Abstract
Recent studies demonstrated that viremia and extraintestinal rotavirus infection are common in acutely infected humans and animals, while systemic diseases appear to be rare. Intraperitoneal infection of newborn mice with rhesus rotavirus (RRV) results in biliary atresia (BA), and this condition is influenced by the host interferon response. We studied orally inoculated 5-day-old suckling mice that were deficient in interferon (IFN) signaling to evaluate the role of interferon on the outcome of local and systemic infection after enteric inoculation. We found that systemic replication of RRV, but not murine rotavirus strain EC, was greatly enhanced in IFN-α/β and IFN-γ receptor double-knockout (KO) or STAT1 KO mice but not in mice deficient in B- or T-cell immunity. The enhanced replication of RRV was associated with a lethal hepatitis, pancreatitis, and BA, while no systemic disease was observed in strain EC-infected interferon-deficient mice. In IFN-α/β receptor KO mice the extraintestinal infection and systemic disease were only moderately increased, while RRV infection was not augmented and systemic disease was not present in IFN-γ receptor KO mice. The increase of systemic infection in IFN-deficient mice was also observed during simian strain SA11 infection but not following bovine NCDV, porcine OSU, or murine strain EW infection. Our data indicate that the requirements for the interferon system to inhibit intestinal and extraintestinal viral replication in suckling mice vary among different heterologous and homologous rotavirus strains, and this variation is associated with lethal systemic disease.
Group A rotaviruses have a segmented double-stranded RNA genome and are members of the Reoviridae family. The virus replicates in mature epithelial cells on the tips of small intestinal villi and causes diarrhea in humans and many other animal species (30). In infants and young children rotavirus-induced severe dehydrating diarrhea results in more than 600,000 deaths annually, mostly in developing countries, and over 2 million hospitalizations each year (45). Rotavirus infection was traditionally considered to be limited to the small intestine; however, recent studies have demonstrated that viremia and extraintestinal infection occur frequently, in both humans and animals (8).
Despite the presence of viremia and extraintestinal infection, rotavirus-induced systemic disease appears to be uncommon. There are reported cases of encephalopathy, myocarditis, hepatitis, pancreatitis, and pneumonia in children with rotavirus infection (11, 14, 22, 24, 25, 31-33, 42, 53, 59), but the causal relation between the rotavirus infection and these manifestations was not established. It has been suggested that rotavirus infection increases the risk of type 1 diabetes by inducing an autoimmune response (26, 34), but others found no increased risk after infection (7). In a murine model, intraperitoneal (i.p.) inoculation of the rhesus rotavirus (RRV) strain into newborn wild-type or severely immune-deficient SCID mice resulted in biliary atresia (BA) and hepatitis (46, 50, 54). Interestingly, these conditions have not been reported in mice infected with homologous murine rotavirus, which replicates most efficiently in mouse intestine, nor other heterologous nonmurine rotavirus strains (1, 54). It has also been reported that SCID children with chronic rotavirus infections show signs of hepatitis, presumably associated with homologous human rotavirus infection (44). It appears that both the virus genetic makeup and the host immune response can play important roles in determining the outcome of systemic rotavirus infection. For instance, the RRV nonstructural protein, NSP3, has been linked to the ability of the virus to spread beyond the intestinal barrier to the mouse liver (41).
The immune determinants of resistance to repeat rotavirus infection and resolution of primary infection have been extensively studied in mice. Both CD8+ T cells and antirotavirus antibody are able to clear primary viral infection in the intestines, but T cells mediate this effect more rapidly (20, 39). Antibody responses, especially the mucosal immunoglobulin A response, as well as CD4+ and CD8+ T cells, are all involved in protection against reinfection, with mucosal antibodies being the primary effectors of protection after natural infection or live viral vaccination (17, 21, 40).
The role of the innate immune system, including the interferon (IFN) response in rotavirus infection, is less well studied. It has been shown that levels of type I and II interferons are elevated in rotavirus-infected children and animals (3, 13, 29, 48, 56). Both type I and II interferons are able to limit rotavirus infection in vitro, if cells are pretreated (6). In addition, in early studies, IFN-α was used to successfully treat rotavirus diarrhea in bovine and porcine models (37, 56). In suckling mice, however, IFNs appear to have little if any effect on the course of diarrhea or virus shedding during rotavirus infection (2, 55). On the other hand, it has recently been shown that rotavirus nonstructural protein NSP1 can interact with interferon regulatory factors 3 and 7 (IRF3 and IRF7) and enhance their degradation (4, 5). These observations suggest that rotaviruses have evolved mechanisms to circumvent interferon's antiviral activity and therefore imply that interferon plays an antiviral role during infection.
There is currently little information that specifically addresses the immune mechanisms involved in restricting systemic rotavirus infection. Since RRV only induces systemic disease in newborn or severely immune-deficient mice, it seems likely that immune mechanisms are necessary to control systemic infection, but it is not clear which components mediate this control. Of note, exogenous IFN-α is effective in preventing and treating biliary and liver disease in RRV-infected newborn mice (47), suggesting that the innate immune response may be important for control of systemic RRV infection. However, Shivakumar et al. presented data indicating that IFN-γ plays a role in enhancing inflammation during RRV-induced biliary atresia, suggesting that IFN also contributes to the pathology during systemic infection (52).
Using IFN signaling-deficient mouse models, we demonstrated that oral RRV infection in suckling mice could result in a prolonged disseminated infection in multiple extraintestinal organs, including liver, bile duct, pancreas, and mesenteric lymph nodes (MLN) and a lethal systemic disease. Systemic disease following infection with other heterologous virus strains was either mild (SA11) or not present (NCDV and OSU). Deficiency in IFN signaling did not affect enteric or systemic replication of the murine rotavirus EC strain, but systemic replication of EW murine virus was augmented slightly. It appears that the host type I and II IFN responses play an important roles in limiting intestinal and extraintestinal infection of some rotavirus strains; however, this effect varies significantly among different strains.
MATERIALS AND METHODS
Virus.
Simian rotaviruses RRV and SA11, bovine strain NCDV, and porcine strain OSU were propagated in the green monkey kidney MA104 cell line. Stock virus titers were determined by plaque assay in MA104 cells and expressed as PFU per ml as described elsewhere (27), and these stocks were used as tissue culture homogenates for infection. The 50% diarrheal dose (DD50) RRV in suckling BALB/c, 129SV, and C57BL/6 mice is 105 PFU/suckling mouse (reference 43 and unpublished data). The DD50 of NCDV and OSU is approximately 106 PFU/suckling mouse. Of note, at the maximal dose level used in this study, although all mice develop diarrhea, unlike RRV and SA11 infection few mice develop a detectable immune response to NCDV or OSU (18). Murine rotavirus EC and EW are virulent wild-type strains that are not tissue culture adapted. The in vivo propagation and infectivity determination of these strains were described previously (10). Briefly, intestines from EC- or EW-infected suckling BALB/c mice were collected and homogenized. Centrifugation-clarified intestinal homogenate was used as virus stock for this study. The infectivity of the murine strains was determined in vivo in suckling BALB/c mice and expressed as the DD50. The infectivity of murine virus was similar in suckling C57/BL6 and 129SV mice (unpublished data).
Mice and oral rotavirus infection.
IFN-α/β receptor knockout mice (IFN-αR KO), interferon α/β and IFN- γ receptor double knockout mice (IFN-αγR KO), and wild-type control 129SV mice were the generous gift of H. Virgin (38). IFN-γ receptor knockout mice (IFN-γR KO), which were on a 129SV background and β2m and Rag1 KO mice, which were on a C57/BL6 background, were purchased from the Jackson Laboratory (Bar Harbor, ME). STAT1 knockout mice (STAT1 KO) mice, which were also on a 129SV background, were purchased from Taconic (Germantown, NY). JHD−/− mice were originally obtained from Genpharm International (Mountain View, CA) (21). All mice were maintained in the Veterinary Medical Unit of the Palo Alto VA Health Care System. All animal studies were approved by the Stanford Institutional Animal Care Committee.
Five-day-old suckling mice were orally inoculated with 2 × 107 PFU of RRV, SA11, NCDV, or OSU (102 DD50) or 104 DD50 of the murine strain EC or EW, and subsequent diarrhea was monitored as described elsewhere (10). The appearance of systemic disease, manifested as lethargy, oily fur, weight loss, and white stools, was recorded. At different times postinfection mice were sacrificed and tissues collected for virus detection and quantification and histology.
Virus titration by plaque assay.
