Abstract
Many viruses escape the cellular immune response by downregulating cell surface expression of major histocompatibility complex (MHC) class I molecules. However, infection of cells with flaviviruses can upregulate the expression of these molecules. In this study we analyzed the expression of MHC class I in K562 and THP-1 human cell lines that were stably transfected with self-replicating subgenomic dengue virus RNA (replicons) and express all the dengue virus nonstructural proteins together. We show that MHC class I expression is upregulated in the dengue virus replicon-expressing cells and that the binding of natural killer (NK) inhibitory receptors to these cells is augmented. This upregulation results in reduced susceptibility of the dengue virus replicon-expressing cells to NK lysis, indicating a possible mechanism for evasion of the dengue virus from NK cell recognition. Visualizing MHC class I expression in replicon-containing K562 and THP-1 cells by confocal microscopy demonstrated aggregation of MHC class I molecules on the cell surface. Finally, replicon-expressing K562 cells manifested increased TAP (transporter associated with antigen processing) and LMP (low-molecular-mass protein) gene transcription, while replicon-expressing THP-1 cells manifested increased NF-κB activity and MHC class I transcription. We suggest that expression of dengue virus nonstructural proteins is sufficient to induce MHC class I upregulation through both TAP-dependent and -independent mechanisms. Additionally, aggregation of MHC class I molecules on the cell membrane also contributes to significantly higher binding of low-affinity NK inhibitory receptors, resulting in lower sensitivity to lysis by NK cells.
Dengue is an emerging arboviral disease caused by infection with dengue virus (DV). DVs are single-stranded, positive-sense RNA viruses belonging to the family Flaviviridae. The genomic RNA comprises a single open reading frame flanked by two untranslated regions. The encoded viral polyprotein is co- and posttranslationally processed by host cell and viral proteases to yield three structural proteins (C, prM, and E) and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). We previously established human cell lines that continuously express self-replicating subgenomic DV RNA (replicons) (16). Flavivirus replicons express all the viral nonstructural proteins together in a way that mimics expression during authentic viral infection and have proved powerful tools for studying the functional roles of nonstructural proteins in RNA and virus replication (17-19). More-recent studies with flavivirus replicons have also provided insight into the effects of viral RNA replication and expression of nonstructural proteins on host cell function, such as inhibition of innate immune responses mediated by the interferon (IFN) system (16, 35).
Natural killer (NK) cells are another important component of the innate first line of defense against viral infection and are activated early in the course of DV infection (4, 38). NK cytolytic activity is downregulated by engagement of major histocompatibility complex (MHC) class I molecules by NK-inhibitory receptors (8, 26). So far, three different types of NK-inhibitory receptors have been identified: the killer cell immunoglobulin (Ig)-like receptor (KIR) family (26), the C-type lectin family (41), and the Ig-like transcript/leukocyte Ig-like receptor (LIR) family (2). Several unrelated viruses have evolved mechanisms to evade NK recognition and killing (8, 25). A number of studies have demonstrated that flavivirus infection upregulates cell surface expression of MHC class I expression (reviewed in references 20, 24, and 36). In part, this appears to be IFN independent (9), but the exact mechanism and especially the viral components involved are unclear. Uncleaved C-prM protein has been implicated in increasing MHC class I expression, and it has been suggested that flavivirus-mediated upregulation of MHC class I may be an incidental consequence of virus assembly rather than a mechanism evolved as a means of immune escape (23).
In the present study we investigated MHC class I expression in DV replicon-expressing human cells and the sensitivity of these cells to lysis by NK cells. DV replicons contain a large in-frame deletion within their structural genes and thus do not express C-prM and E. We present here the first evidence that DV replicon expression is sufficient to enhance membrane expression of MHC class I. Enhanced MHC class I expression in DV replicon-expressing cells is associated with increased binding of specific NK-inhibitory receptors and reduced susceptibility to NK lysis.
MATERIALS AND METHODS
Cells.
The cell lines used in this work were the K562 (human chronic myeloid leukemia) and THP-1 (human monocytic) cell lines stably expressing the DV replicon ΔCprME-PAC2A, as previously described (16). Replicon-containing cell lines were maintained in RPMI medium containing 10% fetal bovine serum (FBS), nonessential amino acids, 1 mM penicillin-streptomycin, and 1 mM sodium pyruvate (all supplements from Gibco, Paisley, United Kingdom) and 3.3 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO). K562 and THP-1 cells without replicons were continuously maintained in the same medium without puromycin. The interleukin-2 (IL-2)-dependent NK-like cell line NK92 was maintained in α-MEM containing 12.5% FBS, 12.5% horse serum, 2 mM l-glutamate, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate (all supplements from Gibco); 100 μM 2-mercaptoethanol, 2 mM folic acid (Sigma), 20 mM myoinositol (Sigma), and 100 IU/ml human IL-2 (Biological Industries, Kibbutz Beit Haemek, Israel). NK92/KIR2DL1-GFP cells (a kind gift from Deborah N. Burshtyn, University of Alberta, Canada) have been described elsewhere (40). YTS/eco and YTS/KIR2DL1 NK-like cell lines (a kind gift from Ofer Mandelboim, The Hebrew University, Israel) have been described elsewhere (6).
Isolation and culture of NK cells.
NK cells were isolated from the peripheral blood of healthy donors using the human NK cell isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). NK purity was assayed using BD Multtest antibody cocktail (anti-CD3/CD16+CD56/CD45/CD19). NK cells were more then 90% CD3− and CD56+. In order to obtain long-term activated polyclonal or, by limiting dilution, clonal NK cells, purified NK cells were cultured on irradiated feeder cells in the presence of 1.0 μg/ml phytohemagglutinin and maintained in Cellgro SCGM serum-free medium (CellGenix, Freiburg, Germany) supplemented with 10% heat-inactivated human plasma obtained from healthy donors, 1 mM sodium pyruvate, 2 mM l-glutamine, MEM nonessential amino acids, 1% penicillin-streptomycin, and 10 mM HEPES (all supplements from Gibco) and 300 IU/ml of human IL-2 (Biological Industries).
Cytotoxicity assay.
The cytotoxic activity of NK lines and clones against the various targets was assessed in 5-h 35S release assays, as previously described (28). In all experiments shown, the spontaneous release was less than 25% of maximal release.
Antibodies and fusion Ig proteins.
The following antibodies were used in this study: phycoerythrin-conjugated anti-CD158a and fluorescein isothiocyanate (FITC)-conjugated anti-CD158b (BD Biosciences PharMingen, San Diego, CA), the pan-HLA-I-specific monoclonal antibody (MAb) W6/32, and the anti-DV NS1 protein-specific MAb 5H5.4 (a kind gift from Paul Young, University of Queensland, Australia [12]). The generation of KIR2DL1-Ig, KIR2DL2-Ig, KIR2DS2-Ig, CD99-Ig, and LIR1-Ig fusion proteins was performed as previously described (1). Briefly, the sequence encoding the extracellular portion of the receptor was amplified by PCR from cDNA isolated from human NK clones. These PCR-generated fragments were cloned into a mammalian expression vector containing the Fc portion of human IgG1. The construct was transfected into COS-7 cells, and the protein produced was purified using a protein G column. In some of the experiments LIR1-Ig and KIR2DL1-Ig produced in HEK 293T cells as previously described (7, 45) were used.
Flow cytometry.
