Abstract
The LTR, v-src, LTR provirus, which arose by the reverse transcription and integration of src mRNA in the H-19 hamster tumor, has been successfully rescued by fusion with chicken fibroblasts infected with Rous-associated virus RAV-1. One rescued virus, E6, acquired 1 kilobase of the 5' end of the gag gene structure. Recombination took place in the region of 15-nucleotide homology exactly between v-src exon (position 7054) and gag (position 1417). This recombination resulted in the alteration of src splice acceptor site sequences, but this site is maintained as a functional splice acceptor site. The nucleotide structure of the long terminal repeat of recombinant E6 virus suggests that it arose by the intermolecular jump of reverse transcription from RAV-1 to src mRNA and then the switch of templates between already depicted regions of homology. The second jump of reverse transcription was apparently an intramolecular event. The acquisition of 1 kilobase of the 5' gag by E6 resulted in maintaining the balance of unspliced and spliced E6 RNAs and assured the replication advantage of rescued E6 virus over rescued F6 virus, the genome of which corresponds to that present in ancestral H-19 cells.
Full text
PDF
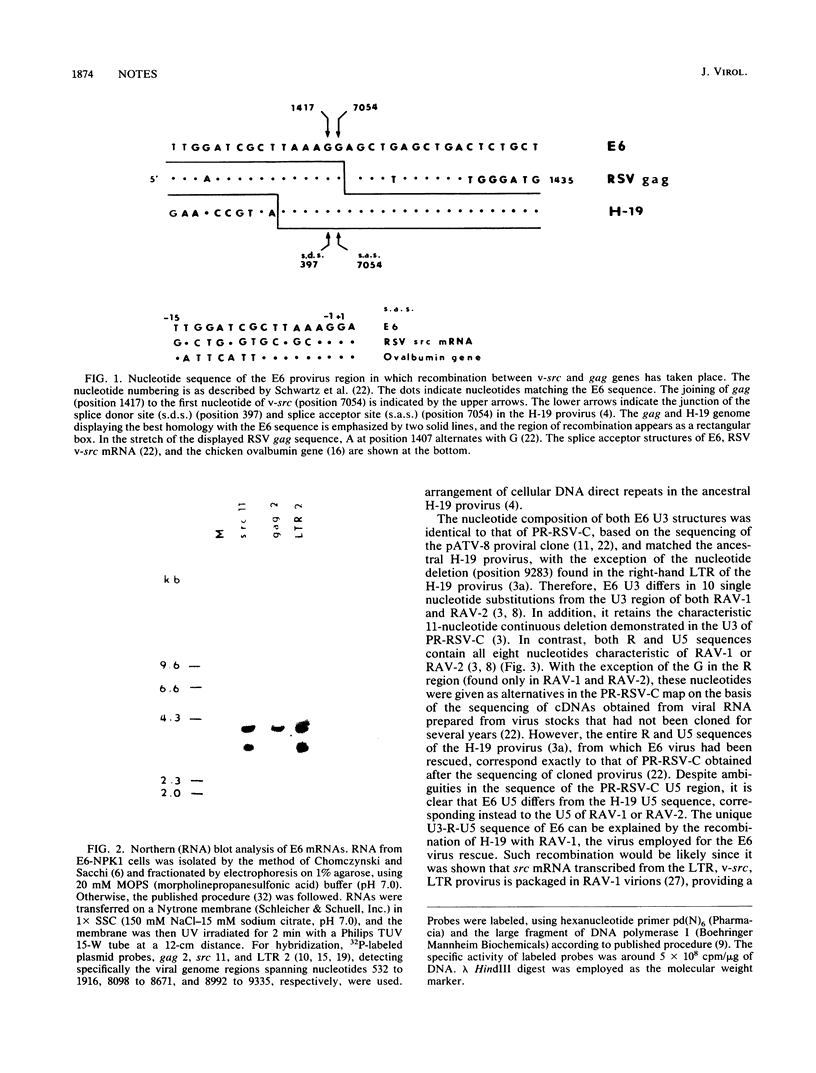
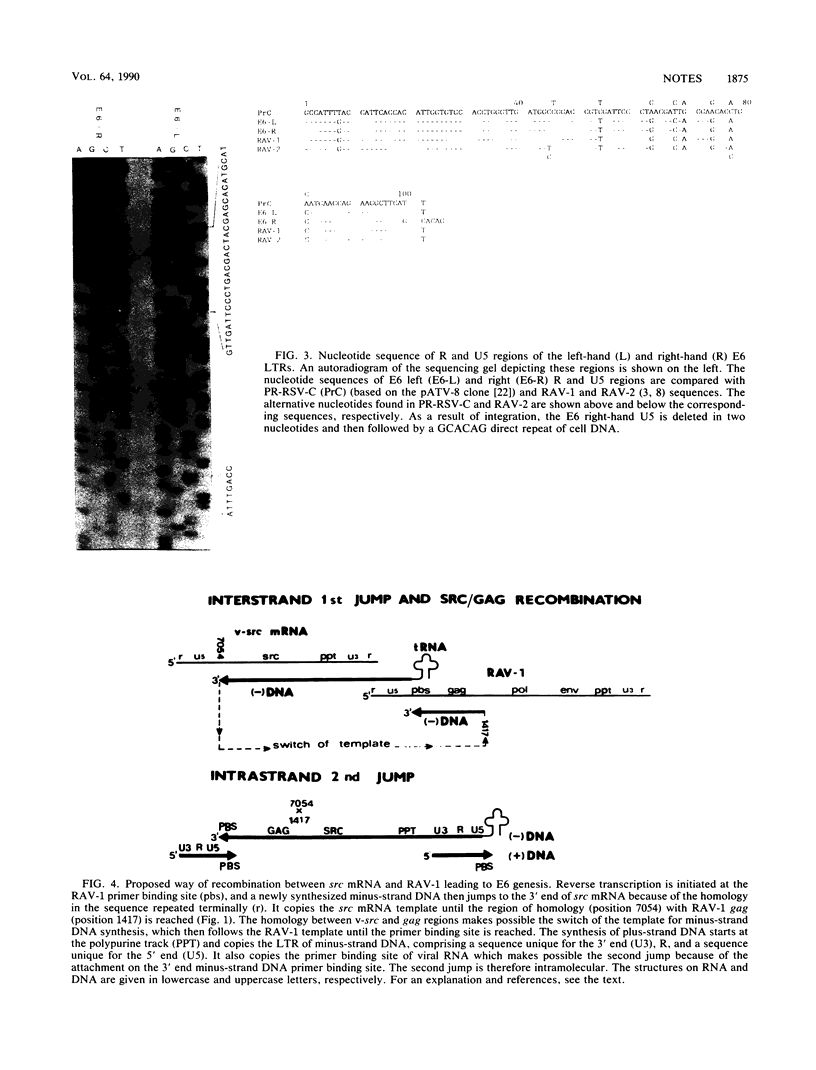
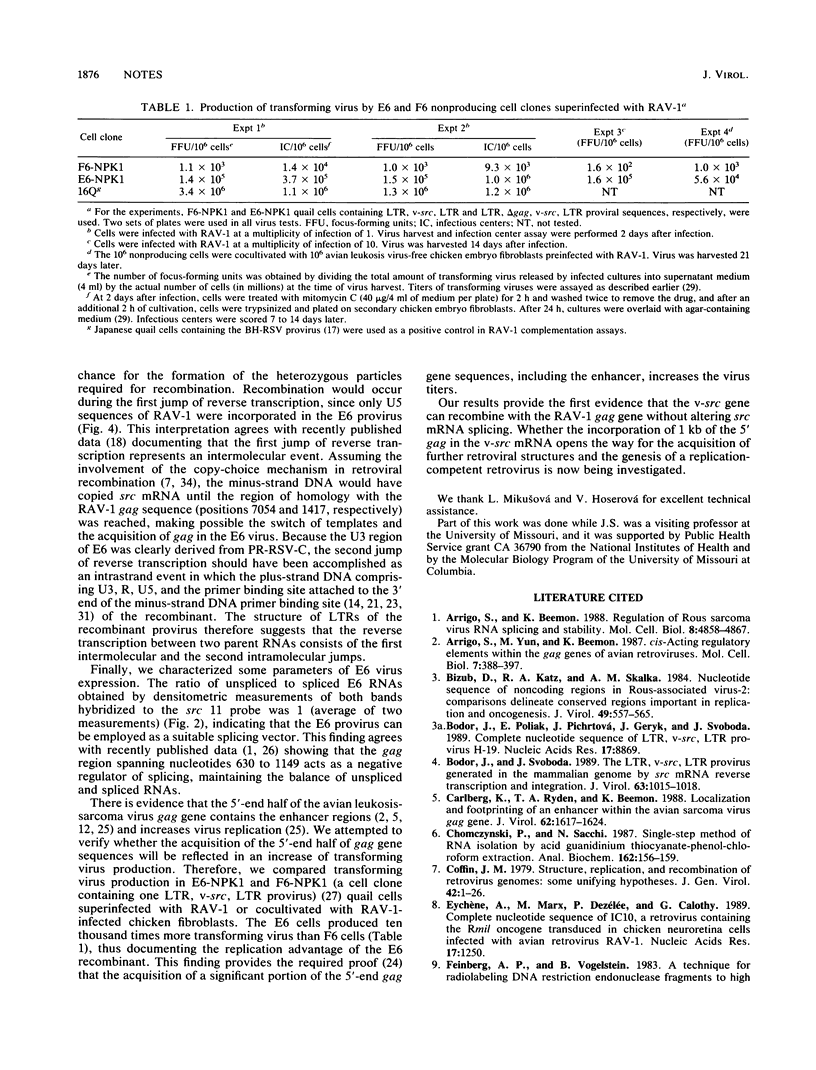

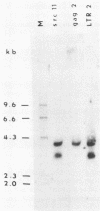
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988 Nov;8(11):4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor J., Poliak E., Pichrtová J., Geryk J., Svoboda J. Complete nucleotide sequence of LTR, v-src, LTR provirus H-19. Nucleic Acids Res. 1989 Nov 11;17(21):8869–8869. doi: 10.1093/nar/17.21.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor J., Svoboda J. The LTR, v-src, LTR provirus generated in the mammalian genome by src mRNA reverse transcription and integration. J Virol. 