the stage of disease. Ann. Neurol. 40611-617. [DOI] [PubMed] [Google Scholar]
- 3.An, S. F., M. Groves, B. Giometto, A. A. Beckett, and F. Scaravilli. 1999. Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol. (Berlin) 98481-487. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, M. G., D. Hauer, D. P. Sharma, S. V. Joag, O. Narayan, M. C. Zink, and J. E. Clements. 1993. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology 195616-626. [DOI] [PubMed] [Google Scholar]
- 5.Andersson, M. A., T. B. Bergstrom, C. Blomstrand, S. H. Hermodsson, C. Hakansson, and G. B. Lowhagen. 1988. Increasing intrathecal lymphocytosis and immunoglobulin G production in neurologically asymptomatic HIV-1 infection. J. Neuroimmunol. 19291-304. [DOI] [PubMed] [Google Scholar]
- 6.Antinori, A., G. Arendt, J. T. Becker, B. J. Brew, D. A. Byrd, M. Cherner, D. B. Clifford, P. Cinque, L. G. Epstein, K. Goodkin, M. Gisslen, I. Grant, R. K. Heaton, J. Joseph, K. Marder, C. M. Marra, J. C. McArthur, M. Nunn, R. W. Price, L. Pulliam, K. R. Robertson, N. Sacktor, V. Valcour, and V. E. Wojna. 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 691789-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10573-585. [DOI] [PubMed] [Google Scholar]
- 8.Banks, W. A., A. J. Kastin, and V. Akerstrom. 1997. HIV-1 protein gp120 crosses the blood-brain barrier: role of adsorptive endocytosis. Life Sci. 61PL119-PL125. [DOI] [PubMed] [Google Scholar]
- 9.Banks, W. A., A. J. Kastin, J. M. Brennan, and K. L. Vallance. 1999. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp. Neurol. 156165-171. [DOI] [PubMed] [Google Scholar]
- 10.Bell, M. D., D. D. Taub, and V. H. Perry. 1996. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience 74283-292. [DOI] [PubMed] [Google Scholar]
- 11.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 12.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 9414637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browning Paul, J., E. J. Wang, M. Pettoello-Mantovani, C. Raker, S. Yurasov, M. M. Goldstein, J. W. Horner, J. Chan, and H. Goldstein. 2000. Mice transgenic for monocyte-tropic HIV type 1 produce infectious virus and display plasma viremia: a new in vivo system for studying the postintegration phase of HIV replication. AIDS Res. Hum. Retrovir. 16481-492. [DOI] [PubMed] [Google Scholar]
- 14.Bush, T. G., N. Puvanachandra, C. H. Horner, A. Polito, T. Ostenfeld, C. N. Svendsen, L. Mucke, M. H. Johnson, and M. V. Sofroniew. 1999. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23297-308. [DOI] [PubMed] [Google Scholar]
- 15.Cann, A. J., J. A. Zack, A. S. Go, S. J. Arrigo, Y. Koyanagi, P. L. Green, S. Pang, and I. S. Chen. 1990. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J. Virol. 644735-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case, S. S., M. A. Price, C. T. Jordan, X. J. Yu, L. Wang, G. Bauer, D. L. Haas, D. Xu, R. Stripecke, L. Naldini, D. B. Kohn, and G. M. Crooks. 1999. Stable transduction of quiescent CD34(+)CD38(−) human hematopoietic cells by HIV-1-based lentiviral vectors. Proc. Natl. Acad. Sci. USA 962988-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiodi, F., J. Albert, E. Olausson, G. Norkrans, L. Hagberg, A. Sonnerborg, B. Asjo, and E. M. Fenyo. 1988. Isolation frequency of human immunodeficiency virus from cerebrospinal fluid and blood of patients with varying severity of HIV infection. AIDS Res. Hum. Retrovir. 4351-358. [DOI] [PubMed] [Google Scholar]