Cells were incubated with various fusion Igs (50 μg/ml) for 1.5 to 2 h at 4°C, washed, and stained with allophycocyanin-conjugated-F(ab′)2 goat anti-human IgG-Fc (109-136-098); Jackson ImmunoResearch, West Grove, PA). Staining and washing buffer consisted of 0.5% (wt/vol) bovine serum albumin and 0.05% sodium azide in phosphate-buffered saline (PBS). During staining with W6/32 or 5H5.4 antibodies, cells were incubated with MAbs for 45 min at 4°C, washed, and stained with FITC-conjugated F(ab′)2 goat anti-mouse IgG (115-096-062); Jackson ImmunoResearch, West Grove, PA). In these experiments, staining and washing buffer consisted of 2% fetal calf serum and 0.05% sodium azide in PBS. Propidium iodide was added prior to reading for exclusion of dead cells. Flow cytometry was performed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA), and fluorescence data were acquired using logarithmic amplification. Data files were acquired and analyzed using BD CELLQuest 3.3 software.
Immunostaining and confocal microscopy.
Cells (2 × 104 to 4 × 104) were centrifuged onto Superfrost Plus slides (Menzel-Glaser, Germany) by the cytospin technique and fixed at −20°C with ethanol-acetone (1:1) for 10 min. Specimens were then blocked in PBS-10% FBS for 1 h at room temperature in order to saturate nonspecific sites. Cells were stained with 20 μg/ml W6/32 or 100 μg/ml fusion Ig protein in PBS-10% FBS for 1 h, washed with PBS, and stained with Cy3-conjugated goat anti-mouse IgG (115-165-003) or Cy5-conjugated F(ab′)2 goat anti-human IgG (109-176-098) secondary antibodies (Jackson ImmunoResearch) in PBS-10% FBS for 1 h at room temperature. Specimens were mounted in the dark using mounting medium (DAKO, Denmark), and slides were examined with a confocal laser scanning Olympus IX81 microscope equipped with the Fluoview SV1000 and imaged using the FV10-ASW-1.5 software.
Reverse transcription-PCR (RT-PCR).
RNA was isolated with the RNeasy system (Qiagen, Valencia, CA), and 1 μg was used to generate cDNA using oligo(dT) primers in the reverse transcription system (Promega Corporation, Madison, WI) in accordance with the manufacturer's instructions. The cDNA was used as a template for PCR amplification of the gene products HLA-A (5′-GAC AGC GAC GCC GCG AGC CA-3′ and 5′-GGC AGC GAC CAC AGC TCC AG-3′; 817 bp), HLA-B (5′-GAC AGC GAC GCC GCG AGT CC-3′ and 5′-AGT AGC GAC CAC AGC TCC GA-3′; 798 bp), HLA-C (5′-GAG ATC ACA CTG ACC TGG CA-3′ and 5′-GAA CAC AGT CAA TGT GGG G-3′; 630 bp), HLA-Cw3 (5′-GAG ATC ACA CTG ACC TGG CA-3′ and 5′-CAC ATT ATG CTA ACA GGA ACG C-3′; 524 bp), HLA-Cw5 (5′-GGA GAC ACA GAA GTA CAA GCG-3′ and 5′-GCC AGG TCA GTG TGA TCT C-3′; 467 bp), TAP1 (transporter associated with antigen processing 1) (5′-ACG TCC ACC CTG AGT GAT TC-3′ and 5′-AGC TTT TCC CTA AAC TTC TGG G-3′; 381 bp), TAP2 (5′-TAC CTG CTC ATA AGG AGG GTG C-3′ and 5′-ATT GGG ATA TGC AAA GGA GAC G-3′; 311 bp), tapasin (5′-AGT TCA ACC CTT TCA GGA GGG CA-3′ and 5′-GAA AGG CAG ACA GGA AA GGC-3′; 385 bp), LMP2 (low-molecular-mass protein 2) (5′-TGT GAT GGG TTC TGA TTC C-3′ and 5′-CAG AGC AAT AGC GTC TGT GG-3′; 448 bp), LMP7 (5′-TCG CCT TCA AGT TCC AGC ATG-3′ and 5′-CCA ACC ATC TTC CTT CAT GTG G-3′; 541 bp), β-actin (5′-TGT TAC CAA CTG GGA CGA CA-3′ and CTG GGT CAT CTT TTC ACG GT-3′; 139 bp), and DV NS1 (5′-CTG AAG TGT GGC AGT GGG ATT-3′ and 5′-AGT GCA CTT TCT ATC CAA TAA CCC-3′; 557 bp). PCR products were amplified with 20, 25, and 30 cycles (60°C), separated by 1.7% agarose gel electrophoresis, and visualized by ethidium bromide staining.
Quantitative real-time PCR.
Quantitative real-time PCR was performed with the ABI 7500 real-time PCR system in accordance with the manufacturer's instructions (Applied Biosystems, Foster City, CA) and analyzed using the 7500 SDS1.2 software. cDNA was amplified using the Absolute Sybr green 6-carboxy-X-rhodamine mix (ABgene, Epsom, United Kingdom) with the TAP1 (5′-GGC GAA GCC CAG AAG TTT A-3′ and 5′-CCC ACT TTC AGC AGC ATA CC-3′), TAP2 (5′-CCT CGA CTC ACC CTC CTT TC-3′ and 5′-TGC ATC CTG GAT CTC CCG A-3′), and β-actin (5′-CCT GGC ACC CAG CAC AAT-3′ and 5′-TGC TTG CTG ATC CAC ATC TGC T-3′) primers. Primers and template concentrations were optimized prior to the real-time PCR analysis of samples for which the data are shown. Each assay included a no-template control and triplicate cDNA samples. The mRNA level in each sample was normalized against the reference gene β-actin mRNA, used as an internal control gene.
Western blots.
Twenty micrograms of whole-cell lysate was loaded on a 10% acrylamide gel. The proteins were transferred to a nitrocellulose blotting membrane (Sartorius) in a Bio-Rad blotting apparatus at 200 V for 1 h. The membrane was blocked with 1% bovine serum albumin, 0.05% Na azide, and 0.5% Tween in PBS for 1 h at room temperature. Incubation with monoclonal 148.3 mouse anti-human TAP1 (diluted 1:500) or monoclonal mouse anti-human β- actin (ICN; catalog no. 69100; diluted 1:20,000) overnight at 4°C was followed by a 1-h incubation with a monoclonal horseradish peroxidase-conjugated sheep anti-mouse IgG (Amersham; catalog no. NA931V; diluted 1:5,000) at room temperature. The membrane was extensively washed, treated with ECL Western blotting detection reagents (Santa Cruz; catalog no. sc-2048), and exposed to film.
NF-κB activation assay.
The NF-κB activation assay was previously described (5). Briefly, a reporter plasmid expressing luciferase through a minimal promoter linked to three copies of the consensus NF-κB-responsive element was used to measure activation of NF-κB. For the positive control, a plasmid expressing p65 (RelA) was cotransfected. Plasmids were transfected by electroporation into 7 × 106 cells. The total DNA was complemented to 40 μg by adding empty plasmids. Enzymatic activity in the cell extract was assayed 24 h after transfection, and the luciferase activity was normalized to the protein concentration. Results are expressed in arbitrary units.
RESULTS
Expression of DV replicons induces upregulation of MHC class I surface expression and augments binding of NK-inhibitory receptors.
