Abstract
The tumor suppressor gene p53 plays a central role in the maintenance of normal cell growth and genetic integrity, while its impact on the Epstein-Barr virus (EBV) life cycle remains elusive. We found that p53 is important for histone deacetylase inhibitor-induced EBV lytic gene expression in nasopharyngeal carcinoma cells. Restoration of p53 in p53-null, EBV-infected H1299 cells augments the potential for viral lytic cycle initiation. Evidence from reporter assays demonstrated that p53 contributes to the expression of the immediate-early viral Zta gene. Further analysis indicated that the DNA-binding ability of p53 and phosphorylation of Ser392 may be critical. This study provides the first evidence that p53 is involved in the regulation of EBV lytic cycle initiation.
Epstein-Barr virus (EBV), a human gammaherpesvirus, is associated with many human malignancies (23). In the EBV life cycle, the process of reactivation switches the viral state from the latent phase to the productive lytic cycle. The reactivation of EBV not only produces infectious viral progeny but also contributes to disease progression. It was demonstrated that EBV lytic replication is critical for the pathogenesis of oral hairy leukoplakia in immunocompromised patients (34). Elevation of antibodies against EBV lytic gene products in the sera of patients with nasopharyngeal carcinoma (NPC) also suggests a correlation between viral reactivation and human cancer (8, 45). In addition, the requirement of lytic gene expression for outgrowth of lymphoblastoid cell lines in a SCID mouse model suggests the importance of reactivation for EBV pathogenesis (16). Therefore, it is important to study the regulation of reactivation of EBV.
Zta and Rta play key roles in initiating EBV reactivation. During the switch from latency to the lytic cycle, the major threshold is to overcome the repression and initiate the activation of the Zta promoter (Zp) or the Rta promoter (Rp). In the latent phase, the EBV genome is bound by histone proteins into a chromatin structure (9); this not only helps to reduce DNA volume but also serves generally to silence transcription. Considering Zp, for example, the myocyte enhancer binding factor 2 family that binds to Zp recruits class II histone deacetylases (HDACs) and thus causes local chromatin condensation to maintain latency (12). Upon reactivation, an increase in histone acetylation of Zp to overcome chromatin repression seems to be one of the key steps toward initiation of expression of Zta (22). Hence, the action of HDAC inhibitor (HDACi)-mediated chromatin remodeling of Zp may account in part for the ability of EBV to reactivate.
Based on previous studies, it seems that p53 acts as a two-edged sword in viral replication. For some DNA viruses, such as polyomaviruses and papillomaviruses, activation of p53 would lead to cell cycle arrest and thus impede viral replication, because viral replication must be supplemented by replicative machineries which are present only in the S-phase cellular environment (25, 30, 37, 40). On the other hand, p53 may participate positively in the replication of other viruses. It has been shown that p53 can enhance adenoviral replication and increase the production of virions (35). For human immunodeficiency virus, lack of p53 inhibits viral replication (31). For herpesviruses, several studies have indicated that p53 is recruited to viral replication compartments, suggesting its possible involvement in viral replication (11, 44, 47). Recently, p53 was also found to be important in permissive human cytomegalovirus (HCMV) replication, probably by affecting the formation of replication foci and hence promoting viral replication (2). According to these findings, it is intriguing to question whether p53 is involved in the progression of the EBV lytic cycle. Data from Kenney's lab indicated that Zta can interact with p53 and that both proteins can regulate the other's biological functions (27, 46). However, the role of p53 in the initiation of EBV reactivation still needs further investigation.
EBV lytic cycle can be induced significantly in p53-positive NPC cell lines but not in p53-null cells.