- 18.Consiglio, A., A. Gritti, D. Dolcetta, A. Follenzi, C. Bordignon, F. H. Gage, A. L. Vescovi, and L. Naldini. 2004. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc. Natl. Acad. Sci. USA 10114835-14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosenza, M. A., M. L. Zhao, Q. Si, and S. C. Lee. 2002. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 12442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallasta, L. M., L. A. Pisarov, J. E. Esplen, J. V. Werley, A. V. Moses, J. A. Nelson, and C. L. Achim. 1999. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1551915-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis, L. E., B. L. Hjelle, V. E. Miller, D. L. Palmer, A. L. Llewellyn, T. L. Merlin, S. A. Young, R. G. Mills, W. Wachsman, and C. A. Wiley. 1992. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 421736-1739. [DOI] [PubMed] [Google Scholar]
- 22.Douek, D. 2007. HIV disease progression: immune activation, microbes, and a leaky gut. Top. HIV Med. 15114-117. [PubMed] [Google Scholar]
- 23.Epstein, L. G., and H. E. Gendelman. 1993. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann. Neurol. 33429-436. [DOI] [PubMed] [Google Scholar]
- 24.Eugenin, E. A., K. Osiecki, L. Lopez, H. Goldstein, T. M. Calderon, and J. W. Berman. 2006. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 261098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3553-564. [PMC free article] [PubMed] [Google Scholar]
- 26.Ford, M. L., T. M. Onami, A. I. Sperling, R. Ahmed, and B. D. Evavold. 2003. CD43 modulates severity and onset of experimental autoimmune encephalomyelitis. J. Immunol. 1716527-6533. [DOI] [PubMed] [Google Scholar]
- 27.Gendelman, H. E., Y. Persidsky, A. Ghorpade, J. Limoges, M. Stins, M. Fiala, and R. Morrisett. 1997. The neuropathogenesis of the AIDS dementia complex. AIDS 11S35-S45. [PubMed] [Google Scholar]
- 28.Gorry, P. R., G. Bristol, J. A. Zack, K. Ritola, R. Swanstrom, C. J. Birch, J. E. Bell, N. Bannert, K. Crawford, H. Wang, D. Schols, E. De Clercq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 7510073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goudsmit, J., E. C. Wolters, M. Bakker, L. Smit, J. Van der Noordaa, E. A. Hische, J. A. Tutuarima, and H. J. Van der Helm. 1986. Intrathecal synthesis of antibodies to HTLV-III in patients without AIDS or AIDS related complex. Br. Med. J. (Clin. Res. ed.) 2921231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halene, S., L. Wang, R. M. Cooper, D. C. Bockstoce, P. B. Robbins, and D. B. Kohn. 1999. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood 943349-3357. [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, S. H., and A. Y. Jong. 2001. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell Microbiol. 3277-287. [DOI] [PubMed] [Google Scholar]
- 32.Kanmogne, G. D., K. Schall, J. Leibhart, B. Knipe, H. E. Gendelman, and Y. Persidsky. 2007. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab. 27123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaul, M., G. A. Garden, and S. A. Lipton. 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410988-994. [DOI] [PubMed] [Google Scholar]
- 34.Kodama, T., K. Mori, T. Kawahara, D. J. Ringler, and R. C. Desrosiers. 1993. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J. Virol. 676522-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenig, S., H. E. Gendelman, J. M. Orenstein, M. C. Dal Canto, G. H. Pezeshkpour, M. Yungbluth, F. Janotta, A. Aksamit, M. A. Martin, and A. S. Fauci. 1986. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 2331089-1093. [DOI] [PubMed] [Google Scholar]
- 36.Kolson, D. L., E. Lavi, and F. Gonzalez-Scarano. 1998. The effects of human immunodeficiency virus in the central nervous system. Adv. Virus Res. 501-47. [DOI] [PubMed] [Google Scholar]
- 37.Kure, K., J. F. Llena, W. D. Lyman, R. Soeiro, K. M. Weidenheim, A. Hirano, and D. W. Dickson. 1991. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum. Pathol. 22700-710. [DOI] [PubMed] [Google Scholar]
