Abstract
Annexin A1 is a member of a phospholipid and calcium binding family of proteins; it is involved in anti-inflammation and in the regulation of differentiation, proliferation, and apoptosis. Here, we show the existence of a functional binding site for the tumor suppressor p53 near the proximal CCAAT box and the fact that the basal expression of annexin A1 in human colon adenocarcinoma cells is driven by p53 at the transcriptional level. Posttranscriptional mechanisms may also play an important role in maintaining constitutive annexin A1 expression. In addition, a p53/NF-Y complex is detected bound to the p53 binding site on its promoter. Butyrate is a natural product of fiber degradation in the colon and a key regulator of colonic epithelium homeostasis. We show that butyrate, a class I and II histone deacetylase inhibitor, induces transcriptional activation of annexin A1 expression correlated with differentiation. The effect of butyrate is mediated through a release of NF-Y from the proximal CCAAT box and an enhancement of p53 binding. The interaction of p53 with the promoter is dependent on p38 MAPK activity either in the absence or in the presence of butyrate. Further, activation of p38 MAPK by this agent is required to increase annexin A1 promoter activity and to increase protein expression.
Annexins are a family of calcium and phospholipid binding proteins, with 12 members in mammals, whose common protein core is responsible for these properties. The N terminus is variable in both length and sequence and mediates most of the differential functional activities assigned to these proteins. These functions include roles in endocytosis and exocytosis, anticoagulant activity, ion channel regulation, interaction with the cytoskeleton, cell proliferation and differentiation, and anti-inflammatory properties (10, 11).
Annexin A1 was the first member of this family to be identified. It presents an N-terminal extension of intermediate length that is subject to regulation by posttranslational modifications, such as phosphorylation, transglutamination, and limited proteolytic cleavage. The main role ascribed to annexin A1 is an anti-inflammatory activity. It was first discovered as a mediator of some of the effects of glucocorticoids through the inhibition of PLA2 activity (21) and has been related to cyclooxygenase and inducible nitric oxide synthase expression (14). Additionally, an active secretion of this protein has been detected, leading to externalized annexin A1 prone to N-terminal proteolysis (31). Secreted annexin A1 inhibits neutrophil and monocyte/macrophage migration to the site of inflammation (34) and modulates T-cell activation and differentiation (5).
A role for annexin A1 in proliferation, apoptosis, and cancer has been reported (11, 32). Several studies have shown that annexin A1 expression is deregulated in several carcinomas and tumor cell lines. Its expression is downregulated, associated with decreased differentiation, in B-cell lymphomas and in different types of solid tumors (i.e., breast, prostate, or thyroid carcinomas), suggesting that annexin A1 may be an essential component for maintenance of the normal epithelia (33, 36, 40, 45). On the other hand, increased expression of annexin A1 has also been described to occur in other types of cancer (i.e., gastric, pancreatic, or esophageal carcinomas) (1, 46, 47). In addition, an increase of annexin A1 expression with differentiation has been reported to occur in normal epithelia (37) and in different cell lines. Macrophage-like differentiation induced in U937 cells with phorbol esters is accompanied by increased annexin A1 expression, as also described to occur in A549 human lung carcinoma cells (19, 41). We have extended these observations to induction of differentiation in colon adenocarcinoma cells. Three different approaches (butyrate treatment, growth in the absence of glucose and in the presence of inosine, and postconfluent growth of Caco-2 cells) all result in an induction of annexin A1 protein expression (13).
Butyrate is a natural product derived from the degradation of dietary fiber by bacteria under anaerobic conditions in the colon. It constitutes the main energy source for colonocytes in vivo, regulates their proliferation, and induces their differentiation along the crypt-villus axis, leading to apoptosis induction through terminal differentiation (39). Butyrate inhibition of class I and II histone deacetylases (HDACs) is thought to be responsible for most of the actions of this agent in normal and transformed cells (7). It has been shown that HDAC inhibitors are able to selectively induce apoptosis in transformed cells more than in normal cells. In fact, several of these inhibitors, including butyrate, are currently undergoing clinical trials for the treatment of both solid and hematopoietic tumors (27). It is therefore of great interest to analyze the mechanisms of action of HDAC inhibitors, especially butyrate, as it is a natural regulator in nonpathological situations. However, it is likely that butyrate has other intracellular targets, among them the intracellular kinase signaling pathways (6, 48).
There are mainly two types of genes that are regulated by butyrate: those containing Sp1/Sp3 sites on their promoters and those containing CCAAT boxes (7, 20). p21 is the gene more widely studied within the former group, and HDAC inhibitors transcriptionally upregulate p21 following both p53-dependent and p53-independent mechanisms (28). These agents act through Sp1 on the p21 promoter without modifying its binding (50). They increase local histone acetylation and recruitment of histone acetyltransferases and decrease the association of HDAC with the promoter (12). On the other hand, the knowledge about the regulation by butyrate of genes containing CCAAT boxes is scarce. In colon cancer SW620 cells, the promoter of the MDR1 gene is activated by butyrate and trichostatin A (TSA), involving NF-Y binding to the CCAAT box (20). However, there is no information about the upstream events that lead to activation of these promoters and whether this regulation is dependent on differentiation.
We have developed a model system with a parental nontumorigenic and poorly differentiated colon adenocarcinoma cell line (BCS-TC2; parental cells) (44) and a butyrate-resistant cell line (BCS-TC2.BR2), derived from the parental cells, established after long-term culture in the presence of 2 mM butyrate. Treatment of parental cells with butyrate induces growth arrest, differentiation, and apoptosis (29). BCS-TC2.BR2 cells are resistant to apoptosis induced by different agents, including butyrate, but are still induced to differentiate upon butyrate treatment (23). Interestingly, they are tumorigenic and slightly more differentiated (23); on the basis of cDNA microarrays, annexin A1 gene expression is upregulated in BCS-TC2.BR2 cells (30). Here, we present further evidence of the role of annexin A1 as a differentiation marker in colon adenocarcinoma cells. We characterize its induction in both parental and butyrate-resistant cells in response to butyrate treatment as well as after treatment with TSA, a more general HDAC inhibitor. We show that annexin A1 basal expression is mainly controlled by a functional cooperation between p53 and NF-Y and that induction by butyrate is dependent on p38 mitogen-activated protein kinase (MAPK) activation.
MATERIALS AND METHODS
Cell culture and treatments.
The establishment and characterization of the human colon adenocarcinoma BCS-TC2 and BCS-TC2.BR2 cell lines have been previously described(23, 44). Routinely, cells were cultured at 37°C under a humidified atmosphere of 5% CO2 and 95% air and were grown in Dulbecco's modified Eagle's medium containing 4.5 g/liter glucose and supplemented with 5% heat-inactivated fetal calf serum, penicillin (50 IU/ml), streptomycin (50 μg/ml), and glutamine (300 μg/ml). BCS-TC2.BR2 cells were routinely maintained in standard growth medium in the presence of 2 mM butyrate. Cell harvesting was performed by trypsinization.
Butyrate (Sigma, Alcobendas, Spain) was prepared in standard culture medium and filter sterilized. Treatment was performed on exponentially growing cells (2 to 3 days after seeding in the absence of butyrate), which were maintained in the presence of the indicated butyrate concentrations for different times. TSA was prepared in dimethyl sulfoxide (DMSO) and added to the cells at 0.5 μM. The specific p38 MAPK inhibitor SB203580 (Sigma) was also dissolved in DMSO and used at different concentrations. Samples containing the same volume of DMSO added into the culture medium were used as controls of TSA or SB203580 treatments.
