Abstract
Recombinant adeno-associated virus vectors based on serotype 6 (rAAV6) efficiently transduce skeletal muscle after intravenous administration and have shown efficacy in the mdx model of muscular dystrophy. As a prelude to future clinical studies, we investigated the biodistribution and safety profile of rAAV6 in mice. Although it was present in all organs tested, rAAV6 was sequestered mainly in the liver and spleen. rAAV6 had a minimal effect on circulating blood cells and caused no apparent hepatotoxicity or coagulation activation. rAAV6 caused some neutrophil infiltration into the liver, with a transient elevation in cytokine and chemokine transcription/secretion. In summary, rAAV6 induces transient toxicity that subsides almost completely within 72 h and causes no significant side effects.
Recombinant adeno-associated virus vectors based on serotype 2 (rAAV2) are used successfully as gene transfer agents. Recently, many new serotypes of AAV were identified (3, 4), and this has driven investigations of other AAV serotypes as vector alternatives. AAV6, a less prevalent serotype that is closely related to AAV1 (10, 25), has shown promise because it can transduce certain tissues more efficiently than AAV2 can, including the lungs, heart, brain, and muscle (6, 7, 9, 14, 15, 22, 36). In particular, AAV6 transduces skeletal muscle throughout the body after intravenous administration and was used to alleviate symptoms in the mdx mouse model of muscular dystrophy (6). This in vivo tropism, possibly due to the use of N-linked sialic acids as attachment receptors (26, 36), has led to its consideration for use in a phase I clinical trial for Duchenne muscular dystrophy.
Based on studies done predominantly with AAV2, rAAV is considered safe, although inflammatory responses are initiated after AAV2 exposure. In vitro, AAV2 upregulates a few immune response genes (30), while in vivo, transient induction of chemokine and cytokine transcription is seen in livers of mice after intravenous delivery (38), indicating activation of innate immunity. This is supported by Kupffer-cell-dependent infiltration of neutrophils and CD11b+ cells into the livers of these mice (38). A complement response to AAV2 has also been shown, with iC3b mediating virus uptake by macrophages and with impaired antibody responses toward AAV2 seen in C3- or complement receptor-deficient mice (37). The last observation suggests a link between the innate immune response to AAV2 and the adaptive immune response, which was previously studied in more detail by Zaiss et al. (37).
Because different AAV serotypes use diverse attachment receptors and differ in the kinetics of virion uncoating (33), it is likely that intracellular signaling and subsequent responses differ after exposure to AAV2 or AAV6, irrespective of the different tissue tropisms. This suggests that safety data obtained with rAAV2 cannot be translated to rAAV6. Therefore, as a prelude to future clinical studies, we investigated early responses after intravenous delivery of 2 × 1012 viral genomes of rAAV6 to C57/BL6 mice. Mice were injected in the tail vein with a previously described rAAV6 vector expressing the human alkaline phosphatase gene from the cytomegalovirus (CMV) promoter (6, 7), and empty particles were eliminated from vector preparations on a cesium chloride gradient. All studies were carried out at a dose known to confer therapeutic levels of skeletal muscle transduction in mice (6), and since leakage of viral particles into the bloodstream and subsequent liver transduction have been observed after intramuscular injection of AAV2 (2, 13), these data are also relevant for other administration routes. All animal studies were performed as previously described for adenovirus (Ad) (31, 32), in accordance with the institutional guidelines set forth by the University of Washington. For comparison, we injected 1011 viral particles of a previously described (17, 29) recombinant Ad serotype 5 (rAd5) vector expressing the green fluorescent protein (GFP) gene from the CMV promoter. We used rAd5 as a positive control because it activates different extracellular, intracellular, and membrane-bound innate immune sensing systems and also activates specialized cells in vivo that are responsible for secretion of proinflammatory cytokines and chemokines that mediate inflammatory cell recruitment (11). This dose of rAd5 has been used in most biodistribution and toxicity studies (11, 16, 28).
rAAV6 in blood.