- 38.Kure, K., W. D. Lyman, K. M. Weidenheim, and D. W. Dickson. 1990. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am. J. Pathol. 1361085-1092. [PMC free article] [PubMed] [Google Scholar]
- 39.Lai, L., N. Alaverdi, L. Maltais, and H. C. Morse III. 1998. Mouse cell surface antigens: nomenclature and immunophenotyping. J. Immunol. 1603861-3868. [PubMed] [Google Scholar]
- 40.Langford, T. D., S. L. Letendre, G. J. Larrea, and E. Masliah. 2003. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 13195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luabeya, M. K., L. M. Dallasta, C. L. Achim, C. D. Pauza, and R. L. Hamilton. 2000. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 26454-462. [DOI] [PubMed] [Google Scholar]
- 42.McArthur, J. C., D. R. Hoover, H. Bacellar, E. N. Miller, B. A. Cohen, J. T. Becker, N. M. Graham, J. H. McArthur, O. A. Selnes, L. P. Jacobson, et al. 1993. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 432245-2252. [DOI] [PubMed] [Google Scholar]
- 43.Navia, B. A., B. D. Jordan, and R. W. Price. 1986. The AIDS dementia complex. I. Clinical features. Ann. Neurol. 19517-524. [DOI] [PubMed] [Google Scholar]
- 44.Nottet, H. S., and H. E. Gendelman. 1995. Unraveling the neuroimmune mechanisms for the HIV-1-associated cognitive/motor complex. Immunol. Today 16441-448. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien, W. A., Y. Koyanagi, A. Namazie, J. Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 34869-73. [DOI] [PubMed] [Google Scholar]
- 46.Osiecki, K., L. Xie, J. H. Zheng, R. Squires, M. Pettoello-Mantovani, and H. Goldstein. 2005. Identification of granulocyte-macrophage colony-stimulating factor and lipopolysaccharide-induced signal transduction pathways that synergize to stimulate HIV type 1 production by monocytes from HIV type 1 transgenic mice. AIDS Res. Hum. Retrovir. 21125-139. [DOI] [PubMed] [Google Scholar]
- 47.Pardridge, W. M. 1999. Blood-brain barrier biology and methodology. J. Neurovirol. 5556-569. [DOI] [PubMed] [Google Scholar]
- 48.Persidsky, Y., and H. E. Gendelman. 2003. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 74691-701. [DOI] [PubMed] [Google Scholar]
- 49.Persidsky, Y., and H. E. Gendelman. 2002. Murine models for human immunodeficiency virus type 1-associated dementia: the development of new treatment testing paradigms. J. Neurovirol. 8(Suppl. 2)49-52. [DOI] [PubMed] [Google Scholar]
- 50.Persidsky, Y., A. Ghorpade, J. Rasmussen, J. Limoges, X. J. Liu, M. Stins, M. Fiala, D. Way, K. S. Kim, M. H. Witte, M. Weinand, L. Carhart, and H. E. Gendelman. 1999. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1551599-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persidsky, Y., J. Limoges, R. McComb, P. Bock, T. Baldwin, W. Tyor, A. Patil, H. S. Nottet, L. Epstein, H. Gelbard, E. Flanagan, J. Reinhard, S. J. Pirruccello, and H. E. Gendelman. 1996. Human immunodeficiency virus encephalitis in SCID mice. Am. J. Pathol. 1491027-1053. [PMC free article] [PubMed] [Google Scholar]
- 52.Persidsky, Y., M. Stins, D. Way, M. H. Witte, M. Weinand, K. S. Kim, P. Bock, H. E. Gendelman, and M. Fiala. 1997. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J. Immunol. 1583499-3510. [PubMed] [Google Scholar]
- 53.Persidsky, Y., J. Zheng, D. Miller, and H. E. Gendelman. 2000. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol. 68413-422. [PubMed] [Google Scholar]
- 54.Petito, C. K., and K. S. Cash. 1992. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann. Neurol. 32658-666. [DOI] [PubMed] [Google Scholar]
