Abstract
We previously showed that herpes simplex virus type 1 (HSV-1) immediate-early (IE) protein ICP27 can posttranscriptionally stimulate mRNA accumulation from a transfected viral late gene encoding glycoprotein C (gC) (K. D. Perkins, J. Gregonis, S. Borge, and S. A. Rice, J. Virol. 77:9872-9884, 2003). We began this study by asking whether ICP27 homologs from other herpesviruses can also mediate this activity. Although the homologs from varicella-zoster virus (VZV) and human cytomegalovirus (HCMV) were inactive, the homolog from bovine herpesvirus 4 (BHV-4), termed HORF1/2, was a very efficient transactivator. Surprisingly, most of the mRNA produced via HORF1/2 transactivation was 225 nucleotides shorter than expected due to the removal of a previously undescribed intron from the gC transcript. We found that the gC mRNA produced in the absence of transactivation was also mostly spliced. In contrast, gC mRNA produced by ICP27 transactivation was predominantly unspliced. Based on these results, we conclude that ICP27 has two distinct effects on the transfected gC gene: it (i) stimulates mRNA accumulation and (ii) promotes the retention of an intron. Interestingly, the spliced transcript encodes a variant of gC that lacks its transmembrane domain and is secreted from transfected cells. As the gC splicing signals are conserved among several HSV-1 strains, we investigated whether the variant gC is expressed during viral infection. We report here that both the spliced transcript and its encoded protein are readily detected in Vero cells infected with three different laboratory strains of wild-type HSV-1. Moreover, the variant gC is efficiently secreted from infected cells. We have designated this alternate form of the protein as gCsec. As the extracellular domain of gC is known to bind heparan sulfate-containing proteoglycans and to inhibit the complement cascade via an interaction with complement component C3b, we speculate that gCsec could function as a secreted virulence factor.
During the productive infection of cells with herpes simplex virus type 1 (HSV-1), the immediate-early (IE) protein ICP27 plays an important role in inducing the expression of the delayed-early (DE) and late (L) genes. However, the 75 or so DE/L genes vary significantly in their dependence upon ICP27 (48, 56, 59, 70, 72). Some, such as the DE gene UL29, do not require ICP27 at all (56), whereas others are either modestly or highly dependent on ICP27. Examples of genes that are highly dependent on ICP27 include the DE gene UL42 (72), encoding a DNA polymerase processivity factor, and the L gene UL44, encoding the membrane glycoprotein gC (56). The determinants that govern whether, and to what extent, a particular HSV-1 DE/L gene requires ICP27 for its expression are not understood. Similarly, the mechanisms by which ICP27 induces responsive genes are not yet completely defined. As ICP27 has been ascribed several regulatory functions, it is quite possible that multiple mechanisms come into play. There is evidence that ICP27 promotes gene expression by increasing transcription rates (35), stimulating mRNA polyadenylation (48), preventing mRNA degradation (5, 12), and enhancing translation (15, 17, 39). Notably, a number of studies have suggested that a key function of ICP27 in regards to inducing viral genes is its ability to promote the nuclear export of viral mRNAs (37, 60, 68). Viral DE/L mRNAs may require a special mechanism for export since they are predominantly unspliced and therefore do not have access to the major cellular pathway for mRNA export, in which export is coupled to pre-mRNA splicing (62). An emerging model is that ICP27, via its RNA-binding activity (33, 50), nucleocytoplasmic shuttling activity (49, 53, 60, 68), and interactions with cellular export factors REF and NXF1 (9, 10), can bind to unspliced viral mRNAs in the nucleus and help mediate their transport to the cytoplasm (10, 37).
In addition to stimulating viral gene expression, ICP27 carries out other regulatory functions during infection. It is required for the activation of NF-κB and the stress-activated protein kinases Jun N-terminal kinase and p38 (26, 27), and it promotes the cytoplasmic accumulation and virion packaging of the IE proteins ICP4 and ICP0 (66, 79, 80). In addition, a well-described function of ICP27 is its ability to inhibit host cell pre-mRNA splicing, contributing to viral shutoff of host cell macromolecular synthesis (7, 24, 25, 41). This effect appears to be due at least in part to ICP27's physical interaction with host cell SR-protein kinase 1 (SRPK1), resulting in the hypophosphorylation of SR protein splicing factors (65).
ICP27 is the only one of the five HSV-1 IE genes that has clear homologs in all characterized mammalian herpesviruses (47). This suggests that ICP27 and its relatives carry out a fundamental function (or functions) in viral replication. In general, the ICP27 homologs that have been studied are similar to ICP27 in that they stimulate viral gene expression, and in some cases this can be shown to occur at a posttranscriptional level (13, 22, 42, 45, 77, 78). Moreover, genetic studies have revealed that these proteins are required for optimal viral growth in cultured cells (21, 23, 44, 64). However, in most cases, ICP27 homologs from heterologous herpesviruses fail to complement the growth of HSV-1 null mutants (51, 78), although the Epstein-Barr virus (EBV) homolog SM shows weak complementation ability (3). Therefore, although ICP27 and its homologs share common functional properties, there are elements of specificity that are not yet understood.
In an attempt to further uncover the mechanism by which ICP27 transactivates target genes, we have focused on the gC gene as a model target. During infection, gC mRNA and protein expression are highly dependent on ICP27 (56, 67). The expression of gC is also strongly dependent on viral DNA replication, making it a “true-late” or γ2 gene (29). We have described a class of viral ICP27 mutants that efficiently replicate their DNA but which do not express gC mRNA (56, 57). This indicates that ICP27's stimulatory effect on gC is distinct from the effect of viral DNA synthesis. Consistent with this view, we found that the intact gC gene, removed from the context of the viral genome, remains responsive to ICP27 in a plasmid cotransfection assay (52).
An interesting finding from our earlier transfection study involved the plasmid pgCΔpro (52). In this construct, the gC gene was placed downstream of the normally powerful human cytomegalovirus (HCMV) IE promoter (Fig. 1A). However, this plasmid expressed very little gC mRNA unless ICP27 was supplied in trans. Using nuclear run-on analyses, we showed that the effect of ICP27 was posttranscriptional. Based on these results, we proposed that there is an element in the body of the gC gene that silences expression and that ICP27 overcomes the effect of this element. We began the present study by asking whether ICP27 homologs from other herpesviruses can also activate the gC gene in pgCΔpro. We identified one homolog, the HORF1/2 protein from bovine herpesvirus 4 (BHV-4), that is quite proficient at inducing gC mRNA expression. Surprisingly, the gC mRNA produced by HORF1/2 transactivation is structurally different than that produced via ICP27 transactivation. Investigation of this phenomenon has led to the discovery of a previously undescribed intron in the gC gene. We show that this intron is utilized in HSV-1-infected cells, leading to the production of a secreted variant of gC.
FIG. 1.
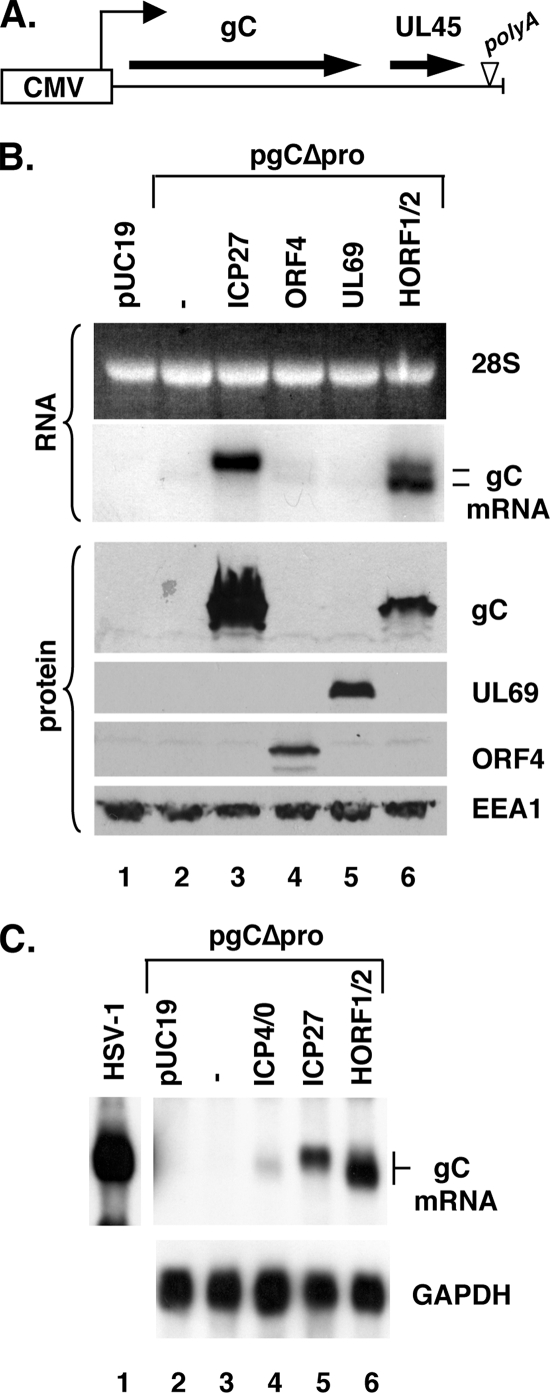
Transactivation of the gC gene in transfected cells by ICP27 or ICP27 homologs. (A) Representation of the modified gC gene residing on plasmid pgCΔpro. Transcription of the gene in this construct is driven by the HCMV IE promoter. The small arrow indicates the transcription start site. The gC and UL45 open reading frames are indicated by the large arrows. Note that the gC and UL45 genes are independently transcribed but share a polyadenylation signal, denoted by the inverted triangle. (B) BHV-4 HORF1/2 transactivates gC expression. Vero cells were transfected with pUC19 only, or with pgCΔpro plus or minus plasmids encoding ICP27 or ICP27 homologs. Three-fourths of the transfection mixture was added to cultures for RNA analysis (top panels), while one-quarter was added to separate cultures for protein analysis (bottom panels). Two days later, total RNA and protein were prepared. The RNA was subjected to Northern blotting using a glyoxal gel. The ethidium bromide stain of the gel shows comparable loading of 28S rRNA. Below, the gC hybridization signal is shown. The bottom panel shows immunoblotting analysis for the antigens indicated. EEA1 is a cellular antigen that serves as a loading control. (C) Transactivation of pgCΔpro by ICP27, ICP4/0, and HORF1/2. Transfections were carried out as for panel B. Total RNA, harvested 2 days posttransfection, was analyzed by Northern analysis for gC (top) and GAPDH (bottom) by using a formaldehyde gel. Lane 1 contains RNA from infected cells; a shorter autoradiographic exposure of this lane is shown.
