Abstract
The death of CD4+ CCR5+ T cells is a hallmark of human immunodeficiency virus (HIV) infection. We studied the plasma levels of cell death mediators and products—tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), Fas ligand, TNF receptor type 2 (TNFR-2), and plasma microparticles—during the earliest stages of infection following HIV type 1 (HIV-1) transmission in plasma samples from U.S. plasma donors. Significant plasma TRAIL level elevations occurred a mean of 7.2 days before the peak of plasma viral load (VL), while TNFR-2, Fas ligand, and microparticle level elevations occurred concurrently with maximum VL. Microparticles had been previously shown to mediate immunosuppressive effects on T cells and macrophages. We found that T-cell apoptotic microparticles also potently suppressed in vitro immunoglobulin G (IgG) and IgA antibody production by memory B cells. Thus, release of TRAIL during the onset of plasma viremia (i.e., the eclipse phase) in HIV-1 transmission may initiate or amplify early HIV-1-induced cell death. The window of opportunity for a HIV-1 vaccine is from the time of HIV-1 transmission until establishment of the latently infected CD4+ T cells. Release of products of cell death and subsequent immunosuppression following HIV-1 transmission could potentially narrow the window of opportunity during which a vaccine is able to extinguish HIV-1 infection and could place severe constraints on the amount of time available for the immune system to respond to the transmitted virus.
A critical event in human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) infection is virus-induced massive CD4+ and CCR5+ T-cell loss involving gut-associated lymphoid tissues (9, 20, 45). Depletion of gut-associated lymphoid tissue CD4 T cells has been documented at peak viral load (VL) in cases of acute SIVmac239 infection (27, 45, 50, 61) as well as within weeks of HIV-1 transmission in humans (9, 26, 51). During acute SIV infection, a high percentage of memory CD4+ T cells are infected (27, 45, 50, 61). While the mechanisms of immune cell death in acute HIV-1 infection are not known, in chronic HIV-1 infection, induction of cell death pathways by HIV Tat, Nef, Vpr, or gp120 proteins (5, 8, 10, 64, 65), HIV-1 infection of CD4+ T cells (26, 45, 51, 61), and uninfected cell death by molecules such as tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (30, 47) may be important.
The time from HIV-1 transmission to establishment of the latently infected pool of CD4 T cells has been termed the window of opportunity within which a preventive HIV-1 vaccine must extinguish the HIV-1 infection (38, 63). The latently infected pool of CD4 T cells is established, at a minimum, by the time of symptomatic acute HIV-1 infection approximately 25 days after transmission), although the earliest time of establishment in humans of the latent CD4 T-cell pool is not known (13, 63). In nonhuman primates, SIV dissemination occurs early after transmission, and, based on the narrow window of time during which postexposure prophylaxis may prevent infection, the SIV latent pool may be established within 2 to 3 days postinfection (1, 21, 62). Adaptive CD4, CD8, and B-cell antibody responses to HIV-1 do not appear during the VL ramp-up phase of acute infection but rather appear in coincidence with the fall in VL and appearance of acute infection symptoms at the end of the window of opportunity (23, 56; G. Tomaras and B. F. Haynes, unpublished observations). Thus, studies of the events that transpire from transmission until the onset of plasma viremia (the eclipse phase) and during the VL load ramp-up phase of acute HIV-1 infection are critical to understanding why immune responses do not occur earlier after HIV-1 transmission and to defining the obstacles a successful vaccine must overcome to extinguish HIV-1 infections.
In this report, we investigate the hypothesis that in addition to gut CD4 T-cell loss, delay in HIV-1-protective immune responses early after HIV-1 transmission may involve the production of elevated levels of immunosuppressive moieties such as TRAIL, TNF receptor type 2 (TNFR-2), and Fas ligand as well as of plasma microparticles (MPs). MPs are small membrane-bound vesicles that are released from the surface of apoptotic cells by exocytic or budding processes; as such, MPs bear cell surface markers and can bind annexin V because of the expression of phosphatidylserine (32-44, 39). MPs, which circulate in the blood under many clinical conditions, are part of a spectrum of subcellular structures that are released from cells and can be distinguished from exosomes, which are released from multivesicular bodies during activation. Unlike MPs, exosomes express endosomal markers. MPs have immunomodulatory activities and can promote immune cell death; exosomes are also immunologically active, can suppress immune responses (20, 34, 42, 55), and have been reported to have been found at elevated levels in cases of chronic HIV-1 infection (4). If elevations in levels of immunosuppressive molecules, coupled with early CD4+ T-cell death, occur early following HIV-1 transmission, then these events could potentially define a protected time during which HIV-1 is able to replicate while anti-HIV-1 T- or B-cell responses are suppressed.
To study the eclipse and early viral ramp-up phases of acute HIV-1 infection, we used archived plasma from donors whose samples were collected before, during, and after the HIV-1 VL ramp-up phase (23). We found elevations in soluble TRAIL levels in plasma soon after the appearance of HIV-1 in plasma, corresponding to ∼17 days following transmission, and later found elevated plasma TNFR-2, Fas ligand, and MP levels at the time of maximum plasma VL. These data implicate TRAIL as an early mediator of cell death in acute HIV-1 infection and provide evidence for the existence of a narrowed window of opportunity during which a HIV-1 vaccine must extinguish the transmitted virus infection.
MATERIALS AND METHODS
Plasma samples.
Seroconversion panels (HIV-1 positive [HIV-1+]/hepatitis C virus− [HCV−]/HBV− [n = 30], HIV-1−/HCV−/HBV+ [n = 10], and HIV-1−/HCV+/HBV− [n = 10]) were obtained from ZeptoMetrix Corporation (Buffalo, NY) (see Methods and Materials in the supplemental material). Each panel consisted of sequential aliquots of plasma (range, 4 to 30 aliquots) collected approximately every 3 days during the time of acute infection with HIV-1 (23). HIV-1−/HCV−/HBV− human plasma samples (n = 25) were obtained from Innovative Research (Southfield, MI). All studies were approved by the Duke University Human Subjects Institutional Review Board.
VL testing.
VL testing of HIV-1 plasma donor panels was performed by Quest Diagnostics (Lyndhurst, NJ) (HIV-1 RNA PCR Ultra). HCV and HBV VL testing was performed by Zeptometrix; select HCV VLs were provided by Philip Norris, Blood Systems Research Institute, San Francisco, CA.
ELISAs for plasma markers of apoptosis.
Enzyme-linked immunosorbent assays (ELISAs) for Fas, Fas ligand, and TRAIL (Diaclone, Besançon Cedex, France) and for TNFR-1 and TNFR-2 (Hycult Biotechnology, Uden, The Netherlands) were performed according to the manufacturer's directions. Plasma was assayed undiluted (TRAIL), diluted 1:10 (TNFR-1, TNFR-2, and Fas), or diluted 1:2 (Fas ligand). Preliminary studies demonstrated no obvious peaks in TNFR-1 and Fas levels, and therefore the studies presented here focus on TRAIL, TNFR-2, and Fas ligand only (see Methods and Materials in the supplemental materials). To analyze temporal relationships of an analyte peak compared to peak plasma VL, we determined how many subjects had a peak TRAIL, TNFR-2, and/or Fas ligand level before, coincident with, or after peak plasma VL. Data showing the relationship of relative plasma MP counts to plasma VLs (see Table S2 in the supplemental material) and the ratio of relative plasma MP counts to plasma VLs (see Table S3 in the supplemental material) are available. Results occurring before peak plasma VL were defined as representing a peak of a least 20% over initial panel sample MP levels within 15 days before maximum plasma VL; results occurring after peak plasma VL were defined as representing a >20% peak occurring within 15 days after maximum plasma VL; results occurring as coincident analyte peaks were defined as those that occurred on the same day as the maximum plasma VL.
