Abstract
Intraembryonic hematopoiesis occurs at two different sites, the floor of the aorta and subaortic patches (SAPs) of the para-aortic splanchnopleura (P-Sp)/aorta-gonad-mesonephros (AGM) region. Notch1 and RBP-jκ are critical for the specification of hematopoietic stem cells (HSCs) in Notch signal-receiving cells. However, the mechanism by which Notch signaling is triggered from the Notch signal-sending cells to support embryonic hematopoiesis remains to be determined. We previously reported that Mind bomb-1 (Mib1) regulates Notch ligands in the Notch signal-sending cells (B. K. Koo, M. J. Yoon, K. J. Yoon, S. K. Im, Y. Y. Kim, C. H. Kim, P. G. Suh, Y. N. Jan, and Y. Y. Kong, PLoS ONE 2:e1221, 2007). Here, we show that intraembryonic hematopoietic progenitors were absent in the P-Sp of Mib1−/− embryos, whereas they were partly preserved in the Tie2-cre; Mib1f/f P-Sps, suggesting that Mib1 plays a role in the endothelium and the SAPs. Interestingly, dll1 and dll4/Jag1 are expressed in the SAPs and the endothelium of the AGM, respectively, where mib1 is detected. Indeed, Notch signaling was activated in the nascent HSCs at both sites. In the P-Sp explant culture, the overexpression of Dll1 in OP9 stromal cells rescued the failed production of hematopoietic progenitors in the Mib1−/− P-Sp, while its activity was abolished by Mib1 knockdown. These results suggest that Mib1 is important for intraembryonic hematopoiesis not only in the aortic endothelium but also in the SAPs.
A wide range of evidence has suggested that hemangioblasts and the hemogenic endothelium are the presumptive precursors to emerging hematopoietic cells within the embryo proper (6, 13, 40). Cytological and histological analyses have proposed that the floor of the aorta in the para-aortic splanchnopleura (P-Sp)/aorta-gonad-mesonephros (AGM) region is the site of the origin of intraembryonic hematopoietic stem cells (HSCs) (15, 24). Recently, several reports suggested that other structures, called subaortic patches (SAPs), below the aortic floor are involved in HSC generation (2, 16, 34). The SAPs are relatively uncharacterized mesenchymal cell layers that express the GATA3 transcription factor and the AA4.1 antigen (34, 45), and they are easily detectable at the peak of intraembryonic HSC production, while they disappear with the close of HSC generation in the AGM at embryonic day 12 (E12) (14, 17). The SAPs harbor long-term reconstituting HSCs that express c-Kit, CD31, and CD41 but not CD45 (2), indicating that the SAPs are another supportive niche for intraembryonic HSC generation in the P-Sp/AGM region.
Notch signaling is a conserved signaling pathway that plays a critical role in the determination of cell fate and the maintenance of progenitors in many developmental systems (1). In mammals, four Notch receptors (Notch1 to Notch4) and five Notch ligands (Deltalike-1 [Dll1], Dll3, Dll4, Jagged-1 [Jag1], and Jag2) have been identified. Notch signaling is initiated through interactions with the Jagged and Delta families of ligands expressed on the neighboring cells, which induce the proteolytic cleavage of Notch receptors and the release of the Notch intracellular domain (3). The Notch intracellular domain translocates to the nucleus and forms a transcriptional activator complex with RBP-jκ, which turns on the Notch target genes. Loss-of-function genetic studies of mice have demonstrated that the Notch1-RBP-jκ pathway is essential for HSC generation in intraembryonic hematopoiesis (4, 18, 29, 46). Although the requirement of Notch signaling for the generation of HSCs has been well studied, the microenvironment that supports intraembryonic HSC generation remains to be elucidated. Therefore, which Notch ligand(s) is involved in the generation of intraembryonic HSCs and what cell type(s) expresses the Notch ligand(s) need to be clarified.
Several lines of evidence have indicated that the endocytosis of Notch ligands in the signal-sending cells is essential for Notch receptor activation in the signal-receiving cells (31, 32). Four E3 ubiquitin ligases, Mind bomb-1 (Mib1), Mib2, Neuralized-1 (Neur1), and Neur2, have been identified as regulators of Notch ligand endocytosis (21, 27, 30, 44, 50). Analyses of the Mib1−/− mice and the zebrafish mib1 mutants revealed that, among the four E3 ubiquitin ligases, Mib1 is essential for the generation of functional Notch ligands and regulates the classical Notch ligands Dll1, Dll4, Jag1, and Jag2 in vertebrates (21, 26, 28). Mib1-deficient cells cannot activate Notch signaling in the adjacent signal-receiving cells, but they still are able to receive Notch signals from the neighboring cells expressing Notch ligands and Mib1 (26). Thus, a coculture system using these Mib1-deficient cells with cells expressing Notch ligand(s) and Mib1 will be a unique and ideal model to identify the Notch ligand(s) in the signal-sending cells for Notch activation.
In this study, to identify the role of Mib1 in embryonic hematopoiesis, we performed P-Sp organ cultures using Mib1−/− embryos and Mib1 conditional knockout embryos with the Tie2-cre transgene (Tie2-cre; Mib1f/f). The generation of hematopoietic progenitors was completely blocked in the Mib1−/− P-Sp but was retained, albeit at a reduced level, in the Tie2-cre; Mib1f/f P-Sp, which has Notch signaling defects in the aortic endothelium (28). These findings suggest that Mib1 regulates intraembryonic hematopoiesis in not only the aortic endothelium but also at another site in the P-Sp region. Dll1 and Dll4/Jag1 are expressed in the SAPs and aortic endothelial cells, respectively, where Mib1 and Notch1 are expressed. Indeed, Notch activation in the nascent HSCs at both sites was detected by analyzing the transgenic Notch reporter mice. Furthermore, OP9 cells overexpressing Dll1 (OP9-Dll1) rescued the defective hematopoietic activity in the Mib1−/− P-Sp, and Mib1 knockdown in OP9-Dll1 cells abolished the rescued hematopoietic activity in the Mib1−/− P-Sp/OP9-Dll1 coculture. Thus, these findings suggest that Notch signaling through Mib1 plays a critical role in the generation of hematopoietic progenitors in both the endothelium and SAPs.
MATERIALS AND METHODS
Mice.
Mib1−/− (26) and Mib1f/f (28) mice were maintained in the POSTECH animal facility. C57BL/6, Tie2-cre (Tek-cre), and ROSA26R mice were purchased from Jackson Laboratories. Transgenic Notch reporter (TNR) mice were a kind gift from N. Gaiano (11). All mouse lines were maintained in specific-pathogen-free conditions at the POSTECH animal facility under institutional guidelines.
P-Sp explant culture.
The P-Sp explant culture was performed as described previously, with a minor modification (53). In brief, embryos were dissected from pregnant females at 9.0 to 9.5 days postcoitum. By convention, the morning the vaginal plug was detected was defined as E0.5. Mib1 genotyping was confirmed by LacZ staining in the yolk sac (YS) and genomic PCR, as described previously (26). P-Sp explants were seeded on OP9-green fluorescent protein (OP9-GFP), OP9-Dll1, or OP9-Jag1 stromal cells in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 10−2 mM 2-mercaptoethanol, 50 ng/ml stem cell factor (SCF; Peprotech), and 5 ng/ml interleukin-3 (IL-3; R&D Systems). To induce T-cell or B-cell lineage differentiation, 5 ng/ml IL-7 (R&D Systems) was added to the medium.
Direct CFC assay.
The P-Sps from E9.5 littermate control and Mib1−/− embryos were digested in 0.1% collagenase (Sigma) in phosphate-buffered saline (PBS) that was supplemented with 10% FBS, 5 ng/ml IL-3, and 50 ng/ml SCF for 1 h at 37°C. The cells (2 × 105 for the control embryos) or whole-cell suspensions (Mib1−/− embryos) were plated in 3 ml methylcellulose (Stem Cell Technologies) supplemented with 10% FBS, 10 ng/ml IL-3, 100 ng/ml SCF, 0.1% monothioglycerol (Sigma), 2 U/ml erythropoietin (R&D Systems), 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech), and 100 ng/ml G-CSF (Peprotech). Colony types were scored at day 7 by morphological appearance and by the Wright-Giemsa staining of each colony. The other colony-forming cell (CFC) assay was performed as follows. Fresh total cells from the YS or cells that had been recovered by gentle pipetting from the P-Sp organ culture were used for the CFC assay. Cells from 1 embryo equivalent of a P-Sp organ culture or 1 × 105 cells from the YS were seeded in methylcellulose supplemented with 100 ng/ml SCF, 10 ng/ml IL-3, and 2 U/ml erythropoietin.