MATERIALS AND METHODS
Cells, viruses, and infections.
Vero (African green monkey kidney cells) and ARPE-19 (human retinal pigment epithelial cells) (14) cell lines were obtained from the American Type Culture Collection. Human diploid fibroblasts, life extended by stable transfection of the gene for the catalytic subunit of human telomerase (4), were obtained from Wade Bresnahan. The wild-type (WT) strains of HSV-1 used were KOS1.1 (32), Patton, and 17. Infections were carried out at a multiplicity of infection (MOI) of 10 PFU per cell in phosphate-buffered saline containing 0.1% glucose and 0.1% heat-inactivated newborn calf serum. Viral absorption was for 1 h at 37°C, at which time the viral inoculum was replaced with 199 medium containing 2% heat-inactivated newborn calf serum, 50 U of penicillin/ml, and 50 μg of streptomycin/ml. All infected cells were incubated thereafter at 37°C.
The viral mutant mLS1 was constructed by a marker transfer technique (56). Briefly, a HindIII-PstI restriction fragment from pgC-mut (described below) was cotransfected into Vero cells with infectious KOS1.1 DNA. Plaques arising from this cotransfection were screened by the following procedure. Infected-cell DNAs were isolated from Vero cells infected with the plaques, and the gC-UL45 region of the HSV-1 genome was PCR amplified. The PCR products were then tested for the loss of a Bsu36I restriction site which is diagnostic for the 3′ splice site mutation (see below). A positive virus isolate was designated mLS1, plaque purified, and expanded into a viral stock. The mutation in mLS1 was confirmed by DNA sequencing.
Plasmids and transfections.
The plasmid pgCΔpro, which has the HSV-1 gC gene under the control of the cytomegalovirus (CMV) IE promoter, has been previously described (52). The plasmids pC27 (49) and pSG1 (20) were used to express ICP27 and ICP0/ICP4, respectively. The plasmid used to express the HCMV UL69 protein was pHM160 (78).
For analysis of varicella-zoster virus (VZV) ORF4, an expression vector was made. To do this, we first obtained pSstIc, a VZV genomic clone containing the ORF4 gene, from Andrew Davison. The ORF4 gene, including its endogenous promoter, was then subcloned as a 3.0-kb PvuII fragment into the SmaI site of pUC19. This plasmid was designated pORF4. pORF4 was digested with TseI, which cuts just upstream of the ORF4 initiation codon, as well as downstream of the coding region. The 3′ recessed ends of the resulting 2.0-kb fragment were repaired with a Klenow fragment, and the fragment was cloned into pUC19 at the SmaI site. This plasmid was designated pORF4Tse. The ORF4 gene was released by digestion with EcoRI and SalI and cloned into the eukaryotic expression vector pCI-neo (Promega) at the EcoRI/SalI sites. This plasmid, designated pORF4Δpro, served as the ORF4 expression construct.
The BHV-4 HORF1/2 gene, homologous to ICP27, was cloned and expressed as follows. HORF1/2 is expressed in BHV-4 from a spliced mRNA (V. L. van Santen, unpublished data) containing the first and second rightward reading frames of the HindIII-O fragment and was named using the EBV naming system. First, the HORF1/2 gene was assembled from cloned BHV-4 strain DN-599 fragments by subcloning the 2.5-kb BHV-4 HindIII-O fragment, isolated from pH 149 (73), and a 2.5-kb HindIII-PvuII subfragment of the BHV-4 HindIII-M fragment, isolated from pH 1 (73), between the HindIII and SmaI sites of pTZ19U. The resulting plasmid (pH149H1HP2.5) contains the 1.6-kb sequence encoding the complete HORF1/2 pre-mRNA, plus approximately 1.8 kb of 5′-flanking sequences and 1.5 kb of 3′-flanking sequences. The sequence of the HORF1/2 gene, including 391 nucleotides 5′ to the transcription start site and 40 nucleotides 3′ to the polyadenylation site, can be found under GenBank accession number U30519. HORF1/2 is expressed as an early gene in infected cells (8) and requires the BHV-4 R transactivator for expression from its own promoter (van Santen, unpublished). Therefore, the HORF1/2 gene was placed under control of the simian virus 40 early promoter in a vector derived from pSV-β-galactosidase (Promega Corporation, Madison, WI). The vector was cleaved with HindIII and BamHI to remove the β-galactosidase coding sequences. The HindIII-EcoRI fragment containing the 3′ portion of the HORF1/2 gene, including polyadenylation signal, was isolated from pH149H1HP2.5 and ligated to the pSV-β-galactosidase-derived vector after repair of the BamHI and EcoRI-cleaved ends with Klenow polymerase. The SmaI fragment beginning approximately 25 bp 3′ to the start of transcription of the HORF1/2 gene was isolated from pH149H1HP2.5 and was cloned into pTZ19U, to add a vector-derived HindIII site near the 5′ end of the HORF1/2 gene. The HindIII fragment containing the 5′ portion of the HORF1/2 gene was then isolated from this intermediate plasmid and inserted into the HindIII site of the pSV-β-galactosidase-derived vector already containing the 3′ portion of HORF1/2, between the simian virus 40 promoter and the 3′ portion of HORF1/2, to generate pSVHORF1/2, the expression vector used in our studies.
To functionally inactivate the intron in pgCΔpro, oligonucleotide-directed mutagenesis was used to alter the strong 3′ splice site, changing the AG dinucleotide at the end of the intron to GG. This was done using the QuikChange site-directed mutagenesis system (Stratagene). The change destroys a Bsu36I site, and thus, potential clones were screened for loss of this site. A positive clone was designated pgCΔpro-mut, and its sequence was confirmed by DNA sequencing. For construction of a virus mutant, the splice site mutation was introduced into the background of pgC (52), giving rise to pgC-mut.
Transfections were done by either of two methods. Some transfections were performed using the calcium phosphate technique, as previously described (52, 55). Alternately, transfections were done using the Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's recommended protocol. For analysis of RNA in 10-cm-diameter dishes, 12 μg of pgCΔpro and 12 μg of transactivator plasmid were used. For analysis of protein in 25-cm2 flasks, 4 μg of pgCΔpro and 12 μg of transactivator plasmid were used in most experiments. However, in the experiment shown in Fig. 4, done with the Lipofectamine 2000 reagent, 4 μg of pgCΔpro and 4 μg of transactivator plasmid were used. For transfections lacking either or both of the above constituents, pUC19 or salmon sperm DNA was substituted in order to maintain the same total amount of DNA in each transfection.
FIG. 4.
The spliced transcript encodes a secreted form of gC. (A) Standard and variant forms of gC. At the top, a diagram of the major characterized form of gC is shown; the black bar represents the transmembrane domain (TM). The C-terminal residues are shown; the sequence of the transmembrane domain is underlined. Below, a diagram of the variant form encoded by the spliced mRNA is shown. The amino acid residues in the alternate C terminus are indicated. (B) Secretion of gC from transfected cells. Vero cells were transfected with the plasmids indicated, and both the cells and media were harvested 2 days posttransfection. Soluble gC from the media was collected by heparin-Sepharose chromatography. Cell-associated gC and the soluble gC from the media were analyzed by immunoblotting. Cell-associated EEA1 was also analyzed as a loading control. The left and right panels show short and long exposures of the immunoblot, respectively.