Previous studies have shown that infection with flaviviruses induces upregulation of MHC class I on virus-infected cells. The majority of these studies have been performed using flaviviruses other than DV and nonhuman cells (reviewed in references 20, 24, and 36). Studies of the effect of DV on MHC class I expression on human cells are lacking, and the mechanism of flavivirus-induced MHC class I upregulation is unclear. In this study, we examined whether MHC class I upregulation can be observed in human cell lines expressing only the nonstructural proteins of DV. K562.ΔCprME-PAC2A cells are K562 cells that continuously express the DV replicon ΔCprME-PAC2A (16). During the course of these experiments, K562.ΔCprME-PAC2A cells were frequently tested for stable replicon expression by RT-PCR of DV NS1 (Fig. 1A) or by staining for NS1 expression on the membrane (data not shown). In order to assess cell surface expression of MHC class I, K562 cells that did and did not contain DV replicons were stained with the pan-HLA class I-specific MAb W6/32 and analyzed using flow cytometry. MHC class I expression was greatly increased on K562.ΔCprME-PAC2A cells compared with K562 cells (Fig. 1B). We next defined whether increased cell surface expression of MHC class I was associated with increased binding of NK-inhibitory receptors. Parental K562 cells express HLA-Cw3 and HLA-Cw5 molecules but not HLA-A or -B molecules (22). Different KIRs recognize different versions of HLA-C: KIR2DL1 recognizes HLA-C characterized by a Lys80 residue, e.g., Cw2, Cw4, Cw5, and Cw6; KIR2DL2 recognizes HLA-C characterized by an Asn80 residue, e.g., Cw1, Cw3, Cw7, and Cw8 (34). As expected, we found that staining with both KIR2DL1-Ig and KIR2DL2-Ig (but not negative control CD99-Ig) was much greater in K562.ΔCprME-PAC2A cells that in K562 cells (Fig. 1C). HLA-Cw3 and HLA-Cw5 are not recognized by other NK receptors such as LIR1, KIR2DS2, and KIR2DS4 (37, 44). Accordingly, staining with these recombinant receptors did not reveal differences between K562 and K562.ΔCprME-PAC2A cells (Fig. 1C for LIR1-Ig). Therefore, DV RNA replication and nonstructural protein expression are sufficient to upregulate MHC class I expression in K562 cells, resulting in enhanced binding of the relevant NK-inhibitory receptors. To further differentiate between viral RNA replication and protein expression, nonstructural DV proteins were individually expressed (NS1, -2A, -2B, -3, -4A, -4B, and -5) or expressed in pairs (NS1-NS2A and NS2B-NS3) in K562 cells. Expression of the single proteins was verified at RNA and protein levels. However, none of the different transfectants showed upregulation of MHC class I surface expression or augmented binding of NK-inhibitory receptors (data not shown). Therefore, this phenomenon probably requires viral RNA replication with or without expression of several nonstructural proteins.
FIG. 1.
Binding of anti-MHC class I and NK-inhibitory receptors to K562 and K562.ΔCprME-PAC2A cells. (A) RT-PCR of K562 and K562.ΔCprME-PAC2A cells showing the expression of the DV replicon in K562.ΔCprME-PAC2A cells. (Top) Product of amplification with DV NS1 primers; (bottom) product of amplification with β-actin primers as a standard. (B) Surface expression of MHC class I in K562 and K562.ΔCprME-PAC2A cells analyzed by flow cytometry with the pan-HLA-I-specific MAb W6/32. Cells were incubated with 10 μg/ml of W6/32, followed by FITC-conjugated anti-Fc secondary antibody. Background staining with the secondary antibody (gray filled histogram) is presented. Mean fluorescence intensities (MFIs) were 2.5 for background staining, 6.7 for K562 cells, and 27.6 for K562.ΔCprME-PAC2A cells. Results are from one representative experiment out of four performed. (C) Binding of different receptor-Igs to K562 and K562.ΔCprME-PAC2A cells. Cells were incubated with the indicated fusion protein for 2 h at 4°C, followed by allophycocyanin-conjugated anti-Fc secondary antibody. MFIs for K562 and K562.ΔCprME-PAC2A cells were, respectively, as follows: CD99-Ig, 3.5 and 3.6; LIR1-Ig, 7.7 and 8.0; KIR2DL1-Ig, 26.8 and 229; KIR2DL2-Ig, 28.9 and 208. Results are from one representative experiment out of four performed.
In order to explore whether these findings can be generalized, the same series of experiments were repeated using a different human cell line, THP-1, with and without the DV replicon. As with the K562 model, THP-1.ΔCprME-PAC2A cells were assessed routinely for replicon expression by RT-PCR of NS1 (Fig. 2A) and surface expression of NS1 (data not shown). THP-1 cells express much higher levels of MHC class I than K562 cells (15, 42) (compare Fig. 2B to 1B), and expression was further increased on THP1 cells expressing DV replicons (Fig. 2B). Unlike K562 cells, THP-1 cells express high levels of HLA-A and HLA-B but not HLA-C. In keeping with this, there was no difference between THP-1 and THP-1.ΔCprME in the binding of recombinant NK-inhibitory receptors that do not interact with HLA-A or HLA-B such as KIR2DL1, KIR2DL2, and KIR2DS2 (Fig. 2C, shown for KIR2DL1 and KIR2DS2). In contrast, there was a surprisingly marked increase in the binding of recombinant LIR1-Ig, but not negative control CD99-Ig, in THP-1.ΔCprME-PAC2A cells compared with THP-1 cells (Fig. 2C). LIR1 interacts with HLA-G, and it also recognizes a broad spectrum of HLA class I molecules, in particular HLA-A and HLA-B, with lower affinity (10, 26, 27). It is unlikely that increased expression of HLA-G accounts for the marked increase in LIR1 binding since the HLA-G mRNA transcript was not detected by RT-PCR in either THP-1 or THP-1.ΔCprME-PAC2A cells (data not shown). We further treated THP-1 cells with IFN-γ and stained the cells for MHC class I expression and with LIR1-Ig. The enhancement of MHC class I expression between THP-1 and IFN-γ-treated THP-1 cells (Fig. 2D, top) was similar to the difference between THP-1 and replicon-expressing THP-1 cells (Fig. 2B). However, the enhancement in staining with LIR1-Ig following IFN-γ treatment (Fig. 2D, bottom) was modest compared to the difference in staining between THP-1 and the replicon-expressing cells (Fig. 2C).
FIG. 2.
Binding of anti-MHC class I and NK-inhibitory receptors to THP-1 and THP-1.ΔCprME-PAC2A cells. (A) RT-PCR of THP-1 and THP-1.ΔCprME-PAC2A cells showing the expression of the DV replicon. (B) Surface expression of MHC class I in THP-1 and THP-1.ΔCprME-PAC2A cells analyzed by flow cytometry with the pan-HLA-specific MAb W6/32. Background staining with the secondary antibody (2Ab) is presented. Mean fluorescence intensities (MFIs) were 2.5 for background staining, 242 for THP-1 cells, and 417 for THP-1.ΔCprME-PAC2A cells. Results are from one representative experiment out of three performed. (C) Binding of different receptor-Igs to THP-1 and THP-1.ΔCprME-PAC2A cells. MFIs for THP-1 and THP-1.ΔCprME-PAC2A cells were, respectively, as follows: CD99-Ig, 2.1 and 3.6; KIR2DS2-Ig, 2.3 and 2.1; KIR2DL1-Ig, 15.9 and 15.8; LIR1-Ig, 5.2 and 78.9. (D) Flow cytometry analysis of MHC class I surface expression (top) and LIR1-Ig staining (bottom) of THP-1 and IFN-γ-treated THP-1 cells. THP-1 cells were treated with 100 U/ml recombinant human IFN-γ for 36 h. Cells were then stained with W6/32 or with secondary antibody only (2Ab) or stained with LIR1-Ig. Results are from one representative experiment out of two performed.