At various times postinfection, suckling mice were anesthetized and decapitated to remove blood and the small intestine, liver, and extrahepatic bile duct and gall bladder, MLN, and pancreas were collected and stored at −70°C. Before assay, twice-freeze-thawed tissue samples were individually weighed and made to 10% (wt/vol) suspensions with media 199 (M199; Gibco/Invitrogen, Grand Island, NY) supplemented with 1,000 U/ml penicillin and 1,000 μg/ml streptomycin without fetal calf serum. Samples were homogenized with a tuberculin syringe and clarified by centrifugation at 17,000 × g for 10 min. The clarified supernatants were used for virus titration on MA104 cells. The plaque assay for virus titration on MA104 cells was described previously (27). Briefly, supernatants from tissue homogenates were activated with trypsin (5 μg/ml; Sigma-Aldrich, St. Louis, MO), serially diluted in M199 without fetal calf serum, and added to six-well plates of MA104 cells. After absorption for 45 min at 37°C in a 5% CO2 incubator, plates were washed with M199 and overlaid with 0.55% SeaKem ME agarose (Cambrex Bio Science, Rockland, ME) in M199 with trypsin (0.5 μg/ml). After 4 days of incubation at 37°C in 5% CO2, plates were stained with neutral red (Sigma-Aldrich). The virus plaques were enumerated and virus titers were expressed as PFU per gram of tissue.
ssQRT-PCR assay.
A single-strand-specific quantitative reverse transcription-PCR (ssQRT-PCR) to detect the presence of noncultivatable EC and EW was described previously (16). Briefly, tissues were collected at different days postinfection and immediately frozen. Frozen tissue samples were individually weighed and total RNA extracted. An ssRT reaction for either positive- or negative-sense RNA was performed separately with primers specific for the EC, EW, and RRV NSP3 gene. Quantitative PCR on the strand-specific RT products was performed with the 7900HS sequence detection system from Applied Biosystems (Foster City, CA) using a QuantiTect SYBR green RT-PCR kit (Qiagen, Inc., Valencia, CA). Cloned virus NSP3 plasmids were used for standards, and extracted virus RNA was used to correct for the differential in the (+) and (−) strand reactions. The levels of virus were expressed as viral RNA minus-strand copy number per milligram of tissue.
Histology and immunohistology.
The small intestine, liver, bile duct, and pancreas of infected wild-type and IFN-αγR KO mice were collected and fixed in 10% formalin for hematoxylin and eosin staining, which was performed by Histo-Tec Laboratory (Hayward, CA). Pathology changes were observed by conventional microscopy, and images were acquired with a Nikon digital camera. For immunofluorescence staining, various tissue samples including liver, bile duct, and pancreas were frozen in optimum cutting temperature compound (Sakura Finetechnical Co., Tokyo, Japan). Six-micrometer-thick sections were cut, air dried, and fixed in a cold acetone-methanol mixture (1:1) for 10 min at −20°C. Tissues were stained with Alexa-594-labeled anti-rotavirus VP6 antibody (clone 255/60 or 1E11, which detected all rotavirus strains used in this study) combined with one of the cell marker antibodies, including Alexa-488-labeled anti-K8 (clone Troma I [35]) and unlabeled anti-K19 (clone Troma III [35]), both from Developmental Studies Hybridoma Bank, Iowa City, IA, fluorescein isothiocyanate (FITC)-labeled anti-Gr-1 (clone RB6-8C5; eBioscience, San Diego, CA), FITC-labeled anti-F4/80 (clone BM8; eBioscience), and FITC-labeled anti-CD11c (clone HL3; BD Bioscience, San Jose, CA). A FITC-labeled donkey anti-rat immunoglobulin G antibody was used as secondary antibody for the unlabeled primary rat monoclonal antibody. The antibody mixture also included RNase A (1:500) and TOTO-3 iodide (Molecular Probes, Eugene, OR) for nucleus staining. Slides were mounted with Aqua Poly/Mount (Polysciences, Warrington, PA). The images were acquired with a Zeiss LSM 510 confocal microscope system (Carl Zeiss MicroImaging, Inc., Thornwood, NY), and images were analyzed using an LSM510 computer program. We also stained selected tissue specimens with anti-NSP4 antibody (B4-2) and found that it regularly colocalized with anti-VP6 staining, indicating that VP6-positive cells in this study represent cells undergoing RV transcription and translation (data not shown).
Statistical analysis.
Statview (SAS Institute, Cary, NC) was used for statistical analysis. Rates of diarrhea systemic disease and percentages of virus-positive tissues were analyzed with Fisher's exact test between groups at each time point. Virus levels in tissues, determined by either plaque assay or ssQRT-PCR, were analyzed with the Mann-Whitney test.
RESULTS
Combined deficiency in type I and II interferon responses does not alter RRV- or EC-induced diarrhea but does increase the risk of systemic disease in suckling mice.
Five-day-old wild-type (129SV) and selected interferon receptor and STAT1 KO mice were orally inoculated with 2 × 107 PFU of RRV. Diarrhea appeared 1 day after infection in all groups and resolved between 7 and 8 days postinfection (p.i.) (Fig. 1A). There were no significant differences in the percentage of mice with diarrhea or the duration of diarrhea among any of the groups (P > 0.05).
FIG. 1.
Occurrence of diarrhea and systemic disease in RRV- or EC-infected wild-type mice and selected IFN receptor KO and STAT1 KO mice. Groups of 8 to 16 5-day-old suckling mice were orally infected with rotavirus. Diarrheal disease was observed daily after infection for RRV-infected (A) or EC-infected (C) mice and is presented as the percentage of mice with diarrhea. There was no statistical difference among all RRV-infected groups and between the two EC-infected groups. (B) Percentage of systemic disease in RRV-infected IFN-αγR KO, IFN-αR KO, and STAT1 KO mice (group size, 9 to 16). Disease was not observed in any RRV-infected IFN-γR KO or wild-type mice or in EC-infected mouse strains. Mice with systemic disease had oily fur and growth retardation. Disease was also manifest by white stools and progressed to severe lethargy or death in approximately 80 to 90% of the IFN-αγR KO and STAT1 KO pups by the time of weaning. Disease in the IFN-αR KO group was milder, and all mice recovered. Symbols: ▪, IFN-αγR KO; •, STAT1 KO; *, IFN-αR KO; ⧫, IFN-γR KO; ▴, wild type. +, P < 0.05 by Fisher's exact test between wild-type mice and IFN-αγR KO or STAT1 KO mice.
The IFN-αγR KO and STAT1 KO mice infected with RRV began to develop systemic signs and symptoms of disease characterized by oily fur, growth retardation, and white stools, which had a similar color and consistency to milk pellets in the stomach, from 4 days p.i. This condition was similar to the previously described symptoms of biliary atresia induced by i.p. injection of RRV in newborn mice (46). By 8 days p.i. 85% of IFN-αγR KO and 91% of STAT1 KO mice showed signs of the systemic disease, which further deteriorated into severe lethargy (Fig. 1B). Mortality began by day 8 p.i., but most mortality occurred 15 days p.i. or later, when the mice began to be weaned. Nearly all mice with severe symptoms were either moribund or dead (data not shown). Mice with mild symptoms (such as a short period of white-colored stool or growth delay) eventually recovered. Mortality or severe lethargy and morbidity among the STAT1 and IFN-αγR KO mice were approximately 80 to 90%. In IFN-αR KO mice, about 40% of mice developed a mild, self-limited, nonlethal illness characterized by modest growth retardation and only several days of white-colored stools (Fig. 1B). These symptoms appeared somewhat earlier than in the IFN-αγR KO mice and disappeared by day 6 p.i. Systemic disease was not observed in either wild-type or IFN-γR KO mice (Fig. 1B). Systemic disease was also not observed in 5-day-old B-cell-deficient JHD mice, CD8+ T-cell-deficient β2m KO mice, or T/B-cell-deficient SCID or Rag-1 KO mice after RRV infection. Of note, while 100% of IFN-αγR KO mice and wild-type mice infected with the murine rotavirus strain EC developed diarrhea, neither the duration of diarrhea nor the amount of virus in the intestine was significantly different between these two groups (P > 0.05) (Fig. 1C and 2A). Signs and symptoms of systemic disease were not observed in any of the EC-infected mice.