RNA analysis.
Total RNA was prepared using the Trizol reagent (Invitrogen) and the protocol supplied by the manufacturer. For preparation of separate nuclear and cytoplasmic RNA fractions, the RNeasy kit (Qiagen) was used. All RNA preparations were treated with RNase-free DNase (Roche Diagnostics Corp.) to remove contaminating DNA. For analysis of poly(A) tails by oligo(dT)-directed RNase H cleavage, poly(A)+ RNA was selected from 250 μg of total transfected cell RNA using the Oligotex mRNA kit (Qiagen). To specifically remove poly(A) tails, the poly(A)+ RNA was treated with oligo(dT) and RNase H as described by Cheung et al. (11).
Two procedures were used for Northern blotting analysis. In some cases, the RNA samples were subjected to electrophoresis through denaturing formaldehyde-agarose gels (40). In other cases, dimethyl sulfoxide-glyoxal treatment was used to denature the RNA. For glyoxal treatment, the RNA samples were incubated in dimethyl sulfoxide-glyoxal by using Glyoxal Sample Loading Dye (Ambion) according to the manufacturer's instructions. The RNA was then electrophoresed at 5 V/cm for ∼2 h on a 1.5% agarose gel in electrophoresis buffer consisting of 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 30 mM Bis-Tris, and 1 mM EDTA, pH 6.5. For both Northern blotting methods, blotting and hybridization were carried out as previously described (52). Several probes were used for detection of gC mRNA. In some cases (Fig. 1C and Fig. 2), the probe was the gel-purified PstI-HindIII 3.0-kb insert of pgC. In others, the probe was the linearized pEcoRI-BamHI-1-1 plasmid (18). For the experiment shown in Fig. 6, either an 813-bp EcoNI-EcoRV fragment or a 216-bp SphI-BamHI fragment (labeled probes 1 and 2, respectively) was used as a probe. The probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was a 1.3-kb EcoRI fragment of mouse GAPDH cDNA. All of the above probes were labeled with 32P by using a random primer-labeling kit (Invitrogen). The probe used for U3 snoRNA was a specific oligonucleotide, 5′ end labeled with 32P by using polynucleotide kinase as described by Cheung et al. (11).
FIG. 2.
The transactivator-specific difference in gC mRNA structure is generated in the nucleus and is not due to poly(A) tail length. (A) Comparison of nuclear and cytoplasmic mRNAs. Vero cells were transfected as for Fig. 1, and both nuclear and cytoplasmic RNAs were isolated at 2 days posttransfection. Ten micrograms of nuclear or cytoplasmic RNA was analyzed by Northern blotting after electrophoresis on formaldehyde gels. The blots were probed for gC (top) or the nucleus-specific U3 snoRNA (bottom). (B) Analysis of the effect of poly(A) tail length. Vero cells were transfected with pgCΔpro and a transactivator plasmid encoding either ICP27 or HORF1/2, and total RNA was harvested at 2 days posttransfection. Equal amounts of RNA were subjected to poly(A)+ RNA purification, and the 3′ poly(A) tails were specifically removed by treatment with oligo(dT) and RNase H (lanes 3 and 4). gC sequences were detected by Northern blotting. As controls, 10 μg of total transfected cell RNA (lanes 1 and 2) or 3 μg of HSV-1-infected cell RNA was also analyzed (lane 5).
FIG. 6.
Analysis of HSV-1 mutant mLS1. (A) Viral yield assays. Vero cells were infected with WT or mLS1 at the MOIs shown. Viral progeny were harvested at 24 hpi, and viral titers were determined by plaque assay on Vero cells. (B) Expression of the spliced gC transcript. RNA was prepared from mock- or HSV-1-infected Vero cells at 7 or 20 hpi and analyzed for gC splicing using the RT-PCR assay described for Fig. 3. Bands arising from unspliced (u) and spliced (s) gC mRNAs are indicated. (C) Expression of the spliced transcript in other cells. Analysis was as for panel B, but life-extended human fibroblasts or ARPE-19 cells were used and RNA was harvested at 16 hpi. (D) Strategy for Northern blotting analysis. The unspliced and spliced forms of the gC transcript (transcripts a and b, respectively) are shown, as is the UL45 transcript (transcript c). (E) Northern blotting. The RNA utilized in panel B was analyzed by Northern blotting using probe 1 (top) or 2 (bottom). For the blot analyzed with probe 2, only the region containing the UL45 transcript is shown. Numbers at left are sizes in kilobases.
Reverse transcription-PCRs (RT-PCRs) were done using the OneStep RT-PCR kit (Qiagen). The forward primer was 5′-GGCTGGCCCTGGTGCTGCCG, and the reverse primer was 5′-CCTTCCCTCCCCCTCCGCATCC. For cloning of products, ethidium bromide-stained DNA bands were excised from agarose gels, purified, and cloned in pCR2.1-TOPO by using a TOPO-TA cloning kit (Invitrogen). DNA sequencing of the inserts was carried out at the University of Minnesota Biomedical Genomics Center.
Protein analysis.
Preparation of protein samples from transfected cells and immunoblotting were carried out as previously described (52). The following antibodies were used for immunoblotting: for gC, a 1:300 dilution of mouse monoclonal antibody (MAb) H1104 (Rumbaugh-Goodwin Institute); for gD, a 1:3,000 dilution of mouse MAb H1103 (Rumbaugh-Goodwin Institute); for VP5, a 1:1,000 dilution of mouse MAb (Abcam); for UL45, a 1:1,000 dilution of rabbit polyclonal antiserum (a gift from Curtis Brandt); for cellular early endosomal antigen 1 (EEA1), a 1:2,500 dilution of a mouse MAb (BD Biosciences, San Jose, CA); for VZV ORF4, a 1:1,000 dilution of rabbit polyclonal antiserum (a gift from Jeff Cohen); and for HCMV UL69, a 1:500 dilution of rabbit polyclonal antiserum (a gift from Wade Bresnahan). Rabbit antiserum specific for peptide immunogen VILGRSRTTHGVEQNASP was produced and supplied by Invitrogen Corporation. It was used at a dilution of 1:1,000. The secondary antibodies used for immunoblot detection were horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit immunoglobulin G, purchased from Jackson ImmunoResearch (West Grove, PA), and were both diluted 1:7,500. Secondary antibodies were detected with enhanced chemiluminescence Western blotting detection reagents (Amersham).
Heparin affinity chromatography.
Transfected or infected cells and media from 25-cm2 flasks were collected, and cell lysates were prepared as described above. To remove cellular debris, cell medium was centrifuged (1,000 × g, 30 min, 4°C). Medium from infected cells was subjected to another centrifugation to remove virions (23,000 × g, 2 h, 4°C). The cleared supernatant was mixed with a 50% solution of heparin-Sepharose beads (Heparin Sepharose 6 Fast Flow; GE Healthcare, Amersham Biosciences) and incubated overnight at 4°C with continuous mixing. The heparin-Sepharose beads with attached proteins were pelleted (200 × g, 3 min, 4°C), washed twice with phosphate-buffered saline containing protease inhibitors (50 μg of TLCK [Nα-p-tosyl-l-lysine chloromethyl ketone 2] per ml and 25 μg of phenylmethylsulfonyl fluoride per ml), and resuspended in 2× sodium dodecyl sulfate-polyacrylamide gel sample buffer. After 10 min of boiling with occasional mixing, the beads were again pelleted and the supernatant fraction was analyzed by immunoblotting.
RESULTS
Transactivation of the HSV-1 gC gene by ICP27 and a BHV-4 homolog.
We previously showed that ICP27 posttranscriptionally stimulates gC mRNA production from plasmid pgCΔpro, which has the HSV-1 gC gene under the control of the strong CMV IE promoter (Fig. 1A) (52). We began this study by asking whether ICP27 homologs from other herpesviruses can also transactivate gC in this assay. Three homologs were analyzed: the ORF4 gene from VZV, an alphaherpesvirus; the UL69 gene from HCMV, a betaherpesvirus; and the HORF1/2 gene from BHV-4, a gammaherpesvirus. To carry out the analysis, pgCΔpro was transfected into Vero cells, either alone or with plasmids encoding ICP27 or its homologs. Total RNA and protein were prepared at 2 days posttransfection, and gC expression was analyzed by Northern blotting (Fig. 1B, top two panels) and immunoblotting (bottom panels). Consistent with our previous results, the CMV IE promoter-driven gC gene was not expressed efficiently in the absence of transactivation (lane 2), whereas coexpression of ICP27 led to readily detectable mRNA and protein (lane 3). Among the ICP27 homologs, both the VZV ORF4 and HCMV UL69 (lanes 4 and 5, respectively) failed to transactivate the gC gene. This was not due to their lack of expression, as both proteins were readily detectable by immunoblotting. In contrast, BHV-4 HORF1/2 efficiently induced gC mRNA and protein (lane 6). Thus, it appears that some but not all ICP27 homologs can stimulate expression of the gC gene in this assay.