Our observations do not reflect clonal selection during the generation of the stable replicon-containing cell lines. First, replicon-expressing cells were propagated from a total population of transfected cells rather than from individual cell clones. Second, the observations of induced MHC class I expression and enhanced binding of the appropriate NK receptors were the same for two cell types, K562 and THP-1. Therefore, DV RNA replication with nonstructural protein expression upregulates MHC class I expression on K562 and THP-1 cells, which is associated with a very marked increase in the binding of the relevant NK-inhibitory receptor.
Visualization of MHC class I upregulation on K562 and THP-1 cells expressing the DV replicon.
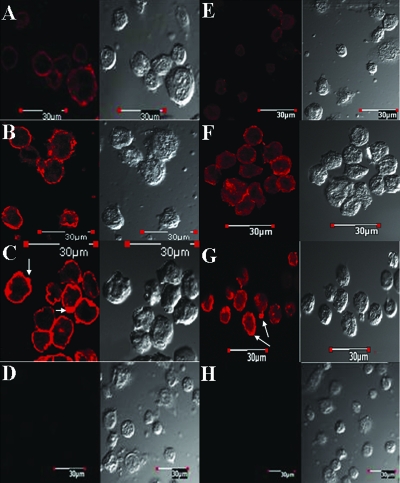
In order to gain a better understanding of the increased MHC class I expression in K562.ΔCprME-PAC2A and THP-1.ΔCprME-PAC2A cells, we visualized the binding of the pan-HLA class I-specific MAb W6/32 using confocal microscopy. Binding of W6/32 at the cell surface was clearly greater in K562.ΔCprME-PAC2A cells than in K562 cells (Fig. 3A), with some areas of particularly intense staining at the cell surface. In accordance, the staining of K562 with KIR2DL1-Ig was negligible whereas K562.ΔCprME-PAC2A cells were positively stained in a pattern similar to that of MHC class I expression (Fig. 3B). THP-1 cells that did not contain DV replicons stained positive with W6/32, consistent with constitutive expression of MHC class I (Fig. 4A). The overall increase in MHC class I expression in THP-1.ΔCprME-PAC2A cells appeared less striking than that in the K562 model, probably due to the high MHC class I expression of parental THP-1 cells (Fig. 2B). Yet, we observed intense focal areas of staining at the cell surface using W6/32 (Fig. 4C). The low-affinity LIR1-Ig stained THP-1 cells poorly (Fig. 4E). However, intense focal areas of staining at the cell surface of THP-1.ΔCprME-PAC2A cells were observed following staining with LIR1-Ig (Fig. 4G). To test whether this apparent aggregation is simply a consequence of MHC-I upregulation, we treated THP-1 cells with IFN-γ and stained with W6/32 and LIR1-Ig. MHC-I upregulation of IFN-γ-treated THP-1 cells was confirmed by flow cytometry (e.g., Fig. 2D, top) and further visualized by confocal microscopy using W6/32 (Fig. 4B). IFN-γ-treated THP-1 cells were also stained positively with LIR1-Ig (Fig. 4F), yet aggregation was more prominent in THP-1.ΔCprME-PAC2A cells (Fig. 4G). These data suggest that DV RNA replication with nonstructural protein expression not only increases surface expression of MHC class I but may cause aggregation of MHC class I molecules at the cell surface. In keeping with this, we noted that the increase in the binding of the low-affinity NK-inhibitory receptor LIR1 to THP-1.ΔCprME-PAC2A cells was much greater than the increase in the binding of the high-affinity anti-HLA class I antibody (W6/32) and the modest increase in the binding of LIR1-Ig to IFN-γ-treated THP-1 cells, as measured by flow cytometry (Fig. 2D, bottom).
FIG. 3.
Confocal analysis of MHC class I expression in K562 cells expressing the DV replicon. K562 and K562.ΔCprME-PAC2A cells were fixed and stained with the pan-HLA-I-specific MAb W6/32 (A) or with KIR2DL1-Ig (B), followed by Cy3-conjugated anti-mouse Fc or Cy5-conjugated anti-human Fc secondary antibodies (2Ab), respectively. Images on the right show the background staining of K562.ΔCprME-PAC2A cells with the appropriate secondary antibody. Cells were analyzed by confocal microscopy.
FIG. 4.
Confocal analysis of MHC class I expression in IFN-γ-treated THP-1 and THP-1 cells expressing the DV replicon. THP-1 cells were treated with 100 U/ml recombinant human IFN-γ for 36 h. THP-1, IFN-γ-treated THP-1, and THP-1.ΔCprME-PAC2A cells were stained with W6/32 antibody followed by Cy3-conjugated anti-mouse Fc (panels A, B, and C, respectively) or with Cy3-conjugated anti-mouse Fc alone (D; shown for THP-1.ΔCprME-PAC2A cells only). THP-1, IFN-γ-treated THP-1, and THP-1.ΔCprME-PAC2A cells were stained with LIR1-Ig followed by Cy3-conjugated anti-human Fc (panels E, F, and G, respectively) or with Cy3-conjugated anti-human Fc alone (H; shown for THP-1.ΔCprME-PAC2A cells only). Shown are representative images from one experiment out of two performed.
Differential lysis of parental and replicon-expressing cells by NK92 and NK92/KIR2DL1-GFP cell lines.
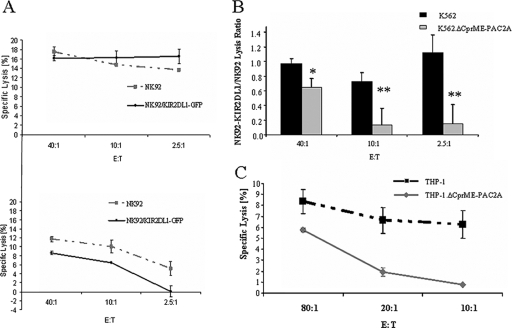
In order to investigate whether enhanced binding of KIR2DL1 to K562.ΔCprME-PAC2A cells altered their susceptibility to NK cell lysis, we compared the cytotoxic activity of NK92 cells stably transfected with the KIR2DL1 receptor (40) to the cytotoxic activity of NK92 parental cells that do not express either KIR2DL1 or KIR2DL2 (14) (data not shown). The cytolytic activities of NK92/KIR2DL1-GFP and NK92 cells against K562 cells were comparable, but a significant reduction of NK92/KIR2DL1-GFP cytotoxicity against K562.ΔCprME-PAC2A cells was observed over a range of effector-to-target cell (E:T) ratios (Fig. 5A and B). Similar results were obtained with the YTS/eco and YTS-KIR2DL1 NK cell lines, although the general lysis activity of these cells against K562 and K562.ΔCprME-PAC2A cells was low (data not shown). Since expression of DV replicons in THP-1 cells resulted in increased binding of LIR1-Ig receptor and since NK92 cells express LIR-1 (21) (data not shown), we tested the ability of NK92 cells to kill THP-1 and THP-1.ΔCprME-PAC2A cells. NK92 displayed lower cytolytic activity against THP-1.ΔCprME-PAC2A cells than against THP-1 cells over a range of E:T ratios (Fig. 5C). These data suggest that upregulation of MHC class I expression by DV replicons specifically inhibits the cytolytic activity of NK-like cell lines expressing the relevant inhibitory receptors.
FIG. 5.