Antibodies.
The primary mouse monoclonal antibodies anti-human p53 (Sigma), AnxA1 (DSHB, Iowa, IA), lamin B1 (MBL, Woburn, MA), and vinculin (hVIN1, Sigma) and rabbit polyclonal antibodies anti-human NF-YA (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-p38 MAPK (Thr180/Tyr182), and p38 MAPK (Cell Signaling, Danvers, MA) were used in Western blot and chromatin immunoprecipitation (ChIP) analyses. Peroxidase-conjugated goat anti-rabbit immunoglobulin G was from Bio-Rad and peroxidase-conjugated goat anti-mouse immunoglobulin G from Pierce (Bonn, Germany). For flow cytometry, R-phycoerythrin-conjugated mouse anti-human dipeptidyl peptidase IV (DPP-IV; PharMingen/BD Biosciences) monoclonal antibody was used.
Western blot analysis.
Total cell protein extracts were obtained by solubilization of the monolayers in 10 mM Tris, pH 8.0, containing 140 mM NaCl, 2% (vol/vol) Triton X-100, and protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 mM pepstatin A, 1 μg/ml leupeptin, 1 mM Na3VO4, 1 mM NaF, 1 mM β-glycerophosphate, and 1 mM dithiothreitol). Nuclear extracts were obtained as follows: harvested cells (20 × 106 to 30 × 106) were washed twice with ice-cold phosphate-buffered saline (PBS), resuspended in 180 μl of ice-cold hypotonic lysis buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA containing protease and phosphatase inhibitors), and incubated on ice for 10 min, followed by addition of 20 μl of Nonidet P-40; after 3 min at room temperature, cells were vortexed and the cytosolic fraction was obtained by centrifugation for 5 min at 2,500 × g. The nuclear pellet was resuspended in 60 μl of high-salt-concentration extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA containing protease and phosphatase inhibitors) and incubated with shaking at 4°C for 1 h. The nuclear extract was centrifuged for 5 min at 16,000 × g and the supernatant stored at −80°C. Protein concentration was determined using the Bradford assay.
Total cell or nuclear protein extracts (20 μg protein) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis after heat denaturing in the presence of 5% β-mercaptoethanol. Proteins were transferred to nitrocellulose membranes and analyzed by Western blotting as described elsewhere (13). Development was performed using the Amersham ECL Western blot system (GE Healthcare, Barcelona, Spain). Films were scanned, and a densitometric analysis was performed to obtain volumograms on a photodocumentation system from UVItec (Cambridge, United Kingdom), using UVIBand version 97 software. Data were normalized against the intensities of the bands corresponding to vinculin (total extracts) or lamin B1 (nuclear extracts) as controls for even protein loading.
Quantitative real-time reverse transcription-PCR.
Total RNA was purified from cell cultures by using an RNAqueous kit (Ambion, Austin, TX) according to the manufacturer's instructions. Each RNA sample was reverse transcribed into cDNAs by using a high-capacity reverse transcription kit from Applied Biosystems (Alcobendas, Spain). Annexin A1 mRNA expression was quantified using a QuantiTect Sybr green PCR kit and a human ANXA1 SG QuantiTect primer assay (QT00078197), both from Qiagen (Hilden, Germany), in a 7900HT fast real-time PCR system from Applied Biosystems. The real-time PCR thermal conditions were 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min each. The relative real-time PCR quantification was based on the ΔΔCT method, using the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene as a reference endogenous gene.
Analysis of colonic-epithelium differentiation markers.
Alkaline phosphatase activity was measured in cell extracts obtained as previously described (29), using a commercially available kit (Spinreact, Barcelona, Spain). One unit of enzyme activity is defined as 1 μmol of substrate hydrolyzed per minute at 37°C. DPP-IV (or CD26) was determined by fluorescence-activated cell sorting analysis; cells untreated or treated with 4 mM butyrate for 48 h were detached by trypsinization and allowed to recover for 30 min in complete culture medium. After being washed twice with PBS, 5 × 105 cells were resuspended in 500 μl PBS containing 0.1% bovine serum albumin. After addition of 10 μl of the appropriate dilution of R-phycoerythrin-conjugated anti-DPP-IV antibody, the cell suspension was incubated for 1 h at 37°C in the dark. Cells were again washed twice with PBS containing 0.1% bovine serum albumin and finally resuspended in 500 μl and analyzed with a FACScan flow cytometer (Becton-Dickinson, San Jose, CA), as described previously (13, 22).
Luciferase reporter assay.
One microgram of DNA was transfected into exponentially growing cells in 24-well plates with Escort IV (Sigma). After 20 h, the medium was replaced by fresh medium containing butyrate or TSA. After 24 h of treatment, cells were lysed and luciferase activity was measured with a Lumat LB 9507 luminometer (Berthold, Germany), using the luciferase reporter gene assay from Roche (Mannheim, Germany).
For dual luciferase assays, 500 ng of the indicated plasmid was transfected together with 500 ng pRL-thymidine kinase (Promega, Alcobendas, Spain) into exponentially growing cells in 24-well plates with Escort IV (Sigma). After 20 h, the medium was replaced by fresh medium and further incubated for 24 h. Firefly and Renilla luciferase activities present in cellular lysates were assayed using the dual luciferase reporter system (Promega). Firefly luciferase data were normalized for the corresponding transfection efficiency by using the Renilla luciferase activity of each sample.
Plasmids containing different fragments of the promoter region of annexin A1 cloned into pGL3-Luc (Promega) were kindly provided by Ma Pilar Fernández (A1H-1 and A1H-3) and S. E. Moss (Ax1.2 and Ax1.3) (9). Construct A1H-5 was obtained as follows: first, the EcoRI/HindIII fragment from A1H-1 was cloned into the pUC19 plasmid, and then, the RsaI/HindIII fragment from this construct was cloned into the SmaI/HindIII sites of pGL3-Luc. Construct A1H-6 was obtained by cloning of the DraI/HindIII fragment of A1H-1 into the SmaI/HindIII sites of pGL3-Luc. The identities of the final constructs were verified by restriction digestion and DNA sequencing (Genomics and Proteomics Center from the Complutense University of Madrid).
ChIP.
ChIP procedures were performed according to reference 38. Briefly, isolated nuclei from formaldehyde-cross-linked cell cultures were lysed and the cross-linked chromatin was sonicated to yield fragments of around 300 bp. Diluted soluble chromatin fragments were precleared with blocked protein G Dynabeads (Invitrogen, Barcelona, Spain) to discard nonspecifically bound chromatin fragments. Immunofractionation of complexes was carried out by adding 2 μg of the corresponding antibodies (anti-p53 or anti-NF-YA) to aliquots containing 50 μg DNA each. The immunocomplexes were recovered by centrifugation after adding blocked protein G Dynabeads and extensively washed. An aliquot of the cross-linked chromatin was treated as described above, but in the absence of the antibody (the n.a. fraction), and the first supernatant, after the immunoprecipitation, was saved as the input fraction. Immunoselected chromatin was eluted, and the formaldehyde cross-linking was reverted. The DNA from all samples was purified with a PCR purification kit (Sigma) and used for PCR analysis using specific primer pairs. PCR fragments were size fractioned by 2% agarose gel electrophoresis, stained with ethidium bromide, and analyzed with a photodocumentation system from UVItec.