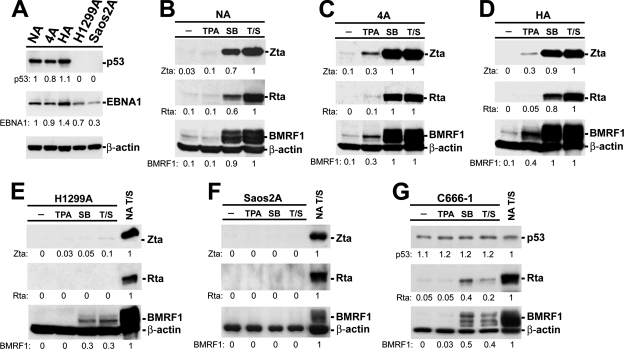
To investigate the potency of EBV lytic cycle progression in different cellular backgrounds, five EBV-infected epithelial cell lines were established as described previously (4). Three NPC cell lines, TW01, TW04, and HONE-1, express p53, and the others are p53-null human cells, including a non-small-cell lung cancer cell line, H1299 (5, 41), and an osteosarcoma cell line, Saos2 (26). Cells infected with a recombinant Akata strain EBV containing a neomycin resistance marker and selected by G418 were designated NA, 4A, HA, H1299A, and Saos2A, respectively (4). As shown in Fig. 1A, expression of the viral latent protein EBNA1 indicates successful infection with EBV, and the expression of p53 was also demonstrated by Western blotting. To examine the viral lytic induction potencies of these cell lines, cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA; phorbol ester), sodium-n-butyrate (SB; HDACi), or TPA plus SB (T/S). The characteristic EB viral lytic proteins, Zta, Rta, and BMRF1, were detected in NA, 4A, and HA cells, indicating viral lytic cycle progression (Fig. 1B to D). Furthermore, viral lytic gene expression could also be observed by using a natural EBV-harboring NPC cell line, C666-1 (6), in which p53 protein was expressed (Fig. 1G). However, in H1299A and Saos2A cells, expression of these viral lytic proteins was hardly induced by chemical manipulation (Fig. 1E and F), suggesting that EBV lytic cycle initiation can be triggered only in a specific cellular environment. We speculated that p53 might play a role in the regulation of the EBV lytic cycle.
FIG. 1.
Characterization of lytic cycle induction potencies in EBV-infected epithelial cell lines. (A) Establishment of EBV-harboring epithelial cells. Five EBV-infected epithelial cell lines, designated NPC-TW01-Akata (NA), NPC-TW04-Akata (4A), HONE-1-Akata (HA), H1299-Akata (H1299A), and Saos2-Akata (Saos2A), were established by infecting cells with recombinant Akata strain EBV and selecting them with G418. Ten micrograms of each cell lysate was resolved by electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred onto a Hybond-C membrane (Amersham Biosciences), and analyzed by immunoblotting to detect the expression of the viral latent protein EBNA1 and p53 (DO-1; Santa Cruz) with their specific antibodies. The luminescence signals were revealed by enhanced chemiluminescence (Perkin-Elmer) following exposure on X-ray films. β-Actin was used as an internal control. The intensity of each EBNA1, p53, and β-actin signal was determined by ImageQuant, and the relative intensities of EBNA1 and p53 (indicated below each panel) were shown after being normalized with their corresponding β-actin intensities and then standardized with the signal of NA cells (lane 1). (B to G) Examination of the lytic potencies of EBV-harboring cells. Cells were treated with 40 ng/ml TPA, 3 mM SB, or T/S or mock treated for 48 h, and then the lytic gene expression of Zta, Rta, and BMRF1 in NA (B), 4A (C), HA (D), H1299A (E), and Saos2A (F) cells and in the EBV-carrying NPC cell line C666-1 (G) was detected by immunoblotting as described above. The relative intensities shown were calculated and standardized with the corresponding T/S intensities (B to D) or with the intensities for positive control NA cells treated with T/S for 48 h (NA T/S) (E to G).
p53 is an essential factor for chemicals, especially HDACi, to induce viral early lytic protein expression.
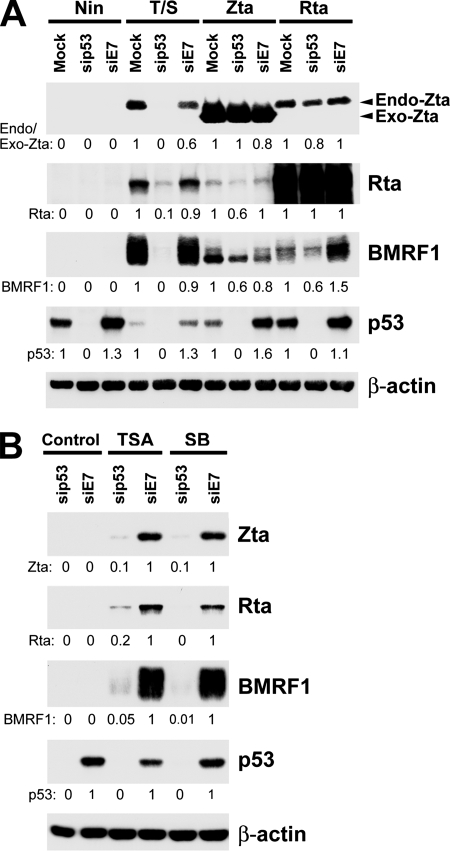
In order to elucidate the involvement of p53 in EBV lytic cycle progression, we used a DNA-based pSUPER small interfering RNA (siRNA) expression approach. Plasmids expressing p53-targeted siRNA (sip53) and an irrelevant control siRNA (siE7) were constructed according to the methods in previous reports (1, 13). As shown in Fig. 2A, sip53 clearly reduced p53 expression in NA cells, and blockade of p53 expression severely impaired T/S-triggered viral lytic gene expression compared to that in cells treated with siE7. These data strongly support our speculation that p53 contributes to successful initiation of the viral lytic cycle in EBV-positive NPC cells. On the other hand, overexpression of exogenous Zta or Rta to provoke EBV lytic activation could still induce detectable expression of endogenous Zta and BMRF1 in sip53-treated cells (Fig. 2A), suggesting that the ability of Zta or Rta to transactivate its downstream genes is not notably affected by the lack of p53 (7, 14). Therefore, our data suggest that p53 might be involved in the initial step of viral reactivation.