Apoptotic MP quantification.
The number of MPs in each plasma sample was determined by flow cytometry with modifications as described previously (10, 13, 18) (see Fig. S2 and Methods and Materials in the supplemental material). An outline of the development of flow cytometric techniques for measurements of plasma MPs is available (see Fig. S2 in the supplemental material).
MP phenotypic analysis.
MPs were analyzed by flow cytometry for cell surface markers as described previously (4,39) (see Fig. S3 and Methods and Materials in the supplemental material). Data showing the effects of freeze-thaw cycles on the phenotype of plasma MPs are available (see Fig. S3 in the supplemental material).
Electron microscopy of plasma MPs.
Eight milliliters of plasma was diluted 1:5 in filtered saline solution, and MPs were pelleted (200,000 × g for 1 h at 4°C). Pellets were washed twice (100,000 × g for 30 min) in 1 ml of saline solution. The MP pellet was resuspended in 500 μl of saline solution and overlaid onto 1 ml of a 40% sucrose solution (in saline solution), and MPs were centrifuged (100,000 × g for 90 min). The pellets were fixed (1% formaldehyde at 4°C overnight), pelleted (100,000 × g for 60 min), and additionally fixed in 4% glutaraldehyde. They were encased in agar, washed in buffer, fixed in 1% osmium tetroxide, washed, and stained in uranyl acetate. Pellets were dehydrated in ethanol followed by propylene oxide, embedded in epoxy resin, and baked overnight. Ultrathin sections were cut using a Reichert Ultracut E ultramicrotome with diamond knives, poststained with uranyl acetate and lead citrate, and examined using a Philips CM12 electron microscope (FEI Co., Hillsboro, OR).
In vitro mucosal B-cell culture model.
Tonsils were obtained with Institutional Review Board-approved protocols as discarded tissues from pediatric and adult patients who underwent tonsillectomy at the Duke University Medical Center. Tonsil cells were cultured in RPMI 1640 supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin, 25 μg/ml gentamicin, 1 μg/ml amphotericin B, and 10% fetal bovine serum (Gemini Bioproducts, West Sacramento, CA) at a density of 1 × 106 cells/ml in total volumes of 1 ml in polystyrene 5-ml round-bottom tubes (BD Biosciences, Mountainview, CA) (17) (see Methods and Materials in the supplemental material). Statistical significance was determined by normalizing the Ig secretion of stimulated cells cultured alone to 100% and comparing the percentages of Ig secretion by stimulated cells cocultured with MPs.
The amount of MPs added to 1 × 106 tonsil cells in a 1-ml culture volume was the amount of MPs derived from treatment of the amount of peripheral blood mononuclear cells (PBMC) that would be in 1 ml of blood. Since the normal blood lymphocyte-monocyte count is approximately 3,000/mm3, this would be 3 × 106 PBMC per ml of blood. We prepared our MPs from 5 × 107 PBMC, tonsil, or Jurkat T cells after treatment of the cells with staurosporine, which induces apoptosis. The MPs were washed twice—a process that reduces the amount of MPs by an order of magnitude such that the remaining MPs would be from approximately 5 × 106 cells in 1 ml. A 100-μl volume of this suspension of MPs (estimated for a 100-μl volume to be from 5 × 105 cells) was added to 106 tonsil cells. Thus, for a clinical situation such as acute HIV-1 infection, where a large percentage of CD4 T cells die, this ratio of MPs to viable tonsil cells in culture is estimated to be within the range of what might be occurring in vivo (i.e., the ratio of MPs from 5 × 105 apoptotic cells to 106 viable cells in vivo).
Statistical analyses.
Statistical analysis was performed using linear mixed-effect models, Student's t tests, and Wilcoxon rank-sum tests as outlined in detail in the figure legends and elsewhere (see Methods and Materials in the supplemental material). The zero time (T0) was estimated using a linear mixed-effects mode for the logarithmic VLs (on a log 10 scale), accounting for censoring at the lower limit of detection of the assay (see Methods and Materials in the supplemental material). Each analysis of the data required use of different sample subsets. For T0 determination (n = 26), 4 of 30 subjects were omitted due to lack of the adequate samples required to calculate T0. To compare analyte levels at their maximum T0 levels in the first sample to those from uninfected donors (n = 26), the same 4 of the 30 subjects were omitted due to inadequate sampling, since four panels had no observed maximum (peak) analyte levels. For determining viral expansion rate and analyte maximum relationships, 11 of the 30 subjects were found to have a true peak VL that demonstrated a postpeak decay. The values for the remaining panels were truncated during ramp-up, prior to attaining peak VL. Data on the distribution of time intervals between donations (interobservation times) and the total data points available from 26 panels for each day relative to first detectable day of viremia are available elsewhere (see Fig. S1 in the supplemental material).
RESULTS
TRAIL, TNFR-2, and Fas ligand plasma levels are elevated during acute HIV-1 infection.
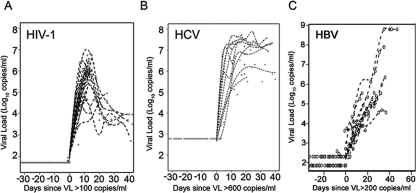
To establish a reference time point to align plasma seroconversion panels in time, the T0 was determined for each of 26 HIV-1+ panels with sufficient time points and for 10 HCV+ and 10 HBV+ plasma donor subjects; this time point was defined as the initial time at which the VL trajectory crossed the lower limit of detection of the assay (100 copies/ml, 600 copies/ml, and 200 copies/ml for HIV-1, HCV, and HBV, respectively) (Fig. 1; see also Fig. S1 in the supplemental material).
FIG. 1.
Plasma VLs of HIV-1-, HCV-, and HBV-infected plasma donor subjects. A total of 26 HIV-1+ seroconversion plasma donor plasma panels (HBV and HCV negative), 10 HBV plasma donor seroconversion panels (HIV-1 negative), and 10 HCV plasma donor seroconversion panels (HIV-1 negative) were studied. Panels demonstrate the kinetics of VL ramp-up in cases of HIV-1 (A), HCV (B), and HBV (C) infection. T0 was defined as the first day that the VL reached 100 copies/ml for HIV-1, 600 copies/ml for HCV, and 200 copies/ml for HBV. T0 values were assigned to only 26 out of 30 panels studied because of time gaps in sampling for four subjects.