Although HORF1/2 transactivated the gC gene, we noted that a substantial fraction of the gC mRNA produced by HORF1/2 transactivation had a somewhat faster electrophoretic mobility than that induced by ICP27 (Fig. 1B, compare lanes 3 and 6). The ICP27-induced transcripts had a mobility consistent with the expected ∼2.7-kb size of polyadenylated gC mRNA (18) (data not shown), whereas the majority of the HORF1/2-induced transcripts migrated as if they were ∼0.2 kb smaller.
We previously showed that expression of the gC gene on pgCΔpro can also be induced by cotransfection of a plasmid encoding both the HSV-1 IE proteins ICP4 and ICP0, which are known transcriptional activators (52). To further investigate the electrophoretic mobility difference noted above, we did a side-by-side comparison of the mRNAs induced from pgCΔpro by ICP27, HORF1/2, and ICP4/0 transactivation. The Northern blotting results indicated that ICP4/0-transactivated gC mRNA (Fig. 1C, lane 4) resembled HORF1/2-transactivated RNA (lane 6) in its increased electrophoretic mobility compared to that of ICP27-transactivated mRNA (lane 5). Thus, it appears that two distinct sets of transactivators (HORF1/2 and ICP4/0) lead to a faster-migrating form of gC mRNA compared to the form induced by ICP27.
As a technical note, we noted over the course of our Northern blotting studies that glyoxal gel electrophoresis (e.g., as performed in the experiment shown in Fig. 1B) was superior to formaldehyde gel electrophoresis (as performed in the experiment shown in Fig. 1C) in resolving the gC mRNA species produced from pgCΔpro. When glyoxal gel electrophoresis was employed, the mRNA arising from HORF1/2 transactivation often resolved into two distinct species, the upper one of which comigrated with the ICP27-transactivated mRNA. In contrast, formaldehyde denaturation failed to resolve the HORF1/2-activated gC mRNA into distinct species, although it was apparent that much of the material had a faster electrophoretic mobility than that of ICP27-transactivated mRNA.
The transactivator-specific difference in gC mRNA is generated in the nucleus and is not due to poly(A) tail length.
We next carried out experiments to determine the basis of the electrophoretic mobility difference in gC mRNA. First, we looked at the nucleocytoplasmic distribution of the mRNAs. Vero cells were transfected with pgCΔpro in the absence or presence of ICP27 or HORF1/2, and cytoplasmic and nuclear RNA fractions were prepared 2 days posttransfection. The RNA samples were analyzed by Northern blotting (Fig. 2A). Analysis of the U3 small nucleolar RNA (bottom panel) confirmed that this nuclear fraction-specific RNA was largely confined to the nuclear fraction, attesting that the cytoplasmic fraction was free of nuclear contamination. Analysis of gC mRNA (top panel) revealed two important points. First, the electrophoretic mobility difference between the ICP27- and HORF1/2-transactivated gC mRNAs was observed in both the nuclear and the cytoplasmic RNA fractions. This suggests that the difference is generated in the nucleus, prior to export of the mRNA to the cytoplasm. Second, quantitation of the signals by phosphorimaging indicated that the ratios of nuclear to cytoplasmic gC RNA were very similar in the two preparations. Specifically, for the ICP27-transactivated mRNA, 65.5% of the total was in the cytoplasm, whereas for the HORF1/2-transactivated RNA, 71.1% was cytoplasmic. This indicates that the difference in gC mRNA electrophoretic mobility is not associated with a difference in the nucleocytoplasmic distribution of the mRNAs.
Ellison et al. reported that HeLa cells infected with certain HSV-1 ICP27 mutants express alpha-globin transcripts with abnormally long and heterogenous poly(A) tails (16). Given this documented effect of ICP27 on transcript size, we speculated that the difference in the mobility of the gC transcripts could be due to poly(A) tail length. To test this, we treated purified whole-cell RNA preparations of transfected cell poly(A)+ RNA with RNase H and oligo(dT) in order to specifically remove the poly(A) tracts (11). The products were then analyzed by Northern analysis (Fig. 2B). The treatment effectively removed poly(A) tails, as demonstrated by the expected increase in electrophoretic mobility of the samples (compare lanes 1 and 2 with lanes 3 and 4, respectively). However, the electrophoretic size difference still remained, indicating that poly(A) tail length is not responsible.
The RNA size difference is due to mRNA splicing.
We next considered whether the electrophoretic mobility difference could be due to mRNA splicing. Early viral mRNA mapping studies from Ed Wagner's laboratory showed that the majority of gC mRNA in infected cells is unspliced but that a small fraction does undergo splicing (18). To investigate possible splicing, we analyzed the predicted gC transcript for potential splice sites by using a splice site predictive neural network tool (NNSPLICE0.9; www.fruitfly.org/seq_tools/splice.html) (54). The results of this analysis are shown in Fig. 3A, with potential splice sites indicated together with their prediction scores (those with a score of 0.4 or higher are considered significant). Strikingly, the prediction tool identified an extremely strong potential 3′ splice acceptor site in the gC transcript (score = 0.99) in the intergenic region between the gC and UL45 genes. This sequence was also recognized as a strong potential 3′ splice site by two other prediction programs (data not shown). This potential splice site was not one of the sites identified earlier by the Wagner lab.
FIG. 3.
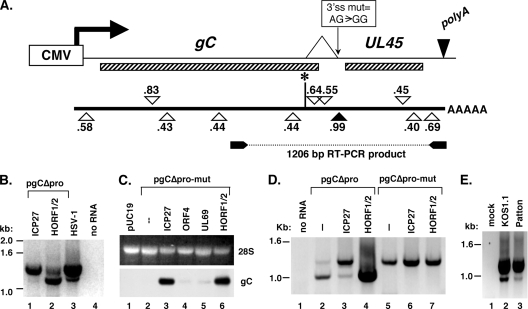
Identification of an intron in the gC transcription unit. (A) Potential splice sites in the gC transcription unit. At the top, a diagram of the gC transcription unit in pgCΔpro is shown, with coding regions designated by the striped bars. Below, possible 5′ and 3′ splice sites, predicted by a splice prediction algorithm, are shown as inverted and regular triangles, respectively, accompanied with prediction scores. Scores above 0.4 are considered to be possible in vivo splice signals; note the very significant score of 0.99 for the 3′ splice site that resides in the gC/UL45 intergenic region (solid triangle). At the bottom, the primers used for RT-PCR analysis are shown as well as the predicted 1,206-bp product expected for unspliced RNA. At the top of the figure, the identified splice is indicated. Also shown is the AG-to-GG mutation which inactivates the 3′ splice site in pgCΔpro-mut. (B) RT-PCRs on transfected cell RNA. Total RNA from Vero cells transfected with pgCΔpro and ICP27 (lane 1) or HORF1/2 (lane 2) was analyzed by RT-PCR using the primers shown in panel A. As a control, HSV-1-infected cell RNA (lane 3) was also analyzed. A negative image of the ethidium bromide-stained gel is shown. (C) Northern analysis of RNA produced by splice site mutant pgCΔpro-mut. Total RNA from Vero cells transfected with pgCΔpro-mut plus or minus transactivating plasmids was analyzed by Northern analysis as described for Fig. 2B. (D) RT-PCR comparison of RNA produced from pgCΔpro and pgCΔpro-mut transfections. Total RNA from Vero cells transfected with the plasmids shown was analyzed as for panel B. (E) Expression of spliced gC mRNA in two WT HSV-1 strains. Vero cells were infected with HSV-1 strain KOS1.1 or Patton. Total RNA was isolated at 8 hpi and analyzed by RT-PCR as for panel B.
To see if this strong splicing signal was utilized in transfected cells, we designed oligonucleotide primers that spanned the region in question and carried out RT-PCR analysis on transfected cell mRNA. Unspliced gC mRNA is predicted to give rise to a 1.2-kb product (Fig. 3A), and indeed, this was the major product seen when HSV-1-infected cell RNA was analyzed (Fig. 3B, lane 3; a negative image of the ethidium bromide-stained gel is shown). This band was also seen when the RNA used was from pgCΔpro-transfected cells in which ICP27 served as the transactivator (lane 1). However, when the RT-PCR was carried out using RNA derived from transfected cells in which HORF1/2 was the transactivator, the major product was ∼1.0 kb, although some 1.2-kb product was also evident (lane 2). To further characterize these products, both the 1.2-kb product from the ICP27-transactivated RNA and the ∼1.0-kb product from the HORF1/2-transactivated RNA were cloned, and three clones of each product were sequenced. The three clones derived from the 1.2-kb band all corresponded to the product expected to have been generated by unspliced gC mRNA. However, the three clones derived from the ∼1.0-kb band all lacked the same 225-nucleotide internal sequence. This deleted sequence appeared to correspond to an intron, as it was bounded on the 5′ end by a GT dinucleotide and on the 3′ end by an AG dinucleotide. The 5′ splice site (marked by the vertical line with asterisk in Fig. 3A) did not correspond to any of the sequences scored as significant by the prediction tool, whereas the 3′ splice site corresponded to the predicted strong 3′ site.