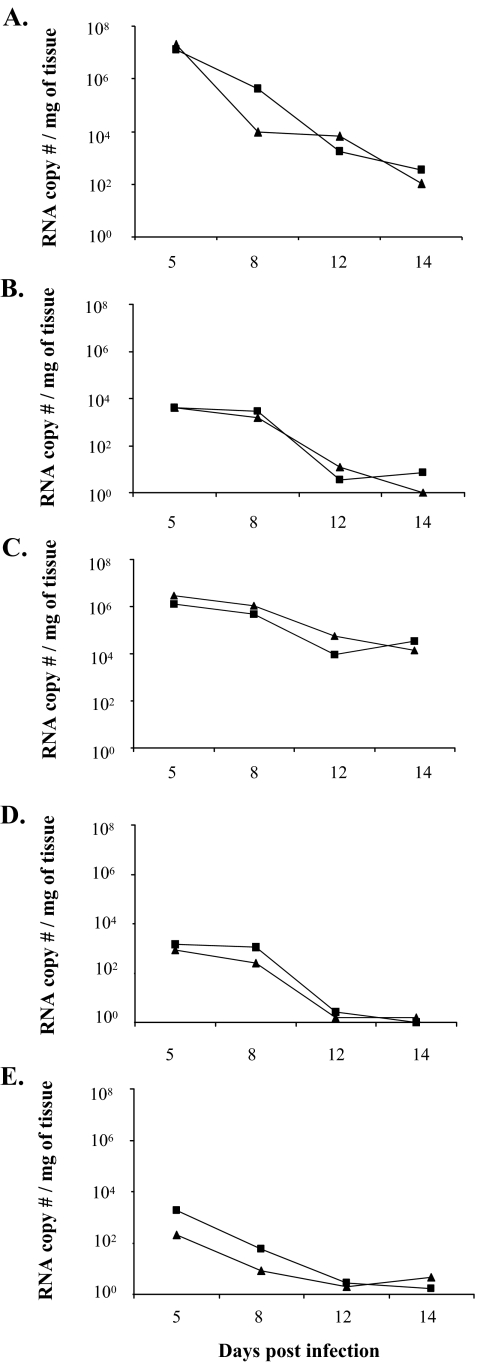
FIG. 2.
Virus levels in intestines and extraintestinal organs after EC infection in 5-day-old wild-type and IFN-αγR KO mice. On selected days p.i., groups of 6 to 15 mice were sacrificed, and virus levels in the indicated tissues were determined by quantitative RT-PCR (see Materials and Methods) and are expressed as viral RNA copy number per mg of tissue. The geometric means of virus RNA copy number per mg of tissue are presented. Tested tissues include intestine (A), liver (B), MLN (C), bile duct (D), and blood (E). Virus was not detected in the pancreas of EC-infected wild-type or IFN-αγR KO mice (data not shown). There were no statistically significant differences at any time point postinfection in any tissues between wild-type and IFN-αγR KO mice. Symbols: ▪, IFN-αγR KO; ▴, wild type.
Intestinal and extraintestinal replication in wild-type, interferon receptor-deficient, and STAT-1-deficient mice infected with RRV or EC.
Because RRV caused a systemic disease in mice with defects in the interferon signaling pathway, we next examined the level of viral replication in selected organs from these mice. Rotavirus could be detected in the small intestines of all groups on days 2 and 5 p.i. (Fig. 3A and B). Titers of virus in the intestine were not significantly different among the different groups of mice on day 2 p.i., but wild-type mice had the lowest titers in the intestine on day 5 p.i., and these were significantly lower than the IFN-αγR KO and STAT1 KO groups (P < 0.05). Other groups were not statistically different. By day 8 and thereafter virus was not detectable in the small intestines of wild-type, IFN-αR KO, or IFN-γR KO mice. On the other hand, over 50% of the IFN-αγR KO and STAT1 KO mice continued to have high levels of virus in their small intestine on day 8 (10,000-fold greater than wild type), and these levels then declined and were not detectable by day 14 (Fig. 3A and B).
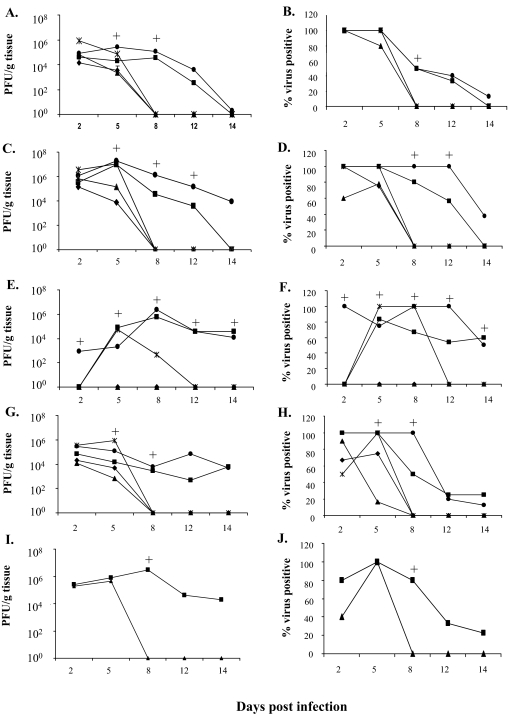
FIG. 3.
Virus titers in intestines and extraintestinal organs following RRV infection in 5-day-old wild-type mice or selected IFN receptor KO and STAT1 KO mice. On different days postinfection, groups of 4 to 10 mice were sacrificed and virus titers in indicated tissues were determined by plaque assay on MA104 cells (see Material and Methods). The graphs on the left (A, C, E, G, and I) show the geometric mean virus titers for different tissues, and the graphs on the right (B, D, F, H, and J) show the percentages of virus-positive tissues on the indicated day. Geometric mean virus titers were calculated from those samples that contained detectable virus. When a mouse in a group became virus negative at any time point, a value of 1 was assigned. On day 8 p.i. or later, the percentage of virus-positive organs in the IFN-αγR KO and STAT1 KO groups declined but the titers in virus-positive animals generally remained high. Tested tissues included intestine (A and B), liver (C and D), pancreas (E and F), MLN (G and H), and bile duct (I and J). +, P < 0.05 for IFN-αγR KO or STAT1 KO mice versus wild-type mice with the Mann-Whitney test (virus titration) or Fisher's exact test (percent virus positive). Statistical analyses were performed with all samples in the groups included for all the tests in this study. See Results for more of the statistical results. Symbols: ▪, IFN-αγ R KO; •, STAT1 KO; ✴, IFN-αR KO; ⧫, IFN-γ R KO; ▴, wild type.
The levels of virus in the intestines of RRV-infected mice, when measured by quantitative real-time RT-PCR, were between 700- and 800-fold lower than in EC-infected mice on days 5 and 8 p.i., respectively (P < 0.001), similar to what has been reported previously (Fig. 4 and data not shown) (16). Of note, there was no significant difference in the amount of EC rotavirus in the intestine of IFN-αγR KO versus wild-type mice at any time point postinfection (P > 0.05) (Fig. 2A).
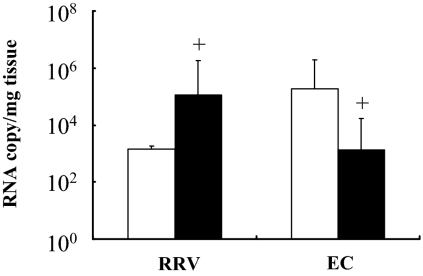
FIG. 4.
Comparison of virus levels in the liver and intestine of RRV-infected versus EC-infected IFN-αγR KO mice. Results shown are the viral RNA copy number per mg of tissue of RRV- or EC-infected intestine (open bars) and liver (solid bars) in IFN-αγR KO mice on day 8 p.i. +, statistically significant difference in virus levels between intestine and liver (P < 0.05 by Mann-Whitney test).
High levels of RRV (similar to peak levels in the intestine) were detected in the liver homogenates of the majority of mice in all groups on days 2 and 5 p.i. (Fig. 3C and D). There were no significant differences in the amount of virus in the liver among the various groups on day 2 p.i., but the titers fell in the wild-type and IFN-γR KO mice by day 5 p.i. and virus was not detectable in livers of any wild-type, IFN-αR KO, and IFN-γR KO mice on day 8 p.i. On the other hand, 80% and 60% of IFN-αγR KO mice were still RRV positive on days 8 and 12, respectively (Fig. 3D) at levels more than 10,000-fold higher than in wild-type mice. By day 14 p.i. RRV was undetectable in the liver of IFN-αγR KO mice (0/10) despite the fact that most of the mice continued to have severe systemic disease and liver pathology (data not shown). The livers of all the STAT1 KO mice were RRV positive on days 8 and 12 p.i. (Fig. 3C and D) with higher virus titers compared to the IFN-αγR KO group (P < 0.05). Unlike the IFN-αγR KO group, 37% (3/8 mice) of STAT1 mice still had virus in the liver on day 14 p.i. Virus was also detected in the livers of EC-infected IFN-αγR KO and wild-type mice at different time points postinfection; however, as with the intestine, there was no significant difference between IFN-αγR KO and wild-type mice at any time point (Fig. 2B) (P > 0.05).