Protein immunoprecipitation.
Samples containing 100 μg of nuclear proteins were incubated with 5 μl antibody against NF-YA for 20 h at 4°C. Protein A-Sepharose CL-4B (50 μl; GE Healthcare) was added, and a 4-h incubation at 4°C was carried out. Immunoprecipitated material was washed four times in PBS, and proteins were eluted from the Sepharose beads. Detection was performed by Western blotting as described above, using the primary mouse monoclonal anti-human p53 or rabbit polyclonal anti-human NF-YA antibodies.
Other procedures.
The differences between the mean values were analyzed using SigmaPlot version 9.01 and SigmaStat version 3.11 (Systat Software, Erkrath, Germany) and using Student's t test (two-tailed). Statistical significance was considered to be achieved at P values of <0.05.
Prediction of the p53 binding sequence in the annexin A1 promoter was carried out using the p53MH program (16) and MatInspector version 7.7.3 with library version 7.0 (4).
RESULTS
Effect of butyrate and TSA on annexin A1 expression and cell differentiation.
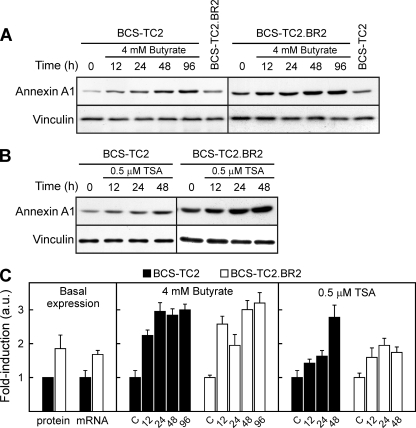
Treatment of BCS-TC2 cells with 4 mM butyrate increased annexin A1 protein levels in a time-dependent manner up to ∼5-fold at 96 h (Fig. 1A). Resistant cells presented slightly higher annexin A1 basal levels than parental cells (1.5- to 2-fold higher) and also showed an increase in annexin A1 levels after treatment with butyrate, but to a lower extent (1.8-fold at 96 h) than the parental ones (Fig. 1A). In order to asses if this effect was mediated through HDAC inhibition, cells were treated with TSA. Figure 1B shows the increase (∼2-fold at 48 h) induced in annexin A1 protein level by 0.5 μM TSA in both cell lines. The same concentration of DMSO employed in TSA treatments was used as a control in these experiments. Control cells did not show variations in annexin A1 levels in culture for up to 96 h in the absence of DMSO or 48 h in its presence, and thus, only time zero controls are shown.
FIG. 1.
Western blot and quantitative real-time reverse transcription-PCR analyses of annexin A1 expression. (A) The effects of 4 mM butyrate treatment for different times in BCS-TC2 and BCS-TC2.BR2 cells were analyzed. The basal expression level of both cell lines is included in the same blots for comparison; controls (0) did not vary with time in culture (not shown). Vinculin expression was analyzed as a control for equal protein loading and used to normalize data. (B) Effect of 0.5 μM TSA treatment. Control cells were incubated with the same concentration of DMSO added in TSA treatment. Representative blots from four independent experiments are shown. (C) Quantitative real-time reverse transcription-PCR was carried out after treatment of cells with 4 mM butyrate and 0.5 μM TSA for different periods of time. Data are normalized to levels for GAPDH as an internal control, and a value of 1 is assigned to the corresponding untreated control in each cell line. The left panel shows a comparison of basal protein and mRNA expression levels in both cell lines, in reference to BCS-TC2 cell levels. Data represent mean values (± standard deviations [SD]) for four different experiments. a.u., arbitrary units.
Annexin A1 mRNA levels have been analyzed by quantitative real-time reverse transcription-PCR (Fig. 1C). BCS-TC2.BR2 cells express 1.6-fold-higher basal mRNA levels than their parental cells. Butyrate induces an increase in mRNA levels in both cell lines (around threefold compared to those of the corresponding controls) prior to the effect detected at the protein level. TSA treatment also induces mRNA expression, but to a lower extent, in both cell lines.
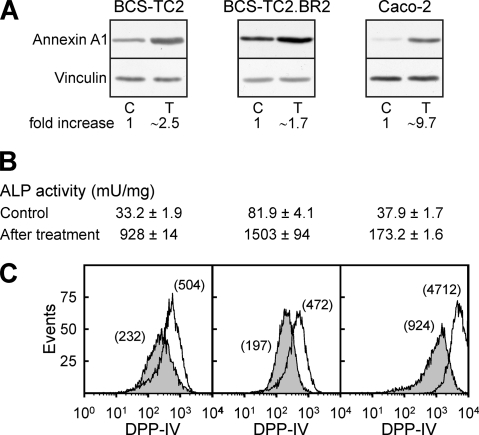
In addition to the increase in annexin A1 expression in colon adenocarcinoma cells (Fig. 2A), butyrate induces differentiation, as observed from alkaline phosphatase activity values (Fig. 2B), not only in BCS-TC2 cells but also in the butyrate-resistant cell line and in other cell lines, such as Caco-2 (28-, 18-, and 5-fold, respectively, after 48 h of treatment with 4 mM butyrate). The cell surface expression levels of the epithelial cell differentiation marker DPP-IV are also increased in the three cell lines (Fig. 2C).
FIG. 2.
Induction of differentiation markers in human colon adenocarcinoma cells after butyrate treatment. Cells were incubated in the presence or absence (control) of 4 mM butyrate for 48 h; afterwards, annexin A1 protein expression was analyzed by Western blotting (A), alkaline phosphatase activity by measuring the hydrolysis of p-nitrophenyl phosphate (B), and DPP-IV surface expression by flow cytometry (C). Blots in panel A and histograms in panel C are representative of four independent experiments; data in panel B correspond to mean values (± SD) for three independent experiments with quadruplicate samples. C, control; T, treated cells.
Effect of butyrate and TSA on annexin A1 promoter activity.
Several fragments from the annexin A1 promoter controlling the expression of firefly luciferase, together with a control plasmid with a strong promoter controlling the expression of Renilla luciferase, were transfected into BCS-TC2 and BCS-TC2.BR2 cells. Figure 3A shows a schematic representation of the promoter fragments used with indication of the potential binding sites for transcription factors, as deduced from the annexin A1 promoter sequence analysis and those described in the literature.
FIG. 3.
Basal annexin A1 promoter activities and effects of butyrate and TSA treatments. (A) Scheme of the different annexin A1 promoter fragments employed in luciferase assays showing the binding sites for transcription factors relative to the transcription start position (+1). GRE, glucocorticoid response element. (B) Promoter activities of the A1H-1, Ax1.3, A1H-5, and A1H-6 constructs in BCS-TC2 and BCS-TC2.BR2 cells measured with dual luciferase assays in exponentially growing cells. (C) Effects of treatment with butyrate or TSA for 24 h on the luciferase activities of different fragments of the annexin A1 promoter in BCS-TC2 and BCS-TC2.BR2 cells. Luciferase activities are expressed as relative induction levels, with the corresponding untreated cells used as a control. Data correspond to mean values (± SD) for three independent experiments performed with triplicate samples.