Flow cytometry analysis.
For surface staining, cell suspensions from the P-Sp, YS, or P-Sp culture were incubated on ice in the presence of various mixtures of labeled antibodies. Anti-CD11b, anti-Ter119, anti-CD45, anti-B220, anti-T-cell receptor β (anti-TCR-β), anti-vascular-endothelial (VE) cadherin, and anti-c-Kit antibodies (Becton Dickinson) were used to detect the various hematopoietic cell lineages and the hemogenic endothelial cells. Flow cytometry analysis was performed in a FACSCalibur with the CELLQUEST program (Becton Dickinson).
OP9 stromal cell lines expressing Notch ligands.
Murine stem cell virus (MSCV)-Dll1-OP9 cells were generated by transducing OP9 cells with an MSCV-puro retroviral vector engineered to express the Dll1 gene. OP9-GFP and OP9-MigR1-Dll1 cells were generously provided by J. C. Zuniga-Pflucker. OP9-Jag1 cells were kindly provided by M. J. Bevan. OP9-GFP, OP9-Dll1, and OP9-Jag1 cells were cultured as monolayers in the OP9 medium (alpha minimum essential medium supplemented with 20% FBS [HyClone]).
Immunohistochemistry.
Wild-type embryos were fixed in 4% paraformaldehyde overnight at 4°C and embedded in optimal-cutting-temperature compound for sectioning (thickness, 15 μm). Frozen sections were immunostained with rat anti-CD31 (BD Biosciences), goat anti-Dll1 (Santa Cruz Biotechnology), rat anti-VE-cadherin (BD Biosciences), rabbit anti-tyrosine hydroxylase (anti-TH; Cell Signaling Technology), monoclonal anti-GATA3 (Santa Cruz Biotechnology), and anti-CD41 (BD Biosciences) antibodies. For GFP detection, TNR embryos were fixed in 4% paraformaldehyde for 2 h at 4°C. Frozen sections were immunostained with rabbit anti-GFP (Molecular Probe) and other antibodies. For immunostaining after 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining, frozen sections were soaked in X-Gal staining buffer overnight at 37°C, fixed, and embedded for sectioning. Sections were incubated with primary antibodies overnight at 4°C.
Fluorescent in situ hybridization.
Fluorescent in situ hybridization was performed as described previously, with a slight modification (46). In brief, wild-type embryos (E10.5) were frozen in optimal-cutting-temperature compound, and 15-μm sections were fixed in 4% paraformaldehyde for 10 min. After an incubation with 3% H2O2 in PBS, the sections were treated with 0.2 M HCl and digested with proteinase K (Roche). Acetylated slides were prehybridized for 1 h and then hybridized overnight at 65°C with digoxigenin-tagged probes and fluorescein-tagged probes. Anti-digoxigenin-peroxidase and anti-fluorescein-peroxidase antibodies (Roche) were used at 1:500 in blocking reagent (Roche). Slides were developed using the tyramide amplification system (TSA-plus cyanine3/fluorescein system; PerkinElmer). Probe information for the Notch ligands and Mib1 can be provided on request.
siRNA inhibition of Mib1 and luciferase assay.
For small interfering RNA (siRNA)-mediated silencing, we used SMART-pool mouse MIB1 siRNA and the siCONTROL Nontargeting siRNA pool (Dharmacon, Inc.). These siRNA duplexes were electroporated into OP9-Dll1 cells using a Microporator apparatus and buffers recommended by the manufacturer (Digital Bio). Thirty-six hours after electroporation (to allow siRNA silencing), Western blot analyses, luciferase assays, and P-Sp cultures were done. For the CBF-luciferase (CBF-Luc) assay, the 8× wild-type or mutant CBF-Luc vectors were transfected into C2C12-Notch1 cells with pRL-TK vector using Lipofectamine (Invitrogen). Luciferase activities were measured with a dual-luciferase kit (Promega).
Image acquisition.
Images were acquired with a Fluoview1000 confocal microscope (Olympus) for embryonic sections. Images from liquid cultures and colony images from the CFC assay were acquired with an Axiovert 200 M microscope (Carl Zeiss) using an AxioCam MRC camera (Carl Zeiss) and the MRGrab1.0 (Carl Zeiss) software.
RESULTS
Early hematopoiesis in the YS is preserved, but intraembryonic hematopoiesis is impaired, in Mib1-deficient mice.
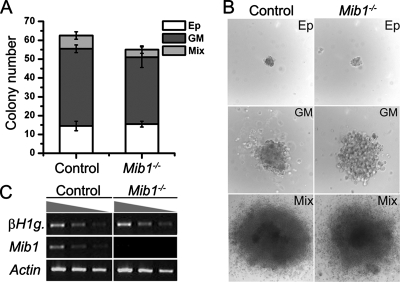
To examine the role of Mib1 in early hematopoiesis, we investigated the CFC activity in the YS of littermate control and Mib1−/− embryos at E8.0 to approximately E8.5. The numbers and sizes of blood cell colonies derived from the Mib1−/− YS were very similar to those from the control YS (Fig. 1A, B). Furthermore, the expression of βH1-globin, the marker of the primitive erythroid lineage that is dominant in the YS (42), was slightly higher in the Mib1−/− YS than that in the control YS (Fig. 1C).
FIG. 1.
Normal hematopoiesis in the YS of Mib1−/− embryos. Cells freshly prepared from littermate control or Mib1−/− YS at E8.0 to ∼8.5 (colonies per 1 × 105 cells) were cultured in semisolid medium for 7 days. (A) The colony number and type from the CFC assay were evaluated. Bars indicate means ± standard deviations of CFCs obtained from each YS in three independent experiments. Ep, primitive erythroid colony; GM, colony; Mix, mixed colony. (B) Representative colony morphologies from the CFC assay are shown. Magnification, ×100. (C) The expression levels of βH1-globin (βH1g.) and Mib1 were analyzed by semiquantitative RT-PCR of the YS cells from control and Mib1−/− embryos at E8.0.
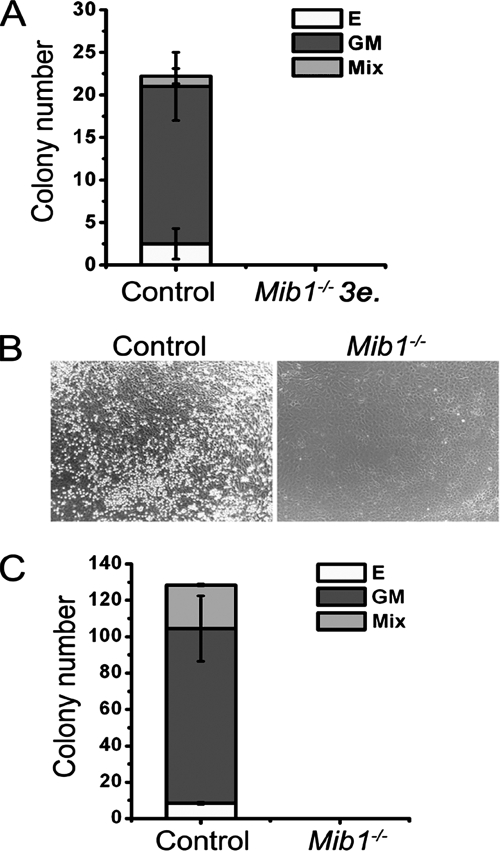
In order to test whether intraembryonic hematopoiesis occurs in the Mib1−/− mice, we performed direct hematopoietic colony assays with fresh cells obtained from the P-Sp of the E9.5 littermate control and Mib1−/− embryos, because Mib1-deficient embryos died between E10.5 and ∼E11.5 (26). Hematopoietic CFCs of the different lineages were generated in cell cultures from the control embryos, whereas no colonies were obtained when cells from three Mib1−/− P-Sps were incubated under the same conditions (Fig. 2A). To exclude the possibility that the defective colony-forming activity in the Mib1−/− P-Sp resulted from the very low numbers of HSCs, we cultured the P-Sp explants on the OP9 stromal cells to expand the number of progenitors. Control P-Sp explants produced round-shaped and nonadherent cells on the OP9 stromal cells (Fig. 2B, left), and nonadherent cells formed hematopoietic colonies in a semisolid culture (Fig. 2C). These nonadherent cells expressed only β-major globin (adult globin), not βH1-globin (embryonic globin) (see Fig. S1 in the supplemental material), and various hematopoietic lineage markers such as myeloid, erythroid, and B-lymphoid markers (see Fig. S2 in the supplemental material), indicating that they were not derived from primitive hematopoietic cells. In contrast, Mib1−/− P-Sp explants failed to develop any hematopoietic cells or hematopoietic colonies under the same culture conditions (Fig. 2B, right, and C). Taken together, these results demonstrate that Mib1 is required for the generation of hematopoietic progenitors in the P-Sp/AGM region but not for hematopoiesis in the YS.