Although virus was detected in the intestines and livers of both RRV- and EC-infected IFN-αγR mice, the ratio of virus levels between these two organs was very different (Fig. 4). On day 8 p.i. the RRV levels in liver as measured by QRT-PCR were 80 times higher than in the intestine of IFN-αγR KO mice (P < 0.05), but in EC-infected mice, intestinal virus levels were 148 times higher than liver virus levels (P < 0.001) (Fig. 4). The ratio of viral RNA between liver and intestine during RRV or EC infection remained different on day 14 postinfection (data not shown). RRV levels in liver and other extraintestinal organs were not significantly influenced by the level of viremia, since the blood levels were very low (below levels in extraintestinal organs) or not detectable, especially on day 8 postinfection or later in both strains of mice by both plaque assay and QRT-PCR detection (data not shown). Prior studies in normal mice had also demonstrated that the level of viral RNA in extraintestinal tissues was substantially higher than seen in the blood (16). These results suggest that RRV replicates to higher levels than EC in extraintestinal organs such as the liver and pancreas (see below) in mice deficient in interferon signaling.
RRV was not detected in the pancreas of wild-type or IFN-γR KO mice at any time after infection (Fig. 3E and F). In IFN-αR KO and IFN-αγR KO mice, virus was detected in the pancreas on day 5 p.i. while virus detection in the pancreas of STAT1 KO mice started on day 2 p.i. On days 5 and 8 p.i., between 70 and 100% of IFN-αR KO, IFN-αγR KO, and STAT1 KO mice had high titers of RRV in the pancreas (Fig. 3E and F) at levels 10,000-fold greater than present in wild-type mice. The virus titers were similar among these groups on day 5 p.i., but by day 8 p.i. virus titers in the pancreas of IFN-αR KO mice were significantly lower than those in the IFN-αγR KO and STAT1 KO groups (P < 0.05). Virus was not detectable in the pancreas of IFN-αR KO mice on day 12 p.i., while 50 to 60% of IFN-αγR KO mice remained virus positive on days 12 and 14 p.i., respectively. All STAT1 KO mice had virus in the pancreas on day 12 p.i., with titers similar to the levels in the virus-positive IFN-αγR KO group. The RRV titers in the pancreas of IFN-αγR and STAT1 KO mice were also significantly higher than those in the intestine, especially at late time points postinfection (P < 0.05) (Fig. 3A versus E). There was no detectable virus in the pancreas of EC-infected IFN-αγR KO or wild-type mice (data not shown) at any time point.
Between 60 and 100% of RRV-infected mice had virus in the MLN on days 2 and 5 p.i. in all groups (Fig. 3G and H). The virus levels were not significantly different among groups on day 2 p.i., but by day 5 p.i. levels of RRV in the MLN of wild-type mice had fallen significantly compared to other groups (P < 0.01) (Fig. 3G). By day 8 p.i., virus was not present in wild-type, IFN-αR KO, or IFN-γR KO mice, but the MLN in 50% of IFN-αγR KO and 100% of STAT1 mice remained strongly positive (Fig. 3H). RRV was generally cleared from the MLN of IFN-αγR KO and STAT1 KO mice by day 12 p.i. In EC-infected mice, virus was detected at high levels in 100% of wild-type and IFN-αγR KO mice at all time points, and there were no significant differences in the levels between the two groups (Fig. 2C).
The biliary tract is a primary target for RRV-induced injury in newborn mice (46). RRV was also detected in the common bile duct of 40 to 100% of orally infected 5-day-old wild-type mice on days 2 and 5 p.i., but unlike newborn mice, RRV became undetectable by day 8 p.i. (Fig. 3I and J). However, 80 to 100% of IFN-αγR KO mice had high titers of RRV in the common bile duct on day 8 p.i. that were more than 10,000-fold higher than those present in the common bile duct of wild-type mice (Fig. 3I and J). The percentage of virus-positive bile ducts fell to 30% and 20% by days 12 and 14 p.i., respectively, but the titers remained higher than concurrent titers in the liver (P < 0.01). In EC-infected mice, virus was readily detected in the common bile duct of both wild-type and IFN-αγR KO mice on days 5 and 8, but the levels were basically identical in the IFN-αγR KO and wild-type mice (Fig. 2D) and these levels were similar to levels in the liver at the same time points (P > 0.05).
Pathological changes in intestine, liver, and pancreas of interferon receptor-deficient mice after RRV infection.
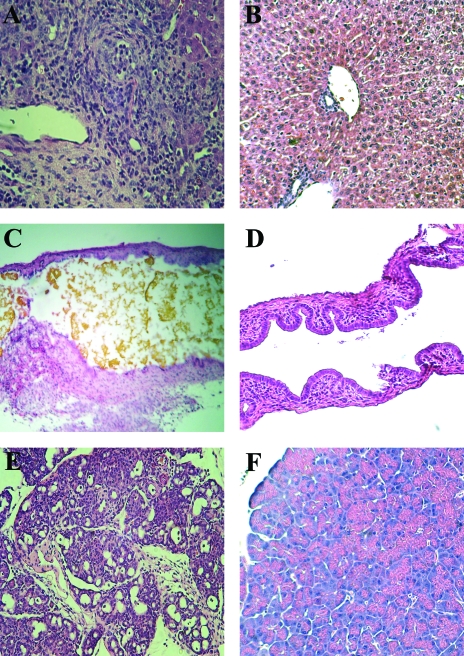
The pathological changes in intestine and extraintestinal organs of RRV-infected IFN-αγR KO and wild-type mice are shown in Fig. 5 and 6. On day 2 p.i. the small intestine of both wild-type and IFN-αγR KO mice showed similar pathological changes typical of rotavirus infection, including swollen microvilli and intestinal epithelial cell vacuolization (Fig. 5A and C). The pathological changes in the small intestinal tissue generally disappeared by day 8 p.i. in both IFN-αγR KO and wild-type groups (Fig. 5B and D). On the other hand, by day 12 p.i., in livers of RRV-infected IFN-αγR KO mice there was a substantial inflammatory response in the portal triad area, with round cell infiltration, especially around intrahepatic bile ducts (Fig. 6A). These changes persisted on day 14 p.i. in IFN-αγR KO mice with systemic disease even though virus was no longer detectable in these livers (data not shown). In addition, the inflammatory reaction could also be observed in the common bile duct, resulting in thickening of the ductal wall and bile duct obstruction as indicated by bile accumulation in the lumen of the common duct and gall bladder (Fig. 6C). There were no pathological changes in the liver (Fig. 6B) or bile ducts (Fig. 6D) of RRV-infected wild-type mice or in EC-infected wild-type or IFN-αγR KO mice at the same time points.
FIG. 5.
Pathological changes in the small intestine of RRV-infected IFN-αγR KO and wild-type mice. Small intestines of RRV-infected IFN-αγR KO and wild-type mice were collected on days 2 and 8 p.i. Specimens were fixed in 10% formalin and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin and observed under a light microscope. Images of small intestines of RRV-infected IFN-αγR KO at day 2 p.i. (A) or day 8 p.i. (B) and RRV-infected wild-type mice at day 2 p.i. (C) and day 8 p.i. (D) are shown. Magnification, ×200.
FIG. 6.
Pathological changes in extraintestinal organs of RRV-infected IFN-αγR KO and wild-type mice. The liver (A), common bile duct (C), and pancreas (E) of IFN-αγR KO mice and liver (B), common bile duct (D), and pancreas (F) of wild-type mice were collected on day 12 after RRV infection and processed as for intestinal samples (see Fig. 5 legend). Sections were observed under a light microscope. Magnification, ×100.
There was edema in the pancreas of RRV infected IFN-αγR KO mice on day 5 postinfection (data not shown) and massive damage and inflammatory cell infiltration in the pancreatic parenchyma by day 12 p.i. (Fig. 6E). The beta islet areas of the pancreas appeared spared (Fig. 6E). The pancreas of RRV-infected wild-type mice appeared normal (Fig. 6F). The pathological changes in RRV-infected STAT1 KO mice were similar to those in IFN-αγR KO mice in all tissues examined, while no changes were observed in the pancreas of any EC-infected mice (data not shown).
Identification of rotavirus-infected target cells in extraintestinal organs based on confocal microscopy and immunofluorescent staining.