Basal activity of A1H-1 promoter fragment was threefold higher in BCS-TC2 cells than in BCS-TC2.BR2 cells (Fig. 3B), whereas annexin A1 levels were slightly higher in the latter (Fig. 1). Thus, posttranscriptional mechanisms must exist to yield this increased protein level in resistant cells. Promoter activity in BCS-TC2.BR2 cells did not vary significantly when the −911/−38 region was deleted (A1H-6). When this fragment in BCS-TC2 cells was assayed, luciferase activity decreased, yielding promoter activity values similar to those obtained with butyrate-resistant cells; this result suggests that this is the minimal activity of this promoter in both cell lines. Repressor elements must exist in the −911/−172 region, as its deletion (Ax1.3) increases luciferase activity in both cell lines. In addition, the putative p53 binding site activates transcription, as deletion of the −172/−95 sequence (A1H-5) significantly decreased luminescence below that of the Ax1.3 fragment (Fig. 3B).
Treatment of both cell lines with 4 mM butyrate or 0.5 μM TSA increased the transcriptional activities of the annexin A1 promoter fragments to different extents (Fig. 3C). In BCS-TC2 cells, the effect of butyrate was greater than that of TSA, while the opposite was true for BCS-TC2.BR2 cells (except in A1H-6). In addition, the effect induced by butyrate was around twofold higher in BCS-TC2 cells than in butyrate-resistant cells whereas the effect of TSA was significantly higher in the latter. The removal of the upstream −911/−396 region (A1H-3) had no effect on the activation induced by butyrate or TSA on both cell lines; however, removal of the −396/−318 and −172/−95 regions further enhanced the activation induced by HDAC inhibitors. Interestingly, the latter region contains a p53 binding site whereas no binding site for already described transcription factors was predicted or has been reported to occur in the −396/−318 region. On the other hand, two regions (−318/−172 and −95/−38), both containing a CCAAT box, seem to play a role in the activation. The effect of the proximal box (located inside a putative butyrate response element) (Fig. 4A) was greater than that of the distal one; deletion of the proximal region reduced the activation induced by butyrate to values below those obtained with the A1H-1 fragment in BCS-TC2 cells, while in BCS-TC2.BR2 cells, this reduction was smaller, with an activation similar to that observed with the A1H-1 fragment (Fig. 3C). As a whole, these data suggest that a CCAAT-binding transcription factor and p53 could be involved in promoter regulation by HDAC inhibitors.
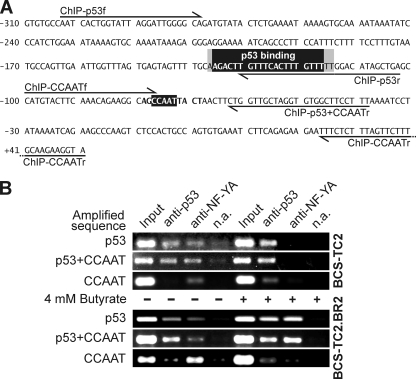
FIG. 4.
ChIP assay of the annexin A1 promoter at the p53 binding site and the proximal CCAAT box. (A) Partial sequence of the annexin A1 promoter region (−310 to +51) showing the p53 binding site (reverse shading, positions −136 to −117, according to the p53MH program; gray shading, additional bases included in the MatInspector prediction) and the proximal CCAAT box (reverse shading, positions −77 to −73). The primers used for PCR amplification of the p53 (ChIP-p53f and ChIP-p53r), p53 plus NF-Y (ChIP-p53f and ChIP-p53+CCAATr), and NF-Y (ChIP-CCAATf and ChIP-CCAATr) binding regions are indicated. (B) Binding of p53 and NF-Y transcription factors to the annexin A1 gene in cells without butyrate (−) or after a 24-h treatment with 4 mM butyrate (+) was studied using antibodies against p53 and NF-YA. The immunoprecipitated samples were analyzed by PCR using the primers given in panel A. As controls for the ChIP experiment, PCR was also carried out with the supernatant of a mock immunoprecipitation without antibodies (Input; diluted 1:100) and with samples obtained under these no-antibody conditions (n.a.).
NF-Y and p53 bind to the annexin A1 promoter.
p53MH and MatInspector predictions indicate the existence of p53 and NF-Y binding sites close to each other on the annexin A1 promoter. Binding of both transcription factors was seen by electrophoretic mobility shift and supershift assays, employing different oligonucleotides within this region (data not shown). In order to confirm the relevance of these observations in vivo, ChIP assays using specific antibodies were performed. These results show that p53 binds to the sequence remarked upon in Fig. 4A and that NF-Y binds to the proximal CCAAT box in BCS-TC2 and BCS-TC2.BR2 cells in the absence of butyrate (Fig. 4B). Whereas p53 bound only directly to its site, the NF-YA subunit was detected bound to the CCAAT box and to the p53 site as well, indicating that a complex between p53 and NF-Y (at least the NF-YA subunit) might be also indirectly recruiting NF-Y to the annexin A1 promoter.
In both cell lines, butyrate treatment (24 h, 4 mM) strongly decreased direct NF-Y binding to the CCAAT box in the annexin A1 promoter. However, in BCS-TC2.BR2 cells, NF-YA still indirectly binds to the promoter through the p53 binding site. Additionally, butyrate induces an increased level of p53 binding to the annexin A1 promoter in parental and resistant cells.
p53 and NF-YA protein expression and immunoprecipitation studies.
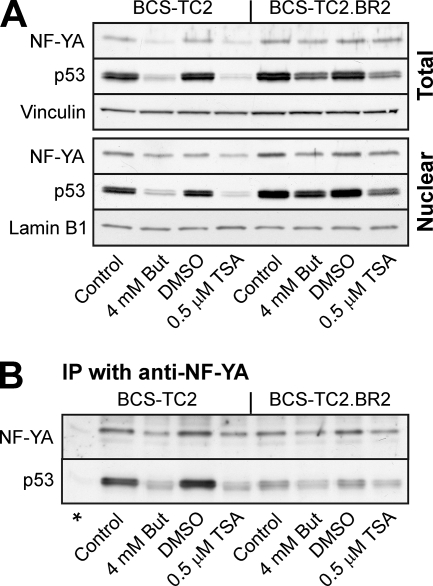
Western blot analysis reveals that butyrate and TSA treatments induced a reduction in NF-YA protein expression in BCS-TC2 cells in both nuclear and whole-cell extracts (Fig. 5A). Resistant cells present slightly higher basal NF-YA levels than their parental cells, and the reductions in expression in response to both agents were smaller. In contrast, p53 protein expression abruptly decreased in parental cells (5.0- and 7.2-fold decreases induced by butyrate and TSA, respectively, in nuclear extracts), while the effects were much less pronounced in the BCS-TC2.BR2 cell line (around 2-fold with both agents). Basal p53 expression was also slightly higher in resistant cells (1.5-fold in nuclear extracts).
FIG. 5.
NF-YA and p53 protein expression and interaction. (A) Western blot analyses for NF-YA and p53 were carried out using total or nuclear cell extracts from BCS-TC2 and BCS-TC2.BR2 cells treated with butyrate or TSA for 24 h. Blots using anti-lamin B1 and anti-vinculin were used as loading controls in nuclear and total extracts, respectively. (B) Immunoprecipitated (IP) material with antibodies against NF-YA in nuclear extracts from BCS-TC2 or BCS-TC2.BR2 cells was probed for the presence of NF-YA and p53 by Western blot analysis with the corresponding antibodies. An immunoprecipitation-negative control without specific antibodies is included (*). Representative blots from four independent experiments are shown.