FIG. 2.
Defective hematopoiesis in the P-Sp region of Mib1−/− embryos. (A) Direct CFC assay using fresh P-Sp cells from E9.5 littermate control and Mib1−/− embryos. Bars indicate means ± standard deviations of CFCs obtained from one control embryo and a pool of three Mib1−/− embryos (3e.) in three independent experiments. (B) P-Sp explants from E9.5 control or Mib1−/− embryos were cultured on OP9 cells for 7 days. Magnification, ×100. (C) CFC activity of the cells recovered from the P-Sp explant culture. Bars indicate means ± standard deviations of CFCs obtained from the P-Sp culture in four independent experiments. E, erythroid colony; GM, GM colony; Mix, mixed colony.
Presence of cells displaying phenotypes of hemogenic endothelial cells in Mib1−/− embryos.
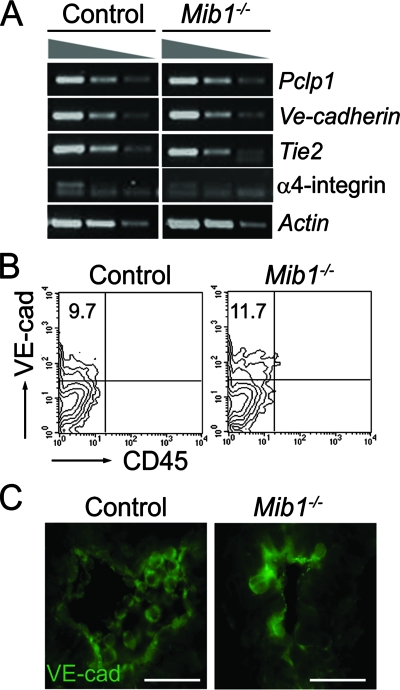
The existence of hemogenic endothelial cells/hemangioblasts as the cellular origin of HSCs in the embryo proper has been suggested (6, 9, 13, 22, 43). To determine whether hemogenic endothelial cells were present in the Mib1−/− embryos, we assessed the expression of hemogenic endothelial cell markers in the P-Sp/AGM region (19, 35, 41, 47, 52). As expected, there was no apparent difference in the expression levels between the littermate control and Mib1−/− P-Sp (Fig. 3A), suggesting that hemogenic endothelial cells exist in the Mib1−/− embryos.
FIG. 3.
Hemogenic endothelial cells in the Mib1−/− embryos. (A) The expression of surface markers of hemogenic endothelial cells was analyzed by semiquantitative RT-PCR to compare the expression levels of the P-Sp of E9.5 control embryos to those of Mib1−/− embryos. (B) Cells from the P-Sp were analyzed by flow cytometry for the expression of VE-cadherin and CD45 gated on the Ter119− populations. A representative from three independent experiments is shown, and the percentages of cells in the upper left quadrant are indicated. (C) Immunostaining with an anti-VE-cadherin antibody on a transverse section of the aorta from E9.5 control and Mib1−/− embryos. The orientation of the aorta is dorsal (up) to ventral (down). Magnification, ×400; scale bars, 50 μm.
It was previously reported that the hemogenic activity is retained in the VE-cadherin+/CD45−/Ter119− cells in both the YS and P-Sp (39). The proportion of VE-cadherin+/CD45−/Ter119− cells in the P-Sp of the Mib1−/− embryos was similar to that of the control embryos (Fig. 3B). Consistently, the expression of VE-cadherin was detected in the fused aorta of the Mib1−/− embryos, although the morphology of the aorta was disorganized (Fig. 3C). These results indicate that while Mib1 is dispensable for the generation of hemogenic endothelial cells/hemangioblasts, it is required for the specification of hematopoietic progenitors from the precursors.
Preserved intraembryonic hematopoiesis in embryos lacking Mib1 in Tie2-positive cells.
It was previously suggested that the floor of the aorta is the presumptive hemogenic site that supports intraembryonic HSC generation (7, 14, 24, 40). To test whether Mib1 activity in the endothelium is necessary for intraembryonic hematopoiesis, we used Tie2-cre; Mib1f/f mice, in which Mib1 is inactivated in the endothelium (28). In order to first examine whether Notch signaling is abrogated in the aortic endothelium of Tie2-cre; Mib1f/f embryos, we generated Tie2-cre; Mib1f/f embryos possessing a Notch reporter transgene by crossing Tie2-cre; Mib1+/f to TNR mice, which express GFP in cells upon Notch/CBF1 activation (11). In E9.5 Tie2-cre; Mib1+/f; TNR embryos, we readily observed GFP signals in the PECAM-positive endothelial cells of the dorsal aorta but not in the Tie2-cre; Mib1f/f; TNR embryos, showing the abrogation of Notch signaling in the endothelium (see Fig. S3 in the supplemental material). Thus, this model provides a valuable genetic model to assess the contribution of Notch signaling in the aortic endothelium for intraembryonic hematopoiesis.
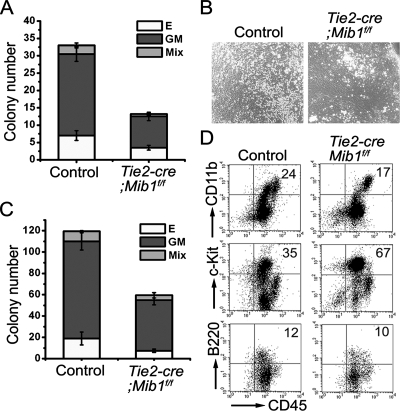
Even though the Notch ligands in the aortic endothelium were not functional in the Tie2-cre; Mib1f/f embryos, the E9.5 P-Sp of the Tie2-cre; Mib1f/f embryos still generated hematopoietic colonies of various lineages in a direct hematopoietic colony assay, although the number of colonies was decreased (Fig. 4A). Consistently, when the P-Sps from the Tie2-cre; Mib1f/f embryos were cultured on OP9 cells, hematopoietic cells were readily generated, but their numbers were reduced compared to those of the control embryos (Fig. 4B). The nonadherent cells from the P-Sp cultures of the control and Tie2-cre; Mib1f/f embryos formed hematopoietic colonies in semisolid medium (Fig. 4C). Furthermore, a flow cytometric analysis of the nonadherent cells from Tie2-cre; Mib1f/f P-Sp cultures revealed that they express various hematopoietic cell markers, such as CD45, c-Kit, CD11b, and B220 (Fig. 4D), indicating the presence of multipotent hematopoietic progenitors in the Tie2-cre; Mib1f/f P-Sp. Most hematopoietic cells from the Tie2-cre; Mib1f/f P-Sp culture had the floxed mib1 allele, indicating that they are not derived from Tie2-positive endothelial cells (data not shown). These results suggest that distinct resources other than the aortic endothelium provide Notch signaling through Mib1 to support the generation of hematopoietic progenitors in the P-Sp/AGM region.
FIG. 4.
Preserved intraembryonic hematopoiesis in the Tie2-cre; Mib1f/f embryos. (A) Direct CFC assay using fresh P-Sp cells from E9.5 littermate control and Tie2-cre; Mib1f/f embryos. Bars represent means ± standard deviations of CFC activities obtained from control and Tie2-cre; Mib1f/f P-Sps in three independent experiments. E, erythroid colony; GM, GM colony; Mix, mixed colony. (B) P-Sp explants from E9.5 control and Tie2-cre; Mibf/f embryos were cultured on OP9 cells for 10 days. Magnification, ×100. (C) The CFC activity of cells from the Tie2-cre; Mib1f/f P-Sp explant culture (in colonies per cultured cells from P-Sp) was reduced compared to that from control P-Sp. Bars indicate the means ± standard deviations of CFCs obtained from the P-Sp culture in three independent experiments. (D) Nonadherent cells were harvested at day 12 and were analyzed for the surface expression of CD45, CD11b, c-Kit, and B220. Note that the nonadherent cells from the Tie2-cre; Mib1f/f P-Sp explants express various hematopoietic cell surface markers. Representative results from three independent experiments are presented, and the percentages of cells in the upper right quadrant are indicated.