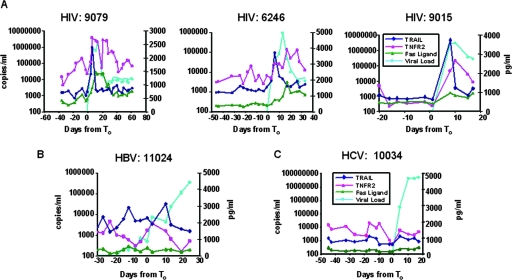
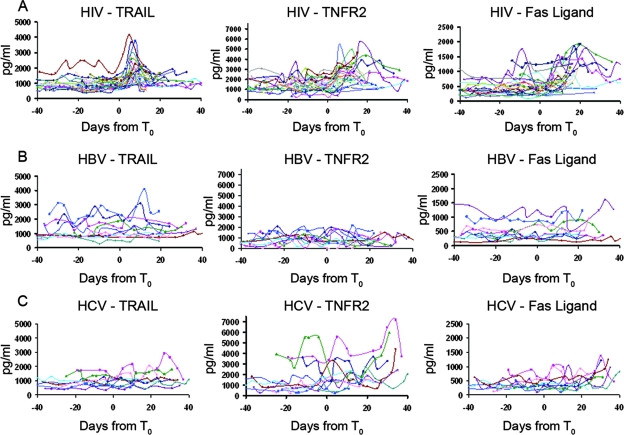
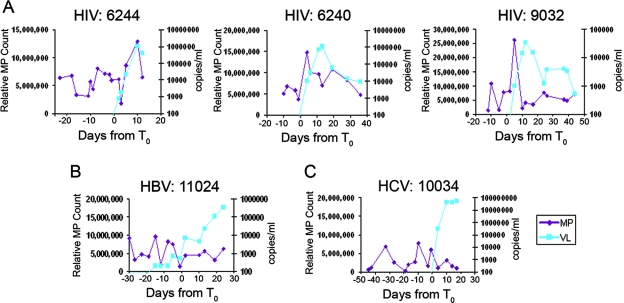
Soluble TRAIL, TNFR-2, and Fas ligand levels in sequential plasma samples from each HIV-1, HBV, and HCV plasma donor were assayed (Fig. 2A, B, and C), and the temporal relationship between an apoptotic analyte peak and the maximum observed plasma VL was determined. We determined the number of subjects that had peaks in plasma cell death analyte levels occurring before (within 15 days before maximum VL), coincident with (same day as maximum VL), or within 15 days after maximum VL (Fig. 3; see also Table S1 in the supplemental material). The majority of acute HIV-1 infection subjects (30 of 30 for TRAIL, 27 of 30 for TNFR-2, and 26 of 30 for Fas ligand) demonstrated peak analyte levels occurring within a 30-day time frame (i.e., 15 days before, at the time of, or within 15 days after the observed maximum VL). Of particular interest, the TRAIL levels of the majority (21 of 30) of the subjects peaked before the observed VL maximum occurred, while TNFR-2 and Fas ligand levels peaked coincidently with VL (Fig. 2A and 3A; see also Table S1 in the supplemental material).
FIG. 2.
Representative plasma donor patient samples analyzed for plasma markers of cell death. (A) TRAIL, TNFR-2, and Fas ligand levels were measured for each plasma sample by ELISA for three representative HIV-1+ subjects. Data for a representative patient with acute HBV are shown in panel B; data for a representative patient with acute HCV are shown in panel C. TRAIL levels are shown in dark blue, TNFR-2 levels in red, Fas ligand levels in green, and VL levels in light blue. Whereas elevations in TRAIL, TNFR-2, and Fas ligand levels were commonly found in samples from acutely HIV-1-infected plasma donors, they were not common in samples from HBV- and HCV-infected subjects (see Table S1 in the supplemental material).
FIG. 3.
Composite data showing TRAIL, TNFR-2, and Fas ligand levels for all subjects throughout the course of acute HIV-1 infection (30 subjects), acute HBV infection (10 subjects), and acute HCV infection (10 subjects). (A) Analyte data from acute HIV-1 infection results show early peaks in plasma TRAIL levels and later elevations in TNFR-2 and Fas ligand levels. (B and C) Only 3 of a total of 20 subjects with acute hepatitis infection (HBV and HCV) had elevations in TRAIL levels, and the late elevations in plasma analyte levels seen in cases of HIV-1 infection were not seen in cases of HBV infection for TNFR-2 or Fas ligand levels; however, elevations in TNFR-2 levels were seen for 6 subjects with acute HCV infection. Elevations were defined as >20% increases in plasma analyte levels within 15 days before or after the observed maximum VL (see Table S1 in the supplemental material).
Hepatitis B and C infections have been reported to induce cell death of hepatocytes (5, 11, 12). Thus, subjects acutely infected with HCV and HBV were also studied, and sporadic elevations in TRAIL, TNFR-2, or Fas ligand levels were found in 1, 2, and 1 of 10 subjects acutely infected with HBV and in 2, 6, and 7 of 10 subjects acutely infected with HCV, respectively (Fig. 2B and C and Fig. 3B and C) (see table S1 in the supplemental material). Analyte peaks, when present, were generally not high and occurred before or coincidently with HBV and HCV maximum VLs. Only three HBV+/HCV+ subjects had elevated TRAIL levels around the time of plasma viremia (Fig. 3B and C) (see table S1 in the supplemental material).
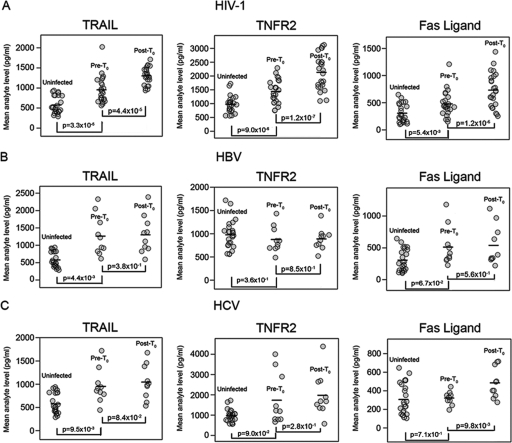
To determine the statistical significance of elevated plasma analyte levels in HIV-1, HBV, and HCV plasma panels, for those plasma panels for which T0 could be established, mean plasma analyte levels of all plasma samples for each patient post-T0 were determined via mixed-effect modeling and compared to mean pre-T0 analyte levels for all samples for each patient. In addition, pre-T0 mean analyte levels were compared with mean analyte levels for uninfected-control plasma samples (Fig. 4A, B, and C). For HIV-1+ plasma donors, the mean post-T0 TRAIL, TNFR-2, and Fas ligand levels were significantly elevated compared to pre-T0 TRAIL, TNFR-2, and Fas ligand levels (P = 4.4 × 10−5, P = 1.2 × 10−7, and P = 1.2 × 10−6, respectively) (Fig. 4A). Interestingly, the HIV-plasma donor pre- T0 analyte levels were also significantly different from analyte levels of uninfected-control plasma samples (for TRAIL, P = 3.3 × 10−4; for TNFR-2, P = 9.0 × 10−4; for Fas ligand, P = 5.4 × 10−3), thus demonstrating the extraordinarily early timing of immune perturbations in acute HIV-1 infection. However, insufficient time points were available to determine the specific pre-T0 time point of elevation for plasma analytes.
FIG. 4.
Pre-T0 and mean post-T0 analyte levels for cohorts of subjects acutely infected with HIV-1, HBV, and HCV, with mean analyte levels compared to those of a cohort of 25 uninfected-control plasma samples. (A) Mean HIV-1 post-T0 analyte levels were significantly elevated compared to pre-T0 mean analyte levels from the same infected subjects; in addition, HIV pre-T0 levels were significantly elevated compared to mean analyte levels from uninfected donors (P values from paired Wilcoxon rank-sum tests). Panels B and C show pre-T0 and post-T0 mean analyte levels for HBV (B) and HCV (C). (B) In HBV results, whereas there was no difference between pre-T0 and post-T0 analyte levels, there was a significant difference in uninfected plasma versus pre-T0 TRAIL levels. (C) In HCV results, post-T0 Fas ligand levels were significantly elevated compared to pre-T0 levels; pre-T0 HCV TRAIL levels were also significantly elevated compared to uninfected plasma control levels.