We took a genetic approach to further study this potential intron. Since the highly conserved AG dinucleotide at the 3′ end of introns is critical for splicing (2), we engineered a derivative of pgCΔpro, called pgCΔpro-mut, in which this dinucleotide was mutated to GG (Fig. 3A). We then tested pgCΔpro-mut in a transfection assay to determine its basal level of expression as well as its response to transactivators (Fig. 3C). The Northern blotting results indicated that the gC gene resident on pgCΔpro-mut resembles the parental gene in both its low basal activity and its ability to be transactivated by either ICP27 or HORF1/2 (but not by VZV ORF4 or HCMV UL69). However, in contrast to the parental gene on pgCΔpro, the RNA made via HORF1/2 transactivation appeared to comigrate in a glyoxal gel with that made via ICP27 transactivation (compare lanes 6 and 3). To investigate this further, another transfection experiment was performed in which pgCΔpro and pgCΔpro-mut were directly compared using the RT-PCR assay. As seen previously, transactivation of the parental gene by ICP27 led to mostly unspliced mRNA (Fig. 3D, lane 3), whereas HORF1/2 transactivation led to predominantly spliced mRNA (lane 4). In contrast, when the 3′ splice site was inactivated by mutation, the RNA produced via either ICP27 or HORF1/2 transactivation was unspliced (lanes 6 and 7). RT-PCR products were also seen in the untransactivated samples (lanes 2 and 5), presumably due to amplification of low-level basal transcripts. Interestingly, the majority of the products from pgCΔpro arose from spliced mRNA (lane 2), indicating that splicing is the preferred default pathway for expression of this gene in Vero cells.
Based on the above analyses, we conclude that the difference in gC mRNA electrophoretic mobility between the ICP27- and HORF1/2-transactivated RNAs is due to the splicing of a previously undescribed 225-nucleotide intron in the gC transcript. This intron is largely retained when the gene is transactivated by ICP27 but is efficiently excised when the gene is transactivated by HORF1/2.
The spliced transcript encodes a secreted form of gC.
Based on the DNA sequence of the gC transcription unit, the removal of the intron is predicted to alter the C terminus of gC (Fig. 4A; see also Fig. 5). Specifically, the splice removes the sequence encoding the last 40 residues of gC and replaces it with a novel sequence specifying 18 alternate residues. Since both sequences initiate with Val-Ile, the variant form is predicted to have 16 novel C-terminal residues substituted for the normal 38 C-terminal residues. The sequence lost includes both the transmembrane and endodomains of the protein. The new sequence is predominantly hydrophilic and is thus not predicted to encode a transmembrane domain. Past mutagenesis studies of gC have shown that removal of gC's transmembrane domain often results in the secretion of gC (30, 31).
FIG. 5.
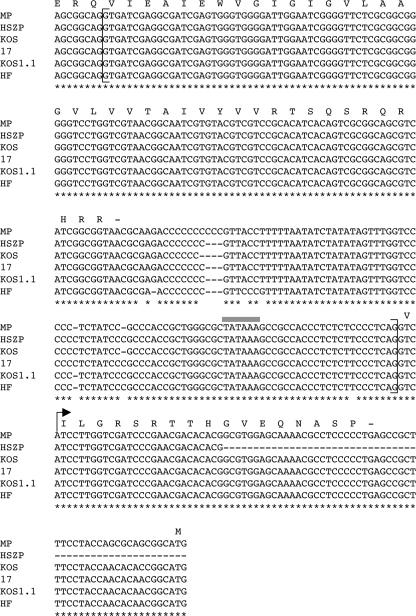
Conservation of gC splicing signals and alternate C-terminal coding sequence in various HSV-1 strains. The DNA sequences of five HSV-1 strains (MP, HSZP, KOS, 17, and HF) and one substrain (KOS1.1) were aligned using the program Clustalw. Note that only a partial sequence of strain HSZP is available in this region. The sequence shown extends from near the 3′ end of the gC coding region to the initiation codon of UL45. Nucleotides conserved in all isolates are denoted by an asterisk. The protein coding region of the C terminus of gC is shown, as well as the alternate C terminus found on the variant form. The brackets denote the 5′ and 3′ splice sites of the intron. Also shown is the UL45 gene TATA box homology (gray bar) and transcription initiation site (arrow).
To test whether the spliced transcript encodes a secreted from of gC, we transfected Vero cells with pgCΔpro or pgCΔpro-mut, plus or minus ICP27 or HORF1/2 as the transactivator. At 2 days posttransfection, both the cells and media were collected. Since gC is known to bind to heparin (28), the medium was incubated with heparin-Sepharose beads, and the stably bound fraction was analyzed by immunoblotting, along with the cell-associated material. Figure 4B shows short and long exposures of the immunoblot (on the left and right, respectively). When the parental gCΔpro plasmid was used, gC was readily detected in the medium from cells in which HORF1/2 served as the transactivator (lane 4). However, in cells transfected with pgCΔpro-mut, in which splicing cannot occur, no secreted gC was detected, despite large amounts of cell-associated gC (lane 7). The long exposure (right) shows that lower levels of secreted gC could be detected from pgCΔpro even when ICP27 was the transactivator (lane 3) or in the absence of transactivation (lane 2). This is consistent with the RT-PCR analysis, which indicated that there is a low level of spliced gC mRNA even in these situations. These results demonstrate that the spliced transcript encodes a secreted form of gC.
WT HSV-1 expresses a spliced gC transcript.
The RT-PCR analysis of infected-cell RNA (Fig. 3B) suggested that the majority of gC transcripts made in HSV-1-infected cells are unspliced. However, the RT-PCR analysis also showed a small amount of RT-PCR product that has a size consistent with splicing of the intron (∼1.0 kb). We investigated this further by looking at a second HSV-1 laboratory strain, Patton, and indeed this strain also showed low levels of a similar small RT-PCR product (Fig. 3E). We excised the major (∼1.2-kb) and minor (∼1.0-kb) KOS1.1 RT-PCR products from an agarose gel and cloned them in Escherichia coli. Two of each type were sequenced. As expected, the clones from the larger product corresponded to those expected to have been generated from unspliced mRNA. The smaller clones, however, were precisely missing the same 225-nucleotide intron that was identified in the transfected cells. Thus, we conclude that a subset of the gC transcripts expressed by WT HSV-1 are spliced in the manner described above.
The fact that gC mRNA splicing may also occur in strain Patton prompted us to ask whether the splicing signals are generally conserved among HSV-1 strains. We assembled and aligned all of the HSV-1 sequences available in the NCBI database from the region of interest (Fig. 5; note that not all of the sequence of strain HSZP is available). We found that both the 5′ and 3′ splicing signals are conserved, including the polypyrimidine tract commonly found upstream of splice acceptor sites. Also conserved is the sequence encoding the alternate gC C terminus (which is largely located in the 5′ untranslated region of the UL45 gene). In contrast, similar splicing signals were not found in HSV-2, nor was a coding sequence resembling the alternate C terminus found (not shown). These results suggest that HSV-1, but not HSV-2, has evolved to express the spliced form of the gC transcript.
As a tool to further investigate the spliced transcript and its function, we engineered the 3′ splice site mutation described above into the HSV-1 genome. The resulting mutant was designated mLS1. In Vero cells, mLS1 replicated similarly to KOS1.1 in growth assays, at both high and low MOIs (Fig. 6A), and produced plaques that were indistinguishable from KOS1.1 plaques in size and morphology (not shown). The replication abilities of mLS1 and KOS1.1 were also compared at both high and low MOIs in life-extended human normal diploid fibroblasts and human retinal pigment epithelial (ARPE-19) cells. In both types of cells, mLS1 replicated as efficiently as did the WT (not shown).
Next, we used the RT-PCR assay to analyze the gC mRNA produced by WT and mLS1 in Vero cells at 7 and 20 h postinfection (hpi). The band representing the spliced mRNA was readily amplified from WT-infected cell RNA, but as expected, was not detected when mLS1-infected cell RNA was used (Fig. 6B). We also examined the expression of the spliced transcript in life-extended human fibroblasts and ARPE-19 cells (Fig. 6C). In both cases, the spliced form of the gC mRNA was produced by the WT HSV-1 but not mLS1. Thus, splicing of the gC transcript occurs in a variety of cultured cells.