FIG. 2.
p53 is an essential factor for chemical induction of EBV lytic cycle initiation. (A) Depletion of p53 abrogates the T/S-induced EBV lytic cycle. NA cells were transfected with plasmids expressing p53-targeted siRNA (sip53) and an irrelevant control siRNA (siE7) for two passages. Cells were then treated with T/S or transfected with Zta- or Rta-expressing plasmids to induce the EBV lytic cycle. “Nin” denotes cells without chemical treatment or transfection. Immunoblotting was performed to examine the expression of p53 and the viral lytic genes for Zta, Rta, and BMRF1. Endo-Zta and Exo-Zta denote Zta proteins from different EBV strains, namely, Akata (endo) and B95.8 (exo). (B) p53 is important for HDACi-induced viral lytic cycle initiation. After transfection with the indicated siRNA, NA cells were induced with 1.25 μM TSA for 24 h or 3 mM SB for 48 h. Cell lysates were then used for an immunoblotting assay to detect the expression of the indicated viral gene products and p53. β-Actin was used as an internal control. Relative intensities of target proteins were determined.
To determine whether p53 is required specifically for the induction of the EBV lytic cycle by TPA or SB alone, we knocked down p53 expression in NA cells and treated them with TPA, SB, or another HDACi, trichostatin A (TSA), alone. Our data indicate that p53 is required for HDACi-mediated lytic induction in NA cells (Fig. 2B). TPA alone could not induce detectable viral gene expression in this system (data not shown); still, we could not exclude the effects of p53 on the TPA-mediated lytic cycle in other cell types.
p53 influences HDACi-mediated Zp activity but does not affect the histone acetylation status of Zp.
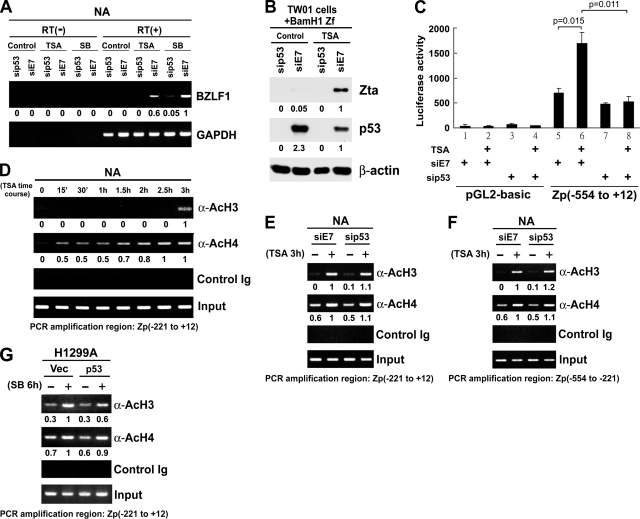
From the data described above, we assumed that p53 is involved in regulating the initial step of the viral lytic cycle. To test this hypothesis, reverse transcription-PCR (RT-PCR) analysis was performed to detect the immediate-early (IE) BZLF1 transcripts. The data in Fig. 3A show that BZLF1 transcripts could not be induced by TSA or SB when p53 was absent, indicating the participation of p53 in transcriptional expression of Zta. Meanwhile, using the entire BamHI Z fragment from the B95.8 strain as a simple induction system in EBV-negative TW01 cells, we also demonstrated that p53 plays an essential role in expression of Zta, which is under the regulation of its own promoter (Fig. 3B). Furthermore, the results of a Zp reporter assay confirmed that TSA-mediated BZLF1 promoter activity was reduced when the expression of p53 was abrogated by siRNA in TW01 cells (Fig. 3C). We also tested whether the activity of Rp was affected in the absence of p53, but TSA cannot induce Rp activation in our system (data not shown). Taken together, these data suggest that the BZLF1 promoter is active only when p53 protein is present.