1989 Feb;63(2):1015–1018. doi: 10.1128/jvi.63.2.1015-1018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K., Ryden T. A., Beemon K. Localization and footprinting of an enhancer within the avian sarcoma virus gag gene. J Virol. 1988 May;62(5):1617–1624. doi: 10.1128/jvi.62.5.1617-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Eychène A., Marx M., Dezélée P., Calothy G. Complete nucleotide sequence of IC10, a retrovirus containing the Rmil oncogene transduced in chicken neuroretina cells infected with avian retrovirus RAV-1. Nucleic Acids Res. 1989 Feb 11;17(3):1250–1250. doi: 10.1093/nar/17.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geryk J., Pichrtova J., Guntaka R. V., Gowda S., Svoboda J. Characterization of transforming viruses rescued from a hamster tumour cell line harbouring the v-src gene flanked by long terminal repeats. J Gen Virol. 1986 Nov;67(Pt 11):2395–2404. doi: 10.1099/0022-1317-67-11-2395. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mitsialis S. A. Cloning of avian tumor virus DNA fragments in plasmid pBR322: evidence for efficient transcription in E. coli from a virus-coded promoter. Gene. 1980 Dec;12(1-2):113–121. doi: 10.1016/0378-1119(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Karnitz L., Faber S., Chalkley R. Specific nuclear proteins interact with the Rous sarcoma virus internal enhancer and share a common element with the enhancer located in the long terminal repeat of the virus. Nucleic Acids Res. 1987 Dec 10;15(23):9841–9859. doi: 10.1093/nar/15.23.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. W., Chow M., Champoux J., Baltimore D. Synthesis of murine leukemia virus plus strong stop DNA initiates at a unique site. J Biol Chem. 1982 Jun 10;257(11):5983–5986. [PubMed] [Google Scholar]