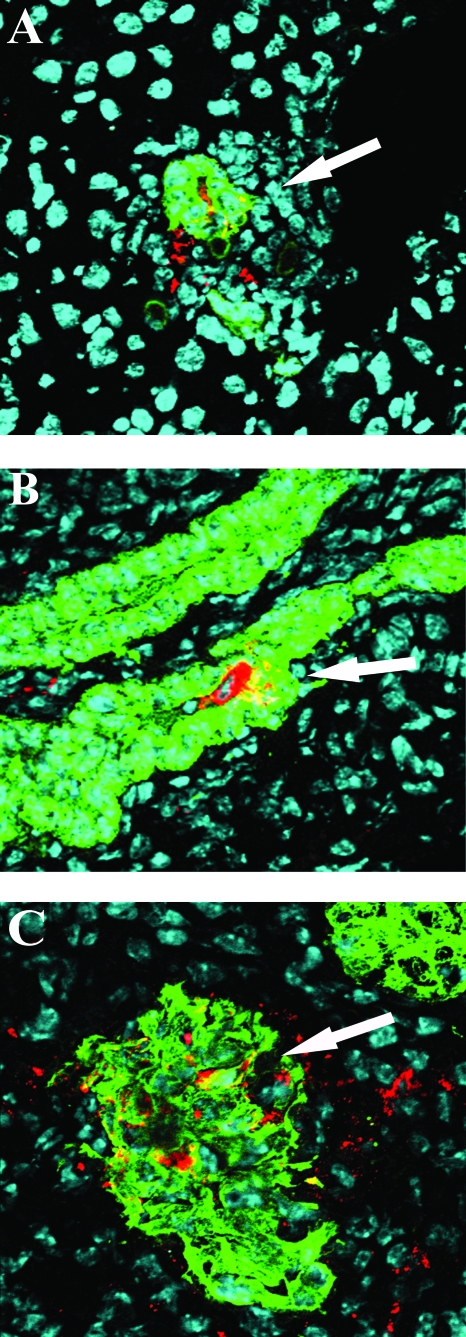
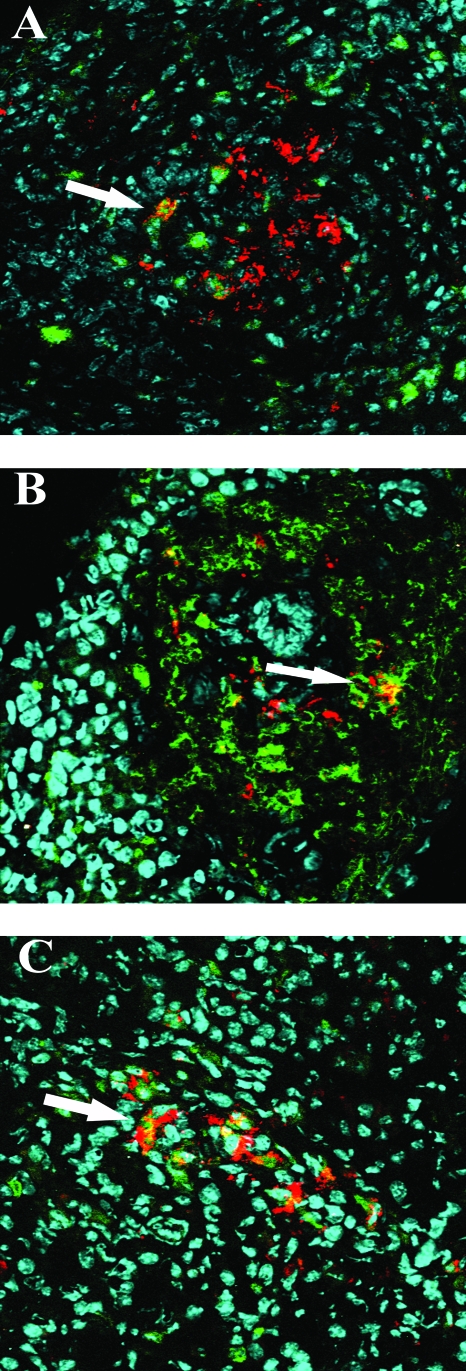
Many studies have previously demonstrated that the mature villus tip cells of the small bowel are the primary targets for rotavirus infection in normal mice. In order to identify the cells that were susceptible to RRV infection in extraintestinal organs, we used confocal microscopy and costained tissues from IFN-αγR KO mice with fluorescent-labeled antibodies to VP6 or NSP4 (data not shown), intermediate filaments K8 (which stains most epithelial cells, including hepatocytes, bile duct epithelial cells, and pancreas acinar cells [35]), K19 (which is specific for liver bile duct cells [35]), neutrophil marker Gr1, macrophage marker F4/80, and dendritic cell marker CD11c. In the livers of RRV-infected IFN-αγR KO mice, viral antigens were found primarily in the portal triads, where inflammatory cells were heavily infiltrated. Viral antigen colocalized within intrahepatic bile duct epithelial cells (Fig. 7A). There was very little if any viral antigen staining in the liver parenchyma, and parenchymal viral antigens did not colocalize with K8 staining (data not shown). Hence, there was no definite evidence that hepatocytes were infected by RRV. In the common bile duct, virus antigen-positive cells were costained with the epithelial marker K19 (Fig. 7B). In the pancreas, viral antigen was detected in K8-positive acinar cells in the parenchyma (Fig. 7C). Viral antigens (both VP6 and NSP4) (data not shown) also colocalized with inflammatory cells in the liver, bile duct, and pancreas, including neutrophils (Fig. 8A), macrophages (Fig. 8B), and dendritic cells (Fig. 8C). Viral antigens were not found in association with either T- or B-cell markers or in fibroblasts (data not shown). We did not detect viral antigens in the liver, bile ducts, or pancreas of RRV-infected wild-type mice or EC-infected mice of either strain (data not shown).
FIG. 7.
Detection of RRV-infected cells in extraintestinal organs using immunofluorescent staining and confocal microscopy. The liver (A), bile duct (B), and pancreas (C) from RRV-infected IFN-αγR KO mice were collected on day 8 p.i. and frozen in OCT. Liver and pancreas sections were costained with anti-VP6 (red) and anti-keratin 8 (green). Bile duct sections were costained with anti-VP6 (red) and anti-keratin 19 (green). Cell nuclei were stained with TOTO-3 iodide (blue). Sections were observed under a confocal microscope. Magnification, ×400. The colocalization of viral antigen and epithelial cell markers is indicated by arrows.
FIG. 8.
Detection of RRV-infected inflammatory cells in extraintestinal organs based on immunofluorescent staining and confocal microscopy. Tissues were costained with anti-VP6 (red) and anti-Gr-1 (A) (liver), F4/80 (B) (liver), and CD11c (C) (MLN) antibodies. Magnification, ×400. The colocalization of viral antigen and selected cell markers is indicated by arrows.
Intestinal and extraintestinal virus titers in interferon receptor-deficient mice infected with rotavirus strains SA11, NCDV, OSU, or EW.
To investigate if the IFN deficiency-mediated enhancement of rotavirus replication in extraintestinal organs was limited to the heterologous simian RRV strain, we orally infected 5-day-old wild-type or IFN-αγR KO mice with an additional simian strain, SA11, a bovine strain, NCDV, or a porcine strain, OSU. SA11 has previously shown to cause mild biliary disease in new born mice (46). There were no significant differences in diarrhea between wild type and IFN-αγR KO mice after SA11, NCDV, or OSU infection, similar to what was seen with RRV (data not shown). SA11-infected IFN-αγR KO mice showed only a mild form of systemic disease characterized by transient white stool, oily fur, and growth retardation (Table 1). The signs and symptoms of disease improved after day 10 postinfection, and no mortality was observed. Systemic disease was not observed in NCDV- or OSU-infected mice. On both days 5 and 8 p.i., SA11 titers in the intestine, livers, and bile ducts of IFN-αγR KO mice appeared higher than in wild-type mice. The differences in intestinal titers on day 5 p.i. and in all three tissues on day 8 p.i. were statistically significant (P < 0.05) (Table 1). SA11 was not detected in MLN and pancreas at any time points in either strain of mice. NCDV and OSU were not detected on day 5 or 8 p.i. in either strain of mice. NCDV but not OSU was detected in intestines of IFN-αγR KO (417 PFU/g) and wild-type (667 PFU/g) mice only on day 2 p.i. The difference was not significant (P < 0.05).
TABLE 1.