FIG. 3.
p53 is a determinant of the HDACi-mediated susceptibility of Zp. (A) Requirement of p53 for HDACi-induced expression of BZLF1 transcripts. NA cells were pretreated with p53 siRNA (sip53) or an irrelevant siRNA (siE7) and then manipulated with TSA for 24 h or SB for 48 h. RT-PCR analysis was performed to measure the BZLF1 transcripts. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was detected as an internal control. RT(+) and RT(−) denote experiments with and without reverse transcriptase in the RT-PCR mix, respectively. The numeric data below the panels are the relative intensities of BZLF1 transcripts, which were determined by ImageQuant quantification and normalized with their corresponding glyceraldehyde-3-phosphate dehydrogenase signals. (B) Confirmation of the requirement of p53 for Zta protein expression from a plasmid containing the BamH1 Z fragment (BamH1 Zf). After transfection with the sip53 and siE7 siRNAs, TW01 cells were transiently transfected with BamH1 Zf, followed by TSA treatment for 24 h. The control represents no drug treatment. Zta protein expression was detected by immunoblotting assay. β-Actin was used as the internal control. The intensities of Zta and p53 relative to that of β-actin are shown. (C) p53 is an essential factor for TSA-induced Zp activation. A reporter construct driven by Zp (positions −554 to +12) and a control vector (pGL2-basic) were used in this luciferase reporter assay. TW01 cells treated with siRNA or TSA are indicated (+). A t test analysis was used to evaluate the statistical significance of the difference in Zp activities in TSA-treated (bar 6) and mock-treated (bar 5) TW01 cells expressing siE7 (P = 0.015). A significant difference in Zp activities between TW01 cells transfected with siE7 (bar 6) and those transfected with sip53 (bar 8) was seen (P = 0.011). (D) TSA promotes Zp histone acetylation status in a time-dependent manner. NA cells treated with TSA for the indicated times were subjected to ChIP assay as described previously (21). Briefly, formaldehyde cross-linked cells were washed with ice-cold phosphate-buffered saline and harvested after centrifugation at 10,000 × g. Pellets were then resuspended in nuclear lysis buffer (50 mM Tris, pH 8.1, 10 mM EDTA, 1% SDS) with Complete protease inhibitor cocktail (Roche). Lysates were sonicated to yield 500- to 1,000-bp DNA fragments. Subsequent lysates were diluted with 10 volumes of ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl, 1 mM dithiothreitol, and 50 μg salmon sperm DNA) and incubated overnight at 4°C with anti-acetylated histone H3 (anti-AcH3; Upstate), anti-AcH4 (Upstate), or nonspecific immunoglobulin G (Dako). The immunocomplexes were precipitated by incubation with salmon sperm DNA and bovine serum albumin preblocked 50% protein A-Sepharose (GE Healthcare), followed by washing sequentially with buffer I (20 mM Tris, pH 8.0, 2 mM EDTA, 150 mM NaCl, 1% Triton, 0.1% SDS), buffer II (20 mM Tris, pH 8.0, 2 mM EDTA, 500 mM NaCl, 1% Triton, 0.1% SDS), buffer III (10 mM Tris, pH 8.1, 0.25 M LiCl, 1 mM EDTA, 1% NP-40, 1% Igepal), and Tris-EDTA buffer. The immunocomplexes were then eluted using buffer containing 1% SDS, 0.25 M NaCl, and 0.1 M NaHCO3. Cross-linked DNAs were then reversed by being heated to 65°C overnight. After being treated with proteinase K, DNAs were extracted with phenol-chloroform, dissolved in Tris buffer (pH 8.0), and analyzed by PCR, using specific primers encompassing positions −221 to +12 of Zp. The ImageQuant-determined intensities of anti-AcH3- and anti-AcH4-captured Zp signals relative to those of their corresponding input controls are shown. (E to G) p53 did not affect TSA-mediated acetylation of H3 and H4 on Zp. p53-depleted NA cells (E and F) and p53-transfected H1299A cells (G) were subjected to histone H3 and H4 acetylation status analysis using ChIP assay. The regions of positions −221 to +12 (E and G) and −554 to −221 (F) of Zp were amplified by PCR. The relative intensities of anti-AcH3- and anti-AcH4-precipitated Zp DNA amounts were provided after being normalized with those of their input controls and standardized with that of TSA-treated siE7 (E and F, lane 2) or SB-treated vector control (G, lane 2).