- Mitsialis S. A., Young J. F., Palese P., Guntaka R. V. An avian tumor virus promoter directs expression of plasmid genes in Escherichia coli. Gene. 1981 Dec;16(1-3):217–225. doi: 10.1016/0378-1119(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H. M. A new replication-defective variant of the Bryan high-titer strain Rous sarcoma virus. Virology. 1977 Apr;77(2):705–721. doi: 10.1016/0042-6822(77)90493-7. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Fiore D. Ordered interstrand and intrastrand DNA transfer during reverse transcription. Science. 1988 Aug 26;241(4869):1064–1069. doi: 10.1126/science.2457948. [DOI] [PubMed] [Google Scholar]
- Pichrtová J., Geryk J., Svoboda J. Structural analysis of proviruses in additional hamster tumour cell lines transformed by provirus II rescued from XC cells and definition of a new cell line harbouring amplified proviruses. Folia Biol (Praha) 1989;35(4):239–253. [PubMed] [Google Scholar]
- Pichrtová J., Svoboda J., Geryk J. Restriction analysis of additional "non-producing" cell clones obtained after transformation of Japanese quail fibroblasts with viruses rescued from cryptovirogenic H-19 cells. Folia Biol (Praha) 1987;33(6):410–417. [PubMed] [Google Scholar]
- Resnick R., Omer C. A., Faras A. J. Involvement of retrovirus reverse transcriptase-associated RNase H in the initiation of strong-stop (+) DNA synthesis and the generation of the long terminal repeat. J Virol. 1984 Sep;51(3):813–821. doi: 10.1128/jvi.51.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Cywinski A., Taylor J. M. Specificity of initiation of plus-strand DNA by Rous sarcoma virus. J Virol. 1984 Nov;52(2):314–319. doi: 10.1128/jvi.52.2.