Differential lysis of parental and replicon-expressing cells by NK92 and NK92/KIR2DL1-GFP cell lines. NK92/KIR2DL1-GFP cell cytotoxicity is impaired against K562 cells expressing the DV replicon. K562 and K562.ΔCprME-PAC2A cells were used as targets in a standard 5-h 35S release assay. (A) Representative assay. (Top) K562; (bottom) K562.ΔCprME-PAC2A. (B) The cytotoxic activity of NK92 cells was compared to the cytotoxic activity of NK92/KIR2DL1-GFP cells, and activity was normalized by dividing target-specific lysis activity of NK92/KIR2DL1-GFP cells by that of NK92 cells. Values are means of two different experiments. *, P < 0.02; **, P < 0.0002 (as analyzed by single-factor analysis of variance). (C) Reduced cytotoxic activity of NK92 cell line against THP-1 cells expressing the DV replicon compared to that of the parental THP-1 cells. THP-1 and THP-1.ΔCprME-PAC2A cells were labeled with 35S and then incubated with NK92 at various E:T ratios for 5 h. The value for each time point is the mean of the percent specific lysis of four separate wells.
Cytolytic activity of primary NK clones against K562 and K562.ΔCprME-PAC2A cells correlates with their KIR2DL1 and KIR2DL2 expression.
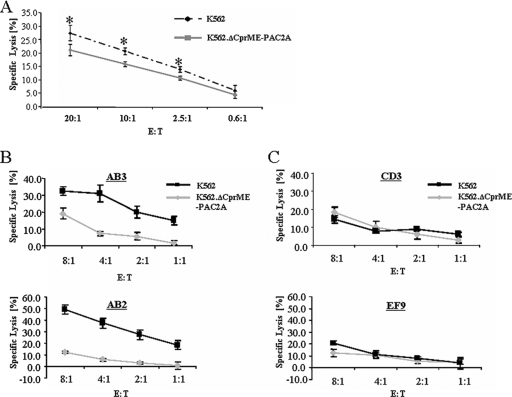
In order to examine whether the cytolytic activity of primary NK cells against cells containing DV replicons was inhibited, we first generated a primary NK line from a healthy donor. The primary NK line expressed both KIR2DL1 (21% positive cells) and KIR2DL2 (40%). The cytolytic activity of the primary NK line against K562.ΔCprME-PAC2A cells was slightly but significantly lower than the lysis of K562 cells over a range of E:T ratios (Fig. 6A). The presence of KIR2DL1 and KIR2DL2 double-negative NK cells (at least 40%) in this NK line could account for the limited reduction in lysis. Therefore, to better explore the role of KIR2DL1 and KIR2DL2, NK clones (19 clones) were selected using specific anti-KIR2DL1 and anti-KIR2DL2 MAbs and tested for their ability to kill K562 and K562.ΔCprME-PAC2A cells. Seven of 11 of the clones expressing either KIR2DL1 or KIR2DL2 or both demonstrated reduced cytolytic activity against K562.ΔCprME-PAC2A cells (e.g., clones AB3 and AB2; Fig. 6B). Furthermore, 6/8 of the clones that were double negative for KIR2DL1 and KIR2DL2 exhibited similar cytolytic activities against K562 and K562.ΔCprME-PAC2A cells (e.g., clones CD3 and EF9; Fig. 6C). These data suggest a strong association between KIR2DL1/2 expression and cytolytic activity against DV replicon-containing K562 cells. The reason why 4/11 of the single/double-positive KIR2DL1/KIR2DL2 cells and 2/8 of the double-negative clones did not kill K562 and K562.ΔCprME-PAC2A cells as expected is not clear. No correlation between the intensity of the relevant receptors on each clone and the deviant behavior of the clone was observed. It is possible that these NK clones express receptors to other proteins that are important in regulating NK cytotoxicity and are either up- or downregulated in DV replicon-containing K562 cells. Another plausible explanation for the noninhibited phenotype of NK clones, stained positively with MAbs to KIR2DL1/KIR2DL2, is that they express the activating KIR2DS1/KIR2DS2 receptors, stained positively by the same MAbs.
FIG. 6.
Lysis of K562 and K562.ΔCprME-PAC2A cells by the primary NK line and NK clones. (A) Reduced cytotoxic activity of the primary NK line against K562 cells expressing the DV replicon. Primary NK cells prepared as described in Materials and Methods were incubated for 5 hours with labeled K562 and K562.ΔCprME-PAC2A cells. Results are from one representative experiment out of two performed. The value for each time point is the mean of the percent specific lysis of four separate wells. *, P < 0.04, as analyzed by single-factor analysis of variance. (B and C) NK clone lysis against K562 and K562.ΔCprME-PAC2A cells. Clones were stained for the presence of KIR2DL1 and KIR2DL2 receptors using anti-KIR2DL1 and anti-KIR2DL2 MAbs. (B) Lysis of K562 and K562.ΔCprME-PAC2A cells by two representative NK clones expressing only KIR2DL2 (AB3) or both KIR2DL1 and KIR2DL2 (AB2). (C) Lysis of K562 and K562.ΔCprME-PAC2A cells by two representative NK clones (CD3 and EF9) that don't express either KIR2DL1 or KIR2DL2. Results for all the lysis assays presented are from one representative experiment out of two performed. The value for each time point is the mean of the percent specific lysis of four separate wells.
Expression of the DV replicon in K562 cells enhances the transcription of immunoproteasome-related genes.
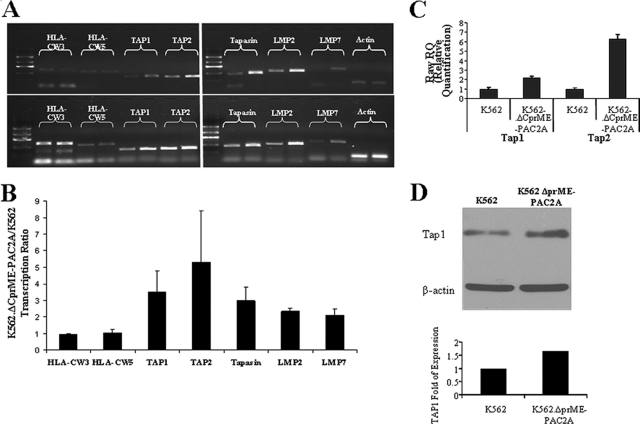
Earlier work has suggested that MHC class I upregulation on the surfaces of flavivirus-infected cells may not be caused by increased transcription of MHC class I itself but rather by enhanced expression of TAP and other immunoproteasome-related proteins (3, 30). In order to determine whether or not DV replicons induced MHC class I upregulation by this mechanism, we examined levels of MHC class I mRNA and mRNAs encoding other known members of the antigen processing and presentation pathway in cells that did and did not contain DV replicons. Although we had observed greatly increased cell surface expression of HLA-Cw3 and HLA-Cw5 on K562.ΔCprME-PAC2A cells compared with K562 cells, HLA-Cw3 and HLA-Cw5 mRNA levels were similar in these two cell lines (Fig. 7A and B). In contrast, significantly enhanced expression of all the immunoproteasome-related genes tested (TAP1, TAP2, tapasin, LMP2, and LMP7 genes) was detected in K562.ΔCprME-PAC2A cells by semiquantitative RT-PCR (Fig. 7A and B). This was confirmed by real-time PCR for TAP1 and TAP2, which demonstrated a 2.2-fold increase in TAP1 and a 6.3-fold increase in TAP2 in K562.ΔCprME-PAC2A cells compared with K562 cells, (Fig. 7C). Protein content analysis for TAP1 revealed similar upregulation (Fig. 7D). Since IFN-γ is a strong inducer of MHC class I and immunoproteasome gene expression (29), we tested supernatants from K562 and K562.ΔCprME-PAC2A cells for the presence of IFN-γ by standard enzyme-linked immunosorbent assay. No IFN-γ was detected, suggesting that upregulation of the immunoproteasome-related genes was not mediated by IFN-γ (data not shown). IFN-α/β are also capable of upregulating MHC class I expression, but, since K562 cells are type I IFN null cells, this can be excluded as an explanation (13). Interestingly, this effect was not observed in THP-1 and THP-1.ΔCprME-PAC2A cells, in which no enhancement of either the mRNA level of immunoproteasome genes or TAP protein content was observed (Fig. 8A and B and data not shown). Yet, HLA-B mRNA levels were greater in THP-1.ΔCprME-PAC2A cells than in THP-1 cells (Fig. 8A and B). Again, no IFN-γ was detected in the supernatants of parental and replicon-expressing THP-1 cells. NF-κB-dependent HLA transcription has been reported in response to flavivirus infection (24). We thus tested NF-κB transcription factor activity in THP-1 and THP-1.ΔCprME-PAC2A cells and observed significant induction of NF-κB activity in THP-1.ΔCprME-PAC2A cells (Fig. 8C). In the K562 model, we observed only a twofold increase in NF-κB activity (Fig. 8D) and no increase in HLA gene transcription (Fig. 7A and B). Taken together, these data show that DV RNA replication with nonstructural gene expression is sufficient to upregulate cell surface expression of MHC class I. However, there is more than one potential mechanism for this effect, which may be specific to MHC subclasses or may vary with cell type.