In contrast, there were no significant differences for HBV in pre-T0 and post-T0 analyte levels for TRAIL, TNFR-2, and Fas ligand (P > 0.05 in each case) (Fig. 4B). There was no elevation of TNFR-2 and Fas ligand levels for HBV panels compared to uninfected plasma results; only pre-T0 TRAIL values for HBV plasmas were significantly elevated compared to the levels seen with uninfected control plasma samples (P = 4.4 × 10−3) (Fig. 4B). For the HCV panels, comparison of pre-T0 and post-T0 means showed significant differences only for Fas ligand elevations. Similarly, for the HCV panels, pre-T0 TRAIL levels were significantly (P = 9.5 × 10−3) elevated compared to uninfected control results, suggesting the presence of early low levels of immune activation or cell death following HCV transmission (Fig. 4C).
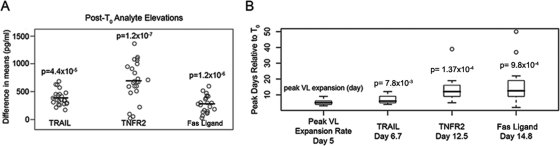
Next, mean post-T0 TRAIL, TNFR-2, and Fas ligand levels for plasma from HIV-infected donors were compared to mean pre-T0 analyte levels by calculating and plotting the differences between pre- and post-T0 analyte means for each subject (Fig. 5A). Mean post-T0 HIV-1 plasma donor analyte levels were again found to be significantly different from the mean analyte levels seen prior to T0 (P = 4.4 × 10−5 for TRAIL, P = 1.2 × 10−7 for TNRF2, and P = 1.2 × 10−6 for Fas ligand [P values from paired Wilcoxon rank-sum test]).
FIG. 5.
Analysis of significance of post-T0 analyte versus pre-T0 analyte level elevations and timing of peak analyte results relative to maximum viral expansion. Panel A shows the post-T0 analyte means minus pre-T0 analyte means of the results obtained with samples from plasma donors with acute HIV-1 infection. These data complement the data shown in Fig. 4A and confirm that for samples of plasma from subjects with acute HIV-1 infection, post-T0 elevations in TRAIL, TNFR-2, and Fas ligand levels were all statistically significant. Panel B shows the timing of analyte peaks relative to maximum viral expansion rates and the first appearance of virus plasma (T0) (see Methods and Materials in the supplemental material). Results are from a paired Wilcoxon signed-rank test, and P values indicate that the two means (i.e., the mean times of the maximum VL expansion and means of each analyte elevation) are significantly different. The delay among analyte peak occurrences after T0 can be described in terms of a mean, a median, and an interquartile range. The arrival time for each analyte maximum is compared with the time of peak viral expansion relative to T0. A P value from the Wilcoxon test is shown above the analyte of interest. The significant P values in panel B indicate that the average day of peak analyte level was significantly different from the average day of peak or maximal rate of viral expansion. Also noted are mean times of peak VL expansion after T0 (day 5), mean peak level of TRAIL after T0 (day 6.7), mean peak level of TNRF2 after T0 (day 12.5), and mean peak level of Fas ligand after T0 (day 14.8). Open circles indicate outlier values.
Third, to analyze the timing of peak analyte levels relative to the rate of viral expansion during VL ramp-up, paired Wilcoxon rank-sum tests were performed on the analyte peak dates relative to the date of peak viral expansion rate for each subject (Fig. 5B) (see Methods and Materials in the supplemental material for details). Data showing the timing of plasma analyte maximum values relative to maximum VL are available (see Table S1 in the supplemental material). The day of the peak viral expansion rate indicates the approximate day following T0 on which the virus was replicating at the maximum rate (see Methods and Materials in the supplemental material). For 24 plasma donors for whom the rate of VL expansion could be calculated, the peak viral expansion rate occurred on mean day 5.5 (median, 5.0 days; interquartile range, 2.3 days) following T0 (Fig. 5B). Plasma donor HIV-1 VL reached its observed maximum an average of 13.9 days after T0 (median, 13 days; interquartile range, 3 days); note that for 19 of 30 subjects, plasma donations were terminated during acute phase ramp-up; their data thus represent truncation prior to the actual peak in viremia. We therefore refer to sampled peaks as observed maxima (see Methods and Materials in the supplemental material). We estimate that the mean time from HIV-1 transmission to appearance of plasma virus is approximately 10 days (14, 16, 18, 24, 25, 46, 59). Thus, for plasma donors the maximal VL occurred a mean of 23.9 days after transmission.
On average, plasma TRAIL levels peaked 1.7 days (median, 0 days; interquartile range, 2.8 days) after the maximal viral expansion rate occurred (day 7.2 after T0 and 6.7 days before maximum VL), while TNFR-2 levels peaked 7.5 days (median, 7.0 days; interquartile range, 7.3 days) after the maximal VL expansion rate occurred (day 13 after T0 and 0.9 days before maximum VL), and Fas ligand levels peaked 9.8 days (median, 7.5 days; interquartile range, 9.0 days) after the maximal VL expansion rate occurred (day 15.3 after T0 and 1.4 days after the maximum VL). Thus, TRAIL levels peaked 1.7 days after the maximum viral expansion rate and well before maximum plasma VL levels, while TNFR-2 and Fas ligand reached peak plasma levels close to the time of highest VL plasma levels.
The mean of the peak plasma TRAIL levels was 2,011 pg/ml, with a range of 886 to 4,138 pg/ml. This level of plasma TRAIL is well within the biologically relevant concentration range for induction of cell death in immune cells (34).
Quantitative flow cytometry analysis of plasma MPs.
Plasma MPs are a normal byproduct of a variety of types of activated or apoptotic cells and arise from the cell surface membrane by an exocytic or budding process related to blebbing, a prominent feature of apoptosis. MPs differ in size and composition from exosomes, which derive from multivesicular bodies (39, 55), although both particle types may occur together (see Fig. S2 in the supplemental material). Samples from 30 plasma donors demonstrated peak MP levels near (within 15 days before or 15 days after T0) the observed maximum VL, and 11 of these 18 peaks occurred immediately before the peak in VL, while 4 of 18 peaked at (or after) the time of maximum VL (Fig. 6A) (see Table S2 in the supplemental material). Five of 10 (50%) HCV donors had similar elevations in MP levels, but only 2 of 10 (20%) HBV panels studied had elevations in MP levels near the maximum VL (Fig. 6B and C).
FIG. 6.
Relative MP counts from plasma samples. (A) Relative MP counts were acquired for each sequential time point for each plasma donor subject (subject numbers above the panels). Data shown are from 3 subjects representing 30 subjects studied. Eighteen of 30 HIV-1 plasma donors had elevations of MP levels within 30 days of the VL maximum value, while 5 of 10 subjects with acute HCV infection and 2 of 10 subjects with acute HBV infection had elevations in MP levels (see Table S1 in the supplemental material). Panel A presents data representing the results obtained with three representative subjects with acute HIV-1 infection. MP data are shown in magenta, and VL data are shown in blue. Panels B and C show representative panels of the results obtained with acute HBV (B)- and HCV (C)-infected subjects with less prominent or no MP level elevations (see Table S1 in the supplemental material). In panels A, B, and C, MP counts are shown in red and VL levels are shown in blue.