Expression of Dll1 in the SAPs.
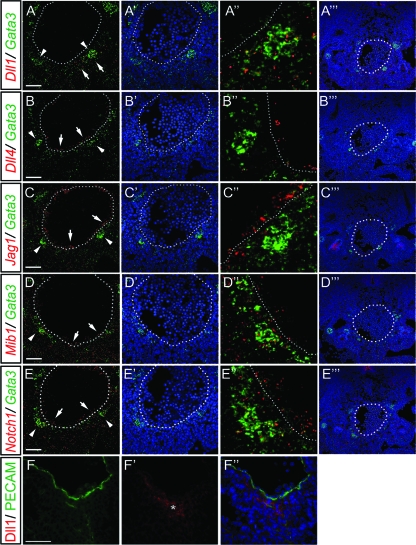
Recently, the SAPs located below the aortic floor have been suggested as another site for intraembryonic HSC generation (2). In the E10.5 Tie2-cre; ROSA26R mice, which express β-galactosidase in cells that have Cre recombinase activity, β-galactosidase activity was observed in the aortic endothelium but not in the SAPs (see Fig. S1 in the supplemental material). Therefore, we speculated that the SAPs are the other site that supports intraembryonic hematopoiesis in the Tie2-cre; Mib1f/f P-Sp. We first examined whether the SAPs exist in the E9.5 Tie2-cre; Mib1f/f embryos. As reported previously (34), the homogeneous signal of GATA3, which is regarded as the presumptive SAP, was detected in the mesenchyme under the dorsal aorta of wild-type and Tie2-cre; Mib1f/f embryos (see Fig. S4 in the supplemental material). To further examine, by double in situ hybridization, whether Mib1 and Notch ligands are expressed in the SAPs, we first marked the SAPs by the expression of GATA3 and TH. While the TH-positive and GATA3-positive sympathetic ganglia were located dorsolaterally from the aorta, the TH-negative and GATA3-positive SAPs were located ventrally from the aorta, as reported previously (34) (see Fig. S6 in the supplemental material). As expected, Mib1 was expressed in both the aortic endothelium and Gata3-expressing SAPs of the E10.5 AGM (Fig. 5D to D″), although it showed broad expression patterns.
FIG. 5.
Notch ligand expression in the AGM. (A to E‴) Fluorescent double in situ hybridization of Gata3 (in green) with either Dll1 (A), Dll4 (B), Jag1 (C), Mib1 (D), or Notch1 (E) (in red) probes on transverse sections from E10.5 wild-type AGM. (A to E) Merged images of the green and red fluorescent signals. (A′ to E′) Merged images of the green and Hoechst signals. (A″ to E″) High-magnification views of panels A to E. (A‴ to E‴) Low-magnification views of merged images of the green, red, and Hoechst signals of panels A to E. (A″)Dll1 is expressed in the mesenchyme ventral to the aorta, which was intermingled with the Gata3 transcripts. (B) Dll4 is detected mainly in the lining of the aorta, presumably the endothelium (arrow) but not in the SAPs (arrowhead). (C) Jag1 is detected in the lining of the aorta and mesenchyme (arrow) but not in the SAPs (arrowhead). (D) Mib1 is detected ubiquitously, including in aorta and SAPs (arrow). (E) Notch1 is detected in the endothelium and the SAPs (arrow). Dotted lines indicate the lining of the aorta. Arrowheads indicate the SAPs. The orientation of the aorta is dorsal (up) to ventral (down). (F to F″) Immunostaining with anti-CD31 (green) and anti-Dll1 (red) antibodies on a transverse section from E10.5 wild-type AGM. The asterisk shows the Dll1-positive patch. Scale bars, 50 μm.
We speculated that Dll1, Dll4, and/or Jag1 of the five mammalian Notch ligands are important for intraembryonic hematopoiesis, because the loss of genes that are critical for embryonic hematopoiesis causes fetal death in embryonic stages (10, 20, 54). Dll4 was expressed predominantly in the aortic endothelium but not in the SAPs, as reported previously (46) (Fig. 5B to B″). Jag1 was expressed in the aortic endothelium and mesenchyme but not in the SAPs (Fig. 5C to C″). Interestingly, Dll1 transcripts were observed in the mesenchymal cells ventral to the aorta, which were intermingled with the Gata3-expressing cells (Fig. 5A to A″). Notch1 transcripts were observed in both the aortic endothelium and SAPs (Fig. 5E to E″). The control sense probe showed no specific signal (data not shown). Immunostaining with Dll1 and CD31 antibodies revealed that Dll1 was expressed in the mesenchyme beneath the aorta but not in the CD31-positive endothelial cells lining the aorta (Fig. 5F to F″). Some Dll1-expressing cells resided around the CD31-positive region below the aorta. Since CD31 is one of the markers that characterize the SAPs (2), this confirms the expression of Dll1 in the SAPs. Taking these results together, Dll1, Mib1, and Notch1 were expressed in the SAPs, while Dll4/Jag1, Mib1, and Notch1 were expressed in the aortic endothelium, suggesting that Dll1 and Dll4/Jag1 are responsible Notch ligands in the SAPs and endothelium, respectively, for intraembryonic hematopoiesis.
Notch activation in the aortic endothelium and the SAPs.
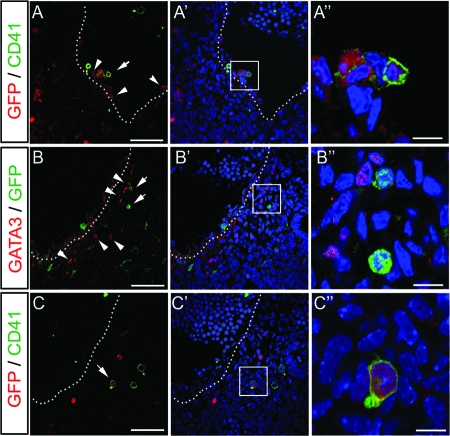
The SAPs harbor long-term reconstituting HSCs that express c-Kit, CD31, and CD41 but not CD45 (2). CD41 recently was found to be the earliest known surface marker of nascent HSCs that distinguishes them from the endothelial lineage during embryogenesis (5, 12, 37). To examine whether Notch signaling is activated in the dorsal aorta and the SAPs, we used E10.5 TNR mice (11). As expected, GFP was expressed in the endothelial cells and the budding cells from the ventral wall of the aorta (Fig. 6A; also see Fig. S7 in the supplemental material). In hematopoietic intra-aortic clusters, GFP was detected in some of the CD41-positive cells, suggesting the involvement of Notch signaling in HSC generation (Fig. 6A to A″; also see Fig. S7 in the supplemental material). Interestingly, GFP-positive cells were found near the GATA3-expressing cells ventral to the dorsal aorta (Fig. 6B to B″; also see Fig. S8 in the supplemental material). In the sequential section, some of the GFP-positive cells coexpressed CD41 in the presumptive SAPs, showing that Notch signaling is active in the nascent HSCs within the SAPs (Fig. 6C to C″; also see Fig. S8 in the supplemental material). The number of GFP and CD41 double-positive cells in the endothelium and mesenchyme was counted throughout the sections of the rostral half of the AGM (see Fig. S7 in the supplemental material), and the ratio of double-positive cells in the endothelium (13.3 ± 3.2)/mesenchyme (4.7 ± 1.2) was 3:1. These results suggest that Notch signaling is involved in the generation of intraembryonic hematopoietic progenitors both in the aortic floor and within the SAPs.
FIG. 6.