FIG. 7.
Transcription and protein content of immunoproteasome-related genes and MHC class I genes in K562 cells expressing the DV replicon. RNA from the parental and replicon-expressing cells was reverse transcribed and amplified by PCR. (A) Representative experiment of RT-PCR amplification with 25 cycles (top) or 30 cycles (bottom) using primers for the indicated genes including the β-actin gene as a standard. For each gene the left lane is the amplification product of K562 cDNA and the right lane is the amplification product of K562.ΔCprME-PAC2A cDNA. Results are from one representative experiment out of three performed. (B) Average of three different experiments like the experiment presented in panel A in which the intensities of the amplification products were quantified and normalized using β-actin. The normalized intensities for K562.ΔCprME-PAC2A cells were divided by the normalized intensities for K562 cells and presented as transcription ratios. (C) Transcription of TAP1 and TAP2 genes in K562 and K562.ΔCprME-PAC2A cells measured by real-time PCR and normalized to the β-actin gene as a reference. Data are presented as raw relative quantification (RQ) ± maximum/minimum RQ and are from one representative experiment out of two performed. Similar results were obtained when results were normalized to those for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and TBP (TATA-binding protein). (D) Western blot of TAP-1 and β-actin. Twenty micrograms of whole-cell lysate was loaded on a 10% acrylamide gel. Transferred proteins were detected with monoclonal 148.3 mouse anti-human TAP1 or monoclonal mouse anti-human β-actin. The densitometry-quantified intensity of the TAP-1 bands was normalized to that for the corresponding β-actin. Expression relative to the normalized TAP-1 intensity of K562 cells is presented.
FIG. 8.
NF-κB activity and transcription of immunoproteasome-related genes and MHC class I genes in THP-1 cells expressing the DV replicon. (A) RT-PCR amplification with 25 cycles using primers for the indicated genes including the β-actin gene as a standard. For each gene the left lane is the amplification product of THP-1 cDNA and the right lane is the amplification product of THP-1.ΔCprME-PAC2A cDNA. Results are from one representative experiment out of two performed. (B) The intensity of the amplification product presented in panel A was quantified and normalized using β-actin. (C) NF-κB activity. A reporter plasmid expressing luciferase through a minimal promoter linked to three copies of the consensus NF-κB-responsive element was used to measure activation of NF-κB. Enzymatic activity in the cell extract was assayed 24 h after transfection. The normalized luciferase activity of THP-1.ΔCprME-PAC2A cells was divided by the normalized luciferase activity of THP-1 and presented relative to NF-κB activity. Results are a summary of two different experiments performed. The bar indicates the standard error (SE). (D) NF-κB activity of K562.ΔCprME-PAC2A cells compared to K562 cells. The experimental procedure is as described for panel C. Results are a summary of two different experiments performed. The bar indicates SE.
DISCUSSION
MHC class I upregulation by flavivirus infection has previously been observed in studies using whole-virus infection (reviewed in references 20, 24, and 36). However, little was known about the viral components that mediate MHC upregulation and the effect of increased MHC class I expression on NK cell function. In the current study, we examined MHC class I expression on cells expressing DV replicons and subsequently the susceptibility of the cells to NK cell lysis. Our results establish that (i) DV replicon expression is sufficient for MHC class I upregulation in two human cell line models, K562 and THP-1 (Fig. 1B and 2B, respectively), (ii) recombinant NK-inhibitory receptors show enhanced binding to replicon-expressing cells that express the corresponding MHC class I molecules (Fig. 1C and 2C), and (iii) NK cell cytolytic activity against replicon-expressing cells is inhibited in accordance with the expression of the corresponding NK-inhibitory receptor (Fig. 5 and 6). Therefore, expression of a DV replicon coding for nonstructural proteins suffices to enhance MHC class I expression that functionally inhibits NK lysis of target cells.
The mechanism of MHC class I upregulation in flavivirus-infected cells has not been fully elucidated. In part, it appears to be IFN independent (9, 20) (data not shown), but the exact mechanism and especially the viral components involved are unclear. Uncleaved C-prM protein has been implicated in increasing MHC class I expression, and it has been suggested that flavivirus-mediated upregulation of MHC class I may be an incidental consequence of virus assembly rather than a mechanism evolved as a means of immune escape (23). Our results using replicon-expressing cells suggest another mechanism for MHC class I upregulation, mediated by expression of one or more DV nonstructural protein and/or viral RNA replication. Single or double expression of DV nonstructural proteins did not affect MHC class I expression (data not shown), suggesting a prominent role for viral RNA replication. In some cell types, flavivirus-induced increases in MHC class I expression were reported to be mediated by TAP (3, 30). For example, West Nile virus (WNV) infection of human skin fibroblast cultures appears to increase LMP-2 and TAP-1 mRNA levels (3), whereas increased TAP activity in WNV-infected HeLa and LCL721 human cell lines occurs without increased TAP mRNA or protein (30). Other studies employing different cell types reported that infection induced NF-κB-dependent transcriptional activation of MHC class I genes (9, 11). In this study, we employed two replicon-expressing cell types, K562 and THP-1, and observed different mechanisms in each cell line. In replicon-containing K562 cells, increased MHC class I expression was associated with increased transcription of immunoproteasome genes, including the TAP1 gene, which was also confirmed at the protein level for TAP-1 (Fig. 7). Modest enhancement of NF-κB activity in the K562 model was not followed by enhanced HLA gene transcription (Fig. 7 and 8). In contrast, immunoproteasome gene transcription was not upregulated in replicon-containing THP-1 cells, but we detected enhanced transcription of the HLA-B gene and significant enhancement of NF-κB activity (Fig. 8). Therefore, in keeping with the preceding literature (23, 24), we found evidence of more than one mechanism of flavivirus-induced upregulation of MHC class I expression operating in different cells. The mechanistic difference could stem from the cell type differences and/or from differences in intensity of replicon expression. The moderately enhanced expression of MHC class I on replicon-containing cells had a profound effect on binding of NK-inhibitory receptors and NK killing. We used confocal microscopy to visualize expression of MHC class I and found the first evidence of flavivirus-induced aggregation of MHC class I molecules at the cell surface (Fig. 3 and 4). IFN-γ-treated THP-1 cells enhanced MHC class I expression but did not manifest a prominent LIR1-Ig binding aggregation phenotype, as did replicon-expressing THP-1 cells (Fig. 4). This correlated with modest staining with LIR1-Ig following treatment with IFN-γ compared to that for replicon-expressing THP-1 cells (Fig. 2). We speculate that aggregation of MHC class I molecules contributes to increased engagement by the low-affinity NK-inhibitory receptors and consequently inhibition of NK killing. The mechanism of MHC class I aggregation is the subject of ongoing studies in our laboratory.