Since the RT-PCR assay examines only a specific region of the gC transcript and is semiquantitative, we also used Northern blotting to more generally compare the gC mRNA species made by WT and mLS1 (Fig. 6D and E). When a probe specific for the 5′ half of the gC transcript was used (probe 1), the two viruses gave very similar patterns at both 7 and 20 hpi (Fig. 6E, top), showing a predominant band that was consistent in size with the major expected ∼2.7-kb species (transcript “a” in Fig. 6D). There was a hint of a slightly smaller band that was present in the WT but not mLS1 RNA sample at 20 hpi (asterisk), which could represent the spliced mRNA (transcript “b”). However, the Northern blotting results were not definitive as to the presence of the spliced transcript. Given that the spliced mRNA is close in size to the unspliced transcript and of lower abundance, this result is perhaps not surprising.
The single nucleotide mutation in the mLS1 mutation resides in the promoter of the UL45 gene, 5 bp upstream of the UL45 transcription start site (Fig. 5). It is therefore possible that this mutation affects the expression of the UL45 gene, which encodes a virion protein that is nonessential for growth in Vero cells (74, 75). To test this, we utilized a second probe (probe 2), which detects the ∼0.9-kb UL45 transcript (Fig. 6E, bottom). The results indicated that both the WT- and mLS1-infected cells produced the UL45 transcript, and there appeared to be slightly more at 7 hpi in the mutant. Thus, at least in Vero cells, this mutation does not negatively affect UL45 mRNA expression and may slightly increase it at early times.
HSV-1 expresses a secreted form of gC.
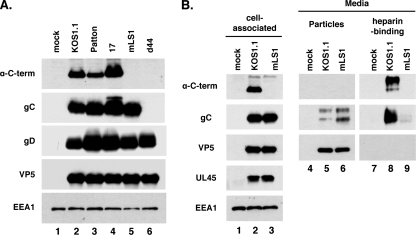
We wanted to test whether the variant form of gC is expressed during HSV-1 infection, as would be predicted from the presence of the spliced mRNA. We also wished to see if this form is secreted, as it is in transfected cells. To aid in this analysis, we obtained antiserum from a rabbit that had been immunized with a peptide corresponding to the 18 residues present on the C terminus of the variant gC. When used in an immunoblotting experiment on total cell protein extracts (Fig. 7A), this antiserum (α-C-term) specifically recognized an ∼85-kDa protein in Vero cells infected with three WT laboratory strains of HSV-1 (KOS1.1, Patton, and 17). As expected, the immunoreactive species was absent in cells infected with mLS1, although normal levels of total gC were produced. Further, no gC of any sort was seen in cells infected with d44, a gC deletion mutant of strain KOS1.1 (52), although glycoprotein D (gD) and VP5 were readily detectable. This experiment demonstrates that the antipeptide serum specifically recognizes the variant form of gC produced by the spliced mRNA. In addition, it shows that three WT laboratory strains of HSV-1 express this protein.
FIG. 7.
The variant form of gC is expressed by multiple HSV-1 strains and is secreted. (A) Detection of variant gC. Vero cells were mock infected or infected with the various strains and mutants indicated, and cellular protein extracts were prepared at 16 hpi. The samples were then analyzed by immunoblotting using an antipeptide serum (α-C-term) specific for the 18 residues predicted to be present on the C terminus of the variant gC. Blots were also probed with MAbs specific for gC, gD, VP5, and EEA1. (B) Secretion of variant gC from HSV-1-infected cells. Cells and media from KOS1.1- or mLS1-infected cells were collected at 16 hpi. From the media, particulate material including virions was collected by centrifugation, and heparin-binding material was isolated by affinity chromatography. The proteins from all three fractions were analyzed by immunoblotting using the antibodies shown.
We next asked whether the variant gC is secreted from HSV-1-infected cells. Vero cells were mock infected or infected with KOS1.1 or mLS1. At 16 hpi, the infected cells were harvested and total cell protein extracts were prepared. At the same time, the medium was collected, and residual cells and debris were removed by low-speed centrifugation. Virions and other particulate material were then pelleted by high-speed centrifugation. The remaining soluble fraction of the medium was subjected to heparin-Sepharose chromatography to isolate soluble gC. All three fractions (cell-associated material, virions/particles from the medium, and heparin-binding material from the medium) were subjected to immunoblotting using various antibodies (Fig. 7B). As expected from the previous experiment, analysis of the cell-associated material showed that KOS1.1 (lane 2) but not mLS1 (lane 3) expressed the variant gC, whereas the two viruses expressed similar amounts of cell-associated gC, as well as VP5. We also analyzed cell-associated UL45 levels and found them to be similar, indicating that the mLS1 mutation does not affect UL45 protein levels at 16 hpi. When the particulate material from the medium was analyzed, KOS1.1 and mLS1 (lanes 5 and 6, respectively) showed similar levels of gC and VP5, suggesting that the two sets of infections had resulted in secretion of comparable levels of virions at this time point. The variant form of gC was not detected in the particulate material, suggesting that it is not virion associated. Importantly, the variant gC was readily detected in the soluble fraction of the medium as a heparin-binding protein (lane 8). Little if any secreted gC was produced by mLS1 (lane 9), indicating that the standard form of gC is not efficiently secreted. We conclude that the variant form of gC, encoded by the spliced transcript, is secreted from HSV-1-infected cells.
DISCUSSION
ICP27 promotes the retention of an intron in the gC transcript.
We have focused on the gC gene as a model to understand the features of particular HSV-1 genes that make their expression dependent on ICP27. In this study, we compared ICP27's ability to stimulate expression of a transfected gC gene to that of three homologs from other herpesviruses. We found that BHV-4 homolog HORF1/2 is also capable of transactivating gC. However, a comparison of the RNA species induced by ICP27 and HORF1/2 has led us to the surprising conclusion that ICP27 not only stimulates the accumulation of gC mRNA but also promotes the retention of a previously undescribed intron. HORF1/2 does not appear to possess this second activity. Based on the comparison of these homologs, it appears that the two regulatory activities, transactivation and the stimulation of intron retention, are functionally separable. An alternate interpretation of our data is that both ICP27 and HORF1/2 can stimulate gC mRNA accumulation but that HORF1/2 possesses an additional activity that promotes splicing. This interpretation, however, is not supported by our observation that in the absence of transactivators, gC mRNA is mostly spliced (Fig. 3D, lane 2). This argues that the major default pathway for gC expression in transfected cells involves splicing.
Although the initial detection of the gC intron was made in the context of transfected cells, our findings are clearly relevant to HSV-1 biology as we readily detect the spliced gC transcript and its encoded protein in infected cells. Moreover, the splicing signals are conserved in all HSV-1 strains for which sequence information is available. Thus, it appears that HSV-1 has evolved to express two versions of the gC transcript: an unspliced one encoding the previously characterized form of gC and a spliced one encoding the variant form. The discovery of a functional intron in an HSV-1 true-late gene is surprising, since, with the exception of the UL15 transcript (46), the predominant forms of HSV-1 DE and L transcripts are unspliced, and ICP27, required for DE and L gene expression, inhibits pre-mRNA splicing. That being said, early studies from Wagner's group reported that a minor fraction of gC transcripts are spliced, using a 5′ donor site that lies within 30 nucleotides of the 5′ end of the mRNA and several alternate 3′ acceptor sites (18). The biological role of these minor spliced RNAs is unclear, as none have been shown to lead to the expression of a protein in virus-infected cells. Neither the intron nor the splice sites that we describe here are ones that were identified in the Wagner study.
Although our results show that ICP27 promotes the retention of the gC intron in transfected cells, is it possible to conclude that ICP27 also regulates gC mRNA splicing during HSV-1 infection? This question is somewhat difficult to address, given that ICP27 is required during infection for significant gC mRNA accumulation in the first place. However, if ICP27 does indeed harbor separable transactivation and intron retention activities as we suggest, then one would predict that it would be possible to identify viral ICP27 mutants that are competent for stimulating gC mRNA accumulation but defective for promoting intron retention (and thus functionally resembling HORF1/2). Consistent with this, we have identified two viral ICP27 N-terminal in-frame deletion mutants that express unusually high ratios of spliced to unspliced gC mRNAs (L. Sedlackova and S. Rice, unpublished data). Thus, although more work needs to be done, it appears that ICP27 does indeed promote the retention of the gC intron during infection.
ICP27 has previously been shown to inhibit cellular mRNA splicing during infection (7, 24, 25, 41) via an interaction with cellular SRPK1 (65) and possibly other splicing factors (6, 7). This regulatory activity of ICP27 is generally thought to function in the shutoff of host gene expression (24, 61). In transfection assays, ICP27 has been shown to inhibit the expression of certain reporter genes that possess introns (63), a result consistent with splicing inhibition. The generally repressive effect of ICP27 on splicing brings up the question of whether the gC intron retention function that we describe simply reflects a nonspecific suppression of splicing. If so, our results suggest that this function of ICP27 may have an additional role in HSV-1 biology, which is to allow the virus to express both spliced and unspliced versions of the gC mRNA. It is conceivable that there are additional HSV-1 DE or L genes that are similarly regulated by ICP27. Alternatively, the ability of ICP27 to promote the retention of the gC intron may be a transcript- or intron-specific effect and not the result of a general inhibition of mRNA splicing. In that case, the ICP27-gC system may be useful as a model to study how a single trans-acting factor can modulate intron retention, which is one form of alternative mRNA splicing (2).
