Abstract
Drosophila innate immunity is controlled primarily by the activation of IMD (immune deficiency) or Toll signaling leading to the production of antimicrobial peptides (AMPs). IMD signaling also activates the JUN N-terminal kinase (JNK) cascade, which is responsible for immune induction of non-antimicrobial peptide immune gene transcription though the transcription factor AP-1. Transcription of the Dopa decarboxylase (Ddc) gene is induced in response to gram-negative and gram-positive septic injury, but not aseptic wounding. Transcription is induced throughout the epidermis and not specifically at the site of infection. Ddc transcripts are detectible within 2 h and remain high for several hours following infection with either gram-negative or gram-positive bacteria. Using Ddc-green fluorescent protein (GFP) reporter gene constructs, we show that a conserved consensus AP-1 binding site upstream of the Ddc transcription start site is required for induction. However, neither the Toll, IMD, nor JNK pathway is involved. Rather, Ddc transcription depends on a previously uncharacterized member of the p38 mitogen-activated protein kinase family, p38c. We propose that the involvement of DDC in a new pathway involved in Drosophila immunity increases the levels of dopamine, which is metabolized to produce reactive quinones that exert an antimicrobial effect on invading bacteria.
Most animal species exhibit the ability to ward off infection. While mammals employ a combination of adaptive and innate immune responses, the majority of organisms use only innate immunity to combat infection. The genetic and molecular techniques available for Drosophila melanogaster have made it an exceptional model for innate immune research (31). Drosophila immunity combines humoral and cellular responses to effect a strong resistance to many microorganisms. The three mechanisms contributing to this resistance are phagocytosis of invading organisms by hemocytes; blood clotting, melanin formation, and opsonization; and transient synthesis of antimicrobial peptides (AMPs) at both the wound site and in the fat body.
Drosophila lives in decaying and fermenting matter and therefore is exposed to a multitude of bacteria, fungi, and viruses that it must defend itself against. The ability to combat natural and septic infections by gram-negative and gram-positive bacteria has been the focus of much study in the last several years. The work has centered on the humoral defense response, involving the synthesis and release of AMPs from the fat body into the hemolymph. Transcription of different but overlapping sets of approximately 20 AMPs (32, 43) is elicited by gram-negative bacteria upon activation of the IMD (immune deficiency) pathway (12, 44, 46) and gram-positive bacteria and fungi following induction of the Toll pathway (45, 68). Each pathway acts through its respective NF-κB transcription factor(s); the Toll pathway uses Dorsal and DIF (33, 50, 55, 61, 66), while the IMD pathway activates Relish (19, 28).
Immune activation of the IMD pathway also causes the induction of the mitogen-activated protein kinase (MAPK) cascade, known as the JUN N-terminal kinase (JNK) pathway, due to a bifurcation at the transforming growth factor (TGF)-activated kinase, TAK1 (9, 73, 80). In Drosophila, the MAPK kinase (MAPKK), Hemipterous (HEP) (23), activates the JNK, Basket (BSK) (9), which can then phosphorylate the heterodimeric transcription factor AP-1 (40, 64), which is composed of a FOS subunit and a JUN subunit (59, 87). Phosphorylation activates AP-1 and causes induction of JNK response genes. JNK signaling is required for proper wound healing in the adult epidermis (21, 63) and the wing disc (8). JNK signaling also activates the transcription of many proteins involved in cytoskeleton remodeling (9), consistent with its role in hemocyte activation in the cellular immune response (86). A role for JNK signaling in AMP gene expression has also been proposed (18, 35); however, further research is needed since JNK repression of AMP synthesis has also been proposed (38).
In addition to the JNK pathway, two other conserved MAPK pathways have potential roles in Drosophila immunity and wound healing. The first, the extracellular signal-regulated kinase (ERK) pathway, has been implicated in the Drosophila embryonic wound response (49). The second is the p38 MAPK family, which has two characterized members (p38a and p38b) that have been implicated in attenuation of the immune response (27). p38 MAPKs are activated by dual phosphorylation by a MAPKK at a characteristic TGY site (51). This site differs from the dual-phosphorylation TPY and TEY motifs of JNK and ERK, respectively.
The primary layer of defense against infection involves the barrier epithelia of the epidermis, including the hypoderm that lies beneath the cuticle, and the epithelial tissues of the trachea and gut. The barrier epithelia are responsible for localized production of AMPs at the site of septic injury (20, 79). While formation of a melanin clot at the wound site is not specifically an immune response, it is essential to prevent hemolymph loss following septic injury. An additional benefit is that the rapid production of the melanized clot entraps bacteria and promotes killing. The melanin produced at the clot site is a direct consequence of the catalytic activation of the zymogen prophenol-oxidase (5), that is stored in the crystal cells (53, 65), to produce active phenol oxidase (PO). PO uses tyrosine to produce the quinones that react nonenzymatically to form melanin (53, 54). Clots form without the action of PO (5, 71); however, PO is required for clot hardening (5, 36). An additional potential role for melanin production at the wound site involves the formation of reactive oxygen species and by-products of melanin production that have cytotoxic antimicrobial properties (58). Interestingly, the JNK cascade is necessary for crystal cell rupture following injury, which leads to melanization at the wound site (4).