NK cells have the potential to control viral infections through recognition and elimination of virus-infected cells (25). NK cells display both activation and inhibitory receptors, which recognize different ligands, and integration of the signals received from these receptors determines NK activity. The role of NK cells in controlling flavivirus infections is not clearly defined. NK cell activity is transiently increased following flavivirus infection in mice (43), and early activation of NK cells in humans has been associated with mild clinical disease (4). Yet, antibody depletion of NK cells in mice did not alter morbidity or mortality after WNV infection (39). It is likely that there is a complex balance between flavivirus-induced activation of NK cells and subversion of NK killing by flavivirus-induced enhanced MHC class I expression on infected cells. The fact that increased MHC class I expression appears to be a general effect of flavivirus infection and may occur by more than one mechanism suggests that this may be an important evolutionary attribute for these viruses. Moreover, upregulation of MHC class I expression has potential disadvantages for the virus, most obviously increased susceptibility to attack by MHC class I restricted cytotoxic T cells (CTL) (11, 33). Indeed, many other viruses have evolved mechanisms to suppress MHC class I expression in order to evade CTL killing (25). One hypothesis is that enhancement of MHC class I expression by flaviviruses enhances the avidity of the CTL-target interaction, recruiting a wider-affinity range of CTL, including low-avidity self-cross-reactive clones. This may divert the CTL system to infected cells in G0 and away from the cycling, most-virus-productive infected cells, which express lower levels of MHC class I (20). Alternatively, it has been proposed that the evolution of these viruses has been most affected by the selective pressure of innate rather than adaptive immunity (24). Flaviviruses such as DV do not establish persistent viral infection in the vertebrate host, and infection of its arthropod vector, which is essential for propagation of the virus, must occur during the short viremic period. Mosquitoes are remarkably resistant to oral infection with DV, and so DV must reach high titers in human blood for further transmission to occur. Our work provides further evidence that DV has evolved mechanisms to evade the major elements of the human innate immune response, in this case NK cell lysis. In combination with other strategies to evade innate immunity, such as subversion if the IFN response (16), this may reflect selective pressure toward viruses that are capable of replicating rapidly and reaching the high blood titers required for transmission to its arthropod vector (24). Subsequent effective engagement of CTL responses, which clear DV and have been implicated in the pathogenesis of dengue hemorrhagic fever (31, 32), may not exert significant evolutionary pressure on DVs if contemporaneously the DV life cycle is continuing within infected mosquitoes. Greater understanding of the intricate relationship between DVs and the human immune system, and in particular the innate immune system, may inform future therapeutic strategies and the development of rationally attenuated vaccine candidates.
ADDENDUM IN PROOF
A recent publication suggests that influenza virus infection augments NK cell inhibition through the membrane-associated reorganization of MHC class I proteins (H. Achdout, I. Manaster, and O. Mandelboim, J. Virol., 4 June 2008, doi:10.1128/JVI.00870-08). This finding together with our results supports a general mechanism implied by certain viruses.
Acknowledgments
This study was supported by grants from the European Commission (INCO-DEV, contract DENFRAME no. 51711) and the World Health Organization (to M.J.).
We thank Inabl Azran for technical assistance.
Footnotes
Published ahead of print on 28 May 2008.
REFERENCES
- 1.Achdout, H., T. I. Arnon, G. Markel, T. Gonen-Gross, G. Katz, N. Lieberman, R. Gazit, A. Joseph, E. Kedar, and O. Mandelboim. 2003. Enhanced recognition of human NK receptors after influenza virus infection. J. Immunol. 171915-923. [DOI] [PubMed] [Google Scholar]
- 2.Allan, D. S., A. J. McMichael, and V. M. Braud. 2000. The ILT family of leukocyte receptors. Immunobiology 20234-41. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, S. J., S. R. Osvath, R. A. Hall, N. J. King, and L. M. Sedger. 2004. Regulation of antigen processing and presentation molecules in West Nile virus-infected human skin fibroblasts. Virology 324286-296. [DOI] [PubMed] [Google Scholar]
- 4.Azeredo, E. L., L. M. De Oliveira-Pinto, S. M. Zagne, D. I. Cerqueira, R. M. Nogueira, and C. F. Kubelka. 2006. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin. Exp. Immunol. 143345-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azran, I., K. T. Jeang, and M. Aboud. 2005. High levels of cytoplasmic HTLV-1 Tax mutant proteins retain a Tax-NF-κB-CBP ternary complex in the cytoplasm. Oncogene 244521-4530. [DOI] [PubMed] [Google Scholar]
- 6.Baba, E., R. Erskine, J. E. Boyson, G. B. Cohen, D. M. Davis, P. Malik, O. Mandelboim, H. T. Reyburn, and J. L. Strominger. 2000. N-linked carbohydrate on human leukocyte antigen-C and recognition by natural killer cell inhibitory receptors. Hum. Immunol. 611202-1218. [DOI] [PubMed] [Google Scholar]
- 7.Bloushtain, N., U. Qimron, A. Bar-Ilan, O. Hershkovitz, R. Gazit, E. Fima, M. Korc, I. Vlodavsky, N. V. Bovin, and A. Porgador. 2004. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 1732392-2401. [DOI] [PubMed] [Google Scholar]
- 8.Cerwenka, A., and L. L. Lanier. 2001. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 141-49. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, Y., N. J. King, and A. M. Kesson. 2004. Major histocompatibility complex class I (MHC-I) induction by West Nile virus: involvement of 2 signaling pathways in MHC-I up-regulation. J. Infect. Dis. 189658-668. [DOI] [PubMed] [Google Scholar]
- 10.Colonna, M., F. Navarro, T. Bellon, M. Llano, P. Garcia, J. Samaridis, L. Angman, M. Cella, and M. Lopez-Botet. 1997. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1861809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas, M. W., A. M. Kesson, and N. J. King. 1994. CTL recognition of west Nile virus-infected fibroblasts is cell cycle dependent and is associated with virus-induced increases in class I MHC antigen expression. Immunology 82561-570. [PMC free article] [PubMed] [Google Scholar]
- 12.Falconar, A. K., and P. R. Young. 1991. Production of dimer-specific and dengue virus group cross-reactive mouse monoclonal antibodies to the dengue 2 virus non-structural glycoprotein NS1. J. Gen. Virol. 72(Pt. 4)961-965. [DOI] [PubMed] [Google Scholar]