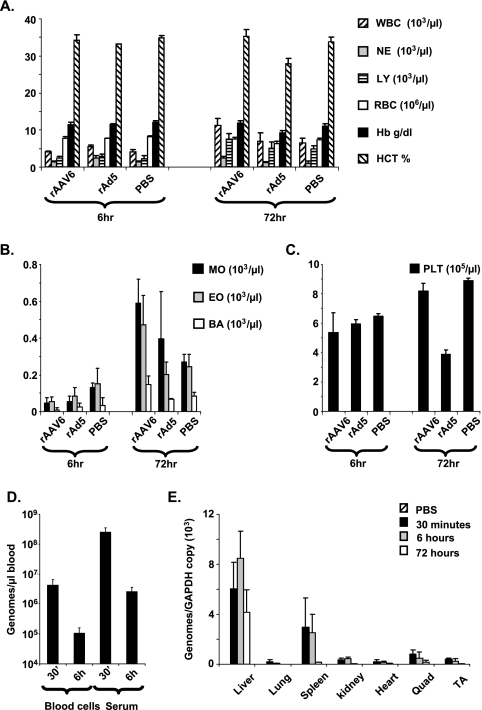
Although AAV genomes have been found in human peripheral blood mononuclear cells and in peripheral blood mononuclear cells from primates that received rAAV2 intravenously (8, 12), the fate of rAAV in circulating blood has not been studied. We first analyzed complete blood counts for mice after intravenous administration of rAAV6, rAd5, or phosphate-buffered saline (PBS) (Department of Laboratory Medicine, University of Washington). After 6 h, all cell types were unaffected by rAAV6 or rAd5 (Fig. 1A to C), but after 72 h, a moderate increase in leukocyte levels (neutrophils, monocytes, eosinophils, and basophils) was seen in rAAV6-injected mice, whereas rAd5-injected mice showed a moderate reduction in erythrocyte, hemoglobin, hematocrit, and platelet levels (Fig. 1A and C). We then looked at vector clearance from blood in serum or blood cell fractions. DNA was isolated from blood cells or serum by use of a DNeasy blood and tissue kit (Qiagen), and primers against regions of the rAAV6 vector genome were used for quantitative PCR (qPCR) analysis (SV40pA [5′-TTTTCACTGCATTCTAGTTGTGGTT-3′] and 3′ITR [5′-CATGCTCTAGTCGAGGTCGAGAT-3′]) as described previously for Ad (31, 32). All reactions were performed in triplicate and carried out in a GeneAmp 5700 instrument (Applied Biosystems). After 30 min, approximately 25% of the input dose could be found in the serum fraction (Fig. 1D), which was >1 log higher than the amount present in blood cells. By 6 h, levels in both the serum and blood cell fractions had declined by 1 to 2 log. In contrast to previous studies with Ad showing comparable amounts of Ad DNA in serum and blood cell fractions (31, 32), rAAV6 associates less with blood cells and persists longer in serum.
FIG. 1.
Blood and tissue analysis. Blood analysis was performed following intravenous delivery of 2 × 1012 viral genomes of rAAV6-CMV-hAP to C57/BL6 mice (n = 7). Measurements were taken from whole blood extracted at 6 and 72 h post-virus delivery. (A) WBC, white blood cells; NE, neutrophils; LY, lymphocytes; RBC, red blood cells; Hb, hemoglobin; HCT, hematocrit. (B) MO, monocyte; EO, eosinophil; BA, basophil. (C) PLT, platelet. rAAV6 sequestration was measured following intravenous delivery of 2 × 1012 viral genomes of rAAV6-CMV-hAP to C57/BL6 mice. (D) Vector genome levels in blood cell and serum fractions were measured at 30 min and 6 h postinjection. (E) Sequestration of vector genomes in organs was also measured at 30 min, 6 h, or 72 h postinjection. Quad, quadriceps; TA, tibialis anterior.
Tissue sequestration.