In addition to the role of PO in wound healing, two other genes, pale (ple) and Dopa decarboxylase (Ddc) (83), have been shown to be essential to wound healing in the Drosophila embryo (49). These genes encode the enzymes tyrosine hydroxylase (TH), which converts tyrosine to dihydroxyphenylalanine (Dopa), and Dopa decarboxylase (DDC), which converts Dopa to dopamine (DA). The neural role of DA is well established; however, DA is also metabolized to produce melanin and sclerotin in epidermal tissues (2, 83), which leads to tanning of the pupal case and adult cuticle and pigmentation of the pharate adult epidermis (81). The transcription factor Grainy-head is responsible for Ddc induction in the embryonic wound response, and it acts through a binding site just upstream of the transcription start site (49). However, deletion of three AP-1 sites and a CREB (an ERK-inducible transcription factor) binding site upstream of Ddc also eliminates expression of Ddc during the wound response, indicating a potential role for the JNK or ERK pathways in this induction. Consistent with the role of a MAPK in Ddc transcriptional induction, injection of the MAPK inhibitor PD98059 into embryos reduced the induction of a Ddc-GFP reporter after aseptic injury.
Evidence suggests that Ddc plays a role in the Drosophila innate immune response in larvae and adults. Defense against the parasitic wasp Leptopilina boulardii requires the production of a melanized capsule that envelops the egg and eventually kills it. Melanotic encapsulation requires the actions of specialized blood cells called lamellocytes (53, 54). Nappi et al. (57) showed that the melanization response was severely compromised in Ddcts2 flies, demonstrating the need for functional DDC in the encapsulation response. Ddc transcription was induced in larvae of Tenebrio molitor infected with Escherichia coli (39), and this corresponded to increased DDC protein and activity levels. In addition, N-β-alanine (NBAD) synthase, an enzyme that converts DA to the sclerotin precursor, NBAD, is induced in the epidermis of T. molitor and Ceratitis capitata following E. coli infection (70). NBAD has potent antimicrobial effects, which can be eliminated by the addition of antioxidants, suggesting that NBAD elicits its killing effects because of spontaneous conversion to cytotoxic quinones. Together, these results suggest a role for DA metabolism in the insect innate immune response. Consistent with this prediction, microarrays performed on flies and SL2 cell culture have identified Ddc as one of the genes induced after both gram-positive and gram-negative bacterial infection, but not fungal infection (9, 17, 34).
In this paper, we investigate the role of Ddc in the Drosophila innate immune response to bacterial infection. We find that Ddc is induced upon septic injury, but not aseptic wounding of larvae and adults, and this response is dependent on a conserved AP-1 consensus binding site upstream of the Ddc transcription start site. However, JNK signaling is not responsible for induction of Ddc transcription; rather, an uncharacterized p38 MAPK, p38c, is responsible. Induction of Ddc occurs throughout the epidermis and is not restricted to the wound site. We propose that DDC activity is required in the epidermis for the synthesis of DA that is metabolized to produce reactive quinones that exert killing effects on invading bacteria.
MATERIALS AND METHODS
Fly stocks, crosses, and infection of organisms.
The stocks used in this study, their sources, and their purposes are shown in Table S1 in the supplemental material (47). All stocks were maintained on a standard cornmeal/molasses medium at room temperature. To obtain Ddcts2 cn/rdo hk Ddcn7 pr cn and Ddcts2cn/Df(2L)130 pr cn flies, Ddcts2 cn/CyO female virgins were crossed to rdo hk Ddcn7 pr cn/CyO or Df(2L)130 pr cn/CyO males, respectively. The progeny were raised at the 18°C permissive temperature. Mutants were identified by their wild-type wings, separated from their curly-winged wild-type siblings, and transferred to the 25°C restrictive temperature for 4 days prior to infection.
The following P-element excision scheme was undertaken to obtain mutants and revertants of p38cKG05834, a strain carrying a P-element marked with a mini-w+ gene: (i) w; p38cKG05834/TM3, Sb ♀s × w/Y; Sb e Δ2-3/TM6, Ubx e ♂s → (ii) yw; PSal89D, Sb/TM6, Ubx e ♀s × w/Y; p38cKG05834/Sb e Δ2-3 ♂s (red eyed) → (iii) yw; PSal89D, Sb/TM6, Ubx e ♀s × yw/Y; p38c*/TM6, Ubx e ♂s (* indicates a P-element excision, identified by the presence of white eyes) → (iv) yw; p38c*/TM6, Ubx e ♀s × yw/Y; p38c*/TM6, Ubx e ♂s → (v) homozygous or balanced stock.
The region of p38c spanning the P-element excision, from the homozygous P-element excision flies, was PCR amplified, cloned into pGEM-T Easy (Promega), and sequenced.
All larvae that were infected were in the mid-third instar, during which time no epidermal Ddc transcription is detectible (M. M. Davis, unpublished observation). Adults that were infected were between 4 and 6 days old, a time when epidermal Ddc transcripts are at minimal levels (14). At least 10 adults and 30 larvae of each genotype were septically injured with a sharpened tungsten needle that was dipped into the pellet obtained from a saturated culture of E. coli DH5α or Staphylococcus aureus ATCC 6538-P. For heat shock experiments, organisms were heat shocked for 1 h at 37°C and allowed to recover at room temperature for 1 h before being septically injured. Adults were injured by being poked in the thorax immediately below the wing, and larvae were wounded in the posterior end. Adults were allowed to recover in a vial containing standard Drosophila medium, and larvae recovered on a piece of sterile water-soaked Whatman paper in a petri dish to prevent desiccation after injury. With the exception of the time course shown in Fig. 5, organisms were allowed to recover for 4 to 6 h following infection. Only the organisms that were alive at harvesting were frozen for subsequent use.