Systemic disease and virus titers in tissues of SA11-infected IFNαγR KO and wild-type mice
| Mice | % with Diseasea | Days p.i. | Virus titer (PFU/g)b
|
||
|---|---|---|---|---|---|
| Intestine | Liver | Bile duct | |||
| IFNαγR KO | 50 | 5 | 3,234* (300-67,000) | 2,965 (670-1,400) | 66,916 (5,700-1,800,000) |
| 8 | 86* (<300-10,000) | 539* (<300-130,000) | 1,656* (<200-56,000) | ||
| Wild type | 0 | 5 | 3 (<300-300) | 152 (<300-50,000) | 389 (<300-330,000) |
| 8 | <300 | <300 | <300 | ||
Maximum rate of disease observed after infection. n = 8 and 7 for IFNαγR KO and wild-type mice, respectively. The disease was less severe than RRV infection.
Geometric mean virus titer, with minimum and maximum titers in parentheses. Number of mice in each group was between 6 and 9. *, P < 0.05 by Mann-Whitney test between IFNαγR KO and wild-type mice at the same time point.
We also infected mice with another murine rotavirus, EW. The kinetics of diarrheal disease after EW infection were similar between wild-type and IFN-αγR KO mice, and no sign of systemic disease was observed in either strain of mice (data not shown). Levels of EW in intestine and MLN of IFN-αγR KO mice were not significantly different from the wild type on day 5 but were approximately 40- and 3-fold higher in the IFN-αγR KO mice in the intestine and MLN on day 8 or 12 p.i., respectively (P < 0.001) (data not shown). The highest detectable EW levels in the liver of wild-type and IFN-αγR KO mice were on day 5 p.i. (640 and 507 copy number/mg tissue for wild-type and IFN-αγR KO mice, respectively) and not statistically different. The EW levels in blood were negligible, and there was no difference between these two strains either (data not shown). Virus was not detected in the pancreas of either strain.
DISCUSSION
Recent evidence clearly demonstrates that rotavirus infection is not limited to intestinal epithelial cells. Viremia and extraintestinal rotavirus replication occur both in humans and many animal species (8). However, distinct systemic diseases or syndromes (other than fever) associated with rotavirus infection seem to be quite rare. Currently the only well-characterized animal model of rotavirus-associated systemic disease is the RRV-induced BA model in newborn mice. A lipopolysaccharide-dependent intussusception model restricted to the gut has recently been described as well (58). The current BA model requires i.p. administration of RRV to newborn mice, which is not the natural route of rotavirus infection. Furthermore, the frequency of BA falls very sharply in mice infected with RRV after the first day of life (12). Previously we used an oral route of infection model in immune-competent 5-day-old suckling mice to demonstrate that rotaviruses undergo a systemic replication phase after either heterologous simian RRV or homologous murine EC infection (16). In this model the systemic phase of rotaviral replication was not associated with apparent sequelae, as seems to be the case in most human infections. We have now expanded this model to study the role of selected host immunological factors, specifically the IFNs, in the modulation of systemic and local viral replication after oral infection by both homologous and heterologous rotaviruses.
In this study, we used oral infection of 5-day-old suckling mice to evaluate the role of IFN in modulating intestinal and systemic infection. We initially infected wild-type or various IFN-deficient KO mice with the heterologous simian rotavirus, RRV, which induces diarrheal disease in suckling mice following relatively high-dose inoculation and also causes BA after i.p. inoculation in newborn mice. We found that between 2 and 5 days postinfection the level of viral replication in the intestine, MLN, liver, and bile duct of immune-competent wild-type and selected IFN-deficient mice was either similar or moderately different. By day 8 p.i. both intestinal and extraintestinal infections had resolved in the wild-type and IFN-γR KO mice without any obvious pathological or symptomatic consequences. However, in mice deficient for both type I and II IFN signaling (IFN-αγR KO and STAT1 KO), virus replication did not resolve and in fact persisted for 12 or more days p.i., suggesting that severe deficiency in IFN signaling delayed the resolution of both enteric and systemic infection. Interestingly, deficiency in both type I and II IFN did not affect the rate, degree, or kinetics of diarrheal disease in the suckling mice, despite the prolongation of infection in the intestines. This lack of effect on diarrhea may be due to the fact that even in the KO mice the level of RRV replication in the intestine is very low compared to EC infection, which causes a longer period of diarrhea.
Of note, RRV infection in mice deficient in both type I and II interferon receptors resulted in severe systemic disease, including liver, biliary, and pancreatic injury and eventual lethality. This systemic disease has similar signs and symptoms to the BA syndrome observed in newborn mice follow i.p. inoculation, with the notable exception that severe pathology in the pancreas has not been previously reported in the BA model. The reason why pancreatic viral replication and disease is observed in the IFNR KO mice model but not in the newborn model is not presently apparent but could be due to the more profound and prolonged deficit in interferon signaling in the knockout mice compared to the newborn animals, in which the deficit in interferon signaling is likely to be only transient. IFN-αR KO mice manifested moderately prolonged viral infection in systemic organs including the pancreas but had only mild systemic disease and no mortality. These findings support the conclusion that the STAT1-dependent IFN signaling responses are critical for controlling systemic replication of the heterologous simian RRV strain. In addition, these findings confirmed previous reports that IFN-α/β is more important than IFN-γ in clearing RRV systemic infection (36). However, in the present study type I and II IFNs appeared to be functioning collaboratively in restricting systemic replication and disease, since knockout of either the IFN-α/β or IFN-γ receptor alone did not result in the full-blown lethal disease syndrome.
The precise basis for the pathological events observed in the double IFNR KO and STAT1 KO mice remains unclear. We have shown that, along with its ability to replicate in the intestinal epithelium, RRV can replicate in biliary epithelial cells of the extra- and intrahepatic bile ducts, pancreatic epithelial cells (but not hepatocytes), and selected inflammatory cells, including dendritic cells and macrophages in IFN-αγR KO mice. It is presently unclear whether biliary and pancreatic epithelia are permissive for many rotavirus strains but only RRV efficiently trafficks to these sites or whether the cellular tropism for RRV is simply broader than other rotavirus strains. We are currently undertaking experiments to explore these two possibilities. In addition, it is not clear if deficiency in type I and II IFN signaling expands the susceptible cell populations in mice. Different cell populations appear to have variable IFN responses after RRV infection (15).
It was previously concluded that the pathogenesis of the systemic disease in the BA model was not specifically the result of viral replication, based on the observation that IFN-γ receptor KO mice had reduced inflammatory responses, reduced lymphocyte infiltration in the liver and bile ducts, and increased long-term survival without a change in virus replication (52). In addition, Huang et al. showed that treating mice with an NF-κB inhibitor significantly reduced BA, which was interpreted as confirming the role of inflammatory cytokines in the pathogenesis of BA (28). However, we did not observe any reduction of inflammation in mice specifically deficient in IFN-γ signaling. Severe inflammation in the liver, biliary tract, and pancreas and severe systemic disease were only observed in mice lacking both type I and II IFN signaling, suggesting that the absence of an IFN response was critical for promoting persistent viral replication and the resultant inflammatory response as well as the severe systemic disease. In fact, in this model it appears that viral replication in extraintestinal sites plays a key role in defining the outcome of infection given the differences in disease severity and viral growth observed between the single and double IFN receptor KO mice strains. The collaboration between type I and II IFNs in controlling viral infection has also been demonstrated in other murine viral infections, such as the mouse cytomegalovirus infection model. (51).
The acquired immune system, including CD8+ T cells and B cells, is critically important for resolving rotavirus infection in the intestine (19, 57). However, the role of these cells in RRV-induced systemic disease appears to be less critical. CD8+ T-cell or B-cell-deficient mice did not develop systemic disease after RRV infection. In fact, 5-day-old orally inoculated Rag1 KO mice, which lack functional T- and B-cell immunity, do not develop systemic disease or have detectable virus in their systemic tissues 12 days after RRV infection. On the other hand, newborn Rag1 KO mice infected i.p. with RRV developed systemic disease and persistent viral infection in the liver, as described in the original BA model (data not shown). This observation is likely due to an inadequate interferon response in the newborn animals, since administration of IFN-α to newborn i.p.-infected mice suppresses BA (47). Presumably, older Rag1 KO mice have developed a more responsive IFN signaling pathway and hence do not develop a systemic illness after i.p. injection, even without a functional acquired immune system. From these findings, it seems apparent that the interferon system plays a critical role in controlling systemic RRV replication and disease.
The effect of the IFN system on restricting replication of the homologous EC murine rotavirus strains in either the intestine or systemically was entirely different from what was observed with the heterologous RRV (or to a lesser extent SA11) strain. It has been reported previously that IFN-α/β or IFN-γ has no effect on diarrheal disease in EC-infected suckling mice (2). In this study diarrheal disease and the viral loads in the intestine and extraintestinal organs were virtually identical in EC-infected IFN-αγR KO and wild-type mice. In addition, systemic disease was not observed in EC-infected IFN-αγR KO mice despite the fact that systemic replication did take place. Interestingly, when the viral loads in the intestines and extraintestinal organs in EC-infected versus RRV-infected mice were compared, we found that RRV replicated more efficiently in extraintestinal organs, especially the liver, bile ducts, and pancreas, while EC replicated far more efficiently in the intestine. The viral genes responsible for these clear differences are currently being investigated.