ChIP assays indicated that p53 and NF-YA presented cooperative binding to DNA. Thus, we analyzed whether these proteins interacted in nuclear extracts from both cell lines by immunoprecipitation using anti-NF-YA antibodies. Figure 5B shows that p53 was detected in the immunoprecipitated material from parental and resistant cells while no protein was detected in the absence of specific antibody. Even though BCS-TC2 cells showed lower p53 protein levels in the nuclear extracts, coimmunoprecipitation with NF-YA yielded a much stronger band than that in butyrate-resistant cells. Butyrate or TSA treatments induced a significant decrease in the amount of p53 immunoprecipitated with NF-YA subunit antibodies in parallel with the decrease in protein levels in the nuclear extracts.
Involvement of the p38 MAPK pathway in annexin A1 regulation.
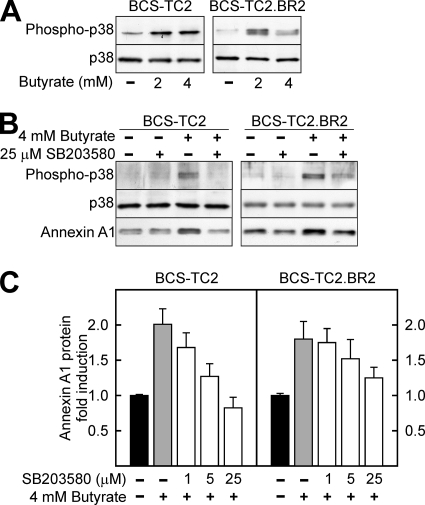
Figure 6A shows that butyrate increased phosphorylation of p38 MAPK in both parental and resistant cells without any change in total protein expression. Incubation with 25 μM SB203580 (a specific p38 MAPK inhibitor) effectively blocked butyrate-induced activation of p38 MAPK (Fig. 6B); in accordance with this inhibition, annexin A1 protein overexpression was prevented even at lower concentrations of the p38 MAPK inhibitor (Fig. 6B and C), with this effect more clearly observed in BCS-TC2 cells than in the butyrate-resistant ones.
FIG. 6.
Influence of p38 MAPK activity in annexin A1 protein induction. (A) p38 MAPK activation after butyrate treatment was assessed by analysis of its phosphorylation in extracts obtained from control and butyrate-treated BCS-TC2 and BCS-TC2.BR2 cells. Total levels of p38 MAPK are shown as a control. (B and C) Involvement of p38 MAPK in annexin A1 protein expression induction by butyrate was assessed after treatment of the cells with the specific inhibitor SB203580 either in the absence or in the presence of butyrate. (B) Representative Western blot using 25 μM SB203580 in the presence or absence of butyrate treatment. (C) Quantification of the effects of different concentrations of SB203580 on annexin A1 expression referred to controls in the absence of butyrate and in the presence of the indicated concentration of p38 MAPK inhibitor. Data in panel C correspond to mean values (± SD) obtained from the densitometric analysis of blots from three independent experiments.
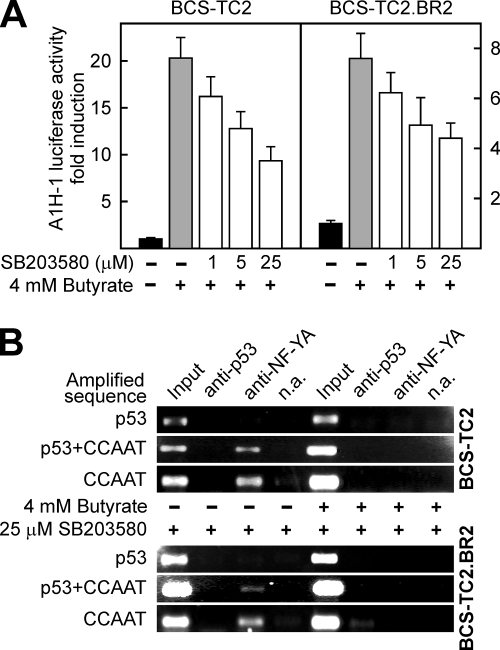
Luciferase experiments showed that the activation of the annexin A1 promoter (A1H-1 fragment) by butyrate was significantly reduced in both BCS-TC2 and BCS-TC2.BR2 cells in the presence of the p38 MAPK inhibitor (Fig. 7A). ChIP assays showed that p53 binding to the annexin A1 promoter is blocked in the presence of SB203580 either in the absence or in the presence of butyrate and in both cell lines (Fig. 7B). NF-Y direct binding to the CCAAT box was unaffected by inhibition of p38 MAPK; butyrate treatment induced reduction of NF-Y binding in a manner similar to that observed in the absence of SB203580 (Fig. 4B and 7B).
FIG. 7.
Effect of p38 MAPK inhibition on butyrate activation of the annexin A1 promoter and in the binding of p53 and NF-Y transcription factors. (A) Luciferase activity was measured in BCS-TC2 and BCS-TC2.BR2 cell homogenates obtained after transfection with the A1H-1 construct and treatment for 24 h with 4 mM butyrate in the presence or absence of 1, 5, or 25 μM SB203580. Activity is expressed as induction with respect to the activity of butyrate-untreated, transfected cells. Data correspond to mean values (± SD) from two independent experiments with triplicate samples. (B) Binding of p53 and NF-Y transcription factors to the annexin A1 gene in cells untreated (−) or treated for 24 h with 4 mM butyrate (+) was analyzed by ChIP in the presence of 25 μM SB203580, using antibodies against p53 and NF-YA. The immunoprecipitated samples were analyzed by PCR using the primers given in Fig. 4A. As controls for the ChIP experiment, PCR was also carried out with the input fraction and with samples obtained in the absence of specific antibodies (n.a.).
DISCUSSION
Annexin A1 function has been mainly ascribed to the modulation of immune response, but there is growing evidence for the involvement of this protein in proliferation, differentiation, and apoptosis (11). Despite its wide tissue distribution and the role of annexin A1 in crucial cellular events, very little is known about the regulation of its expression. Its induction by glucocorticoids still remains a controversial issue, but it has been shown that the putative glucocorticoid response element on the annexin A1 promoter does not mediate this induction (41). It has also been described that phorbol esters and interleukin 6 upregulate annexin A1 protein levels through transcriptional mechanisms involving C/EBP-β (42). Regarding the effect of HDAC inhibitors on the expression of annexin A1, there is only one recent report, to our knowledge, on the effect of the cyclopeptide FR235222 on the annexin A1 protein levels in leukemia cells (35) and one reporting the effect of depsipeptide (FK228), which induces annexin A1 mRNA through an increase in histone acetylation and recruitment of C/EBP-α to the annexin A1 promoter, specifically in AML1/ETO-positive acute myeloblastic leukemia cell lines (43). However, no studies have been reported to date on the effect of butyrate (the main natural modulator of colonic epithelium homeostasis) on the expression of this annexin. Here, we describe how butyrate regulates annexin A1 transcription and protein expression through the activation of the p38 MAPK pathway and the involvement of p53 and NF-Y in this process.