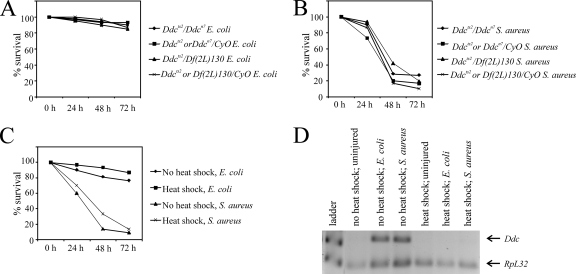
FIG. 5.
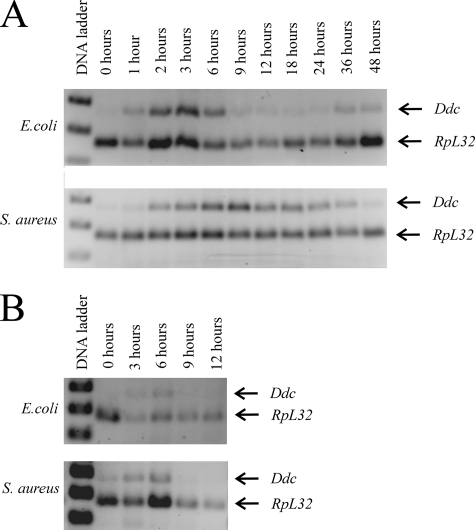
Induction of Ddc transcription in adults occurs shortly after septic injury with live (A) or heat-killed (B) E. coli or S. aureus.
Plasmid construction and the creation of transgenic lines.
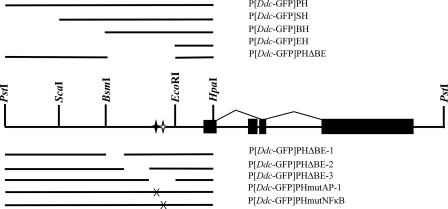
The P-element plasmids P[Ddc-GFP]PH, P[Ddc-GFP]SH, P[Ddc-GFP]BH, and P[Ddc-GFP]EH (Fig. 1) have been described elsewhere (15). The P[Ddc]PHΔBE, P[Ddc]PHΔBE1, P[Ddc]PHΔBE2, P[Ddc]PHΔBE3, P[Ddc]PHmutNFκB, and P[Ddc]PHmutAP-1 constructs in pBluescript SK(+) were all created by inverse PCR using phosphorylated primer pairs (see Table S2 in the supplemental material, which lists the PCR primers and oligonucleotides used in this study) ΔBE3-F and ΔBE1-R, ΔBE1-F and ΔBE1-R, ΔBE2-F and ΔBE2-R, ΔBE3-F and ΔBE3-R, NFκB-F and NFκB-R, and AP1-F and AP1-R, respectively, on PH in pBluescriptSK(+). The resulting products were gel purified and ligated intramolecularly to create the respective plasmids. The breakpoints of the deletions and site specific mutations were all confirmed by sequencing. The Ddc region contained within each plasmid was then liberated by digestion with BamHI and KpnI and cloned into similarly digested pGreen Pelican (3) to create P[Ddc-GFP]PHΔBE, P[Ddc-GFP]BE-1, P[Ddc-GFP]BE-2, P[Ddc-GFP]BE-3, P[Ddc-GFP]mutNFκB, and P[Ddc-GFP]mutAP-1 (Fig. 1).
FIG. 1.
Schematic diagram of the P[Ddc-GFP] reporter constructs used in this study. The epidermis-specific splice pattern shown above the line is situated on the 7.6-kb PstI restriction fragment that is sufficient for proper developmental expression of Ddc. The second exon is nerve specific. The sequence that drives GFP expression is depicted in the lines above and below the diagram of the Ddc genomic region. Relevant restriction enzyme sites, used to create the larger deletions, are shown. The black and white stars represent the conserved AP-1 and NF-κB sites, respectively; X indicates an induced mutation. The P[Ddc-GFP]PHmutNFκB construct contains a 5-bp mutation in the putative NF-κB binding site, and the P[Ddc-GFP]PHmutAP-1 construct contains a 5-bp mutation in the putative AP-1 site.
To create the P[Ddc-RNAi]pSymp and P[Ddc-RNAi]pWIZ constructs, a 490-bp region of the Ddc coding sequence within the 4th exon was amplified with the DDC-NotIF and DDC-NotIR and DDC-XbaIF and DDC-XbaIR primer pairs. The DDC-NotI primers add NotI sites to facilitate cloning into pSymp (22), and the DDC-XbaI primers add XbaI sites to facilitate cloning into pWIZ (42). Both pSymp and pWIZ express the cloned region under the control of a UAS promoter, facilitating Gal4 induction of the transgene. The PCR fragments were gel purified and cloned into pGEM-T Easy (Promega) to create Ddc-NotI in pGEM-T Easy and Ddc-XbaI in pGEM-T Easy. The Ddc fragment was then liberated from Ddc-NotI in pGEM-T Easy by NotI digestion and cloned into similarly digested pSymp to create P[Ddc-RNAi]pSymp. To create P[Ddc-RNAi]pWIZ, the XbaI fragment from Ddc-XbaI in pGEM-T Easy was first cloned into the AvrII site of pWIZ and then cloned again into the NdeI site of the resulting plasmid. Both plasmids were sequenced to confirm the orientation of the Ddc fragment inserted into each site.