Notch signaling in the AGM. (A to A″) Immunostaining with anti-CD41 (in green) and anti-GFP (in red) antibodies on transverse sections from E10.5 TNR AGM. Notch reporter activity in the TNR AGM is observed in endothelial cells and hematopoietic cells budding from the aorta wall (in red; arrowheads). In hematopoietic clusters of the aorta, Notch signaling is activated in some CD41-positive cells (arrow). (B to B″) Immunostaining with anti-GFP (in green) and anti-GATA3 (in red) antibodies on a transverse section from an E10.5 TNR embryo. GFP-positive cells (arrows) are located near the GATA3-expessing cells (arrowheads). (C to C″) Immunostaining with anti-CD41 (in green) and anti-GFP (in red) antibodies on a section sequential to that of panel B. Dotted lines indicate the lining of the aorta. (A″ to C″) High-magnification views of each of the small square regions of panels A′ to C′. Scale bars for panels A to C, 50 μm; scale bars for panels A″ to C″, 10 μm.
Hematopoietic activity of Mib1−/− P-Sp is restored by OP9 cells expressing Dll1.
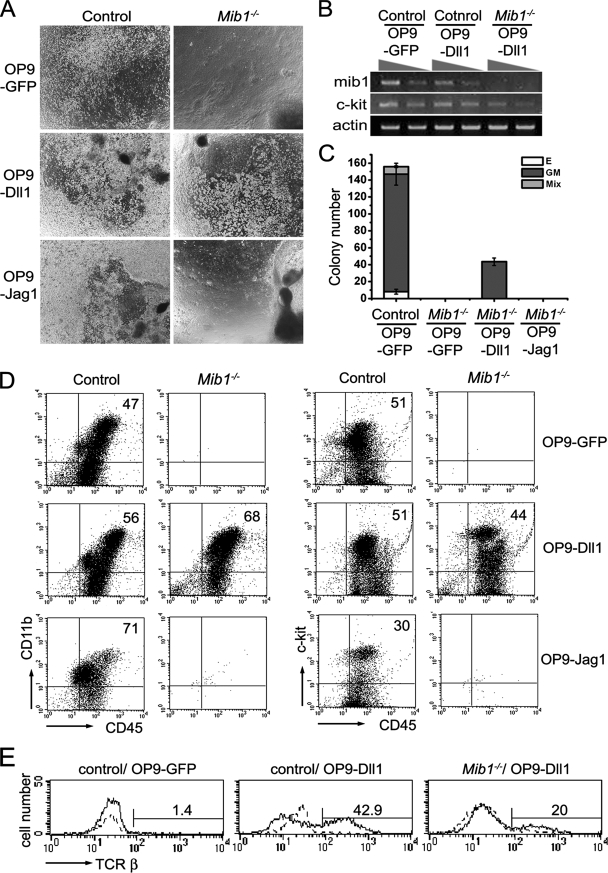
Based on our observations, we sought to test whether Dll1, the ligand expressed in the SAPs, could restore the hematopoietic activity in the Mib1−/− P-Sp explant culture on OP9 cells. The OP9 cells endogenously expressed Jag1 and Mib1 but not other Notch ligands (see Fig. S9 in the supplemental material). Since the OP9 cells did not support hematopoietic activity in the Mib1−/− P-Sp explant culture, the Jag1 endogenously expressed in the OP9 cells might be insufficient to activate Notch signaling in the Mib1−/− P-Sp to produce hematopoietic cells. Thus, the Mib1−/− P-Sp/OP9 culture system overexpressing Notch ligands will provide an excellent model to identify a responsible Notch ligand for the generation of intraembryonic hematopoietic progenitors in the P-Sp/AGM.
To test whether Dll1 restores the hematopoietic activity of Mib1−/− P-Sp, we generated OP9 cells overexpressing the full-length cDNA for murine Dll1 using MSCV-puro vector (OP9-MSCV-Dll1). A high level of expression of Dll1 in the OP9-MSCV-Dll1 cells was detected through reverse transcription-PCR (RT-PCR) and Western blot analyses, although the expression level was lower than that in the other Dll1-expressing OP9 cell line (OP9-MigR1-Dll1) (49) (see Fig. S10 in the supplemental material). We confirmed that Dll1 in both OP9-MSCV-Dll1 and OP9-MigR1-Dll1 readily triggers Notch signals in the neighboring C2C12 cells that express Notch1 receptors (C2C12-Notch1) by using luciferase reporter assay (see Fig. S10 in the supplemental material). OP9 cells separately expressing GFP (OP9-GFP) and Jag1 (OP9-Jag1) were used as a negative control and a Jag1-overexpressing control, respectively. While the OP9-GFP and OP9-Jag1 stromal cells did not support the hematopoietic activity in the Mib1−/− P-Sp explant culture, the OP9-MSCV-Dll1 cells restored the defective hematopoietic activity in the Mib1−/− P-Sp ( Fig. 7A). Although the restoration was incomplete compared to that of the control P-Sp, the OP9-MSCV-Dll1 coculture readily generated numerous small, round, and nonadherent cells from the Mib1−/− P-Sp. The repopulated hematopoietic cells from the Mib1−/− explant culture on the OP9-MSCV-Dll1 cells expressed the c-kit (hematopoietic stem cell marker) transcript but not the Mib1 transcript (Fig. 7B), indicating that the rescued hematopoiesis is not due to contamination by wild-type cells.
FIG. 7.
Restored hematopoietic activity of Mib1−/− P-Sp by OP9 cells expressing Dll1. (A) P-Sp explants from littermate control and Mib1−/− embryos were cultured on OP9-GFP, OP9-MSCV-Dll1 (OP9-Dll1), or OP9-Jag1 stromal cells for 10 days in the presence of SCF and IL-3. The white clumps are hematopoietic cells, and the dark background is the OP9 stromal cells. Black bodies are P-Sp explants. Magnification, ×40. (B) The expression levels of Mib1 and c-kit were analyzed by RT-PCR in the cells recovered from the Mib1−/− P-Sp by the OP9-MSCV-Dll1 cell cocultures. (C) The nonadherent cells from the P-Sp cocultures were harvested at day 12 and plated into the semisolid medium. Bars indicate the means ± standard deviations of CFCs obtained from the P-Sp culture in three independent experiments. E, erythroid colony; GM, GM colony; Mix, mixed colony. (D) The nonadherent cells were harvested at day 12 and were analyzed for the surface expression of CD45, CD11b, and c-Kit. Representative results from three independent experiments are presented, and the percentages of cells in the upper right quadrant are indicated. (E) P-Sp explants from control and Mib1−/− embryos were cultured on OP9-GFP and OP9-MSCV-Dll1 cells in the presence of IL-7, SCF, and IL-3. The nonadherent cells were harvested at day 16 and were analyzed for the surface expression of TCR-β. The profiles indicated by the dotted lines represent cells stained without primary antibody. Percentages reflect cells considered positive.
On day 10 of culture, the nonadherent cells were collected and seeded into a semisolid medium for the CFC assay. These cells generated GM colonies (Fig. 7C). Even though erythroid and mixed colonies were not produced in the rescued cell populations, the existence of GM colonies suggests that hematopoietic cells were generated. In addition, the flow cytometric analysis revealed that the repopulated cells expressed hematopoietic cell surface markers, such as CD45, CD11b, and c-Kit (Fig. 7D), although their expression profiles were slightly different between the control and Mib1−/− P-Sp explants. In addition, the Mib1−/− P-Sp produced hematopoietic cells expressing TCR-β (around 20%) on OP9-MSCV-Dll1 cells in the presence of IL-7 (Fig. 7E), indicating the presence of lymphopoietic precursors. OP9-MigR1-Dll1 also rescued the hematopoietic defect in the Mib1−/− P-Sp culture, as did OP9-MSCV-Dll1 cells, although the differentiation pattern of hematopoietic cells was a little different (Fig. 8D; also see Fig. S10 in the supplemental material), which might be due to differences in the expression level of Dll1. Taken together, these results show that Dll1 can readily send Notch signals to produce multipotent hematopoietic progenitors.
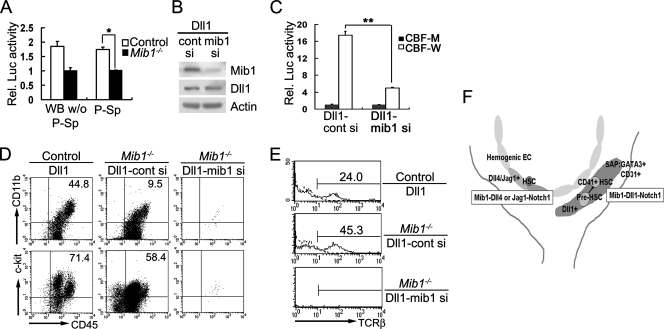
FIG. 8.