To gain insight into the regulation of annexin A1 gene expression, we analyzed the basal activity of this promoter. According to luciferase, electrophoretic mobility shift, and ChIP assays, p53 activates annexin A1 expression through its binding site upstream of the proximal CCAAT box. In BCS-TC2 cells, p53 is mutated in one allele, leading to its accumulation; this mutant form (R282W) does not impair transcriptional activity of wild-type p53 (25), and therefore, a high level of annexin A1 expression is induced. Caco-2 cells show a much lower basal annexin A1 expression than BCS-TC2 or BCS-TC2.BR2 cells (13); this difference could arise from the occurrence of a truncated, inactive form of p53 in Caco-2 cells (8). Thus, this regulation is found to have great importance when annexin A1 expression in cancer cells is considered, as an aberrant upregulation of this annexin could be secondary to alterations in p53. Additionally, posttranscriptional mechanisms must take place in BCS-TC2.BR2 cells to obtain higher annexin A1 levels than those in parental cells. These mechanisms add further complexity to the regulation of the expression of this protein.
In contrast to basal activity, luciferase assays suggest that annexin A1 promoter activation by butyrate depends on CCAAT boxes, mainly the proximal one. Several genes involved in growth, apoptosis, and differentiation have been shown to be upregulated in response to HDAC inhibitors through NF-Y binding to CCAAT boxes (15, 17, 20). In addition, postconfluent, spontaneous differentiation of Caco-2 cells has revealed that NF-Y is essential for the expression of several differentiation markers (3). All these data suggest a role for the NF-Y transcription factor as a mediator of butyrate and TSA actions as well as in cell differentiation.
However, in vitro luciferase data are in apparent contradiction to in vivo ChIP results regarding the role of NF-Y and p53 in promoter activity regulation. For BCS-TC2 cells, in vitro assays support an inhibitory role for p53 in NF-Y-dependent activation. Functional cooperation between p53 and NF-Y has been described to occur on several promoters of genes involved in the cell cycle as well as in endoplasmic reticulum stress. In contrast to annexin A1, these promoters present several CCAAT boxes and no p53 binding site. In these genes, the induction of p53 by DNA damage leads to promoter activity repression through cooperation with bound NF-Y (18, 24). Taking into account that p53 seems to activate basal transcription of the annexin A1 gene, it is difficult to envision how butyrate treatment could dramatically change its behavior. This effect could be, on the contrary, derived from the removal of the p53 binding in the transfected constructs, which may render the CCAAT box more accessible to CCAAT-binding factors activated after treatment with HDAC inhibitors. This could explain the strong decrease in butyrate-induced activation after the removal of the CCAAT box. An alternative explanation is that the removal of the proximal CCAAT box, close to the TATA box, induces an artifact in the in vitro assays, impairing the binding of the transcriptional initiator complex to the truncated promoter construct.
The data from ChIP assays indicate that, even though NF-Y may be important for annexin A1 basal expression (either binding directly to the CCAAT box or indirectly forming a complex with p53), butyrate upregulation of this gene involves the release of NF-Y from the CCAAT box and this release is independent of the butyrate-induced p38 MAPK activation. It has been previously shown that p21 represses NF-Y binding to DNA through inhibition of NF-YA phosphorylation by cdk2 (49). Interestingly, we have previously described that the expression of the cell cycle inhibitor p21 after butyrate treatment increases in BCS-TC2 and BCS-TC2.BR2 cells (23). This could explain why NF-Y does not bind the annexin A1 promoter directly after butyrate treatment.
We have demonstrated the presence of a functional p53 binding site near the proximal CCAAT box, and ChIP analysis strongly suggests that a complex between p53 and NF-Y is binding through the p53 site and not to the CCAAT box. In the absence of butyrate, this complex is more abundant in BCS-TC2 cells than in the butyrate-resistant ones, suggesting that in parental cells NF-Y binds mainly to the promoter through the p53 site. Upon butyrate treatment, a switch is observed in BCS-TC2 cells and no NF-Y is detected in the promoter, either directly bound or bound through p53.
However, in butyrate-treated BCS-TC2.BR2 cells NF-Y is still present at the p53 binding location associated with this transcription factor. This different behavior must be related to butyrate resistance in BCS-TC2.BR2 cells. Even if the interaction between p53 and NF-Y is detected in both cell lines after butyrate treatment, one could speculate that a higher p53 acetylation status in butyrate-sensitive cells may foster its binding to the promoter on its own in preference to the complex with NF-Y. On this idea, it has been recently described that p53 acetylation could disrupt p53/NF-Y interaction on promoters of proapoptotic genes, leading to their activation (2). This model could also explain the increased activation of the promoter observed in BCS-TC2 in luciferase assays compared to that in butyrate-resistant cells, even when a significant decrease in p53 levels is observed.
Despite the differences in basal activity and quantitative effects of butyrate and TSA on the annexin A1 promoter, the effects of both HDAC inhibitors are qualitatively similar in BCS-TC2 cells and BCS-TC2.BR2 butyrate-resistant cells. This result agrees with our previous data indicating that BCS-TC2.BR2 cells are resistant to apoptosis but not to the differentiation induced by this agent (23), reinforcing the role of annexin A1 as a differentiation marker in these cells.
We also wanted to gain insight into the signaling pathways involved in annexin A1 modulation. It has been previously described that butyrate induces phosphorylation of p38 MAPK (48); moreover, this MAPK, but not extracellular signal-regulated kinase, has been shown to play an important role in regulation of Caco-2 cell differentiation by butyrate (6). Considering these data, we checked the possible implication of p38 MAPK on the regulation of annexin A1 transcription by butyrate. We demonstrate that annexin A1 upregulation depends on p38 MAPK activation induced by butyrate. Direct NF-Y binding to the promoter through the proximal CCAAT box is independent of p38 MAPK, but p53 binding is almost completely blocked by its inhibition. Phosphorylation at Ser 33 or 46 (targets of p38 MAPK in the N-terminal domain of p53) (http://www.biology.bnl.gov/cellbio/anderson.html) could then be required for its binding to the promoter. However, inhibition of p38 MAPK is not able to reduce A1H-1 promoter activation by butyrate to values for nontreated cells, even at 25 μM SB203580. Additional modifications and/or factors must thus contribute to butyrate effects. Increased phosphorylation of p53, together with a potential increase in the acetylation status due to inhibition of HDACs, may be at least partially responsible for the butyrate-induced transcriptional activation.
Regarding p38 MAPK activation upstream events, it has been described that butyrate is able to activate protein kinase Cδ and, interestingly, that this isoform specifically activates p38 MAPK (26). Additionally, phorbol ester-induced differentiation involves an increase in annexin A1 expression in several cell types, such as macrophages and keratinocytes (41). These agents act through activation of protein kinase C, further supporting the role of these proteins in the regulation of annexin A1 expression in response to differentiating stimuli.
In summary, here we show that annexin A1 promoter activity is controlled by a functional cooperation between p53 and factors binding to the proximal CCAAT box. Differences in p53 status may underlie the changes in annexin A1 expression reported to occur in several models and cancer types. The induction of the expression of this protein by butyrate involves both p38 MAPK-dependent p53 activation and p38 MAPK-independent release of NF-Y on its promoter. Taking into account the role of p38 MAPK in the differentiation program induced by butyrate in colon epithelial cells, our results reinforce the role of annexin A1 as a marker of this process. The mechanism of regulation of annexin A1 provides the basis for the action of butyrate on a wide range of genes and will help to elucidate the role of this protein in colonic epithelial cells.
Acknowledgments
This work was supported by grant BFU2005-02671 from the DGI, Ministerio de Educación y Ciencia (Spain).