DNA solutions (0.4 μg/μl DNA in 0.5× phosphate-buffered saline and 10% glycerol) containing transgenic constructs were injected into embryos of the genotype w; Sb e Δ2-3/TM6, Ubx e. Surviving adults were crossed to yw flies, and progeny with the w+ phenotype were collected and mated to produce homozygous or balanced stocks.
RNA extraction and RT-PCR.
RNA was extracted from pools of organisms using TRIzol, treated with amplification-grade DNase I (Invitrogen) according to manufacturer's instructions, and then extracted with phenol-chloroform and then chloroform. Semiquantitative Ddc and Ddc-GFP reverse transcription PCRs (RT-PCRs) were performed as detailed elsewhere (14, 15). Synthesis of p38c-specific cDNA was initiated using p38c-1 (see Table S2 in the supplemental material) on 150 ng of isolated RNA. The first-strand reaction was co-reverse transcribed with RPL-32-1, a primer specific for the ribosomal gene RpL32, which served as a loading control. A 3-μl aliquot of the resulting cDNA mixture was combined with p38c forward and reverse primers (p38c-2F and p38c-2R) and amplified for six cycles. The program was then stopped, RpL32 gene-specific primers (RPL32-F and RPL32-R) were added, and the program was allowed to continue for 23 more cycles. After an initial denaturing step of 2 min, the final PCR conditions for p38c consisted of 29 cycles of 95°C for 1 min, 60°C for 1 min, and 73°C for 1 min.
Under these conditions, the amount of each product was proportional to input RNA concentration (see Fig. S1 in the supplemental material). The band intensity was quantified using Image J (1). Using 150 ng of input RNA, the amount of Ddc and Ddc-GFP product was proportional to the number of cycles for 24 to 27 cycles, the amount of RpL32 product was proportional to the number of cycles for 22 to 25 cycles, and the amount of p38c product was proportional to the number of cycles for 27 to 30 cycles.
For the nonquantitative RT-PCR on the p38a and p38c transcripts (see Fig. 9B and C), greater than 1 μg of total RNA was reversed transcribed using Superscript II reverse transcriptase (Invitrogen) using the primers p38a-R and p38c-1, respectively. The cDNA was then used as a template for a PCR using the p38a-F and p38a-R primer pair for p38a and the p38c-2F and p38c-2R primer pair for p38c. The final PCR was 30 cycles of 95°C for 1 min, 60°C for 1 min, and 73°C for 1 min.
FIG. 9.
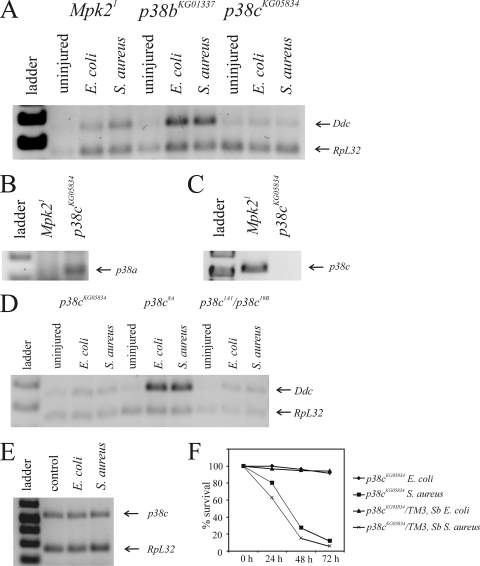
p38c MAPK is required for immune induction of Ddc. (A) Ddc RT-PCR on RNA obtained from uninjured and infected Mpk21, p38bKG01337, and p38cKG05834 adult flies. (B) p38a transcript analysis in Mpk21 and p38cKG05834 mutant flies. (C) p38c transcript analysis in Mpk21 and p38cKG05834 mutant flies. (D) Ddc RT-PCR on RNA obtained from uninjured and infected p38cKG05834, p38c8A (revertant of p38cKG05834), and p38c1A1/p38c19B. (E) p38c transcription is not immune inducible. (F) Survival of p38cKG05834homozygous flies and their p38cKG05834/TM3, Sb wild-type siblings following septic injury with E. coli and S. aureus.
Quantitative real-time PCR.
RNA (2 μg) was reverse transcribed with random primers (Applied Biosystems). The cDNA mixture was diluted 1:20, and a 2.5-μl aliquot was used as a template for quantitative real-time PCR using Ddc (DDC-RT1 and DDC-RT2)- or RpL32 (RPL32-RT1 and RPL32-RT2)-specific primer pairs and ABI Power Sybr green PCR master mix according to the manufacturer's instructions (Applied Biosystems). Applied Biosystems StepOne software was used for quantifying the transcripts.
Production of recombinant proteins.
The constructs made up of dFRA (Drosophila fos) and dJRA (Drosophila jun) in pBluescript KS(+) were a generous gift from Robert Tjian. These plasmids were used as templates for in vitro transcription and translation using the TNT T3 coupled reticulocyte lysate system (Promega) according to the manufacturer's instructions.
Electrophoretic mobility shift assays.