Our original intent in studying the gC gene was to determine why it is so strongly dependent on ICP27 for the accumulation of its mRNA. It is worth considering, then, whether the intron plays a role in the gene's ability to be transactivated by ICP27. It was found that inactivation of the 3′ splice site by a point mutation did not alter the basal expression of the gene in transfected cells or its ability to be transactivated by ICP27 (Fig. 3C). This argues that the intron is not important in transactivation. Consistent with this, deletion of a region in the 5′ region of the gC transcription unit leads to a significant level of constitutive gC mRNA expression and a corresponding decrease in ICP27 dependence (K. Perkins, J. Meyer, A. Strain, L. Sedlackova, and S. Rice, unpublished data). These studies, which are ongoing, suggest that the gC gene harbors a silencing element in its 5′ half, distinct from the intron, and that the effects of this sequence can be overcome by ICP27.
Functional similarities and differences among ICP27 and its homologs.
Mammalian, avian, and reptilian herpesviruses encode a common set of ∼40 evolutionarily conserved core proteins that play fundamental roles in viral regulation, replication, and structure (47). ICP27 is the only one of the five HSV-1 IE proteins which is a member of this core group. ICP27 and its homologs appear to share common functions, in that most are activators of viral gene expression. However, since ICP27 homologs from other herpesviruses do not efficiently complement the growth of HSV-1 ICP27 deletion mutants, it is still unclear whether this family of proteins shares common functions. Our results are relevant to this issue. We find that BHV-4 HORF1/2, but not VZV ORF4 or HCMV UL69, can stimulate gC gene expression in transfected cells. Thus, this particular function is not generally conserved among ICP27 homologs. The reasons behind this are presently obscure. The results do not correlate with evolutionary relatedness or host specificity, as HSV-1 and VZV are both human alphaherpesviruses whereas BHV-4 is a distantly related gammaherpesvirus of the rhadinovirus subfamily (1).
Since VZV ORF4 and HCMV UL69 fail to stimulate gC gene expression in our assay, our data do not bear on whether these proteins possess the ability to promote intron retention. However, our data suggest that HORF1/2 does not possess this function. This finding is consistent with reporter gene assays showing that HORF1/2 does not down-modulate the expression of reporter genes bearing introns or the activity of a BHV-4 transactivator expressed from its intron-containing gene (V. L. van Santen, unpublished data). In contrast, at least two gammaherpesvirus homologs of ICP27, the SM protein of EBV and the herpesvirus saimiri ORF57 protein, are similar to ICP27 in their ability to inhibit expression of some intron-containing genes (58, 76, 77).
HSV-1 expresses a variant, secreted form of gC.
The spliced gC transcript that we have identified encodes a previously unidentified variant form of gC. This species has an alternate C terminus, lacks a transmembrane domain, and is efficiently secreted from infected cells. We therefore propose to designate it as gCsec. Likely, gCsec has not been previously identified because its molecular size is very close to that of the well-characterized form of gC. However, at least two prior studies have provided evidence for secretion of gC, in both transfected (19) and infected (71) cells. Although we have not yet examined the posttranslational modification status of gCsec, we find that the secreted form migrates significantly more slowly than its corresponding cell-associated form. Thus, it seems likely that gCsec is glycosylated, as has been shown for other truncated derivatives of gC (30).
What role does gCsec play in HSV-1 biology? The virion/cell-associated form of gC is known to have at least two important functions (reviewed in reference 69). First, it is an important cell attachment protein by virtue of its ability to bind to cell surface heparan sulfate (HS)-containing proteoglycans. Second, gC binds to and inhibits the C3b component of complement and thus can protect virions and infected cells from host complement. Both the HS- and C3b-binding activities are known to reside in the ectodomain of gC and thus would be expected to be present on gCsec. Consistent with this, we have shown that gCsec binds to heparin, an HS analog. Although we have not yet examined the ability of gCsec to bind to C3b, other soluble truncated derivatives of gC do so (38). Thus, it seems quite feasible that gCsec could function as a soluble complement inhibitor. Vaccinia virus and other poxviruses also encode soluble complement inhibitors, as does at least one other herpesvirus, murine gammaherpesvirus 68 (34, 36). It is also possible that the ability of gCsec to interact with cell surface proteoglycans on uninfected or infected cells could affect viral pathogenesis in vivo. Although gC is dispensable for growth of the virus in culture, it has been shown that a gC-null virus is significantly attenuated in its ability to replicate and cause disease in infected mice and guinea pigs (43). We speculate that gCsec contributes to these pathogenic effects. This possibility could be readily addressed using mLS1 or a similar mutant.
Acknowledgments
We thank Curtis Brandt for HSV-1 UL45 antiserum, Wade Bresnahan for HCMV UL69 antiserum and human fibroblasts, Jeff Cohen for VZV ORF4 antiserum, Andrew Davison for an ORF4 plasmid clone, Alistair McGregor for HSV-1 strain 17, Ian Mohr for HSV-1 strain Patton, and Thomas Stamminger for a UL69 expression construct. Thanks are also due to Leslie Schiff and the members of the Rice, Schiff, and Bresnahan laboratories for many helpful discussions.
This research was supported by NIH grant R01-AI42737. Keith D. Perkins was supported by NIH predoctoral training award T32-AI07421.
Footnotes
Published ahead of print on 21 May 2008.
REFERENCES
- 1.Ackermann, M. 2006. Pathogenesis of gammaherpesvirus infections. Vet. Microbiol. 113211-222. [DOI] [PubMed] [Google Scholar]
- 2.Black, D. L. 2003. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72291-336. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, J. L., S. Swaminathan, and S. J. Silverstein. 2002. The Epstein-Barr virus SM protein is functionally similar to ICP27 from herpes simplex virus in viral infections. J. Virol. 769420-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 7410816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, C. R., M. S. Nakamura, J. D. Mosca, G. S. Hayward, S. E. Straus, and L. P. Perera. 1995. Herpes simplex virus trans-regulatory protein ICP27 stabilizes and binds to 3′ ends of labile mRNA. J. Virol. 697187-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant, H. E., D. A. Matthews, S. Wadd, J. E. Scott, J. Kean, S. Graham, W. C. Russell, and J. B. Clements. 2000. Interaction between herpes simplex virus type 1 IE63 protein and cellular protein p32. J. Virol. 7411322-11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant, H. E., S. E. Wadd, A. I. Lamond, S. J. Silverstein, and J. B. Clements. 2001. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J. Virol. 754376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, L. Y., and V. L. van Santen. 1992. Immediate-early, early, and late RNAs in bovine herpesvirus-4-infected cells. Virology 191909-920. [DOI] [PubMed] [Google Scholar]
- 9.Chen, I. H., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 793949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, I. H., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 7612877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung, P., K. S. Ellison, R. Verity, and J. R. Smiley. 2000. Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells. J. Virol. 742913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corcoran, J. A., W. L. Hsu, and J. R. Smiley. 2006. Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene. J. Virol. 809720-9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defechereux, P., S. Debrus, L. Baudoux, B. Rentier, and J. Piette. 1997. Varicella-zoster virus open reading frame 4 encodes an immediate-early protein with posttranscriptional regulatory properties. J. Virol. 717073-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn, K. C., A. E. Aotaki-Keen, F. R. Putkey, and L. M. Hjelmeland. 1996. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 62155-169. [DOI] [PubMed] [Google Scholar]
- 15.Ellison, K. S., R. A. Maranchuk, K. L. Mottet, and J. R. Smiley. 2005. Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J. Virol. 794120-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison, K. S., S. A. Rice, R. Verity, and J. R. Smiley. 2000. Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J. Virol. 747307-7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontaine-Rodriguez, E. C., and D. M. Knipe. 2008. Herpes simplex virus ICP27 increases translation of a subset of viral late mRNAs. J. Virol. 823538-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frink, R. J., R. Eisenberg, G. Cohen, and E. K. Wagner. 1983. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J. Virol. 45634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh-Choudhury, N., M. Butcher, E. Reid, and H. P. Ghosh. 1994. Effect of tunicamycin and monensin on the transport to the cell surface and secretion of a viral membrane glycoprotein containing both N- and O-linked sugars. Biochem. Cell Biol. 7220-25. [DOI] [PubMed] [Google Scholar]