HDACi has been shown to induce histone acetylation of the BRLF1 promoter (3). One report also suggests that HDACs may contribute to the silencing of Zp (12). We sought to test whether HDACi induces nucleosomal acetylation of Zp and whether p53 influences this process. In our experiments, a chromatin immunoprecipitation (ChIP) assay showed that TSA promotes acetylation of histones H3 and H4 in the Zta promoter region in a time-dependent manner (Fig. 3D). However, it seemed that the TSA-mediated nucleosomal acetylation status of Zp does not change, regardless of the presence of p53 (Fig. 3E, F, and G), suggesting that p53 might not affect the activity of Zp via alteration of its acetylation status.
Modifications of p53 protein affect the expression of the EBV lytic transactivator Zta.
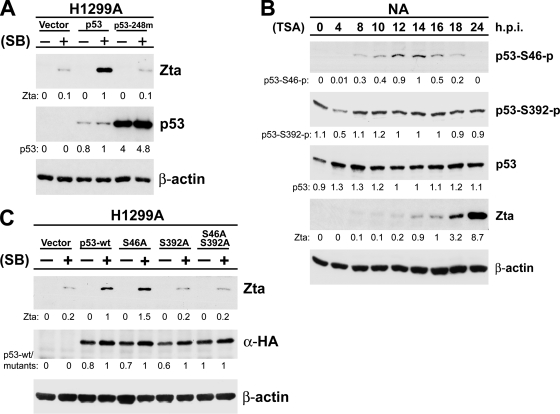
To verify the requirement of p53 for EBV lytic cycle initiation, plasmids expressing wild-type p53 and its DNA-binding mutant (p53-248m) were introduced into p53-null H1299A cells. Restoration of p53, but not the DNA-binding mutant, augmented Zta expression following SB treatment (Fig. 4A), confirming the critical role of p53 in HDACi-induced EBV lytic cycle initiation. The failure of the p53 mutant to augment induction indicated the importance of the DNA-binding ability of p53 for EBV Zta expression.
FIG. 4.
Posttranslational modification of p53 protein and its impact on the induction of EBV Zta expression. (A) Restoration of p53 augments SB-mediated Zta expression. H1299A cells were transiently transfected with p53-expressing plasmid (p53) and a DNA-binding mutant, p53-R248Q (p53-248m), for 24 h, followed by SB treatment (+) or mock treatment (−) for an additional 48 h. Gene expression was detected by an immunoblotting assay using antibodies against Zta and p53. (B) Examination of the phosphorylation status of p53 protein upon TSA treatment. NA cells treated with TSA for the indicated times were extracted with RIPA buffer, followed by an immunoblotting assay using antibodies against specific phosphorylation residues (p53 sampler kit; Cell Signaling). Phosphorylation patterns of Ser46 and Ser392 are shown as p53-S46-p and p53-S392-p. (C) Influence of p53 phosphorylation mutants on viral Zta expression. Serial expression constructs of pcDNA3 (vector), namely, hemagglutinin (HA)-wild-type p53 (p53-wt), HA-p53S46A (S46A), HA-p53S392A (S392A), and HA-p53S46A/S392A (S46A392A), were transiently transfected into H1299A cells. Cells were induced with SB for an additional 48 h. Gene expression was analyzed by an immunoblotting assay using antibodies against Zta, HA, and β-actin. The relative intensities of analyzed protein signals were obtained as described in the legend to Fig. 1.
In other situations, the functions of p53 can be regulated by numerous posttranslational modifications (42). We examined the dynamic modification patterns of the p53 protein by using antibodies against phospho-Ser6, -9, -15, -20, -37, -46, and -392 and acetylated Lys382 of p53 with TSA-treated NA cells. However, only phosphorylation of Ser46 and Ser392 was detected (Fig. 4B). Phosphorylation of Ser46 peaked at 12 to 14 h, while Ser392 remained phosphorylated throughout the process. In order to determine the importance of these modifications for EBV lytic induction, constructs with substitutions of Ser46 and Ser392 were generated and transfected into H1299A cells. Our data showed that when Ser392 was replaced with alanine, p53 lost its ability to support SB-induced expression of Zta (Fig. 4C).
In this study, we found that the role of p53 in regulating EBV lytic replication differs from that for HCMV. For EBV, the major effect of the presence of p53 is to determine the initial expression of the EBV IE Zta gene. In the case of HCMV, lack of p53 expression retards HCMV replication but does not affect HCMV IE gene expression (2), suggesting multiple functional activities of p53 in human herpesvirus replication.