Mobility shift assays were carried out using a 26-bp double-stranded oligonucleotide probe with a 2-bp extension. The 28-base single-stranded oligonucleotides were individually end labeled with [γ-32P]ATP using T4 kinase (Invitrogen) according to the manufacturer's instructions and annealed. The wild-type probe was obtained by annealing the oligonucleotide pair AP1-A and AP1-B (see Table S2 in the supplemental material), and the probe containing the mutated AP-1 site was obtained by annealing AP1M-A and AP1M-B.
The recombinant proteins or reticulocyte lysate mix was incubated in binding buffer (20 mM HEPES-KOH [pH 7.9], 50 mM KCl, 4 mM MgCl2, 4 mM spermidine, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.05% NP-40 and 20% glycerol) in the presence of 2 μg of the nonspecific competitor poly(dI-dC) (Amersham) and specific cold competitor, where applicable, for 5 min at room temperature. Two nanograms of radiolabeled probe was added to the reaction mixture and allowed to bind at room temperature for 15 min. Binding mixtures were fractionated on a 6% polyacrylamide gel in 0.5× Tris-borate-EDTA running buffer, and the products were visualized by autoradiography.
RESULTS
Ddc transcription is induced in the Drosophila innate immune response.
Microarray studies of Drosophila immunity have identified Ddc as an immune-inducible gene (9, 17, 34). To confirm that Ddc transcription is induced by bacterial infection, we performed semiquantitative RT-PCR on uninjured, aseptically injured, and septically injured mid-third-instar larvae and adult flies, harvested 4 h after treatment (Fig. 2A). The Ddc forward and reverse PCR primers are anchored in the first and third exons of Ddc, respectively, and the PCR conditions are such that we never amplify the larger product that contains the nerve-specific second exon (Fig. 1). Transcription of Ddc is induced when larvae or adults are infected with E. coli (gram negative) or S. aureus (gram positive) and when organisms are aseptically injured with a large needle (data not shown); however, little Ddc transcript is detectible when organisms are aseptically injured with a small needle or left untreated (Fig. 2A). We find that if organisms are septically injured with a small needle, Ddc transcription is induced, whereas if that needle is used for aseptic injury, Ddc transcription is absent. We attribute Ddc induction following aseptic injury with a large needle to infection with natural flora on the Drosophila cuticle.
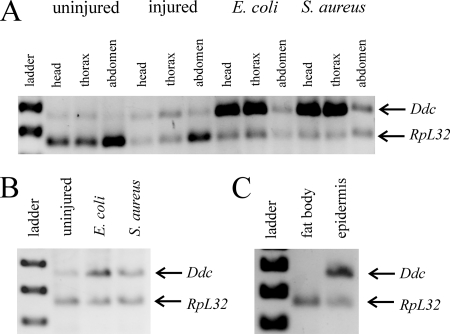
FIG. 2.
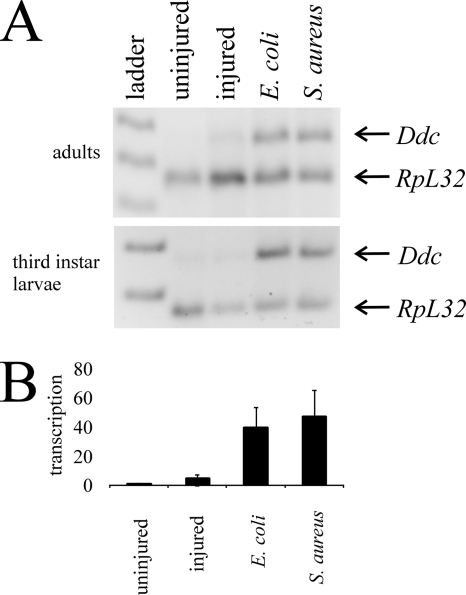
Immune induction of Ddc transcription following septic injury. (A) Semiquantitative RT-PCR was carried out on RNA obtained from adult flies and mid-third-instar larvae. Gene-specific transcripts in this and subsequent figures were separated on a 2% agarose gel and are shown with a co-reverse-transcribed and PCR-amplified RpL32 loading control. Transcripts from organisms septically injured with E. coli (lane 4) or S. aureus (lane 5) are compared to aseptically injured (lane 3) or uninjured (lane 2) organisms. In this and subsequent figures, the 1-kb Plus DNA ladder (Invitrogen) was used as a size standard. (B) Quantitative real-time PCR of Ddc transcripts in the same samples confirms that Ddc is induced following septic injury with E. coli and S. aureus, but not following aseptic injury.
To confirm that our method of semiquantitative RT-PCR was valid, we used real-time PCR to amplify the same samples examined in Fig. 2A. Similarly, we found that Ddc transcription was induced following septic injury with either E. coli or S. aureus, but Ddc transcript was not detected following aseptic injury or in control organisms (Fig. 2B). This confirms the validity of our semiquantitative RT-PCR analyses, which are presented in the remainder of this article.
Systemic Ddc transcription is localized to the epidermis.
Having confirmed that Ddc is induced in the immune response, we next set out to identify which tissues express Ddc. To do this, we aseptically and septically injured adult flies and 4 h later separated their heads, thoraxes, and abdomens for RNA isolations. We find that Ddc transcription is induced strongly in the head and thorax and less so in the abdomen of the flies that were septically injured with either E. coli or S. aureus (Fig. 3A). This induction was absent in flies that were uninjured or aseptically injured. This suggests that Ddc transcription is induced throughout the organism and is not restricted to the site of infection.