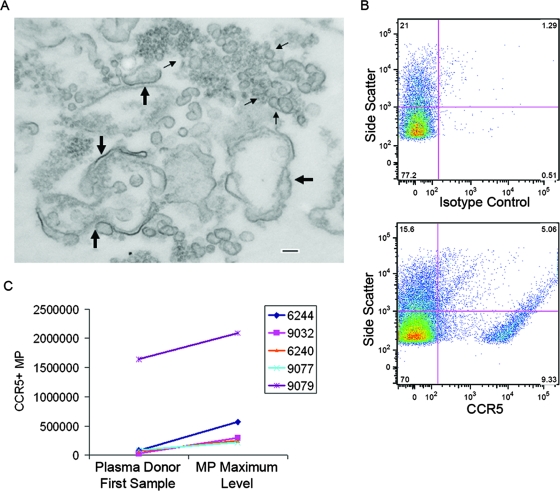
The morphology of MPs at the time of peak VL was studied by electron microscopy using sucrose gradient-purified MPs from the time of both peak VL and peak MP levels (from day 10; see Fig. 6A), and the MPs were found to be heterogeneous in size, ranging from 10 nm to 1,000 nm (Fig. 7A). In view of this size range, these preparations may have contained both MPs and exosomes.
FIG. 7.
Morphology and CCR5 expression of plasma MPs in cases of acute HIV-1 infection. Panel A shows an electron micrograph of plasma MPs purified from a subject (6244) with acute HIV-1 infection at the time of peak MP and VL levels (see Fig. 4A). The micrograph shows plasma MPs that were pelleted by ultracentrifugation and purified over a sucrose pad. Large arrows indicate double-membrane MPs 100 nm to 1 μm in size. Small arrows indicate particles 30 to 100 nm in size (exosomes). Bar, 100 nm. Panel B shows flow cytometry panels obtained by labeling plasma MPs with either an isotype control (upper panel) or an anti-CCR5 monoclonal antibody (lower panel). In this sample from the time of peak VL and MP levels, 13% of MPs were CCR5+. Panel C shows comparisons of the absolute numbers of CCR5+ MPs (percentages of CCR5+ MPs determined by phenotypic flow analyses multiplied by the relative MP count) from five different seroconversion panels for the first plasma sample and at the time of peak MP levels.
Phenotypic characterization of plasma MPs.
Most studies that have included phenotypic analysis of MPs have used fresh plasma that was processed within hours (39), whereas the plasma donor samples in this study had been frozen and thawed at least twice. We found that two freeze/thaw cycles markedly decreased the percentages of CD3+, CD45+, CD61+, and annexin V+ MPs (see Fig. 3 in the supplemental material). Thus, unable to accurately quantitate the phenotype of plasma MPs in archived plasma donor samples, we analyzed MPs for annexin V and CCR5 expression in a qualitative manner to determine whether MPs expressing annexin V or CCR5 were present at the time of peak MP levels for five plasma donor peak MP samples versus VL-negative first-panel samples (Fig. 7B and C). Both annexin V+ and CCR5+ MPs were present in samples from plasma donors; for annexin V, the mean positive percentage of MPs at the MP peak was 12% (range, 2.3% to 38.0%), and for CCR5+ MPs, the positive mean percentage at the MP peak was 5.7% (range, 1.1% to 12.6%). There was a trend toward higher MP numbers at the time of MP peak compared to the first-panel sample results for CCR5+ MPs (Fig. 7C) but not for annexin V+ MPs (data not shown).
While the average peak HIV-1 VL level was 1,421,628 copies/ml, the average total MP peak level was 606,881,733/ml. Thus, at the times of maximum VL and MP levels, the average number of MPs was 427 times larger than the average number of virions (see Table 3 in the supplemental material).
MP-induced B-cell suppression in vitro.
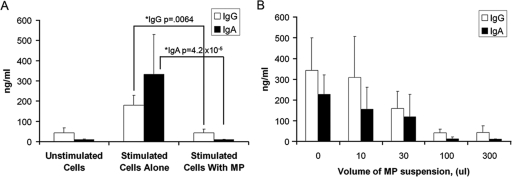
While plasma MPs have been known to have potent suppressive effects on macrophages and dendritic cells (32, 35), only one study has suggested that MPs may inhibit B-cell activation (42). We were particularly interested in apoptotic T-cell MP effects on human memory B-cell activation, since, for protection against infection, a rapid virus-induced memory B-cell response after transmission may be necessary. To determine whether PBMC-derived or tonsil leukocyte-derived MPs could be suppressive with respect to memory B-cell activation, we used a memory B-cell Ig induction assay with pokeweed mitogen (PWM) and class B oCpG oligonucleotides (17). The addition of staurosporine-induced PBMC-derived apoptotic MPs to PWM-stimulated tonsil cell cultures reduced total IgG and IgA production by 70.8% ± the standard error of the mean (SEM) for IgG (P = 6.4 × 10−3) and 94.2% ± SEM for IgA (P = 4 × 10−5) (Fig. 8A); B-cell suppression by T-cell MPs was dose dependent (Fig. 8B). Similar results were observed when apoptotic MPs were generated from Jurkat T cells (data not shown).
FIG. 8.
Peripheral-blood-derived MPs induce suppression of PWM/oCpG oligonucleotide-stimulated tonsil memory B-cell production. (A) Tonsil cells obtained from healthy donors were cultured alone or in the presence of PWM and oCpG with or without PBMC-derived MPs. The addition of 100 μl of purified PBMC-derived MPs induced reduced production of both total IgG and IgA. Data are representative of the results of five experiments and are presented as means ± SEMs. Panel B shows dose-dependent suppression of IgG production induced by increasing amounts of PBMC MPs. Data represent the means ± SEMs of the results of three separate experiments.
Analyses using mock MP preparations to determine any effect of staurosporine on Ig production were performed; the preparations consisted of media alone (without cells) containing the same concentration of staurosporine that remained in the preparation of MP harvested from staurosporine-treated cells. Since 1 μM staurosporine was added to 5 ml of cultured cells to induce apoptosis and since the MPs harvested from the 5 ml of cells were pelleted and then rinsed in 1,000 μl of medium, the staurosporine was diluted to 0.001 μM. Thus, we added 0.001 μM staurosporine to PWM- and oCpG-stimulated B cells and measured total IgG and IgA production. There were no differences in Ig production levels between stimulated B cells cultured in the presence of 0.001 μM staurosporine and those cultured in the absence of staurosporine. Experiments were also performed to assess the viability of the B cells in coculture with MP. No differences in viability were observed between B cells cultured with and without MP after 0, 1, 2, 3, 4, and 5 days in culture.
DISCUSSION
A major finding in this study was the early appearance of elevations of TRAIL levels in plasma from donors following HIV-1 transmission, suggesting that the TRAIL/DR5 pathway is a key pathway in HIV-1-induced cell death immediately following HIV-1 transmission. An alpha interferon (IFN-α) TRAIL/DR5 pathway for CD4+ T-cell apoptosis has been proposed for chronic HIV-1 infection based on in vitro studies and studies of HIV-1+ progressor tonsillar tissues (29, 30, 47). CD4+ T cells from infected subjects are more sensitive to TRAIL-mediated apoptosis than are CD4+ T cells from uninfected subjects due to upregulated TRAIL receptor DR5 levels (29, 30, 36, 47). HIV-1 gp120 induces monocyte and plasmacytoid dendritic cell IFN-α in vitro (29), which in turn induces CD4+ T-cell and monocyte/macrophage TRAIL (29, 30, 47). HIV-1 Tat has also been suggested to induce TRAIL in vitro as a mechanism of bystander killing of CD4+ T cells (65).