- 55.Petitto, J. M., Z. Huang, J. Lo, and W. J. Streit. 2003. IL-2 gene knockout affects T lymphocyte trafficking and the microglial response to regenerating facial motor neurons. J. Neuroimmunol. 13495-103. [DOI] [PubMed] [Google Scholar]
- 56.Ricardo-Dukelow, M., I. Kadiu, W. Rozek, J. Schlautman, Y. Persidsky, P. Ciborowski, G. D. Kanmogne, and H. E. Gendelman. 2007. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J. Neuroimmunol. 18537-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin, L. L., and J. M. Staddon. 1999. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 2211-28. [DOI] [PubMed] [Google Scholar]
- 58.Sasseville, V. G., W. Newman, S. J. Brodie, P. Hesterberg, D. Pauley, and D. J. Ringler. 1994. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am. J. Pathol. 14427-40. [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma, D. P., M. C. Zink, M. Anderson, R. Adams, J. E. Clements, S. V. Joag, and O. Narayan. 1992. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J. Virol. 663550-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivas, S., T. Watanabe, C. S. Lin, C. M. William, Y. Tanabe, T. M. Jessell, and F. Costantini. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun, J., T. Soos, V. N. Kewalramani, K. Osiecki, J. H. Zheng, L. Falkin, L. Santambrogio, D. R. Littman, and H. Goldstein. 2006. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 801850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toggas, S. M., E. Masliah, E. M. Rockenstein, G. F. Rall, C. R. Abraham, and L. Mucke. 1994. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367188-193. [DOI] [PubMed] [Google Scholar]
- 63.Veszelka, S., Z. Urbanyi, T. Pazmany, L. Nemeth, I. Obal, N. T. Dung, C. S. Abraham, G. Szabo, and M. A. Deli. 2003. Human serum amyloid P component attenuates the bacterial lipopolysaccharide-induced increase in blood-brain barrier permeability in mice. Neurosci. Lett. 35257-60. [DOI] [PubMed] [Google Scholar]
- 64.Wang, E. J., M. Pettoello-Mantovani, C. M. Anderson, K. Osiecki, D. Moskowitz, and H. Goldstein. 2002. Development of a novel transgenic mouse/SCID-hu mouse system to characterize the in vivo behavior of reservoirs of human immunodeficiency virus type 1-infected cells. J. Infect. Dis. 1861412-1421. [DOI] [PubMed] [Google Scholar]
- 65.Wang, E. J., J. Sun, M. Pettoello-Mantovani, C. M. Anderson, K. Osiecki, M. L. Zhao, L. Lopez, S. C. Lee, J. W. Berman, and H. Goldstein. 2003. Microglia from mice transgenic for a provirus encoding a monocyte-tropic HIV type 1 isolate produce infectious virus and display in vitro and in vivo upregulation of lipopolysaccharide-induced chemokine gene expression. AIDS Res. Hum. Retrovir. 19755-765. [DOI] [PubMed] [Google Scholar]
- 66.Wiley, C. A., C. L. Achim, C. Christopherson, Y. Kidane, S. Kwok, E. Masliah, J. Mellors, L. Radhakrishnan, G. Wang, and V. Soontornniyomkij. 1999. HIV mediates a productive infection of the brain. AIDS 132055-2059. [DOI] [PubMed] [Google Scholar]
- 67.Williams, K. C., and W. F. Hickey. 2002. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu. Rev. Neurosci. 25537-562. [DOI] [PubMed] [Google Scholar]
- 68.Wu, D. T., S. E. Woodman, J. M. Weiss, C. M. McManus, T. G. D'Aversa, J. Hesselgesser, E. O. Major, A. Nath, and J. W. Berman. 2000. Mechanisms of leukocyte trafficking into the CNS. J. Neurovirol. 6(Suppl. 1)S82-S85. [PubMed] [Google Scholar]
- 69.Xaio, H., W. A. Banks, M. L. Niehoff, and J. E. Morley. 2001. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 89636-42. [DOI] [PubMed] [Google Scholar]
- 70.Zhou, H., B. M. Lapointe, S. R. Clark, L. Zbytnuik, and P. Kubes. 2006. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 1778103-8110. [DOI] [PubMed] [Google Scholar]