Mib1 functions for Notch signaling in the P-Sp and OP9 stromal cells. (A) Defective Notch triggering in the Mib1−/− P-Sp. Cells from littermate control (white bar) and Mib1−/− (black bar) embryos were cocultured with C2C12-Notch1 cells transfected with 8× CBF-Luc and Renilla luciferase vectors. Forty-eight hours after coculture, luciferase activities were measured and normalized to Renilla luciferase activity. Data are presented as the change (n-fold) in the induction of luciferase activity relative to that of C2C12-Notch1 alone (means ± standard deviations are given). *, P < 0.002. WB w/o P-Sp, whole body without P-Sp. (B) Immunoblot of Mib1 protein in OP9-MigR1-Dll1 (Dll1) cells 36 h after microporation with control (cont si) or Mib1 (mib1 si) siRNA. Dll1 expression was not affected. (C) OP9-MigR1-Dll1 cells treated with control (cont si) or Mib1 (mib1 si) siRNA were cocultured with C2C12-Notch1 cells transfected with 8× wild-type CBF-Luc (CBF-W) or mutant CBF-Luc (CBF-M) vector. Twenty-four hours after coculture, luciferase activities were measured. **, P < 0.001. (D and E) P-Sp explants from control and Mib1−/− embryos were cultured on OP9-MigR1-Dll1 (Dll1) cells treated with either control siRNA (cont si) or Mib1 siRNA (mib1 si) in the presence of IL-7 for 12 (D) and 20 (E) days. The nonadherent cells were analyzed for the surface expression of CD45, CD11b, and c-Kit (D) and TCR-β (E). (D) Representative results from three independent experiments are presented, and the percentages of cells in the upper right quadrant are indicated. (E) The profiles indicated by the dotted lines represent cells stained without primary antibody, and percentages reflect cells considered positive. (F) Model for Mib1 function in the generation of hematopoietic progenitors of the aortic endothelium and SAPs. Mib1 is ubiquitously expressed in endothelial cells and mesenchymal cells within the P-Sp/AGM. Although Mib1 is widely expressed, Notch ligands such as Dll1, Dll4, and Jag1 are distinctively expressed. In the aortic endothelium, Mib1 regulates Dll4/Jag1 and activates Notch signaling for the specification of HSCs from hemogenic endothelium. At the SAPs, Mib1 regulates Dll1 to activate Notch signaling in pre-HSCs (the presumptive hematopoietic stem cells). Notch signaling induces the commitment to the CD41-positive nascent HSCs.
Mib1 regulates Notch signaling in the P-Sp and OP9 stromal cells.
To evaluate the Mib1 function for Notch signaling in the P-Sp, primary cells dissociated from P-Sps were cocultured with C2C12-Notch1 cells transfected with a CBF-Luc vector carrying RBP-Jκ binding sites. Control cells readily activated Notch signaling in the C2C12-Notch1 cells, as expected. In contrast, Mib1−/− cells cannot trigger Notch signals in the C2C12-Notch1 cells (Fig. 8A), indicating that Mib1 is required for Notch signaling in the P-Sps.
To further examine whether Mib1 regulates Dll1 function in the OP9-Dll1 cells, we transfected Mib1 siRNA duplexes into the OP9-MigR1-Dll1 cells. Mib1 protein was significantly reduced 36 h after microporation in the OP9-MigR1-Dll1 cells treated with Mib1 siRNA (Fig. 8B). When C2C12-Notch1 cells were cocultured with the OP9-MigR1-Dll1 cells treated with Mib1 siRNA, CBF-Luc reporter activity was markedly reduced compared to that of control siRNA-treated cells (Fig. 8C), indicating that Mib1 is required for Notch signaling through regulating Dll1 function.
Moreover, on the OP9-MigR1-Dll1 cells treated with control siRNA, hematopoietic cells were produced from Mib1−/− P-Sp explants at day 10, while on the OP9-MigR1-Dll1 cells treated with Mib1 siRNA, hematopoietic cells were hardly detectable (Fig. 8D, E). Taken together, these results demonstrate that Mib1 functionally regulates Notch ligands in both the P-Sp of the embryo and OP9 stromal cells for the generation of hematopoietic progenitors.
DISCUSSION
We have shown that Mib1 is essential for the generation of intraembryonic hematopoietic progenitors in both the endothelium and SAPs of the P-Sp/AGM region, and we suggest a novel mechanism by which the Mib1-Dll1-Notch1 pathway regulates intraembryonic hematopoiesis in the SAPs of the P-Sp/AGM region.
In intraembryonic hematopoiesis, two distinctive sites, the floor of the aorta and the SAPs of P-Sp/AGM, have been suggested as the origins of HSC emergence (15, 23). Based on our current and previous studies, Mib1 was expressed in the aortic endothelium of E10.5 AGM and could bind to Dll4, Jag1, and Jag2, which are the Notch ligands expressed in the aortic endothelium of the AGM (26, 46). We previously reported that Tie2-cre; Mib1f/f embryos have defects in arterial cell fate determination, thus showing that Mib1 actually regulates the function of Dll4 in the aortic endothelium (28). In this study, we found that Notch activity was abrogated in the dorsal aorta of Tie2-cre; Mib1f/f embryos. Intriguingly, the hematopoietic activity in the Tie2-cre; Mib1f/f P-Sp culture was always significantly lower than that of the control P-Sp culture, suggesting the loss of Notch signaling in the endothelium that contributes to intraembryonic hematopoiesis. Indeed, we found that Notch signaling is activated in the budding cells and the hematopoietic clusters of the ventral aorta through the TNR mouse analysis, although we could not clarify whether the hematopoietic cells in the endothelium are generated from the hemogenic endothelium or migrated from the SAPs. Collectively, we suggest that Mib1 regulates the generation of hematopoietic progenitors in the aortic endothelium, possibly through Dll4 and/or Jag1 [Mib1-Dll4(Jag1)-Notch1 in the aortic endothelium].
Recently, several studies of SAPs, another hemogenic site, have generated evidence that the SAPs have hematopoietic potential (2). However, the signaling molecules, such as the members of the Notch signaling pathway, involved in HSC generation at the site of SAPs have not been identified. In this study, we found that the hematopoietic activity was reduced but retained in the Tie2-cre; Mib1f/f P-Sp. Since Cre-mediated excision efficiently occurs in the dorsal aorta of Tie2-cre mice, as reported previously (33), and Notch activity was completely abrogated in E9.5 Tie2-cre; Mib1f/f embryos, the retained hematopoietic activity might not result from the incomplete excision of mib1 in the endothelium. Furthermore, the defective hematopoietic activity of E9.5 Mib1−/− P-Sp was rescued by OP9-Dll1, indicating that the temporal window of the Mib1 requirement for the development of HSCs succeeds Tie2 expression. This is different from that of stem cell leukemia, which is necessary for hematopoietic development prior to Tie2 expression (48). Since Cre activity via the Tie2 promoter was not detected in the SAPs, we speculated that Notch signaling through Mib1 is required for the generation of hematopoietic progenitors in the SAPs. Similarly to our observation, a recent study showed the incomplete blockage of hematopoietic activity in Tie2-cre; Runx1f/f embryos, which also suggested the role of SAPs as a reason for the preserved hematopoietic activity (33). Since Runx1 is a well-known downstream mediator of Notch signaling in intraembryonic hematopoiesis (4, 38), this strengthens the possibility that Notch signaling is involved in the generation of intraembryonic hematopoietic progenitors in the SAPs. Indeed, Notch signaling components, such as Dll1, Mib1, and Notch1, were expressed in the SAPs, and Notch signaling reporter activity was observed in the SAPs. Importantly, Notch signaling activity was detected in the CD41-positive nascent HSCs within the SAPs, suggesting the role of Notch signaling in the generation of hematopoietic progenitors in the SAPs. This is consistent with the recent report that the appearance of CD41-positive cells is dependent on Notch signaling in zebrafish (25). Finally, we demonstrated the hematopoietic role of Dll1 in SAPs through the coculture of OP9-Dll1 cells with Mib1−/− P-Sp. These data suggest that Mib1 regulates Dll1 in the SAPs to generate the hematopoietic progenitor [Mib1-Dll1-(Notch1) in the SAPs].