FIG. 3.
Ddc transcription is induced in the epidermis. (A) Semiquantitative RT-PCR from RNA isolated from heads, thoraxes, and abdomens of uninjured, aseptically injured, and septically injured adult flies. (B) RT-PCR of RNA isolated from mid-third-instar domK08108 larvae infected with E. coli or S. aureus. (C) RT-PCR on RNA isolated from fat body and epidermal tissues dissected from mid-third-instar larvae infected with E. coli or S. aureus.
Since Ddc transcription is induced throughout the adult organism, the three most likely locations of transcriptional induction are in the hemocytes, the fat body, or the epidermis. In order to determine if Ddc transcription is induced in the hemolymph, we examined the induction of Ddc transcription in larvae of domino mutants that fail to develop functional hemocytes (10). We were unable to assess Ddc induction in adult domK08108 flies, as these mutants die at pupariation when large numbers of hemocytes are necessary for phagocytosis at the onset of metamorphosis. However, we find that Ddc transcription is induced normally in domK08108 larvae (Fig. 3B, lanes 3 and 4) and that these mutants appear to have a high basal level of Ddc transcription (lane 2), likely due to chronic infection caused by the lack of hemocyte-derived cellular defenses.
To eliminate the possibility that the fat body cells could adhere to the epidermis preparations, we obtained epidermal tissue from larvae. At this stage, the fat bodies are much more defined than in adults (41) and were easily separated from the epidermis. Tissues were isolated by dissection from mid-third-instar larvae 4 h after septic injury, and semiquantitative RT-PCR was performed on the RNA obtained from these samples. The result clearly shows that Ddc transcription is induced in the epidermal tissues and not the fat body (Fig. 3C).
Reduction of DDC activity has no effect on adult survival following infection.
To determine if DDC activity within the epidermis is required for survival in the Drosophila immune response, we infected Ddcts2/Ddcn7 and Ddcts2/Df(2L)130 mutants with E. coli and S. aureus and tracked their survival for 3 days (Fig. 4). The Ddcn7 allele is a null mutation, and Df(2L)130 is a deficiency of the locus; both are embryonic lethal. The Ddcts2 mutation is a temperature-sensitive mutation, and Ddcts2/Df(2L)130 flies have 2 to 3% of the DDC activity detectible in wild-type flies (85). We find that there is no difference in the survival rates of Ddcts2/Ddcn7 (Fig. 4A) and Ddcts2/Df(2L)130 flies (Fig. 4B) compared to their wild-type siblings. Thus, we conclude that Ddc induction at 2 to 3% of normal has no effect on 3-day adult survival following infection with E. coli and S. aureus.
FIG. 4.
Reduction of DDC activity does not affect survival following septic injury. Survival of Ddc mutant flies and Ddc-RNAi-expressing flies after infection with E. coli or S. aureus was tracked for 3 days. (A and B) Survival of the straight-winged heteroallelic Ddc mutant combinations was compared to curly-winged wild-type sibling survival following septic injury with E. coli or S. aureus. (C) Survival of hs-Gal4; P[Ddc-RNAi]pWIZ flies following heat shock (1 h at 37°C, 1 h of recovery) and no heat shock (control) after septic injury with E. coli or S. aureus. (D) Semiquantitative RT-PCR shows that Ddc transcripts are degraded in flies following heat shock induction of the Ddc-RNAi construct, but Ddc transcripts are detected in control (no heat shock) flies infected with E. coli and S. aureus.
In an attempt to eliminate DDC activity completely, we employed RNA interference (RNAi) to degrade Ddc transcripts. To confirm that our Ddc-RNAi constructs were functional, we expressed the UAS-driven RNAi constructs under the control of an Act5C-Gal4 driver. We found that all of the Act5C-Gal4; P[Ddc-RNAi]pWIZ flies died at pupariation, a stage when a high level of DDC activity is required for the rapid tanning of the pupal case. However, it is unlikely that there is a complete loss of DDC activity in these flies, since Ddc null mutants are embryonic lethal (84). Act5C-Gal4; P[Ddc-RNAi]pSymp flies developed normally, suggesting that expression of this construct did not destroy all of the Ddc transcripts, so we eliminated these flies from further analysis. We infected flies of the genotype hs-Gal4; P[Ddc-RNAi]pWIZ with or without heat shock and found that there was no difference in lethalities between heat-shocked and control flies following infection (Fig. 4C). Degradation of Ddc transcripts following heat shock was confirmed by semiquantitative RT-PCR (Fig. 4D). It appears that reduction in DDC activity in these flies does not affect adult survival.
The time courses of Ddc transcription are different following gram-negative and gram-positive infections.