Enhanced intestinal and systemic infection in interferon signaling-deficient mice was not restricted to RRV. Simian SA11, which has been shown to cause mild BA after i.p. administration in newborn mice, also induced mild systemic disease in IFN-αγR KO mice. As was seen with RRV, SA11 has a growth advantage in systemic organs of mice deficient in interferon signaling. Interestingly, SA11 was not detected in MLN or the pancreas in IFN-αγR KO mice, suggesting that SA11 is less well adapted to replicate systemically than RRV. Enhanced systemic replication in the absence of interferon is not, however, a universal characteristic of heterologous rotavirus infection of suckling mice. Neither NCDV nor OSU could be detected in the intestine or extraintestinal organs of wild-type or IFN-αγR KO mice between days 5 and 12 postinfection. Only a very low amount of NCDV was detected in intestines on day 2 p.i., and there was no difference between wild-type and IFN-αγR KO mice, indicating that NCDV and OSU have minimal abilities to replicate in the intestine or systemic organs of mice irrespective of the interferon system. We observed systemic disease in IFN-αγR KO mice infected with 100-fold less RRV, suggesting that the difference of NCDV, OSU, and RRV in inducing systemic infection is not determined primarily by inoculating dose. Thus, it appears that IFNs are required to control systemic infection of those strains of heterologous viruses that are able to replicate efficiently in intestine and extraintestinal organs, since depletion of both types I and II IFN significantly diminished the clearance of these viruses. For those heterologous rotaviruses that have minimal abilities to replicate in the mouse intestine, the IFN antiviral function may not be needed.
Different homologous rotaviruses also have somewhat variable responses to the absence of IFN signaling. The wild-type murine rotavirus EW, which has similar pathogenicity to EC virus in suckling mice, causes diarrheal disease with a very small infectious dose and spreads efficiently to noninoculated littermates (9). The absence of IFN response did not alter the percentage or duration of diarrhea between wild-type and IFN-αγR KO mice after EW infection or the peak levels of intestinal virus on day 5 p.i. However, intestinal levels of EW were significantly higher in IFN-αγR KO mice between days 8 and 12 p.i., suggesting that the deficiency in the IFN response does enhance homologous EW replication to a modest degree, although much less than the effects on RRV (or SA11) replication. Taken together, the effects of interferon signaling on the replication of the two homologous murine rotavirus strains, EC and EW, were substantially less than the effects on the two simian heterologous viruses, RRV and SA11. Whether this is an inherent difference between homologous and heterologous rotavirus infection requires additional studies. Of note, enhanced intestinal replication of the EW strain was previously reported in adult but not in suckling STAT1 KO mice (55). This apparent discrepancy could be due to the fact that fecal samples from multiple time points were pooled before testing in the previous study, which might have masked the differences in virus shedding between normal and STAT1 KO mice in the suckling period.
Several recent studies have revealed that rotavirus NSP1 can act as an E3 ubiquitin ligase that promotes degradation of IRF3 and IRF7 and inhibits the cellular type I IFN response in vitro (4, 5) after rotavirus infection. NSP1 has also been associated with the rotavirus host range restriction in vivo (9). It seems possible, given our observations, that murine rotaviruses, such as EC and EW, have evolved highly effective mechanisms (perhaps mediated by NSP1) to counteract the IFN antiviral effects in the mouse host. If this were the case, further deletion of IFN signaling, as seen in the KO mice, would be expected to have little effect on EC replication. Homologous infection in the gut may be controlled by other host defense mechanisms, such as adaptive immunity, since EC infection in suckling SCIDs, Rag1, or Rag2 KO mice results in chronic fecal shedding but does not induce systemic disease such as hepatitis or pancreatitis (49). In addition, it seems possible that RRV, and to a lesser degree SA11, has the ability, in the absence of interferon, to replicate efficiently in a subset of epithelial cells in the pancreas and biliary tree, which are not highly permissive for murine viruses. On the other hand, the heterologous rotaviruses, such as RRV or SA11, are less effective in suppressing the mouse IFN responses than the murine strains. If this were the case, removal of host IFN signaling would be expected to augment the systemic growth of these viruses. NSP1 from different virus strains has been recently shown to have different affinities for and efficiencies to degrade IRF3 in vitro (23). We are currently investigating the genetic basis for the sensitivity of rotavirus to IFN deficiency and the difference between homologous virus NSP1 and heterologous virus NSP1 in inhibiting host IFN responses in the murine model.
Acknowledgments
We sincerely thank Herbert W. Virgin IV for his kind gift of IFN-α/β and IFN-α/β/γ receptor KO and wild-type mice.
This study was supported in part by a VA Merit Award and NIH grants R01 AI021362-24 and P30DK56339.
Footnotes
Published ahead of print on 21 May 2008.
REFERENCES
- 1.Allen, S. R., M. Jafri, B. Donnelly, M. McNeal, D. Witte, J. Bezerra, R. Ward, and G. M. Tiao. 2007. Effect of rotavirus strain on the murine model of biliary atresia. J. Virol. 811671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel, J., M. A. Franco, H. B. Greenberg, and D. Bass. 1999. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19655-659. [DOI] [PubMed] [Google Scholar]
- 3.Azim, T., M. H. Zaki, G. Podder, N. Sultana, M. A. Salam, S. M. Rahman, K. Sefat e, and D. A. Sack. 2003. Rotavirus-specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J. Med. Virol. 69286-295. [DOI] [PubMed] [Google Scholar]
- 4.Barro, M., and J. T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. USA 1024114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barro, M., and J. T. Patton. 2007. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J. Virol. 814473-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass, D. M. 1997. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology 11381-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomqvist, M., S. Juhela, S. Erkkila, S. Korhonen, T. Simell, A. Kupila, O. Vaarala, O. Simell, M. Knip, and J. Ilonen. 2002. Rotavirus infections and development of diabetes-associated autoantibodies during the first 2 years of life. Clin. Exp. Immunol. 128511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blutt, S. E., and M. E. Conner. 2007. Rotavirus: to the gut and beyond! Curr. Opin. Gastroenterol. 2339-43. [DOI] [PubMed] [Google Scholar]
- 9.Broome, R. L., P. T. Vo, R. L. Ward, H. F. Clark, and H. B. Greenberg. 1993. Murine rotavirus genes encoding outer capsid proteins VP4 and VP7 are not major determinants of host range restriction and virulence. J. Virol. 672448-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207143-153. [DOI] [PubMed] [Google Scholar]
- 11.Conway, S. P. 1988. Rotavirus encephalitis. Arch. Dis. Child. 63224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czech-Schmidt, G., W. Verhagen, P. Szavay, J. Leonhardt, and C. Petersen. 2001. Immunological gap in the infectious animal model for biliary atresia. J. Surg. Res. 10162-67. [DOI] [PubMed] [Google Scholar]
- 13.De Boissieu, D., P. Lebon, J. Badoual, Y. Bompard, and C. Dupont. 1993. Rotavirus induces alpha-interferon release in children with gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1629-32. [DOI] [PubMed] [Google Scholar]
- 14.De La Rubia, L., M. I. Herrera, M. Cebrero, and J. C. De Jong. 1996. Acute pancreatitis associated with rotavirus infection. Pancreas 1298-99. [DOI] [PubMed] [Google Scholar]
- 15.Douagi, I., G. M. McInerney, A. S. Hidmark, V. Miriallis, K. Johansen, L. Svensson, and G. B. Karlsson Hedestam. 2007. Role of interferon regulatory factor 3 in type I interferon responses in rotavirus-infected dendritic cells and fibroblasts. J. Virol. 812758-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenaux, M., M. A. Cuadras, N. Feng, M. Jaimes, and H. B. Greenberg. 2006. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J. Virol. 805219-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 687766-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, N., P. T. Vo, D. Chung, T. V. Vo, Y. Hoshino, and H. B. Greenberg. 1997. Heterotypic protection following oral immunization with live heterologous rotaviruses in a mouse model. J. Infect. Dis. 175330-341. [DOI] [PubMed] [Google Scholar]
- 19.Franco, M. A., N. Feng, and H. B. Greenberg. 1996. Rotavirus immunity in the mouse. Arch. Virol. Suppl. 12141-152. [DOI] [PubMed] [Google Scholar]
- 20.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 697800-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco, M. A., C. Tin, and H. B. Greenberg. 1997. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J. Virol. 714165-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujinaga, S., K. Kaneko, Y. Ohtomo, M. Takada, K. Kobayashi, M. Tada, and Y. Yamashiro. 2005. Acute renal failure due to obstructive uric acid stones associated with rotavirus gastroenteritis. Pediatr. Nephrol. 20239-240. [DOI] [PubMed] [Google Scholar]
- 23.Graff, J. W., J. Ewen, K. Ettayebi, and M. E. Hardy. 2007. Zinc-binding domain of rotavirus NSP1 is required for proteasome-dependent degradation of IRF3 and autoregulatory NSP1 stability. J. Gen. Virol. 88613-620. [DOI] [PubMed] [Google Scholar]
- 24.Grech, V., V. Calvagna, A. Falzon, and A. Mifsud. 2001. Fatal, rotavirus-associated myocarditis and pneumonitis in a 2-year-old boy. Ann. Trop. Paediatr. 21147-148. [DOI] [PubMed] [Google Scholar]
- 25.Grunow, J. E., S. F. Dunton, and J. L. Waner. 1985. Human rotavirus-like particles in a hepatic abscess. J. Pediatr. 10673-76. [DOI] [PubMed] [Google Scholar]
- 26.Honeyman, M. C., B. S. Coulson, N. L. Stone, S. A. Gellert, P. N. Goldwater, C. E. Steele, J. J. Couper, B. D. Tait, P. G. Colman, and L. C. Harrison. 2000. Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 491319-1324. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino, Y., R. G. Wyatt, H. B. Greenberg, J. Flores, and A. Z. Kapikian. 1984. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J. Infect. Dis. 149694-702. [DOI] [PubMed] [Google Scholar]
- 28.Huang, L., W. Z. Gu, X. M. Si, M. F. Wei, and J. X. Feng. 2007. Expression of NF-κB in rotavirus-induced damage to the liver and biliary tract in neonatal mice. Hepatobiliary Pancreat Dis. Int. 6188-193. [PubMed] [Google Scholar]
- 29.Jiang, B., L. Snipes-Magaldi, P. Dennehy, H. Keyserling, R. C. Holman, J. Bresee, J. Gentsch, and R. I. Glass. 2003. Cytokines as mediators for or effectors against rotavirus disease in children. Clin. Diagn. Lab. Immunol. 10995-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2002. Rotaviruses, 4th ed., vol. 2. Lippincott-Raven, Philadelphia, PA.