We found that the impaired hematopoietic activities in the Mib1−/− P-Sp culture were rescued by overexpressing Dll1 on the OP9 cells, but we could not verify whether transplantable HSCs were generated. Previously, it was reported that the cultured P-Sp cells on the OP9 cells cannot reconstitute mouse bone marrow, even when injected into busulfan-pretreated new-born mice (36, 38). In this study, in spite of alternative transplantation trials using the short-term cultured P-Sp cells in Rag1−/− mice, we could not detect any engraftment of the cultured P-Sp cells, even in the wild type (data not shown). Although we could not detect the transplantable long-term HSCs because of the limitations of the OP9/P-Sp culture model, the OP9-Dll1 cells generated multipotent hematopoietic progenitors from the Mib1−/− P-Sp, which could differentiate into myeloid as well as lymphoid lineages.
When the Mib1−/− P-Sp was cultured on the OP9-Dll1 cells, the repopulated cells developed mostly into GM colonies but not erythroid and mixed colonies. Mixed colonies represent the existence of multipotent progenitors. Since the rescued cell populations from Mib1−/− P-Sp via the OP9-Dll1 coculture were much smaller than those of the control P-Sp, mixed colonies might not be found due to the minute number of precursors. However, flow cytometric analyses showed that myeloid and lymphoid cells, two representative lineages, were rescued by the OP9-Dll1 cells, indicating that multipotent progenitors were generated. From the aspect of an erythroid colony, Dll1 reportedly inhibited erythroid maturation (51). Actually, we also found very few erythroid colonies when the control P-Sp was cultured on the OP9-Dll1 cells (see Fig. S10 in the supplemental material). The inhibition of erythroid colony formation appeared to be dependent on the expression level of Dll1 in the OP9 stromal cells. Therefore, it is possible that the OP9-Dll1 cells affect the differentiation of erythroid progenitors, which results in the absence of erythroid colonies.
Recently, de Pooter and colleagues reported that Dll1 inhibited myelopoiesis from embryonic stem cells and hematopoietic progenitors (8). In this study, we used two independent lines, OP9-MSCV-Dll1 (generated by ourselves) and OP9-MigR1-Dll1 (49), as Dll1-overexpressing stromal cells for the P-Sp coculture. Although both cell lines restored the defective hematopoiesis of Mib1−/− P-Sp, it seems that their effects on myeloid development from hematopoietic progenitors were different. This may be due to the different levels of Dll1 expression in these cell lines. Indeed, the OP9-MigR1-Dll1 cells were more potent to activate CBF1-luciferase constructs than the OP9-MSCV-Dll1 cells. In our culture system using OP9-MigR1-Dll1 cells, however, myeloid development from P-Sp was not strongly suppressed compared to that from HSCs of fetal liver (data not shown). This might be due to the difference between P-Sp and isolated stem cells as the hematopoietic sources or different culture conditions. How Dll1 affects the differentiation of HSCs originating from P-Sp remains to be determined.
Our study suggests a model in which Mib1 is important for the Notch signaling of intraembryonic hematopoiesis in both the aortic endothelium and SAPs of P-Sp/AGM (Fig. 8F). In the floor of the aorta, Mib1 may regulate Dll4 or Jag1 in the endothelium, which would activate Notch1 signaling in the hemogenic endothelial cells to generate HSCs. At the SAPs, Mib1 may regulate Dll1, which would activate Notch signaling in unknown mesenchymal precursors and convert them to CD41-positive nascent HSCs. Further studies of various genetic models using conditional Mib1 knockout mice, as well as detailed studies of the intraembryonic hematopoiesis of Notch ligand mutants, will be helpful to clarify the cell-to-cell interactions and ligand-receptor interactions controlling intraembryonic hematopoiesis.
Supplementary Material
Acknowledgments
This work was supported by grants from the Vascular System Research Center of KOSEF.
We are grateful to J. C. Zuniga-Pflucker for the OP9-GFP and OP9-Dll1 cells and M. J. Bevan for OP9-Jag1 cells. We thank Isabelle Godin for providing the Gata3 probe.
Footnotes
Published ahead of print on 27 May 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284770-776. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand, J. Y., S. Giroux, R. Golub, M. Klaine, A. Jalil, L. Boucontet, I. Godin, and A. Cumano. 2005. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc. Natl. Acad. Sci. USA 102134-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J. R. Doedens, A. Cumano, P. Roux, R. A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell 5207-216. [DOI] [PubMed] [Google Scholar]
- 4.Burns, C. E., D. Traver, E. Mayhall, J. L. Shepard, and L. I. Zon. 2005. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 192331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbel, C., and J. Salaun. 2002. AlphaIIb integrin expression during development of the murine hemopoietic system. Dev. Biol. 243301-311. [DOI] [PubMed] [Google Scholar]
- 6.de Bruijn, M. F., X. Ma, C. Robin, K. Ottersbach, M. J. Sanchez, and E. Dzierzak. 2002. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16673-683. [DOI] [PubMed] [Google Scholar]
- 7.de Bruijn, M. F., N. A. Speck, M. C. Peeters, and E. Dzierzak. 2000. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 192465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Pooter, R. F., T. M. Schmitt, J. L. de la Pompa, Y. Fujiwara, S. H. Orkin, and J. C. Zuniga-Pflucker. 2006. Notch signaling requires GATA-2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J. Immunol. 1765267-5275. [DOI] [PubMed] [Google Scholar]
- 9.Dieterlen-Lièvre, F., and A. Cumano. 1998. Cellular and molecular events that govern the development of the hematopoietic and immune system in the embryo. Dev. Comp. Immunol. 22249-252. [DOI] [PubMed] [Google Scholar]
- 10.Duarte, A., M. Hirashima, R. Benedito, A. Trindade, P. Diniz, E. Bekman, L. Costa, D. Henrique, and J. Rossant. 2004. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 182474-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan, A. W., F. M. Rattis, L. N. DiMascio, K. L. Congdon, G. Pazianos, C. Zhao, K. Yoon, J. M. Cook, K. Willert, N. Gaiano, and T. Reya. 2005. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6314-322. [DOI] [PubMed] [Google Scholar]
- 12.Ferkowicz, M. J., M. Starr, X. Xie, W. Li, S. A. Johnson, W. C. Shelley, P. R. Morrison, and M. C. Yoder. 2003. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 1304393-4403. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, S. T., M. Ogawa, T. Yokomizo, Y. Ito, and S. Nishikawa. 2003. Putative intermediate precursor between hematogenic endothelial cells and blood cells in the developing embryo. Dev. Growth Differ. 4563-75. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Porrero, J. A., I. E. Godin, and F. Dieterlen-Lievre. 1995. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. (Berlin) 192425-435. [DOI] [PubMed] [Google Scholar]
- 15.Godin, I., and A. Cumano. 2002. The hare and the tortoise: an embryonic haematopoietic race. Nat. Rev. Immunol. 2593-604. [DOI] [PubMed] [Google Scholar]
- 16.Godin, I., and A. Cumano. 2005. Of birds and mice: hematopoietic stem cell development. Int. J. Dev. Biol. 49251-257. [DOI] [PubMed] [Google Scholar]
- 17.Godin, I., J. A. Garcia-Porrero, F. Dieterlen-Lievre, and A. Cumano. 1999. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J. Exp. Med. 19043-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadland, B. K., S. S. Huppert, J. Kanungo, Y. Xue, R. Jiang, T. Gridley, R. A. Conlon, A. M. Cheng, R. Kopan, and G. D. Longmore. 2004. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 1043097-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara, T., Y. Nakano, M. Tanaka, K. Tamura, T. Sekiguchi, K. Minehata, N. G. Copeland, N. A. Jenkins, M. Okabe, H. Kogo, Y. Mukouyama, and A. Miyajima. 1999. Identification of podocalyxin-like protein 1 as a novel cell surface marker for hemangioblasts in the murine aorta-gonad-mesonephros region. Immunity 11567-578. [DOI] [PubMed] [Google Scholar]