An important question is the following: why do plasma TRAIL levels peak earlier after HIV-1 transmission than do plasma Fas ligand, TNFR-2, and MP levels? Plasma elevations of TRAIL, Fas ligand, and TNFR-2 levels occur in cases of chronic HIV-1 infection and can be induced by immune cell activation, cell death, or both (3, 28, 31, 33). Stacey et al. have found a burst of IFN-α production in samples from the same plasma donors that coincides with the timing of the TRAIL peak levels seen in this study (A. Stacey, E. Haygreen, E. Taylor, J. Heitman, M. Lebedeva, P. Norris, P. Borrow, and the NIAID Center for HIV/AIDS Vaccine Immunology, submitted for publication). Thus, the elevations in plasma TRAIL that precede the maximum VL may be due to early apoptosis or may result from immune activation and plasmacytoid dendritic cell production of IFN-α in response to rising VL. The later appearance of elevated plasma Fas ligand, TNFR-2, and MP levels may occur as a result of, or in response to, massive cell death, as this peak comes at an time analogous to that of the cell death peak documented in studies of experimental SIV infection of rhesus macaques (27, 45, 50, 61).
An alternative interpretation of TRAIL, TNFR-2, Fas ligand, and MP plasma level elevations could be that the degree of cell death seen in cases of HIV-1 infection might be the result of early immune activation and induction of anti-HIV immune responses leading to apoptosis-induced cross-priming of HIV-1 antigens (19). However, since most HIV-1-infected subjects ultimately do not control the HIV-1 infection, it is likely that the early events we have observed in experiments using samples from plasma donors acutely infected with HIV-1 represent a pathological rather than a salutary cascade of events that is a cause of, or is associated with, virus-induced CD4+ T-cell death. In samples from HBV-infected subjects treated with IFN-α, soluble TNFR-2 plasma levels rise following IFN-α infusion, and this peak coincides with a peak in liver enzyme levels indicative of hepatocytolysis (49). Thus, an increase in soluble TNFR-2 levels in cases of HBV infection is likely one of the pathways involved in HBV infection-related liver cell necrosis (49). Similarly, it is possible that plasma IFN-α and TNF-α level spikes that occur at the same time as TRAIL level spikes in samples from plasma donors in cases of acute HIV-1 infection (Stacey et al., submitted) induce TRAIL- and TNFR-2-mediated cell death in CD4+ T cells, perhaps in an attempt to clear HIV-1. However, the magnitude of the cell death induced by HIV-1 and the resulting loss of CD4+ T cells, coupled with cell death product-mediated immunosuppression, likely contributes to ineffective immune responses.
These data are also of interest for studies of cell death plasma analytes and MPs in cases of HBV and HCV infection. Both HBV and HCV are associated with hepatocyte apoptosis during stages in cases of chronic viral hepatitis infection (11, 40, 41, 49). We found no significant elevations of plasma TRAIL, TNFR-2, or Fas ligand levels in HBV in comparisons of pre-T0 with post-T0 levels (Fig. 4B), but we did find significant post-T0 elevations in levels of plasma Fas ligand in cases of acute HCV infection (Fig. 4C). Of the three acute viral infection syndromes, acute HIV-1 infection showed the most clearly demonstrable changes in soluble plasma cell death molecules.
Several studies have suggested that the process of gut CD4+ T-cell loss occurs very early in cases of SIV and HIV-1 infection. Veazey noted the onset of CD4+ gut T-cell loss as early as 7 days after SIV infection (61). In investigations of human subjects, Guadalupe et al. (26), Brenchley et al. (9), and Mehandru and colleagues (51) studied 2, 1, and 9 patients, respectively, during the first month of HIV-1 infection and found depletion of gut CD4+ T cells.
The eclipse phase of HIV-1 infection (from transmission until the appearance of plasma viremia) is estimated to be 10 days in length, with a range of 7 to 21 days (14, 16, 18, 24, 25, 46, 59). The time from the appearance of HIV-1 viremia to the time of the first antibody response and symptomatic HIV-1 infection (and establishment of the latent pool) is approximately 15 days (13, 16, 18, 25). Thus, the maximal window of opportunity for effective preventive HIV-1 vaccination without cell death-induced immune suppression is approximately 25 days. With mediators of apoptosis and immune suppression present as early as day 17 following transmission (after a 10-day average eclipse phase plus a TRAIL peak at 7 days after T0), the window of opportunity may be as narrow as approximately 14 to 17 days.
The presence of TRAIL and TNFR-2 and of elevated MP levels during this early period of acute HIV-1 infection suggests a number of potential mechanisms of immunosuppression. First, direct HIV-1 infection results in loss of a substantial proportion of CD4+ T cells, although the numbers of infected cells do not account for all CD4+ T-cell depletion (9, 26, 51). Second, TRAIL induces bystander killing in uninfected CD4+ T cells that had previously interacted with gp120 and had upregulated DR5 TRAIL receptors (28-30, 47). Indeed, Miura et al. showed that administration of an anti-TRAIL monoclonal antibody to HIV-1-infected hu-PBL-NOD-SCID mice markedly reduced CD4+ T-cell apoptosis (52).
Third, suppression of immune responses can be mediated by T-cell MPs (32, 34, 35). CXCR4+ and CCR5+ MPs can transfer coreceptors to coreceptor-negative cells, making them susceptible to infection by HIV-1 (48, 57). Phagocytosis of MPs by macrophages releases TGF-β, prostaglandin E2, and interleukin-10 (IL-10), which can inhibit antigen-specific T- and B-cell responses (20, 35, 42). In this regard, Estes et al. have shown dramatic increases in lymph node TGF-β and IL-10 levels on day 12 following SIV infection (22). Importantly, we have demonstrated that PBMC and tonsillar cell MPs can directly inhibit memory B-cell activation (Fig. 8).
Fourth, both Fas ligand and TRAIL are incorporated into MPs (37, 53). Fas ligand-expressing MPs can directly induce apoptosis in nearby cells (20, 37, 53), and activated T cells can be the target of Fas ligand-mediated proapoptotic microvesicles (53). Salvato et al. recently suggested that treatment of SIV-infected macaques with a monoclonal antibody against Fas ligand attenuates disease and may lead to elevated antibody responses to SIV (58). However, in interpreting mechanisms of cell death and immunosuppression by MPs, it should be noted that preparations of MPs may contain exosomes, depending on the conditions for isolation and centrifugation (Fig. 7A). Although exosome release usually results from activation rather than apoptosis, both vesicle types may be present together in plasma, making it difficult to identify definitively the basis for immunological activity of a vesicle preparation. With induction of apoptosis by staurosporine, however, MPs appear to be the predominant vesicle type, and it is likely that MPs are responsible for the observed B-cell-suppressive activity seen in vitro (Fig. 8). In the setting of HIV-1 infection, where both activation and apoptosis occur, however, MPs and exosomes may act concomitantly, with exosomes suppressing immune responses (2, 7, 15, 60) and MPs contributing to both immune suppression and cell death (20, 32, 34, 35, 39, 42, 55).
Finally, elevations of plasma TRAIL, TNFR-2, and Fas ligand levels may be predictors of the IL-10-mediated immune exhaustion that eventually occurs with HIV-1 infections (6). In this regard, Norris et al. and Stacey and colleagues have demonstrated IL-10 production in plasma donors soon after the peak in VL (54; Stacey et al., submitted).