While reducing DDC activity did not decrease the survival of infected flies, our results do show that Ddc transcription is induced in the immune response. We set out to determine the transcriptional profile of Ddc expression following infection with E. coli or S. aureus. Since Ddc transcript levels are high at the second to third moults and at pupariation during normal development (M. M. Davis, unpublished observation), we were unable to determine the time course of Ddc transcription following septic injury of third-instar larvae and chose, therefore, to analyze adults. Flies were septically injured with live (Fig. 5A) or heat-killed (Fig. 5B) bacteria and harvested at various time points. RT-PCR on RNA isolated from pools of adults collected at the indicated time points reveals that Ddc transcripts are first detectible about 1 h after infection with live (Fig. 5A, top gel) or heat-killed (Fig. 5B, top gel) E. coli. Levels peak within 3 h, remain high until 6 h after infection, and drop thereafter. The profile of Ddc transcription is different in response to septic injury with S. aureus (Fig. 5A, bottom gel). In this case, Ddc transcripts are first detected 2 h after infection; levels peak by 9 h and drop slowly thereafter. Similar to infection with live S. aureus, flies septically injured with heat-killed S. aureus (Fig. 5B, bottom gel) induce Ddc transcription, and transcripts are detectible 3 and 6 h after infection. However, Ddc transcripts are not detectible 9 and 12 h after infection with heat-killed S. aureus. Thus, a sustained response to S. aureus requires continued infection by live bacteria.
A conserved AP-1 binding site is necessary to induce Ddc transcription.
To determine which regulatory sequence was responsible for Ddc induction in the immune response, we created Ddc-GFP reporter constructs containing various amounts of the Ddc 5′ regulatory region (Fig. 1). Cuticular autofluorescence made confocal images of our epidermal preparations nearly impossible to analyze, so we employed semiquantitative RT-PCR to examine the expression of the reporter constructs. First, we infected flies bearing the constructs that contain the large deletions, P[Ddc-GFP]SH, P[Ddc-GFP]BH, and P[Ddc-GFP]EH, and compared reporter gene induction in these flies to expression in P[Ddc-GFP]PH-bearing flies (Fig. 6A, top gel). For all Ddc-GFP constructs, we examined expression following infection in a minimum of three independent transgenic lines. We find that the reporter gene is induced normally following both E. coli and S. aureus infection in flies bearing the P[Ddc-GFP]PH, P[Ddc-GFP]SH, and P[Ddc-GFP]BH constructs. However, GFP transcription is not induced in flies bearing the P[Ddc-GFP]EH construct. This suggests that the sequence necessary for Ddc induction is located between the BsmI and EcoRI restriction sites (Fig. 1). To confirm this, we infected a transgenic line bearing a construct, P[Ddc-GFP]PHΔBE, that deleted only this region (Fig. 1), and found that the reporter gene could not be induced following septic injury with E. coli or S. aureus. For each construct-bearing line, the wild-type copy of the endogenous Ddc gene was induced (Fig. 6A, bottom gel), showing that the immune response was triggered normally. To further narrow down the region responsible for Ddc induction, we created transgenic flies bearing reporter constructs that contained a deletion of part of the region between the BsmI and EcoRI sites. These were called P[Ddc-GFP]ΔBE-1, P[Ddc-GFP]ΔBE-2, and P[Ddc-GFP]ΔBE-3 (Fig. 1). We find that reporter gene transcription is immune inducible in flies bearing P[Ddc-GFP]ΔBE-1 and P[Ddc-GFP]ΔBE-2, but not in flies carrying P[Ddc-GFP]PHΔBE-3 (Fig. 6B, top gel). We conclude that a sequence contained within the 302-bp region, missing in P[Ddc-GFP]PHΔBE-3, is necessary for the induction of Ddc transcription in the innate immune response.
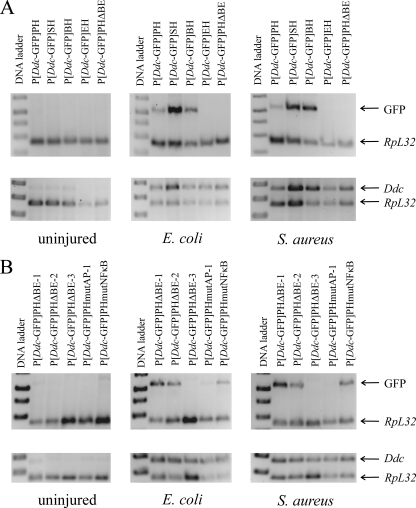
FIG. 6.
Mapping the region upstream of Ddc that is required for immune inducibility. Semiquantitative RT-PCR of Ddc-GFP and endogenous Ddc transcripts was carried out on RNA isolated from adult flies bearing GFP reporter genes. Ddc-GFP or Ddc transcripts were co-reverse transcribed and PCR amplified with an RpL32 loading control. (A) Deletions localizing the response element to the region from BsmI to EcoRI (Fig. 1). (B) The region from BsmI to EcoRI contains an AP-1 site that is necessary for immune inducibility of Ddc.
We aligned this 302-bp region (using the BLAT program; http://genome.ucsc.edu/) (37) with nine other sequenced Drosophila genomes. The sequence was also scanned for the presence of binding sites for known transcription factors found in the database TESS (72). This analysis revealed two conserved binding sites that could be involved in the Drosophila innate immune response (Fig. 7). These sites include a 7-bp AP-1 binding site and an 8-bp NF-κB binding site. The NF-κB binding site most closely resembles the recently defined Rel-specific binding site, not the Dorsal/DIF-specific sequence (11).
FIG. 7.
Conserved AP-1 and NF-κB sites lie within the region deleted in P[Ddc-GFP]BE-3. Alignment of a portion of the Ddc region deleted in P[Ddc-GFP]BE-3 from 10 different Drosophila species reveals a conserved consensus AP-1 (underlined and bold) and a consensus NF-κB site (capital letters and bold) embedded in a less-conserved area. Deviations from the D. melanogaster sequence are italicized.