How does p53 regulate the activity of EBV Zp? One possibility is that p53 binds directly to a responsive element in the Zp region. However, according to sequence analysis, there is no consensus p53-binding site present in that region. Nonetheless, a reporter assay using Zp (positions −554 to +12) (Fig. 3C) suggested a possible p53-influencing region on Zp, and this may rule out the importance of a putative p53-binding site which is present in the first intron of BZLF1. Alternatively, we hypothesized that p53 may regulate Zp via indirect binding to an Sp1 site (24, 36, 43), as these sites are abundant on Zp. However, we could not find any clear evidence for the direct binding of p53 to Zp, using either a ChIP assay or a DNA-affinity precipitation assay (data not shown). Because of the lack of evidence indicating direct binding of p53 to Zp, and certainly a p53 DNA-binding domain is required to regulate Zp (Fig. 4A), we wondered whether p53 might affect Zp by activating or repressing expression levels of some other critical transcription factors whose activities are influenced directly by p53. HDACi regulates gene expression in part by promoting nucleosomal acetylation of promoter regions, but we did not find a detectable alteration of TSA-mediated Zp acetylation in the absence of p53 (Fig. 3E to G). This suggests strongly to us that p53 may affect the activity of Zp by promoting the recruitment of other transcriptional regulators. Meanwhile, by immunoblot screening, we detected phosphorylation of the p53 protein at Ser46 and Ser392 during treatment with HDACi (Fig. 4B). Additional p53 mutation and restoration assays with p53-null H1299A cells supported the importance of the Ser392 residue in triggering the expression of EBV Zta (Fig. 4C). This evidence suggests that some kinases, such as p38, CK2, and CDK9, which are involved in the regulation of Ser392 residues (15, 17, 33), may participate in p53-mediated initiation of the EBV lytic cycle.
Although mutations of p53 have been reported for more than 50% of human cancers, they are found rarely in NPC (19, 28, 32, 39). This study provides evidence that p53 is a pivotal factor that helps EBV by overcoming the silencing of the IE gene promoter Zp and thus promotes the onset of lytic replication. Meanwhile, a close relationship between EBV reactivation and the development of NPC has been demonstrated, but the biological interaction has not been explored (8). If viral reactivation contributes positively to NPC progression and p53 protein is needed for the initiation of EBV lytic replication, tumor cells may effectively be selected for retention of p53 activity.
It is found frequently that p53 accumulates in NPC biopsies, while its function remains elusive (29, 38). This is the first report to imply that the accumulated p53 protein in NPC cells may have biological significance in the EBV life cycle. Considering that several DNA-damaging or apoptosis-promoting agents have been shown to promote EBV reactivation (10, 18, 20) and given the downstream interaction of p53 within these mechanisms, we postulate that proapoptotic signaling may be favorable for EBV lytic induction. The detailed involvement of p53 in the regulation of viral reactivation requires further elucidation.
Acknowledgments
This work was supported by the National Science Council (grant NSC 96-3112-B-002-005) and the National Health Research Institute (grants NHRI-EX 96-9419BI and NHRI-EX 97-9726BI).
We are deeply indebted to Sheau-Yann Shieh (Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan) for kindly providing us with the plasmids expressing hemagglutinin-wild-type and hemagglutinin-mutant p53. We thank Tim J. Harrison (Wohl Virion Centre, University College London) and Yao Chang (Division of Clinical Research, National Health Research Institutes, Taiwan) for valuable discussions and for reviewing the manuscript critically.
Footnotes
Published ahead of print on 21 May 2008.