We thank S. E. Moss (Institute of Ophtalmology, University College of London, United Kingdom) and M. P. Fernandez (Dept. of Biochemistry, University of Oviedo, Spain) for providing annexin A1 promoter constructs and G. López-Rodas and L. Franco (Dept. of Biochemistry, University of Valencia) for their assistance and discussion of the ChIP assays. We are also grateful to Ricardo Ramos (Unidad de Genómica, Parque Científico de Madrid) for his assistance with quantitative real-time PCR. The anti-human annexin A1 EH17a monoclonal antibody developed by J. D. Ernst was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD, and maintained by The University of Iowa, Dept. of Biological Sciences, Iowa City, IA.
Footnotes
Published ahead of print on 9 June 2008.
REFERENCES
- 1.Bai, X. F., X. G. Ni, P. Zhao, S. M. Liu, H. X. Wang, B. Guo, L. P. Zhou, F. Liu, J. S. Zhang, K. Wang, Y. Q. Xie, Y. F. Shao, and X. H. Zhao. 2004. Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J. Gastroenterol. 101466-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benatti, P., V. Basile, D. Merico, L. I. Fantoni, E. Tagliafico, and C. Imbriano. 2008. A balance between NF-Y and p53 governs the pro- and anti-apoptotic transcriptional response. Nucleic Acids Res. 361415-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevilacqua, M. A., M. C. Faniello, B. Iovine, T. Russo, F. Cimino, and F. Costanzo. 2002. Transcription factor NF-Y regulates differentiation of CaCo-2 cells. Arch. Biochem. Biophys. 40739-44. [DOI] [PubMed] [Google Scholar]
- 4.Cartharius, K., K. Frech, K. Grote, B. Klocke, M. Haltmeier, A. Klingenhoff, M. Frisch, M. Bayerlein, and T. Werner. 2005. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 212933-2942. [DOI] [PubMed] [Google Scholar]
- 5.D'Acquisto, F., A. Merghani, E. Lecona, G. Rosignoli, K. Raza, C. D. Buckley, R. J. Flower, and M. Perretti. 2007. Annexin 1 modulates T-cell activation and differentiation. Blood 1091095-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel, C., O. Schroder, N. Zahn, T. Gaschott, and J. Stein. 2004. p38 MAPK signaling pathway is involved in butyrate-induced vitamin D receptor expression. Biochem. Biophys. Res. Commun. 3241220-1226. [DOI] [PubMed] [Google Scholar]
- 7.Davie, J. R. 2003. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 1332485S-2493S. [DOI] [PubMed] [Google Scholar]
- 8.Djelloul, S., M. E. Forgue-Lafitte, B. Hermelin, M. Mareel, E. Bruyneel, A. Baldi, A. Giordano, E. Chastre, and C. Gespach. 1997. Enterocyte differentiation is compatible with SV40 large T expression and loss of p53 function in human colonic Caco-2 cells. Status of the pRb1 and pRb2 tumor suppressor gene products. FEBS Lett. 406234-242. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly, S. R., and S. E. Moss. 1998. Functional analysis of the human annexin I and VI gene promoters. Biochem. J. 332681-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerke, V., C. E. Creutz, and S. E. Moss. 2005. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 6449-461. [DOI] [PubMed] [Google Scholar]
- 11.Gerke, V., and S. E. Moss. 2002. Annexins: from structure to function. Physiol. Rev. 82331-371. [DOI] [PubMed] [Google Scholar]
- 12.Gui, C. Y., L. Ngo, W. S. Xu, V. M. Richon, and P. A. Marks. 2004. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 1011241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzmán-Aránguez, A., N. Olmo, J. Turnay, E. Lecona, P. Perez-Ramos, I. López de Silanes, and M. A. Lizarbe. 2005. Differentiation of human colon adenocarcinoma cells alters the expression and intracellular localization of annexins A1, A2, and A5. J. Cell Biochem. 94178-193. [DOI] [PubMed] [Google Scholar]
- 14.Hannon, R., J. D. Croxtall, S. J. Getting, F. Roviezzo, S. Yona, M. J. Paul-Clark, F. N. Gavins, M. Perretti, J. F. Morris, J. C. Buckingham, and R. J. Flower. 2003. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 17253-255. [DOI] [PubMed] [Google Scholar]
- 15.Hirose, T., Y. Sowa, S. Takahashi, S. Saito, C. Yasuda, N. Shindo, K. Furuichi, and T. Sakai. 2003. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene 227762-7773. [DOI] [PubMed] [Google Scholar]
- 16.Hoh, J., S. Jin, T. Parrado, J. Edington, A. J. Levine, and J. Ott. 2002. The p53MH algorithm and its application in detecting p53-responsive genes. Proc. Natl. Acad. Sci. USA 998467-8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, W., S. Zhao, S. Ammanamanchi, M. Brattain, K. Venkatasubbarao, and J. W. Freeman. 2005. Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1.NF-Y complex. J. Biol. Chem. 28010047-10054. [DOI] [PubMed] [Google Scholar]
- 18.Imbriano, C., A. Gurtner, F. Cocchiarella, S. Di Agostino, V. Basile, M. Gostissa, M. Dobbelstein, G. Del Sal, G. Piaggio, and R. Mantovani. 2005. Direct p53 transcriptional repression: in vivo analysis of CCAAT-containing G2/M promoters. Mol. Cell. Biol. 253737-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isacke, C. M., R. A. Lindberg, and T. Hunter. 1989. Synthesis of p36 and p35 is increased when U-937 cells differentiate in culture but expression is not inducible by glucocorticoids. Mol. Cell. Biol. 9232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, S., and K. W. Scotto. 1998. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol. Cell. Biol. 184377-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, S. W., H. J. Rhee, J. Ko, Y. J. Kim, H. G. Kim, J. M. Yang, E. C. Choi, and D. S. Na. 2001. Inhibition of cytosolic phospholipase A2 by annexin I. Specific interaction model and mapping of the interaction site. J. Biol. Chem. 27615712-15719. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Conejo, T., N. Olmo, J. Turnay, J. Navarro, and A. Lizarbe. 1996. Characterization of tumorigenic sub-lines from a poorly tumorigenic human colon-adenocarcinoma cell line. Int. J. Cancer 67668-675. [DOI] [PubMed] [Google Scholar]
- 23.López de Silanes, I., N. Olmo, J. Turnay, G. González de Buitrago, P. Pérez-Ramos, A. Guzmán-Aránguez, M. García-Díez, E. Lecona, M. Gorospe, and M. A. Lizarbe. 2004. Acquisition of resistance to butyrate enhances survival after stress and induces malignancy of human colon carcinoma cells. Cancer Res. 644593-4600. [DOI] [PubMed] [Google Scholar]
- 24.Manni, I., G. Mazzaro, A. Gurtner, R. Mantovani, U. Haugwitz, K. Krause, K. Engeland, A. Sacchi, S. Soddu, and G. Piaggio. 2001. NF-Y mediates the transcriptional inhibition of the cyclin B1, cyclin B2, and cdc25C promoters upon induced G2 arrest. J. Biol. Chem. 2765570-5576. [DOI] [PubMed] [Google Scholar]