Thus, the production of high levels of biologically active plasma mediators and byproducts of cell death during the first 2 to 3 weeks of HIV-1 transmission encourages the notion that the window of opportunity during which a preventive vaccine can work may be shorter than previously thought, placing considerable constraints on the time available for development of robust anti-HIV-1 immunity following transmission. Preventive vaccine candidates may need to target HIV-1 molecules that induce apoptosis and may need to be designed to induce protective immune responses to HIV-1 that would be at maximum inhibitory levels at the time of transmission or that could be boosted within hours to days as a secondary immune response to extinguish HIV-1 infection before HIV-1-induced immune perturbations occur.
Supplementary Material
Acknowledgments
Supported by NIH grants AI-0678501 (the Center for HIV/AIDS Vaccine Immunology), AI64518 (the Duke Center for AIDS Research), and AI29168.
We thank Kelly Soderberg, Elizabeth Petzold, and Janet Mueller for program support; Bette Korber, Linda Harris, Dongfeng Li, and Doug Grove for technical and computational assistance; Joseph Sodroski, Myron Cohen, Beatrice Hahn, Andrew McMichael, Persephone Borrow, and Stuart Shapiro for discussions and manuscript review; Munir Alam for editorial assistance and discussions; and Kim R. McClammy for expert secretarial assistance.
Footnotes
Published ahead of print on 28 May 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abel, K., D. M. Rocke, B. Chohan, L. Fritts, and C. J. Miller. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 7912164-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Admyre, C., S. M. Johansson, K. R. Qazi, J. J. Filén, R. Lahesmaa, M. Norman, E. P. Neve, A. Scheynius, and S. Gabrielsson. 2007. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 1791969-1978. [DOI] [PubMed] [Google Scholar]
- 3.Aukrust, P., N. B. Liabakk, F. Muller, E. Lien, T. Espevik, and S. S. Froland. 1994. Serum levels of tumor necrosis factor-alpha (TNF alpha) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J. Infect. Dis. 169420-424. [DOI] [PubMed] [Google Scholar]
- 4.Aupeix, K., B. Hugel, T. Martin, P. Bischoff, H. Lill, J. L. Pasquali, and J. M. Freyssinet. 1997. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J. Clin. Investig. 991546-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badley, A. D., A. A. Pilon, A. Landay, and D. H. Lynch. 2000. Mechanisms of HIV-associated lymphocyte apoptosis. Blood 962951-2964. [PubMed] [Google Scholar]
- 6.Blackburn, S. D., and E. J. Wherry. 2007. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 15143-146. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard, N., D. Lankar, F. Faure, A. Regnault, C. Dumont, G. Raposo, and C. Hivroz. 2002. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 1683235-3241. [DOI] [PubMed] [Google Scholar]
- 8.Boya, P., A. L. Pauleau, D. Poncet, R. A. Gonzalez-Polo, N. Zamzami, and G. Kroemer. 2004. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 1659178-189. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase, A., Y. Zhou, and R. F. Siliciano. 2006. HIV-1-induced depletion of CD4+ T cells in the gut: mechanism and therapeutic implications. Trends Pharmacol. Sci. 274-7. [DOI] [PubMed] [Google Scholar]
- 11.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1329-60. [DOI] [PubMed] [Google Scholar]
- 12.Chou, A. H., H. F. Tsai, Y. Y. Wu, C. Y. Hu, L. H. Hwang, P. I. Hsu, and P. N. Hsu. 2005. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J. Immunol. 1742160-2166. [DOI] [PubMed] [Google Scholar]
- 13.Chun, T. W., D. Engel, M. M. Berrey, T. Shea, L. Corey, and A. S. Fauci. 1998. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 958869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark, S. J., M. S. Saag, W. D. Decker, S. Campbell-Hill, J. L. Roberson, P. J. Veldkamp, J. C. Kappes, B. H. Hahn, and G. M. Shaw. 1991. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N. Engl. J. Med. 324954-960. [DOI] [PubMed] [Google Scholar]
- 15.Clayton, A., J. P. Mitchell, J. Court, M. D. Mason, and Z. Tabi. 2007. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 677458-7466. [DOI] [PubMed] [Google Scholar]
- 16.Cooper, D. A., A. A. Imrie, and R. Penny. 1987. Antibody response to human immunodeficiency virus after primary infection. J. Infect. Dis. 1551113-1118. [DOI] [PubMed] [Google Scholar]
- 17.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286111-122. [DOI] [PubMed] [Google Scholar]
- 18.Daar, E. S., T. Moudgil, R. D. Meyer, and D. D. Ho. 1991. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N. Engl. J. Med. 324961-964. [DOI] [PubMed] [Google Scholar]
- 19.den Haan, J. M., and M. J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13437-441. [DOI] [PubMed] [Google Scholar]
- 20.Distler, J. H., L. C. Huber, S. Gay, O. Distler, and D. S. Pisetsky. 2006. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity 39683-690. [DOI] [PubMed] [Google Scholar]
- 21.Emau, P., Y. Jiang, M. B. Agy, B. Tian, G. Bekele, and C. C. Tsai. 2006. Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention. AIDS Res. Ther. 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes, J. D., Q. Li, M. R. Reynolds, S. Wietgrefe, L. Duan, T. Schacker, L. J. Picker, D. I. Watkins, J. D. Lifson, C. Reilly, J. Carlis, and A. T. Haase. 2006. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J. Infect. Dis. 193703-712. [DOI] [PubMed] [Google Scholar]
- 23.Fiebig, E. W., D. J. Wright, B. D. Rawal, P. E. Garrett, R. T. Schumacher, L. Peddada, C. Heldebrant, R. Smith, A. Conrad, S. H. Kleinman, and M. P. Busch. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 171871-1879. [DOI] [PubMed] [Google Scholar]
- 24.Gaines, H., M. von Sydow, P. O. Pehrson, and P. Lundbegh. 1988. Clinical picture of primary HIV infection presenting as a glandular-fever-like illness. BMJ 2971363-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaines, H., M. von Sydow, A. Sonnerborg, J. Albert, J. Czajkowski, P. O. Pehrson, F. Chiodi, L. Moberg, E. M. Fenyo, B. Asjo, et al. 1987. Antibody response in primary human immunodeficiency virus infection. Lancet i1249-1253. [DOI] [PubMed] [Google Scholar]
- 26.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 28.Herbeuval, J. P., A. Boasso, J. C. Grivel, A. W. Hardy, S. A. Anderson, M. J. Dolan, C. Chougnet, J. D. Lifson, and G. M. Shearer. 2005. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood 1052458-2464. [DOI] [PubMed] [Google Scholar]
- 29.Herbeuval, J. P., J. C. Grivel, A. Boasso, A. W. Hardy, C. Chougnet, M. J. Dolan, H. Yagita, J. D. Lifson, and G. M. Shearer. 2005. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 1063524-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbeuval, J. P., and G. M. Shearer. 2007. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123121-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hober, D., S. Benyoucef, A. S. Delannoy, D. de Groote, F. Ajana, Y. Mouton, and P. Wattre. 1996. High plasma level of soluble tumor necrosis factor receptor type II (sTNFRII) in asymptomatic HIV-1-infected patients. Infection 24213-217. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann, P. R., J. A. Kench, A. Vondracek, E. Kruk, D. L. Daleke, M. Jordan, P. Marrack, P. M. Henson, and V. A. Fadok. 2005. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J. Immunol. 1741393-1404. [DOI] [PubMed] [Google Scholar]