- 31.Kehle, J., C. Metzger-Boddien, F. Tewald, M. Wald, J. Schuurmann, and G. Enders. 2003. First case of confirmed rotavirus meningoencephalitis in Germany. Pediatr. Infect. Dis. J. 22468-470. [PubMed] [Google Scholar]
- 32.Keidan, I., I. Shif, G. Keren, and J. H. Passwell. 1992. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr. Infect. Dis. J. 11773-775. [PubMed] [Google Scholar]
- 33.Kirton, A., K. Busche, C. Ross, and E. Wirrell. 2005. Acute necrotizing encephalopathy in Caucasian children: two cases and review of the literature. J. Child Neurol. 20527-532. [DOI] [PubMed] [Google Scholar]
- 34.Knip, M., and H. K. Akerblom. 1999. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107(Suppl. 3)S93-S100. [DOI] [PubMed] [Google Scholar]
- 35.Ku, N. O., X. Zhou, D. M. Toivola, and M. B. Omary. 1999. The cytoskeleton of digestive epithelia in health and disease. Am. J. Physiol. 277G1108-G1137. [DOI] [PubMed] [Google Scholar]
- 36.Kuebler, J. F., G. Czech-Schmidt, J. Leonhardt, B. M. Ure, and C. Petersen. 2006. Type-I but not type-II interferon receptor knockout mice are susceptible to biliary atresia. Pediatr. Res. 59790-794. [DOI] [PubMed] [Google Scholar]
- 37.Lecce, J. G., J. M. Cummins, and A. B. Richards. 1990. Treatment of rotavirus infection in neonate and weanling pigs using natural human interferon alpha. Mol. Biother. 2211-216. [PubMed] [Google Scholar]
- 38.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeal, M. M., K. S. Barone, M. N. Rae, and R. L. Ward. 1995. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology 214387-397. [DOI] [PubMed] [Google Scholar]
- 40.McNeal, M. M., J. L. VanCott, A. H. Choi, M. Basu, J. A. Flint, S. C. Stone, J. D. Clements, and R. L. Ward. 2002. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J. Virol. 76560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 766502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nigro, G. 1991. Pancreatitis with hypoglycemia-associated convulsions following rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 12280-282. [DOI] [PubMed] [Google Scholar]
- 43.Offit, P. A., and K. I. Dudzik. 1989. Noninfectious rotavirus (strain RRV) induces an immune response in mice which protects against rotavirus challenge. J. Clin. Microbiol. 27885-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oishi, I., T. Kimura, T. Murakami, K. Haruki, K. Yamazaki, Y. Seto, Y. Minekawa, and H. Funamoto. 1991. Serial observations of chronic rotavirus infection in an immunodeficient child. Microbiol. Immunol. 35953-961. [DOI] [PubMed] [Google Scholar]
- 45.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen, C., D. Biermanns, M. Kuske, K. Schakel, L. Meyer-Junghanel, and H. Mildenberger. 1997. New aspects in a murine model for extrahepatic biliary atresia. J. Pediatr. Surg. 321190-1195. [DOI] [PubMed] [Google Scholar]
- 47.Petersen, C., E. Bruns, M. Kuske, and P. von Wussow. 1997. Treatment of extrahepatic biliary atresia with interferon-alpha in a murine infectious model. Pediatr. Res. 42623-628. [DOI] [PubMed] [Google Scholar]
- 48.Qi, T., L. Xie, Y. Wang, J. Wang, H. Chen, and L. Zhou. 2002. Dynamic variation of serum and stool level of interleukin-2, interleukin-6 and interferon-alpha in children with rotavirus enteritis and its relation to clinical manifestations. Zhongh. Shi Yan He Lin Chuang Bing Xue Za Zhi. 16270-273. (In Chinese.) [PubMed] [Google Scholar]
- 49.Riepenhoff-Talty, M., T. Dharakul, E. Kowalski, S. Michalak, and P. L. Ogra. 1987. Persistent rotavirus infection in mice with severe combined immunodeficiency. J. Virol. 613345-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riepenhoff-Talty, M., K. Schaekel, H. F. Clark, W. Mueller, I. Uhnoo, T. Rossi, J. Fisher, and P. L. Ogra. 1993. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 33394-399. [DOI] [PubMed] [Google Scholar]
- 51.Salazar-Mather, T. P., C. A. Lewis, and C. A. Biron. 2002. Type I interferons regulate inflammatory cell trafficking and macrophage inflammatory protein 1alpha delivery to the liver. J. Clin. Investig. 110321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shivakumar, P., K. M. Campbell, G. E. Sabla, A. Miethke, G. Tiao, M. M. McNeal, R. L. Ward, and J. A. Bezerra. 2004. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J. Clin. Investig. 114322-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.St. Geme, J. W., III, and D. Hyman. 1988. Hepatic injury during rotavirus infections. J. Pediatr. 113952-953. [DOI] [PubMed] [Google Scholar]
- 54.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vancott, J. L., M. M. McNeal, A. H. Choi, and R. L. Ward. 2003. The role of interferons in rotavirus infections and protection. J. Interferon Cytokine Res. 23163-170. [DOI] [PubMed] [Google Scholar]
- 56.Vanden Broecke, C., A. Schwers, L. Dagenais, A. Goossens, M. Maenhoudt, P. P. Pastoret, and J. Werenne. 1984. Interferon response in colostrum-deprived newborn calves infected with bovine rotavirus: its possible role in the control of the pathogenicity. Ann. Rech. Vet. 1529-34. [PubMed] [Google Scholar]
- 57.Ward, R. L. 1996. Mechanisms of protection against rotavirus in humans and mice. J. Infect. Dis. 174(Suppl. 1)S51-S58. [DOI] [PubMed] [Google Scholar]
- 58.Warfield, K. L., S. E. Blutt, S. E. Crawford, G. Kang, and M. E. Conner. 2006. Rotavirus infection enhances lipopolysaccharide-induced intussusception in a mouse model. J. Virol. 8012377-12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong, C. J., Z. Price, and D. A. Bruckner. 1984. Aseptic meningitis in an infant with rotavirus gastroenteritis. Pediatr. Infect. Dis. 3244-246. [DOI] [PubMed] [Google Scholar]