The kinetics of early rAAV6 sequestration/biodistribution have not been investigated fully. To address this, we used qPCR to analyze vector genome levels in tissues after injection. DNAs were isolated from tissues by use of a DNeasy blood and tissue kit (Qiagen), and qPCR for vector genomes was performed as described above. Levels of vector were standardized using primers against exon 1 of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (mGAPDH F [5′-ACCCAGAAGACTGTGGATGG-3′] and mGAPDH R [5′-GCAGCACGTCAGATCCACTA-3′]). Quantification revealed that while significant levels of rAAV6 were found in skeletal muscle, the majority of rAAV6 was sequestered by the liver and spleen (Fig. 1E). Nevertheless, the kinetics of rAAV6 DNA clearance from the liver and spleen were different. In the liver, rAAV6 levels did not decline significantly between 30 min and 72 h, whereas the vast majority of rAAV6 was cleared from the spleen by 72 h, indicating different rates of vector degradation in these organs. Overall, the biodistribution of rAAV6 was not very different from that previously seen for rAAV2 (5, 23, 35), which raises questions about the role of the primary attachment receptors heparan sulfate proteoglycans and N-linked sialic acids in dictating the in vivo tropism of rAAV. Notably, for Ad, it was recently found that the primary determinant for liver infection is not the attachment receptor but the ability to complex with vitamin K-dependent coagulation factors in blood (21, 27). Similar mechanisms of transduction cannot be excluded for rAAV.
Hepatotoxicity and coagulation.
Intravenous delivery of rAd vectors causes increases in serum alanine aminotransferase (ALT) levels (17), indicative of hepatotoxicity, or sCD62p and d-dimer levels, indicative of activation of coagulation (32). Six and 72 h after rAAV6 administration, we analyzed ALT (Biotron Diagnostics Inc., Hemet, CA) and sCD62p (R&D Systems, Minneapolis, MN) levels by enzyme-linked immunosorbent assay. ALT levels showed no significant increase compared to those in PBS controls, supporting previous observations for rAAV2 (38), while levels of sCD62p were also not elevated significantly (data not shown). This indicates that there is no systemic hepatotoxicity or activation of coagulation within 72 h of rAAV6 administration.
Chemokine and cytokine activation.
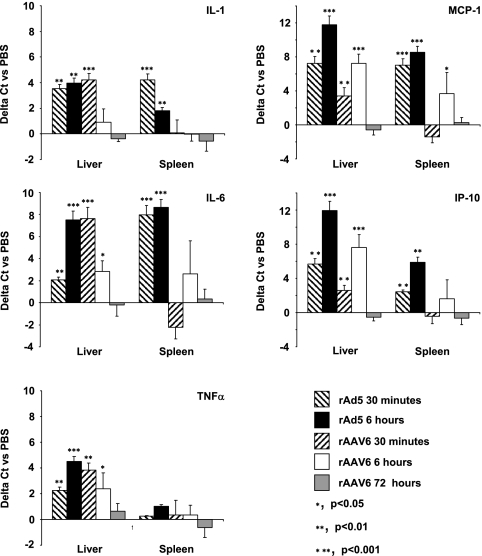
To assess activation of the innate immune system by rAAV6, we first looked at transcription levels for the cytokines/chemokines interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), macrophage chemoattractant protein 1 (MCP-1), and IP-10 after delivery. We chose the genes for these cytokines/chemokines because they are activated after intravenous rAd5 or rAAV2 delivery (18, 38). Transcription was measured in the liver and spleen, the main sites of rAAV6 sequestration, by quantitative reverse transcription-PCR analysis of mRNA levels (Fig. 2). RNAs were isolated from livers and spleens by use of an RNeasy Protect mini kit (Qiagen), and 1 μg of total RNA was converted to cDNA by use of a first-strand cDNA synthesis kit (Fermentas). Primers against exonic gene regions of the murine cytokines/chemokines IL-1β (mIL-1 βF [5′TGGTGTGTGACGTTCCCATT-3′] and mIL-1 