- 25.Marutani, M., H. Tonoki, M. Tada, M. Takahashi, H. Kashiwazaki, Y. Hida, J. Hamada, M. Asaka, and T. Moriuchi. 1999. Dominant-negative mutations of the tumor suppressor p53 relating to early onset of glioblastoma multiforme. Cancer Res. 594765-4769. [PubMed] [Google Scholar]
- 26.McMillan, L., S. K. Butcher, J. Pongracz, and J. M. Lord. 2003. Opposing effects of butyrate and bile acids on apoptosis of human colon adenoma cells: differential activation of PKC and MAP kinases. Br J. Cancer 88748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minucci, S., and P. G. Pelicci. 2006. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer 638-51. [DOI] [PubMed] [Google Scholar]
- 28.Nakano, K., T. Mizuno, Y. Sowa, T. Orita, T. Yoshino, Y. Okuyama, T. Fujita, N. Ohtani-Fujita, Y. Matsukawa, T. Tokino, H. Yamagishi, T. Oka, H. Nomura, and T. Sakai. 1997. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J. Biol. Chem. 27222199-22206. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, J. M., N. Olmo, J. Turnay, M. T. López-Conejo, and M. A. Lizarbe. 1997. Differentiation of BCS-TC2 human colon adenocarcinoma cells by sodium butyrate: increase in 5′-nucleotidase activity. Eur. J. Clin. Investig. 27620-628. [DOI] [PubMed] [Google Scholar]
- 30.Olmo, N., J. Turnay, E. Lecona, M. Garcia-Diez, B. Llorente, A. Santiago-Gomez, and M. A. Lizarbe. 2007. Acquisition of resistance to butyrate induces resistance to luminal components and other types of stress in human colon adenocarcinoma cells. Toxicol. In Vitro 21254-261. [DOI] [PubMed] [Google Scholar]
- 31.Omer, S., D. Meredith, J. F. Morris, and H. C. Christian. 2006. Evidence for the role of adenosine 5′-triphosphate-binding cassette (ABC)-A1 in the externalization of annexin 1 from pituitary folliculostellate cells and ABCA1-transfected cell models. Endocrinology 1473219-3227. [DOI] [PubMed] [Google Scholar]
- 32.Parente, L., and E. Solito. 2004. Annexin 1: more than an anti-phospholipase protein. Inflamm. Res. 53125-132. [DOI] [PubMed] [Google Scholar]
- 33.Patton, K. T., H. M. Chen, L. Joseph, and X. J. Yang. 2005. Decreased annexin I expression in prostatic adenocarcinoma and in high-grade prostatic intraepithelial neoplasia. Histopathology 47597-601. [DOI] [PubMed] [Google Scholar]
- 34.Perretti, M., and F. N. Gavins. 2003. Annexin 1: an endogenous anti-inflammatory protein. News Physiol. Sci. 1860-64. [DOI] [PubMed] [Google Scholar]
- 35.Petrella, A., C. W. D'Acunto, M. Rodriquez, M. Festa, A. Tosco, I. Bruno, S. Terracciano, M. Taddei, L. G. Paloma, and L. Parente. 2008. Effects of FR235222, a novel HDAC inhibitor, in proliferation and apoptosis of human leukaemia cell lines: Role of Annexin A1. Eur. J. Cancer 44740-749. [DOI] [PubMed] [Google Scholar]
- 36.Petrella, A., M. Festa, S. F. Ercolino, M. Zerilli, G. Stassi, E. Solito, and L. Parente. 2006. Annexin-1 downregulation in thyroid cancer correlates to the degree of tumor differentiation. Cancer Biol. Ther. 5643-647. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigo, J. P., J. M. Garcia-Pedrero, M. V. Gonzalez, M. P. Fernandez, C. Suarez, and A. Herrero. 2004. Expression of annexin A1 in normal and chronically inflamed nasal mucosa. Arch. Otolaryngol. Head Neck Surg. 130211-215. [DOI] [PubMed] [Google Scholar]
- 38.Sandoval, J., J. L. Rodríguez, G. Tur, G. Serviddio, J. Pereda, A. Boukaba, J. Sastre, L. Torres, L. Franco, and G. López-Rodas. 2004. RNAPol-ChIP: a novel application of chromatin immunoprecipitation to the analysis of real-time gene transcription. Nucleic Acids Res. 32e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheppach, W., and F. Weiler. 2004. The butyrate story: old wine in new bottles? Curr. Opin. Clin. Nutr. Metab. Care 7563-567. [DOI] [PubMed] [Google Scholar]
- 40.Shen, D., F. Nooraie, Y. Elshimali, V. Lonsberry, J. He, S. Bose, D. Chia, D. Seligson, H. R. Chang, and L. Goodglick. 2006. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum. Pathol. 371583-1591. [DOI] [PubMed] [Google Scholar]
- 41.Solito, E., C. de Coupade, L. Parente, R. J. Flower, and F. Russo-Marie. 1998. Human annexin 1 is highly expressed during the differentiation of the epithelial cell line A 549: involvement of nuclear factor interleukin 6 in phorbol ester induction of annexin 1. Cell Growth Differ. 9327-336. [PubMed] [Google Scholar]
- 42.Solito, E., C. de Coupade, L. Parente, R. J. Flower, and F. Russo-Marie. 1998. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine 10514-521. [DOI] [PubMed] [Google Scholar]
- 43.Tabe, Y., L. Jin, R. Contractor, D. Gold, P. Ruvolo, S. Radke, Y. Xu, Y. Tsutusmi-Ishii, K. Miyake, N. Miyake, S. Kondo, A. Ohsaka, I. Nagaoka, M. Andreeff, and M. Konopleva. 2007. Novel role of HDAC inhibitors in AML1/ETO AML cells: activation of apoptosis and phagocytosis through induction of annexin A1. Cell Death Differ. 141443-1456. [DOI] [PubMed] [Google Scholar]
- 44.Turnay, J., N. Olmo, J. G. Gavilanes, J. Benítez, and M. A. Lizarbe. 1990. Establishment and characterization of a new human colon adenocarcinoma cell line: BCS-TC2. Cytotechnology 375-88. [DOI] [PubMed] [Google Scholar]
- 45.Vishwanatha, J. K., E. Salazar, and V. K. Gopalakrishnan. 2004. Absence of annexin I expression in B-cell non-Hodgkin's lymphomas and cell lines. BMC Cancer 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, K. L., T. T. Wu, E. Resetkova, H. Wang, A. M. Correa, W. L. Hofstetter, S. G. Swisher, J. A. Ajani, A. Rashid, S. R. Hamilton, and C. T. Albarracin. 2006. Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin. Cancer Res. 124598-4604. [DOI] [PubMed] [Google Scholar]
- 47.Wu, C. M., Y. S. Lee, T. H. Wang, L. Y. Lee, W. H. Kong, E. S. Chen, M. L. Wei, Y. Liang, and T. L. Hwang. 2006. Identification of differential gene expression between intestinal and diffuse gastric cancer using cDNA microarray. Oncol. Rep. 1557-64. [PubMed] [Google Scholar]
- 48.Yonezawa, T., Y. Kobayashi, and Y. Obara. 2007. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell. Signal. 19185-193. [DOI] [PubMed] [Google Scholar]
- 49.Yun, J., H. D. Chae, T. S. Choi, E. H. Kim, Y. J. Bang, J. Chung, K. S. Choi, R. Mantovani, and D. Y. Shin. 2003. Cdk2-dependent phosphorylation of the NF-Y transcription factor and its involvement in the p53-p21 signaling pathway. J. Biol. Chem. 27836966-36972. [DOI] [PubMed] [Google Scholar]
- 50.Zhao, Y., S. Lu, L. Wu, G. Chai, H. Wang, Y. Chen, J. Sun, Y. Yu, W. Zhou, Q. Zheng, M. Wu, G. A. Otterson, and W. G. Zhu. 2006. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21Waf1/Cip1. Mol. Cell. Biol. 262782-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]