- 13.Gengrinovitch, S., B. Berman, G. David, L. Witte, G. Neufeld, and D. Ron. 1999. Glypican-1 is a VEGF165 binding proteoglycan that acts as an extracellular chaperone for VEGF165. J. Biol. Chem. 27410816-10822. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, N., A. M. Scharenberg, D. N. Burshtyn, N. Wagtmann, M. N. Lioubin, L. R. Rohrschneider, J. P. Kinet, and E. O. Long. 1997. Negative signaling pathways of the killer cell inhibitory receptor and Fc gamma RIIb1 require distinct phosphatases. J. Exp. Med. 186473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, P. Q., R. J. Tuma-Warrino, M. A. Bryan, K. G. Mitchell, D. E. Higgins, S. C. Watkins, and R. D. Salter. 2004. Escherichia coli expressing recombinant antigen and listeriolysin O stimulate class I-restricted CD8+ T cells following uptake by human APC. J. Immunol. 1721595-1601. [DOI] [PubMed] [Google Scholar]
- 16.Jones, M., A. Davidson, L. Hibbert, P. Gruenwald, J. Schlaak, S. Ball, G. R. Foster, and M. Jacobs. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 795414-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khromykh, A. A., M. T. Kenney, and E. G. Westaway. 1998. trans-complementation of flavivirus RNA polymerase gene NS5 by using Kunjin virus replicon-expressing BHK cells. J. Virol. 727270-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khromykh, A. A., P. L. Sedlak, K. J. Guyatt, R. A. Hall, and E. G. Westaway. 1999. Efficient trans-complementation of the flavivirus Kunjin NS5 protein but not of the NS1 protein requires its coexpression with other components of the viral replicase. J. Virol. 7310272-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khromykh, A. A., and E. G. Westaway. 1997. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J. Virol. 711497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King, N. J., and A. M. Kesson. 2003. Interaction of flaviviruses with cells of the vertebrate host and decoy of the immune response. Immunol. Cell Biol. 81207-216. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan, S. E., and D. N. Burshtyn. 2005. Killer cell Ig-like receptor-dependent signaling by Ig-like transcript 2 (ILT2/CD85j/LILRB1/LIR-1). J. Immunol. 1755006-5015. [DOI] [PubMed] [Google Scholar]
- 22.Le Bouteiller, P., A. Barakonyi, J. Giustiniani, F. Lenfant, A. Marie-Cardine, M. Aguerre-Girr, M. Rabot, I. Hilgert, F. Mami-Chouaib, J. Tabiasco, L. Boumsell, and A. Bensussan. 2002. Engagement of CD160 receptor by HLA-C is a triggering mechanism used by circulating natural killer (NK) cells to mediate cytotoxicity. Proc. Natl. Acad. Sci. USA 9916963-16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobigs, M., A. Mullbacher, and E. Lee. 2004. Evidence that a mechanism for efficient flavivirus budding upregulates MHC class I. Immunol. Cell Biol. 82184-188. [DOI] [PubMed] [Google Scholar]
- 24.Lobigs, M., A. Mullbacher, and M. Regner. 2003. MHC class I up-regulation by flaviviruses: immune interaction with unknown advantage to host or pathogen. Immunol. Cell Biol. 81217-223. [DOI] [PubMed] [Google Scholar]
- 25.Lodoen, M. B., and L. L. Lanier. 2005. Viral modulation of NK cell immunity. Nat. Rev. Microbiol. 359-69. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Botet, M., and T. Bellon. 1999. Natural killer cell activation and inhibition by receptors for MHC class I. Curr. Opin. Immunol. 11301-307. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Botet, M., M. Llano, F. Navarro, and T. Bellon. 2000. NK cell recognition of non-classical HLA class I molecules. Semin. Immunol. 12109-119. [DOI] [PubMed] [Google Scholar]
- 28.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 4091055-1060. [DOI] [PubMed] [Google Scholar]
- 29.Momburg, F., and G. J. Hammerling. 1998. Generation and TAP-mediated transport of peptides for major histocompatibility complex class I molecules. Adv. Immunol. 68191-256. [DOI] [PubMed] [Google Scholar]
- 30.Momburg, F., A. Mullbacher, and M. Lobigs. 2001. Modulation of transporter associated with antigen processing (TAP)-mediated peptide import into the endoplasmic reticulum by flavivirus infection. J. Virol. 755663-5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mongkolsapaya, J., W. Dejnirattisai, X. N. Xu, S. Vasanawathana, N. Tangthawornchaikul, A. Chairunsri, S. Sawasdivorn, T. Duangchinda, T. Dong, S. Rowland-Jones, P. T. Yenchitsomanus, A. McMichael, P. Malasit, and G. Screaton. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9921-927. [DOI] [PubMed] [Google Scholar]
- 32.Mongkolsapaya, J., T. Duangchinda, W. Dejnirattisai, S. Vasanawathana, P. Avirutnan, A. Jairungsri, N. Khemnu, N. Tangthawornchaikul, P. Chotiyarnwong, K. Sae-Jang, M. Koch, Y. Jones, A. McMichael, X. Xu, P. Malasit, and G. Screaton. 2006. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J. Immunol. 1763821-3829. [DOI] [PubMed] [Google Scholar]
- 33.Mullbacher, A., and M. Lobigs. 1995. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity 3207-214. [DOI] [PubMed] [Google Scholar]
- 34.Porgador, A., O. Mandelboim, N. P. Restifo, and J. L. Strominger. 1997. Natural killer cell lines kill autologous β2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 9413140-13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi, S. L., Q. Zhao, V. K. O'Donnell, and P. W. Mason. 2005. Adaptation of West Nile virus replicons to cells in culture and use of replicon-bearing cells to probe antiviral action. Virology 331457-470. [DOI] [PubMed] [Google Scholar]
- 36.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 809349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saulquin, X., L. N. Gastinel, and E. Vivier. 2003. Crystal structure of the human natural killer cell activating receptor KIR2DS2 (CD158j). J. Exp. Med. 197933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shresta, S., J. L. Kyle, P. R. Beatty, and E. Harris. 2004. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J. mice. Virology 319262-273. [DOI] [PubMed] [Google Scholar]
- 39.Shrestha, B., M. A. Samuel, and M. S. Diamond. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standeven, L. J., L. M. Carlin, P. Borszcz, D. M. Davis, and D. N. Burshtyn. 2004. The actin cytoskeleton controls the efficiency of killer Ig-like receptor accumulation at inhibitory NK cell immune synapses. J. Immunol. 1735617-5625. [DOI] [PubMed] [Google Scholar]
- 41.Tormo, J., K. Natarajan, D. H. Margulies, and R. A. Mariuzza. 1999. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature 402623-631. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchiya, S., M. Yamabe, Y. Yamaguchi, Y. Kobayashi, T. Konno, and K. Tada. 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 26171-176. [DOI] [PubMed] [Google Scholar]
- 43.Vargin, V. V., and B. F. Semenov. 1986. Changes of natural killer cell activity in different mouse lines by acute and asymptomatic flavivirus infections. Acta Virol. 30303-308. [PubMed] [Google Scholar]
- 44.Vilches, C., and P. Parham. 2002. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 20217-251. [DOI] [PubMed] [Google Scholar]
- 45.Zilka, A., G. Landau, O. Hershkovitz, N. Bloushtain, A. Bar-Ilan, F. Benchetrit, E. Fima, T. H. van Kuppevelt, J. T. Gallagher, S. Elgavish, and A. Porgador. 2005. Characterization of the heparin/heparan sulfate binding site of the natural cytotoxicity receptor NKp46. Biochemistry 4414477-14485. [DOI] [PubMed] [Google Scholar]