βR [5′CAGCACGAGGCTTTTTTGTTG-3′]), IL-6 (mIL-6F [5′ACAAGTCGGAGGCTTAATTACACAT-3′] and mIL-6R [5′AATCAGAATTGCCATTGCACAA-3′]), TNF-α (mTNFαF [5′GGCTGCCCCGACTACGT-3′] and mTNFαR [5′GACTTTCTCCTGGTATGAGATAGCAA-3′]), MCP-1 (mMCP-1F [5′GAGCATCCACGTGTTGGCT-3′] and mMCP-1R [5′TGGTGAATGAGTAGCAGCAGGT-3′]), and IP-10 (mIP-10F [5′CCAGTGAGAATGAGGGCCATA-3′] and mIP-10R [5′CTCAACACGTGGGCAGGAT-3′]) were used for quantitative reverse transcription-PCR analysis. Isolated RNAs from livers and spleens of rAd5-injected animals were used as positive controls for induction of IL-1β, IL-6, TNF-α, MCP-1, and IP-10 gene transcription. In the liver, rAAV6 induced significant levels of gene transcription for IL-1β, IL-6, TNF-α, MCP-1, and IP-10. While IL-1β, IL-6, and TNF-α mRNA levels were highest after 30 min, MCP-1 and IP-10 mRNA levels peaked after 6 h. This might indicate that MCP-1 and IP-10 transcription is activated by the cytokines IL-1β, IL-6, and TNF-α, although this is not currently clear. In the spleen, rAAV6 induced only MCP-1, IL-6, and IP-10 transcription, after 6 h. Compared to that after rAd5 injection, splenic cytokine/chemokine transcription was significantly lower after rAAV6 injection, indicating that the spleen is less involved in mediating innate toxicity from rAAV6. Importantly, all cytokine/chemokine transcription levels returned to normal within 72 h of rAAV6 injection.
FIG. 2.
Induction of cytokine/chemokine transcription. Proinflammatory cytokine/chemokine transcription was measured following intravenous delivery of 2 × 1012 viral genomes of rAAV6-CMV-hAP or 1011 viral particles of Ad5-CMV-GFP to C57/BL6 mice. Induction of IL-1β, IL-6, TNF-α, MCP-1, or IP-10 gene transcription is shown as the change in cycle threshold (ΔCT) for qPCR versus that for PBS-injected control animals. For each cytokine/chemokine, time point, and vector, the average of the test ΔCT values was calculated and divided by the ΔCT value for PBS-injected animals. P values are for test groups versus PBS-injected animals.
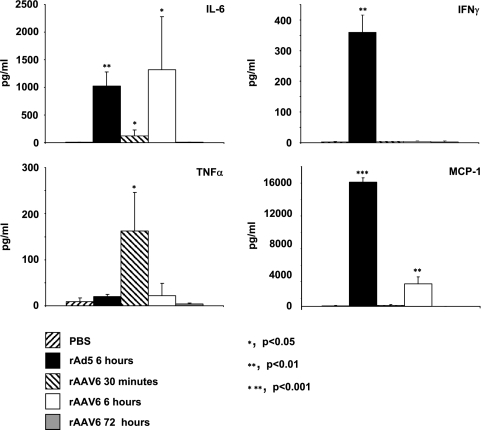
We next looked at the levels of circulating cytokines/chemokines in the sera of rAAV6-injected animals (Fig. 3), as previously described (31, 32), since serum cytokine/chemokine levels upon rAAV administration have not been studied. For IL-6, levels increased significantly after 30 min and 6 h, with the highest raise seen at 6 h. Levels at 6 h were comparable to those seen for rAd5, which is notable since significant levels of IL-6 induction were seen after 6 h in the fatal rAd5 clinical trial (24). However, upon rAAV6 exposure, IL-6 levels were normal by 72 h, whereas IL-6 remained elevated throughout the rAd5-related adverse event. For TNF-α, levels raised significantly after 30 min, with no induction seen for Ad or rAAV6 at any other time point measured. For gamma interferon, no change was seen at any time point tested. For MCP-1, rAAV6 induced a significant rise in serum levels only after 6 h, although the levels were lower than those seen for rAd5-injected animals. In general, these data suggest that although the levels are lower than those for rAd5-injected animals, cytokine/chemokine transcription and expression are induced in animals injected with rAAV6 vectors.
FIG. 3.