- 33.Hosaka, N., N. Oyaizu, M. H. Kaplan, H. Yagita, and S. Pahwa. 1998. Membrane and soluble forms of Fas (CD95) and Fas ligand in peripheral blood mononuclear cells and in plasma from human immunodeficiency virus-infected persons. J. Infect. Dis. 1781030-1039. [DOI] [PubMed] [Google Scholar]
- 34.Huang, Y., N. Erdmann, H. Peng, S. Herek, J. S. Davis, X. Luo, T. Ikezu, and J. Zheng. 2006. TRAIL-mediated apoptosis in HIV-1-infected macrophages is dependent on the inhibition of Akt-1 phosphorylation. J. Immunol. 1772304-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh, M. L., V. A. Fadok, and P. M. Henson. 2002. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Investig. 10941-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeremias, I., I. Herr, T. Boehler, and K. M. Debatin. 1998. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur. J. Immunol. 28143-152. [DOI] [PubMed] [Google Scholar]
- 37.Jodo, S., S. Xiao, A. Hohlbaum, D. Strehlow, A. Marshak-Rothstein, and S. T. Ju. 2001. Apoptosis-inducing membrane vesicles. A novel agent with unique properties. J. Biol. Chem. 27639938-39944. [DOI] [PubMed] [Google Scholar]
- 38.Johnston, M. I., and A. S. Fauci. 2007. An HIV vaccine—evolving concepts. N. Engl. J. Med. 3562073-2081. [DOI] [PubMed] [Google Scholar]
- 39.Jy, W., L. L. Horstman, J. J. Jimenez, Y. S. Ahn, E. Biro, R. Nieuwland, A. Sturk, F. Dignat-George, F. Sabatier, L. Camoin-Jau, J. Sampol, B. Hugel, F. Zobairi, J. M. Freyssinet, S. Nomura, A. S. Shet, N. S. Key, and R. P. Hebbel. 2004. Measuring circulating cell-derived microparticles. J. Thromb. Haemost. 21842-1851. [DOI] [PubMed] [Google Scholar]
- 40.Kallinowski, B., K. Haseroth, G. Marinos, C. Hanck, W. Stremmel, L. Theilmann, M. V. Singer, and S. Rossol. 1998. Induction of tumour necrosis factor (TNF) receptor type p55 and p75 in patients with chronic hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 111269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplanski, G., V. Marin, T. Maisonobe, A. Sbai, C. Farnarier, P. Ghillani, X. Thirion, J. M. Durand, J. R. Harle, P. Bongrand, J. C. Piette, and P. Cacoub. 2002. Increased soluble p55 and p75 tumour necrosis factor-alpha receptors in patients with hepatitis C-associated mixed cryoglobulinaemia. Clin. Exp. Immunol. 127123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Köppler, B., C. Cohen, D. Schlondorff, and M. Mack. 2006. Differential mechanisms of microparticle transfer to B cells and monocytes: anti-inflammatory properties of microparticles. Eur. J. Immunol. 36648-660. [DOI] [PubMed] [Google Scholar]
- 43.Krysko, D. V., K. D'Herde, and P. Vandenabeele. 2006. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 111709-1726. [DOI] [PubMed] [Google Scholar]
- 44.Lapinski, T. W., O. Kowalczuk, D. Prokopowicz, and L. Chyczewski. 2004. Serum concentration of sFas and sFasL in healthy HBsAg carriers, chronic viral hepatitis B and C patients. World J. Gastroenterol. 103650-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 46.Little, S. J., A. R. McLean, C. A. Spina, D. D. Richman, and D. V. Havlir. 1999. Viral dynamics of acute HIV-1 infection. J. Exp. Med. 190841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lum, J. J., A. A. Pilon, J. Sanchez-Dardon, B. N. Phenix, J. E. Kim, J. Mihowich, K. Jamison, N. Hawley-Foss, D. H. Lynch, and A. D. Badley. 2001. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J. Virol. 7511128-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mack, M., A. Kleinschmidt, H. Bruhl, C. Klier, P. J. Nelson, J. Cihak, J. Plachy, M. Stangassinger, V. Erfle, and D. Schlondorff. 2000. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6769-775. [DOI] [PubMed] [Google Scholar]
- 49.Marinos, G., N. V. Naoumov, S. Rossol, F. Torre, P. Y. Wong, H. Gallati, B. Portmann, and R. Williams. 1995. Tumor necrosis factor receptors in patients with chronic hepatitis B virus infection. Gastroenterology 1081453-1463. [DOI] [PubMed] [Google Scholar]
- 50.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 51.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miura, Y., N. Misawa, N. Maeda, Y. Inagaki, Y. Tanaka, M. Ito, N. Kayagaki, N. Yamamoto, H. Yagita, H. Mizusawa, and Y. Koyanagi. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monleón, I., M. J. Martinez-Lorenzo, L. Monteagudo, P. Lasierra, M. Taules, M. Iturralde, A. Pineiro, L. Larrad, M. A. Alava, J. Naval, and A. Anel. 2001. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J. Immunol. 1676736-6744. [DOI] [PubMed] [Google Scholar]
- 54.Norris, P. J., B. L. Pappalardo, B. Custer, G. Spotts, F. M. Hecht, and M. P. Busch. 2006. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res. Hum. Retrovir. 22757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piccin, A., W. G. Murphy, and O. P. Smith. 2007. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21157-171. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z. M. Ma, L. Compton, G. Napoe, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 799228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rozmyslowicz, T., M. Majka, J. Kijowski, S. L. Murphy, D. O. Conover, M. Poncz, J. Ratajczak, G. N. Gaulton, and M. Z. Ratajczak. 2003. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 1733-42. [DOI] [PubMed] [Google Scholar]
- 58.Salvato, M. S., C. C. Yin, H. Yagita, T. Maeda, K. Okumura, I. Tikhonov, and C. D. Pauza. 2007. Attenuated disease in SIV-infected macaques treated with a monoclonal antibody against Fas L. Clin. Dev. Immunol. 200793462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schacker, T., A. C. Collier, J. Hughes, T. Shea, and L. Corey. 1996. Clinical and epidemiologic features of primary HIV infection. Ann. Intern. Med. 125257-264. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, D. D., S. Akyol, and C. Gercel-Taylor. 2006. Pregnancy-associated exosomes and their modulation of T cell signaling. J. Immunol. 1761534-1542. [DOI] [PubMed] [Google Scholar]
- 61.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 62.Weiler, A. M., Q. Li, L. Duan, M. Kaizu, K. L. Weisgrau, T. C. Friedrich, M. R. Reynolds, A. T. Haase, and E. G. Rakasz. 2008. Genital ulcers facilitate rapid viral entry and dissemination following intravaginal inoculation with cell-associated SIVmac239. J. Virol. 824154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong, J. S., and R. F. Siliciano. 2007. Biology of early infection and impact on vaccine design, chapter 3, p. 17-22. In W. C. Koff, P. Kahn, and I. D. Gust (ed.), AIDS vaccine development: challenges and opportunities. Caister Academic Press, Norfolk, VA.
- 64.Xu, X. N., G. R. Screaton, F. M. Gotch, T. Dong, R. Tan, N. Almond, B. Walker, R. Stebbings, K. Kent, S. Nagata, J. E. Stott, and A. J. McMichael. 1997. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exp. Med. 1867-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang, Y., I. Tikhonov, T. J. Ruckwardt, M. Djavani, J. C. Zapata, C. D. Pauza, and M. S. Salvato. 2003. Monocytes treated with human immunodeficiency virus Tat kill uninfected CD4+ cells by a tumor necrosis factor-related apoptosis-induced ligand-mediated mechanism. J. Virol. 776700-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.