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Chang L. J., Cripe T. P., Turek L. P. Efficient transformation by Prague A Rous sarcoma virus plasmid DNA requires the presence of cis-acting regions within the gag gene. J Virol. 1987 Nov;61(11):3401–3409. doi: 10.1128/jvi.61.11.3401-3409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Fogarty S. J. Multiple regions in the Rous sarcoma virus src gene intron act in cis to affect the accumulation of unspliced RNA. J Virol. 1989 Apr;63(4):1669–1676. doi: 10.1128/jvi.63.4.1669-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M. Synthesis and processing of avian sarcoma retrovirus RNA. Adv Virus Res. 1988;35:1–38. doi: 10.1016/s0065-3527(08)60707-1. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Dvorák M., Guntaka R., Geryk J. Transmission of (LTR, v-src, LTR) without recombination with a helper virus. Virology. 1986 Sep;153(2):314–317. doi: 10.1016/0042-6822(86)90035-8. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Lhoták V., Geryk J., Saule S., Raes M. B., Stehelin D. Characterization of exogenous proviral sequences in hamster tumor cell lines transformed by Rous sarcoma virus rescued from XC cells. Virology. 1983 Jul 15;128(1):195–209. doi: 10.1016/0042-6822(83)90330-6. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Machala O., Deozánek T. Rescue of Rous sarcoma virus in mixed cultures of virogenic mammalian and chicken cells, treated and untreated with Sendai virus and detected by focus assay. J Gen Virol. 1968 May;2(3):461–464. doi: 10.1099/0022-1317-2-3-461. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Bishop J. M., Varmus H. E. Structure of a replication intermediate in the synthesis of Rous sarcoma virus DNA in vivo. J Virol. 1982 Apr;42(1):337–341. doi: 10.1128/jvi.42.1.337-341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]