Serum cytokine/chemokine levels. Proinflammatory cytokine/chemokine levels were measured following intravenous delivery of 2 × 1012 viral genomes of rAAV6-CMV-hAP or 1011 viral particles of Ad5-CMV-GFP to C57/BL6 mice. Levels of IL-1β, IL-6, TNF-α, MCP-1, and IFN-γ were measured by enzyme-linked immunosorbent assay or cytometric bead array in serum taken 6 h after vector injection. P values are for test groups versus PBS-injected animals.
Histopathology.
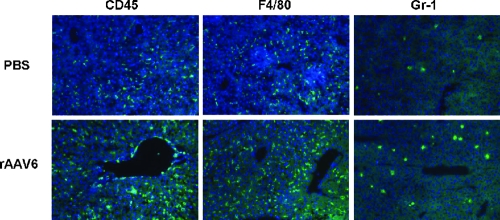
Intravenous injection of rAAV2 induces transient Kupffer cell-dependent infiltration of neutrophils and CD11b-positive cells in the liver after 1 h (38). Using previously described methods (31, 32), cryo-sections of livers from rAAV6-injected mice were stained with antibodies against leukocytes (CD45), monocytes/macrophages (F4/80), granulocytes/neutrophils (Gr-1), and cytotoxic T cells (CD8) (Fig. 4; data not shown). Positive cells were counted in 20 random 1-mm2 areas per tissue section (three per animal). While there was no significant difference in numbers of CD45-, F4/80-, and CD8-positive cells between rAAV6 and control mice at any time point (data not shown), the number of Gr-1-positive cells was significantly increased 6 h after rAAV6 injection (23 ± 7 cells/mm2 versus 10 ± 3 cells/mm2 [rAAV6-injected mice versus control mice]). By 72 h, no difference in the number of Gr-1-positive cells was seen.
FIG. 4.
Inflammation in liver 6 h after infusion of rAAV6 or PBS. Liver sections were stained for CD45, F4/80, and Gr-1.
Currently, the significance of the transient hepatic neutrophil infiltration and cytokine/chemokine expression induced by rAAV is unclear. After rAd5 injection, neutrophils associate with rAd5 in the liver (1), and neutrophil depletion reduces Ad-induced hepatotoxicty (20). This is likely linked to adaptive immune responses toward rAd5-infected cells and to elimination of transgene expression (1). After rAAV2 injection, however, the levels of hepatic neutrophil infiltration are lower than those after rAd5 injection, as are the level and duration of induced cytokine/chemokine expression (38). This is possibly a reason why rAAV, at least in mice, mediates significantly longer transgene expression than rAd5 does after intravenous delivery, since the “weak” innate immune response seen is not sufficient to help prime optimal adaptive immune responses. Whether this is true remains to be determined.
In summary, rAAV6 persists relatively long in the blood, which might explain its ability to efficiently transduce skeletal muscle. Despite this persistence, shortly after intravenous injection a large amount is sequestered in the liver and spleen. Understanding and avoiding this unspecific sequestration may enable us to reduce the effective rAAV6 dose required for muscle transduction, which in turn might bring a clinical application of this vector closer to reality. Regarding safety, intravenous rAAV6 injection causes only a transient elevation of serum cytokines, which are most likely produced by liver cells. The only pathophysiological consequences of this, however, were a minimal increase in circulating leukocyte levels after 72 h and a moderate increase in liver neutrophil numbers. It is worth noting that for our study, empty rAAV6 particles were separated from full (genome-containing) particles prior to intravenous delivery, meaning that the input particle dose was equivalent to the genome number. In prior studies of rAAV2-mediated toxicity, empty and full particles were not separated prior to administration, causing the actual particle number administered to be significantly higher than the indicated genome number. This is particularly significant because empty rAAV2 particles induce transcriptional changes at similar levels to those induced by full particles (30) and may influence the levels of toxicity seen after administration. It is also notable that these studies with mice cannot necessarily be translated to larger animals or humans. This is demonstrated by findings that intravenous rAAV2 application results in long-term transgene expression in mice and dogs but not in humans (19). The loss of transgene expression in humans is attributed to an adaptive anti-rAAV2 capsid immune response, which is affected by biodistribution and innate responses shortly after vector administration. Furthermore, a recent study showed an immunomediated decline in transgene expression after intravenous injection of rAAV6 in dogs, while the same vector conferred long-term transgene expression in mice (34). Clearly, more toxicity studies with rAAV6 in large animals and humans are required, but our finding that rAAV6 is safe in mice opens the avenue for these studies.