- 20.Goldin, A. L., R. M. Sandri-Goldin, M. Levine, and J. C. Glorioso. 1981. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J. Virol. 3850-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruffat, H., J. Batisse, D. Pich, B. Neuhierl, E. Manet, W. Hammerschmidt, and A. Sergeant. 2002. Epstein-Barr virus mRNA export factor EB2 is essential for production of infectious virus. J. Virol. 769635-9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, Z., E. Marendy, Y. D. Wang, J. Yuan, J. T. Sample, and S. Swaminathan. 2007. Multiple roles of Epstein-Barr virus SM protein in lytic replication. J. Virol. 814058-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han, Z., and S. Swaminathan. 2006. Kaposi's sarcoma-associated herpesvirus lytic gene ORF57 is essential for infectious virion production. J. Virol. 805251-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardwicke, M. A., and R. M. Sandri-Goldin. 1994. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J. Virol. 684797-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 687790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hargett, D., T. McLean, and S. L. Bachenheimer. 2005. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J. Virol. 798348-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hargett, D., S. Rice, and S. L. Bachenheimer. 2006. Herpes simplex virus type 1 ICP27-dependent activation of NF-κB. J. Virol. 8010565-10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 651090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland, L. E., K. P. Anderson, C. Shipman, Jr., and E. K. Wagner. 1980. Viral DNA synthesis is required for the efficient expression of specific herpes simplex virus type 1 mRNA species. Virology 10110-24. [DOI] [PubMed] [Google Scholar]
- 30.Holland, T. C., F. L. Homa, S. D. Marlin, M. Levine, and J. Glorioso. 1984. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J. Virol. 52566-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homa, F. L., D. J. Purifoy, J. C. Glorioso, and M. Levine. 1986. Molecular basis of the glycoprotein C-negative phenotypes of herpes simplex virus type 1 mutants selected with a virus-neutralizing monoclonal antibody. J. Virol. 58281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes, R. G., Jr., and W. H. Munyon. 1975. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J. Virol. 16275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingram, A., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Immediate early protein IE63 of herpes simplex virus type 1 binds RNA directly. J. Gen. Virol. 771847-1851. [DOI] [PubMed] [Google Scholar]
- 34.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 89628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean, S., K. M. LeVan, B. Song, M. Levine, and D. M. Knipe. 2001. Herpes simplex virus 1 ICP27 is required for transcription of two viral late (gamma2) genes in infected cells. Virology 283273-284. [DOI] [PubMed] [Google Scholar]
- 36.Kapadia, S. B., H. Molina, V. van Berkel, S. H. Speck, and H. W. Virgin. 1999. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 737658-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 205769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostavasili, I., A. Sahu, H. M. Friedman, R. J. Eisenberg, G. H. Cohen, and J. D. Lambris. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 1581763-1771. [PubMed] [Google Scholar]
- 39.Larralde, O., R. W. Smith, G. S. Wilkie, P. Malik, N. K. Gray, and J. B. Clements. 2006. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J. Virol. 801588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lengyel, J., C. Guy, V. Leong, S. Borge, and S. A. Rice. 2002. Mapping of functional regions in the amino-terminal portion of the herpes simplex virus ICP27 regulatory protein: importance of the leucine-rich nuclear export signal and RGG box RNA-binding domain. J. Virol. 7611866-11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindberg, A., and J. P. Kreivi. 2002. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 294189-198. [DOI] [PubMed] [Google Scholar]
- 42.Lischka, P., Z. Toth, M. Thomas, R. Mueller, and T. Stamminger. 2006. The UL69 transactivator protein of human cytomegalovirus interacts with DEXD/H-Box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol. Cell. Biol. 261631-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubinski, J. M., L. Wang, A. M. Soulika, R. Burger, R. A. Wetsel, H. Colten, G. H. Cohen, R. J. Eisenberg, J. D. Lambris, and H. M. Friedman. 1998. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J. Virol. 728257-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majerciak, V., N. Pripuzova, J. P. McCoy, S. J. Gao, and Z. M. Zheng. 2007. Targeted disruption of Kaposi's sarcoma-associated herpesvirus ORF57 in the viral genome is detrimental for the expression of ORF59, K8α, and K8.1 and the production of infectious virus. J. Virol. 811062-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik, P., D. J. Blackbourn, and J. B. Clements. 2004. The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor. J. Biol. Chem. 27933001-33011. [DOI] [PubMed] [Google Scholar]
- 46.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 691531-1574. [DOI] [PubMed] [Google Scholar]
- 47.McGeoch, D. J., F. J. Rixon, and A. J. Davison. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 11790-104. [DOI] [PubMed] [Google Scholar]
- 48.McGregor, F., A. Phelan, J. Dunlop, and J. B. Clements. 1996. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J. Virol. 701931-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mears, W. E., and S. A. Rice. 1998. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242128-137. [DOI] [PubMed] [Google Scholar]
- 50.Mears, W. E., and S. A. Rice. 1996. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J. Virol. 707445-7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriuchi, H., M. Moriuchi, H. A. Smith, and J. I. Cohen. 1994. Varicella-zoster virus open reading frame 4 protein is functionally distinct from and does not complement its herpes simplex virus type 1 homolog, ICP27. J. Virol. 681987-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins, K. D., J. Gregonis, S. Borge, and S. A. Rice. 2003. Transactivation of a viral target gene by herpes simplex virus ICP27 is posttranscriptional and does not require the endogenous promoter or polyadenylation site. J. Virol. 779872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phelan, A., and J. B. Clements. 1997. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J. Gen. Virol. 783327-3331. [DOI] [PubMed] [Google Scholar]
- 54.Reese, M. G., F. H. Eeckman, D. Kulp, and D. Haussler. 1997. Improved splice site detection in Genie. J. Comput. Biol. 4311-323. [DOI] [PubMed] [Google Scholar]
- 55.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 623814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 641704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice, S. A., and V. Lam. 1994. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J. Virol. 68823-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruvolo, V., E. Wang, S. Boyle, and S. Swaminathan. 1998. The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc. Natl. Acad. Sci. USA 958852-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandri-Goldin, R. M. 2006. The functions and activities of herpes simplex virus protein ICP27, a multifunctional regulator of gene expression, p. 65-83. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: molecular and cellular biology. Caister Academic Press, Norwich, Norfolk, United Kingdom.
- 62.Sandri-Goldin, R. M. 2004. Viral regulation of mRNA export. J. Virol. 784389-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6848-863. [DOI] [PubMed] [Google Scholar]
- 64.Sato, B., M. Sommer, H. Ito, and A. M. Arvin. 2003. Requirement of varicella-zoster virus immediate-early 4 protein for viral replication. J. Virol. 7712369-12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sciabica, K. S., Q. J. Dai, and R. M. Sandri-Goldin. 2003. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 221608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sedlackova, L., and S. A. Rice. 2008. Herpes simplex virus type 1 immediate-early protein ICP27 is required for efficient incorporation of ICP0 and ICP4 into virions. J. Virol. 82268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 18674-86. [DOI] [PubMed] [Google Scholar]
- 68.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 719188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spear, P. G. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. 6401-410. [DOI] [PubMed] [Google Scholar]
- 70.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 749916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiedemann, K. H., H. Hampl, and K. O. Habermehl. 1988. Release of a virus coded glycoprotein from herpes simplex virus type 1 infected cells. Mol. Biol. Rep. 1329-33. [DOI] [PubMed] [Google Scholar]
- 72.Uprichard, S. L., and D. M. Knipe. 1996. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J. Virol. 701969-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Santen, V. L., and L. Y. Chang. 1992. Cloning and mapping of EcoRI, HindIII, and PstI fragments of bovine herpesvirus 4 (DN-599) genome. Intervirology 3444-52. [DOI] [PubMed] [Google Scholar]
- 74.Visalli, R. J., and C. R. Brandt. 1993. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 29167-178. [DOI] [PubMed] [Google Scholar]
- 75.Visalli, R. J., and C. R. Brandt. 1991. The HSV-1 UL45 gene product is not required for growth in Vero cells. Virology 185419-423. [DOI] [PubMed] [Google Scholar]
- 76.Whitehouse, A., M. Cooper, K. T. Hall, and D. M. Meredith. 1998. The open reading frame (ORF) 50a gene product regulates ORF 57 gene expression in herpesvirus saimiri. J. Virol. 721967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitehouse, A., M. Cooper, and D. M. Meredith. 1998. The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level. J. Virol. 72857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkler, M., S. A. Rice, and T. Stamminger. 1994. UL69 of human cytomegalovirus, an open reading frame with homology to ICP27 of herpes simplex virus, encodes a transactivator of gene expression. J. Virol. 683943-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu, Z., W. Cai, and P. A. Schaffer. 1994. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J. Virol. 683027-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu, Z., and P. A. Schaffer. 1995. Intracellular localization of the herpes simplex virus type 1 major transcriptional regulatory protein, ICP4, is affected by ICP27. J. Virol. 6949-59. [DOI] [PMC free article] [PubMed] [Google Scholar]