- 20.Hrabĕ de Angelis, M., J. McIntyre II, and A. Gossler. 1997. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386717-721. [DOI] [PubMed] [Google Scholar]
- 21.Itoh, M., C. H. Kim, G. Palardy, T. Oda, Y. J. Jiang, D. Maust, S. Y. Yeo, K. Lorick, G. J. Wright, L. Ariza-McNaughton, A. M. Weissman, J. Lewis, S. C. Chandrasekharappa, and A. B. Chitnis. 2003. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell 467-82. [DOI] [PubMed] [Google Scholar]
- 22.Jaffredo, T., R. Gautier, A. Eichmann, and F. Dieterlen-Lievre. 1998. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 1254575-4583. [DOI] [PubMed] [Google Scholar]
- 23.Jaffredo, T., W. Nottingham, K. Liddiard, K. Bollerot, C. Pouget, and M. de Bruijn. 2005. From hemangioblast to hematopoietic stem cell: an endothelial connection? Exp. Hematol. 331029-1040. [DOI] [PubMed] [Google Scholar]
- 24.Jordan, H. E. 1917. Aortic cell clusters in vertebrate embryos. Proc. Natl. Acad. Sci. USA 3149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kissa, K., E. Murayama, A. Zapata, A. Cortes, E. Perret, C. Machu, and P. Herbomel. 2008. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood 1111147-1156. [DOI] [PubMed] [Google Scholar]
- 26.Koo, B. K., H. S. Lim, R. Song, M. J. Yoon, K. J. Yoon, J. S. Moon, Y. W. Kim, M. C. Kwon, K. W. Yoo, M. P. Kong, J. Lee, A. B. Chitnis, C. H. Kim, and Y. Y. Kong. 2005. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development 1323459-3470. [DOI] [PubMed] [Google Scholar]
- 27.Koo, B. K., K. J. Yoon, K. W. Yoo, H. S. Lim, R. Song, J. H. So, C. H. Kim, and Y. Y. Kong. 2005. Mind bomb-2 is an E3 ligase for Notch ligand. J. Biol. Chem. 28022335-22342. [DOI] [PubMed] [Google Scholar]
- 28.Koo, B. K., M. J. Yoon, K. J. Yoon, S. K. Im, Y. Y. Kim, C. H. Kim, P. G. Suh, Y. N. Jan, and Y. Y. Kong. 2007. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS ONE 2e1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumano, K., S. Chiba, A. Kunisato, M. Sata, T. Saito, E. Nakagami-Yamaguchi, T. Yamaguchi, S. Masuda, K. Shimizu, T. Takahashi, S. Ogawa, Y. Hamada, and H. Hirai. 2003. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18699-711. [DOI] [PubMed] [Google Scholar]
- 30.Lai, E. C., G. A. Deblandre, C. Kintner, and G. M. Rubin. 2001. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1783-794. [DOI] [PubMed] [Google Scholar]
- 31.Le Borgne, R., A. Bardin, and F. Schweisguth. 2005. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development 1321751-1762. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., and N. E. Baker. 2004. The roles of cis-inactivation by Notch ligands and of neuralized during eye and bristle patterning in Drosophila. BMC Dev. Biol. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Z., M. J. Chen, T. Stacy, and N. A. Speck. 2006. Runx1 function in hematopoiesis is required in cells that express Tek. Blood 107106-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manaia, A., V. Lemarchandel, M. Klaine, I. Max-Audit, P. Romeo, F. Dieterlen-Lievre, and I. Godin. 2000. Lmo2 and GATA-3 associated expression in intraembryonic hemogenic sites. Development 127643-653. [DOI] [PubMed] [Google Scholar]
- 35.Marshall, C. J., R. L. Moore, P. Thorogood, P. M. Brickell, C. Kinnon, and A. J. Thrasher. 1999. Detailed characterization of the human aorta-gonad-mesonephros region reveals morphological polarity resembling a hematopoietic stromal layer. Dev. Dyn. 215139-147. [DOI] [PubMed] [Google Scholar]
- 36.Matsuoka, S., K. Tsuji, H. Hisakawa, M. Xu, Y. Ebihara, T. Ishii, D. Sugiyama, A. Manabe, R. Tanaka, Y. Ikeda, S. Asano, and T. Nakahata. 2001. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood 986-12. [DOI] [PubMed] [Google Scholar]
- 37.Mikkola, H. K., Y. Fujiwara, T. M. Schlaeger, D. Traver, and S. H. Orkin. 2003. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101508-516. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa, M., M. Ichikawa, K. Kumano, S. Goyama, M. Kawazu, T. Asai, S. Ogawa, M. Kurokawa, and S. Chiba. 2006. AML1/Runx1 rescues Notch1-Null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood 1083329-3334 [DOI] [PubMed] [Google Scholar]
- 39.Nishikawa, S. I., S. Nishikawa, H. Kawamoto, H. Yoshida, M. Kizumoto, H. Kataoka, and Y. Katsura. 1998. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8761-769. [DOI] [PubMed] [Google Scholar]
- 40.North, T. E., M. F. de Bruijn, T. Stacy, L. Talebian, E. Lind, C. Robin, M. Binder, E. Dzierzak, and N. A. Speck. 2002. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16661-672. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa, M., M. Kizumoto, S. Nishikawa, T. Fujimoto, H. Kodama, and S. I. Nishikawa. 1999. Expression of alpha4-integrin defines the earliest precursor of hematopoietic cell lineage diverged from endothelial cells. Blood 931168-1177. [PubMed] [Google Scholar]
- 42.Palis, J., S. Robertson, M. Kennedy, C. Wall, and G. Keller. 1999. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 1265073-5084. [DOI] [PubMed] [Google Scholar]
- 43.Palis, J., and M. C. Yoder. 2001. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29927-936. [DOI] [PubMed] [Google Scholar]
- 44.Pavlopoulos, E., C. Pitsouli, K. M. Klueg, M. A. Muskavitch, N. K. Moschonas, and C. Delidakis. 2001. Neuralized encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell 1807-816. [DOI] [PubMed] [Google Scholar]
- 45.Petrenko, O., A. Beavis, M. Klaine, R. Kittappa, I. Godin, and I. R. Lemischka. 1999. The molecular characterization of the fetal stem cell marker AA4. Immunity 10691-700. [DOI] [PubMed] [Google Scholar]
- 46.Robert-Moreno, A., L. Espinosa, J. L. de la Pompa, and A. Bigas. 2005. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intraembryonic hematopoietic cells. Development 1321117-1126. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez, M. J., A. Holmes, C. Miles, and E. Dzierzak. 1996. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity 5513-525. [DOI] [PubMed] [Google Scholar]
- 48.Schlaeger, T. M., H. K. Mikkola, C. Gekas, H. B. Helgadottir, and S. H. Orkin. 2005. Tie2Cre-mediated gene ablation defines the stem-cell leukemia gene (SCL/tal1)-dependent window during hematopoietic stem-cell development. Blood 1053871-3874. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt, T. M., and J. C. Zuniga-Pflucker. 2002. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17749-756. [DOI] [PubMed] [Google Scholar]
- 50.Song, R., B. K. Koo, K. J. Yoon, M. J. Yoon, K. W. Yoo, H. T. Kim, H. J. Oh, Y. Y. Kim, J. K. Han, C. H. Kim, and Y. Y. Kong. 2006. Neuralized-2 regulates a Notch ligand in cooperation with Mind bomb-1. J. Biol. Chem. 28136391-36400. [DOI] [PubMed] [Google Scholar]
- 51.Tachikawa, Y., T. Matsushima, Y. Abe, S. Sakano, M. Yamamoto, J. Nishimura, H. Nawata, R. Takayanagi, and K. Muta. 2006. Pivotal role of Notch signaling in regulation of erythroid maturation and proliferation. Eur. J. Haematol. 77273-281. [DOI] [PubMed] [Google Scholar]
- 52.Takakura, N., X. L. Huang, T. Naruse, I. Hamaguchi, D. J. Dumont, G. D. Yancopoulos, and T. Suda. 1998. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 9677-686. [DOI] [PubMed] [Google Scholar]
- 53.Takakura, N., T. Watanabe, S. Suenobu, Y. Yamada, T. Noda, Y. Ito, M. Satake, and T. Suda. 2000. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102199-209. [DOI] [PubMed] [Google Scholar]
- 54.Xue, Y., X. Gao, C. E. Lindsell, C. R. Norton, B. Chang, C. Hicks, M. Gendron-Maguire, E. B. Rand, G. Weinmaster, and T. Gridley. 1999. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 8723-730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.