REFERENCES
- 1.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296550-553. [DOI] [PubMed] [Google Scholar]
- 2.Casavant, N. C., M. H. Luo, K. Rosenke, T. Winegardner, A. Zurawska, and E. A. Fortunato. 2006. Potential role for p53 in the permissive life cycle of human cytomegalovirus. J. Virol. 808390-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, L. K., and S. T. Liu. 2000. Activation of the BRLF1 promoter and lytic cycle of Epstein-Barr virus by histone acetylation. Nucleic Acids Res. 283918-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y., C. H. Tung, Y. T. Huang, J. Lu, J. Y. Chen, and C. H. Tsai. 1999. Requirement for cell-to-cell contact in Epstein-Barr virus infection of nasopharyngeal carcinoma cells and keratinocytes. J. Virol. 738857-8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J. Y., W. D. Funk, W. E. Wright, J. W. Shay, and J. D. Minna. 1993. Heterogeneity of transcriptional activity of mutant p53 proteins and p53 DNA target sequences. Oncogene 82159-2166. [PubMed] [Google Scholar]
- 6.Cheung, S. T., D. P. Huang, A. B. Hui, K. W. Lo, C. W. Ko, Y. S. Tsang, N. Wong, B. M. Whitney, and J. C. Lee. 1999. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int. J. Cancer 83121-126. [DOI] [PubMed] [Google Scholar]
- 7.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 53243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien, Y. C., J. Y. Chen, M. Y. Liu, H. I. Yang, M. M. Hsu, C. J. Chen, and C. S. Yang. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 3451877-1882. [DOI] [PubMed] [Google Scholar]
- 9.Dyson, P. J., and P. J. Farrell. 1985. Chromatin structure of Epstein-Barr virus. J. Gen. Virol. 661931-1940. [DOI] [PubMed] [Google Scholar]
- 10.Feng, W. H., B. Israel, N. Raab-Traub, P. Busson, and S. C. Kenney. 2002. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 621920-1926. [PubMed] [Google Scholar]
- 11.Fortunato, E. A., and D. H. Spector. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 722033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruffat, H., E. Manet, and A. Sergeant. 2002. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 3141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, A. H., and K. A. Alexander. 2003. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J. Virol. 776066-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 622274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann, R., D. J. Craik, G. Pierens, R. E. Bolger, and L. Otvos, Jr. 1998. Phosphorylation of the C-terminal sites of human p53 reduces non-sequence-specific DNA binding as modeled with synthetic peptides. Biochemistry 3713755-13764. [DOI] [PubMed] [Google Scholar]
- 16.Hong, G. K., M. L. Gulley, W. H. Feng, H. J. Delecluse, E. Holley-Guthrie, and S. C. Kenney. 2005. Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J. Virol. 7913993-14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horikawa-Miura, M., N. Matsuda, M. Yoshida, Y. Okumura, T. Mori, and M. Watanabe. 2007. The greater lethality of UVB radiation to cultured human cells is associated with the specific activation of a DNA damage-independent signaling pathway. Radiat. Res. 167655-662. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, C. H., M. Hergenhahn, S. E. Chuang, P. Y. Yeh, T. C. Wu, M. Gao, and A. L. Cheng. 2002. Induction of Epstein-Barr virus (EBV) reactivation in Raji cells by doxorubicin and cisplatin. Anticancer Res. 224065-4071. [PubMed] [Google Scholar]
- 19.Hwang, J. K., and C. T. Lin. 1997. Co-localization of endogenous and exogenous p53 proteins in nasopharyngeal carcinoma cells. J. Histochem. Cytochem. 45991-1003. [DOI] [PubMed] [Google Scholar]
- 20.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 752400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka, T., and M. A. Lazar. 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol. Cell. Biol. 235122-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins, P. J., U. K. Binne, and P. J. Farrell. 2000. Histone acetylation and reactivation of Epstein-Barr virus from latency. J. Virol. 74710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieff, E. R., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2541-2548. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Wiliams & Willkins, Philadelphia, PA.