Acknowledgments
We thank Paul Gregorevic for helpful discussions.
This work was supported by NIH grant U54HD47175 and by a pilot award from the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Cotter, M. J., and D. A. Muruve. 2005. The induction of inflammation by adenovirus vectors used for gene therapy. Front. Biosci. 101098-1105. [DOI] [PubMed] [Google Scholar]
- 2.Favre, D., N. Provost, V. Blouin, G. Blancho, Y. Cherel, A. Salvetti, and P. Moullier. 2001. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol. Ther. 4559-566. [DOI] [PubMed] [Google Scholar]
- 3.Gao, G., L. H. Vandenberghe, and J. M. Wilson. 2005. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 5285-297. [DOI] [PubMed] [Google Scholar]
- 4.Gao, G. P., Y. Lu, X. Sun, J. Johnston, R. Calcedo, R. Grant, and J. M. Wilson. 2006. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J. Virol. 806192-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonin, P., and C. Gaillard. 2004. Gene transfer vector biodistribution: pivotal safety studies in clinical gene therapy development. Gene Ther. 11(Suppl. 1)S98-S108. [DOI] [PubMed] [Google Scholar]
- 6.Gregorevic, P., J. M. Allen, E. Minami, M. J. Blankinship, M. Haraguchi, L. Meuse, E. Finn, M. E. Adams, S. C. Froehner, C. E. Murry, and J. S. Chamberlain. 2006. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 12787-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorevic, P., M. J. Blankinship, J. M. Allen, R. W. Crawford, L. Meuse, D. G. Miller, D. W. Russell, and J. S. Chamberlain. 2004. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman, Z., E. Mendelson, F. Brok-Simoni, F. Mileguir, Y. Leitner, G. Rechavi, and B. Ramot. 1992. Detection of adeno-associated virus type 2 in human peripheral blood cells. J. Gen. Virol. 73961-966. [DOI] [PubMed] [Google Scholar]
- 9.Halbert, C. L., J. M. Allen, and A. D. Miller. 2001. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 756615-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halbert, C. L., A. D. Miller, S. McNamara, J. Emerson, R. L. Gibson, B. Ramsey, and M. L. Aitken. 2006. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: implications for gene therapy using AAV vectors. Hum. Gene Ther. 17440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman, Z. C., D. M. Appledorn, and A. Amalfitano. 2008. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 1321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez, Y. J., J. Wang, W. G. Kearns, S. Loiler, A. Poirier, and T. R. Flotte. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 738549-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog, R. W., E. Y. Yang, L. B. Couto, J. N. Hagstrom, D. Elwell, P. A. Fields, M. Burton, D. A. Bellinger, M. S. Read, K. M. Brinkhous, G. M. Podsakoff, T. C. Nichols, G. J. Kurtzman, and K. A. High. 1999. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat. Med. 556-63. [DOI] [PubMed] [Google Scholar]
- 14.Huszthy, P. C., A. Svendsen, J. M. Wilson, R. M. Kotin, P. E. Lonning, R. Bjerkvig, and F. Hoover. 2005. Widespread dispersion of adeno-associated virus serotype 1 and adeno-associated virus serotype 6 vectors in the rat central nervous system and in human glioblastoma multiforme xenografts. Hum. Gene Ther. 16381-392. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, H., D. Lillicrap, S. Patarroyo-White, T. Liu, X. Qian, C. D. Scallan, S. Powell, T. Keller, M. McMurray, A. Labelle, D. Nagy, J. A. Vargas, S. Zhou, L. B. Couto, and G. F. Pierce. 2006. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 108107-115. [DOI] [PubMed] [Google Scholar]
- 16.Kay, M. A., F. Graham, F. Leland, and S. L. Woo. 1995. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology 21815-819. [PubMed] [Google Scholar]