- 24.Lagger, G., A. Doetzlhofer, B. Schuettengruber, E. Haidweger, E. Simboeck, J. Tischler, S. Chiocca, G. Suske, H. Rotheneder, E. Wintersberger, and C. Seiser. 2003. The tumor suppressor p53 and histone deacetylase 1 are antagonistic regulators of the cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol. Cell. Biol. 232669-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepik, D., I. Ilves, A. Kristjuhan, T. Maimets, and M. Ustav. 1998. p53 protein is a suppressor of papillomavirus DNA amplificational replication. J. Virol. 726822-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masuda, H., C. Miller, H. P. Koeffler, H. Battifora, and M. J. Cline. 1987. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc. Natl. Acad. Sci. USA 847716-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauser, A., S. Saito, E. Appella, C. W. Anderson, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J. Virol. 7612503-12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasrin, N., K. Taiba, N. Hannan, M. Hannan, and S. al-Sedairy. 1994. A molecular study of EBV DNA and p53 mutations in nasopharyngeal carcinoma of Saudi Arab patients. Cancer Lett. 82189-198. [DOI] [PubMed] [Google Scholar]
- 29.Niedobitek, G., A. Agathanggelou, P. Barber, L. A. Smallman, E. L. Jones, and L. S. Young. 1993. p53 overexpression and Epstein-Barr virus infection in undifferentiated and squamous cell nasopharyngeal carcinomas. J. Pathol. 170457-461. [DOI] [PubMed] [Google Scholar]
- 30.Pampin, M., Y. Simonin, B. Blondel, Y. Percherancier, and M. K. Chelbi-Alix. 2006. Cross talk between PML and p53 during poliovirus infection: implications for antiviral defense. J. Virol. 808582-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauls, E., J. Senserrich, B. Clotet, and J. A. Este. 2006. Inhibition of HIV-1 replication by RNA interference of p53 expression. J. Leukoc. Biol. 80659-667. [DOI] [PubMed] [Google Scholar]
- 32.Porter, M. J., J. K. Field, J. C. Lee, S. F. Leung, D. Lo, and C. A. Van Hasselt. 1994. Detection of the tumour suppressor gene p53 in nasopharyngeal carcinoma in Hong Kong Chinese. Anticancer Res. 141357-1360. [PubMed] [Google Scholar]
- 33.Radhakrishnan, S. K., and A. L. Gartel. 2006. CDK9 phosphorylates p53 on serine residues 33, 315 and 392. Cell Cycle 5519-521. [DOI] [PubMed] [Google Scholar]
- 34.Resnick, L., J. S. Herbst, D. V. Ablashi, S. Atherton, B. Frank, L. Rosen, and S. N. Horwitz. 1988. Regression of oral hairy leukoplakia after orally administered acyclovir therapy. JAMA 259384-388. [PubMed] [Google Scholar]
- 35.Royds, J. A., M. Hibma, B. R. Dix, L. Hananeia, I. A. Russell, A. Wiles, D. Wynford-Thomas, and A. W. Braithwaite. 2006. p53 promotes adenoviral replication and increases late viral gene expression. Oncogene 251509-1520. [DOI] [PubMed] [Google Scholar]
- 36.Schavinsky-Khrapunsky, Y., M. Huleihel, M. Aboud, and A. Torgeman. 2003. Role of protein kinase C and the Sp1-p53 complex in activation of p21(WAF-1) expression by 12-O-tetradecanoylphorbol-13-acetate in human T cells. Oncogene 225315-5324. [DOI] [PubMed] [Google Scholar]
- 37.Shadan, F. F., L. M. Cowsert, and L. P. Villarreal. 1994. n-Butyrate, a cell cycle blocker, inhibits the replication of polyomaviruses and papillomaviruses but not that of adenoviruses and herpesviruses. J. Virol. 684785-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheu, L. F., A. Chen, H. H. Tseng, F. J. Leu, J. K. Lin, K. C. Ho, and C. L. Meng. 1995. Assessment of p53 expression in nasopharyngeal carcinoma. Hum. Pathol. 26380-386. [DOI] [PubMed] [Google Scholar]
- 39.Spruck, C. H., III, Y. C. Tsai, D. P. Huang, A. S. Yang, W. M. Rideout III, M. Gonzalez-Zulueta, P. Choi, K. W. Lo, M. C. Yu, and P. A. Jones. 1992. Absence of p53 gene mutations in primary nasopharyngeal carcinomas. Cancer Res. 524787-4790. [PubMed] [Google Scholar]
- 40.Staib, C., J. Pesch, R. Gerwig, J. K. Gerber, U. Brehm, A. Stangl, and F. Grummt. 1996. p53 inhibits JC virus DNA replication in vivo and interacts with JC virus large T-antigen. Virology 219237-246. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, T., H. Suzuki, T. Hida, Y. Sekido, Y. Ariyoshi, and R. Ueda. 1991. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene 61775-1778. [PubMed] [Google Scholar]
- 42.Toledo, F., and G. M. Wahl. 2006. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 6909-923. [DOI] [PubMed] [Google Scholar]
- 43.Torgeman, A., N. Mor-Vaknin, E. Zelin, Z. Ben-Aroya, M. Lochelt, R. M. Flugel, and M. Aboud. 2001. Sp1-p53 heterocomplex mediates activation of HTLV-I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate that is antagonized by protein kinase C. Virology 28110-20. [DOI] [PubMed] [Google Scholar]
- 44.Wilcock, D., and D. P. Lane. 1991. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature 349429-431. [DOI] [PubMed] [Google Scholar]
- 45.Zeng, Y., L. G. Zhang, Y. C. Wu, Y. S. Huang, N. Q. Huang, J. Y. Li, Y. B. Wang, M. K. Jiang, Z. Fang, and N. N. Meng. 1985. Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int. J. Cancer 36545-547. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Q., D. Gutsch, and S. Kenney. 1994. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol. Cell. Biol. 141929-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong, L., and G. S. Hayward. 1997. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J. Virol. 713146-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]