- 17.Lieber, A., C. Y. He, I. Kirillova, and M. A. Kay. 1996. Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J. Virol. 708944-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 718798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manno, C. S., G. F. Pierce, V. R. Arruda, B. Glader, M. Ragni, J. J. Rasko, M. C. Ozelo, K. Hoots, P. Blatt, B. Konkle, M. Dake, R. Kaye, M. Razavi, A. Zajko, J. Zehnder, P. K. Rustagi, H. Nakai, A. Chew, D. Leonard, J. F. Wright, R. R. Lessard, J. M. Sommer, M. Tigges, D. Sabatino, A. Luk, H. Jiang, F. Mingozzi, L. Couto, H. C. Ertl, K. A. High, and M. A. Kay. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12342-347. [DOI] [PubMed] [Google Scholar]
- 20.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10965-976. [DOI] [PubMed] [Google Scholar]
- 21.Parker, A. L., S. N. Waddington, C. G. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 1082554-2561. [DOI] [PubMed] [Google Scholar]
- 22.Pleger, S. T., P. Most, M. Boucher, S. Soltys, J. K. Chuprun, W. Pleger, E. Gao, A. Dasgupta, G. Rengo, A. Remppis, H. A. Katus, A. D. Eckhart, J. E. Rabinowitz, and W. J. Koch. 2007. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation 1152506-2515. [DOI] [PubMed] [Google Scholar]
- 23.Ponnazhagan, S., P. Mukherjee, M. C. Yoder, X. S. Wang, S. Z. Zhou, J. Kaplan, S. Wadsworth, and A. Srivastava. 1997. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene 190203-210. [DOI] [PubMed] [Google Scholar]
- 24.Raper, S. E., N. Chirmule, F. S. Lee, N. A. Wivel, A. Bagg, G. P. Gao, J. M. Wilson, and M. L. Batshaw. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80148-158. [DOI] [PubMed] [Google Scholar]
- 25.Rutledge, E. A., C. L. Halbert, and D. W. Russell. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seiler, M. P., A. D. Miller, J. Zabner, and C. L. Halbert. 2006. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum. Gene Ther. 1710-19. [DOI] [PubMed] [Google Scholar]
- 27.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 797478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2004. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 785368-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 742567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stilwell, J. L., and R. J. Samulski. 2004. Role of viral vectors and virion shells in cellular gene expression. Mol. Ther. 9337-346. [DOI] [PubMed] [Google Scholar]
- 31.Stone, D., Y. Liu, Z. Y. Li, S. Tuve, R. Strauss, and A. Lieber. 2007. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol. Ther. 152146-2153. [DOI] [PubMed] [Google Scholar]
- 32.Stone, D., Y. Liu, D. Shayakhmetov, Z. Y. Li, S. Ni, and A. Lieber. 2007. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 814866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, C. E., T. A. Storm, Z. Huang, and M. A. Kay. 2004. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 783110-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, L., J. Figueredo, R. Calcedo, J. Lin, and J. M. Wilson. 2007. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum. Gene Ther. 18185-194. [DOI] [PubMed] [Google Scholar]
- 35.Watson, G. L., J. N. Sayles, C. Chen, S. S. Elliger, C. A. Elliger, N. R. Raju, G. J. Kurtzman, and G. M. Podsakoff. 1998. Treatment of lysosomal storage disease in MPS VII mice using a recombinant adeno-associated virus. Gene Ther. 51642-1649. [DOI] [PubMed] [Google Scholar]
- 36.Wu, Z., A. Asokan, and R. J. Samulski. 2006. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14316-327. [DOI] [PubMed] [Google Scholar]
- 37.Zaiss, A. K., M. J. Cotter, L. R. White, S. A. Clark, N. C. Wong, V. M. Holers, J. S. Bartlett, and D. A. Muruve. 2008. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 822727-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaiss, A. K., Q. Liu, G. P. Bowen, N. C. Wong, J. S. Bartlett, and D. A. Muruve. 2002. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 764580-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]