To determine if either of these sites was necessary for Ddc transcriptional induction in the innate immune response, we used site-directed mutagenesis (see Materials and Methods and see Table S2 in the supplemental material) to mutate 5 bases within each site to create P[Ddc-GFP]PHmutNFκB and P[Ddc-GFP]PHmutAP-1 (Fig. 1). When transgenic flies bearing the P[Ddc-GFP]PHmutNFκB construct were infected with either E. coli or S. aureus, reporter gene transcription was induced (Fig. 6B, top gel). This suggests that the NF-κB site plays no role in Ddc immune induction. The importance of an intact AP-1 site is apparent from the lack of reporter gene transcription in P[Ddc-GFP]PHmutAP-1 flies following infection with either bacterium. In both cases, septic injury induced transcription of the endogenous Ddc gene normally in reporter-bearing flies (Fig. 6B, bottom gel). An intact AP-1 site was also necessary for larval transcriptional induction (data not shown).
AP-1 can bind its putative site in vitro.
Loss of reporter gene induction in P[Ddc-GFP]PHmutAP-1 suggests that the JNK-activated transcription factor complex, AP-1, may be involved in the activation of Ddc transcription in the innate immune response. To determine if AP-1 could bind its putative binding site upstream of Ddc, we employed an electrophoretic mobility shift assay (see Fig. S2 in the supplemental material). We find that the JUN/FOS heterodimer can bind the consensus AP-1 binding site, while neither subunit can independently bind the site. Retardation of the probe is eliminated by addition of a cold competitor and is absent when the probe is mutated, demonstrating the specificity of the binding reaction.
Neither Toll, IMD, nor JNK signaling is involved in Ddc immune induction.
To determine if the AP-1 binding site was a target of JNK signaling, in vivo, we employed the bipartite GAL4/UAS system to express wild-type and dominant-negative forms of Drosophila MAPKK, HEP, and the JNK BSK, as well as JUN and FOS under the control of a heat shock promoter. In each case, we infected organisms that had been heat shocked for 1 h and then allowed to recover for 1 h prior to infection. Control organisms were not heat shocked. Expression of hep (UAS-hep), bsk (UAS-bsk), fos (UAS-fra), or jun (UAS-jra) did not precociously induce Ddc transcription in the absence of infection, although Ddc was immune inducible in these flies (see Fig. S3 in the supplemental material). Furthermore, expression of a constitutively active HEP (UAS-hepAct) did not precociously induce Ddc transcription (see Fig. S3A in the supplemental material). Finally, expression of dominant-negative forms of bsk (UAS-bskDN), jun (UAS-junDN), or fos (UAS-fraDN) did not eliminate immune induction of Ddc transcription (see Fig. S3B, C, and D in the supplemental material).
Ectopic expression of these JNK pathway components failed to indicate a role for JNK signaling in the Ddc immune response. Consistent with this observation, Ddc was shown not to be a JNK-inducible gene (9). However, since a conserved AP-1 binding site is necessary for Ddc immune induction, we thought it advisable to assess the effects of a JNK loss-of-function mutant on Ddc inducibility. Tak1 mutant flies, in which the mutation eliminates both IMD and JNK signaling (73, 80), activate immune transcription of Ddc normally (Fig. 8A). This experiment provides strong evidence that the JNK pathway and its transcription factor complex, AP-1, are not involved in the induction of Ddc in the innate immune response. The identity of the transcription factor that acts through the AP-1 consensus site to effect Ddc induction remains unknown.
FIG. 8.
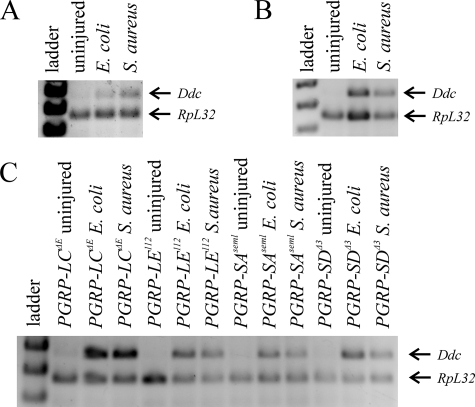
Immune inducibility of Ddc is unaffected in JNK, ERK, and PGRP loss-of-function mutants. Ddc RT-PCR on RNA obtained from uninjured and septically injured Tak1 (A), rl1 (B), or PGRP (C) mutant flies.
We also infected mutants in the receptors responsible for detecting bacterial infection, the peptidoglycan recognition proteins (PGRPs). PGRP-LC and PGRP-LE are the major activators of the IMD pathway (25, 78), while PGRP-SA and PGRP-SD are responsible for induction of the Toll pathway (7, 56). We find that Ddc transcription is induced normally in PGRP-LCΔE, PGRP-LE112, PGRP-SAseml, and PGRP-SDΔ3 mutants following E. coli and S. aureus infection (Fig. 8C). We also infected mutants of additional components of the IMD pathway. These included ird5KG08072 and ird5EY02434, both of which have a P-element insertion in the first exon of the ird5 gene, which encodes the β subunit of the IKK complex (48, 67, 74). This complex is essential for IMD signaling downstream of TAK1. In addition, we infected flies bearing a P-element insertion in the gene encoding the IMD-induced NF-κB transcription factor, Relish, RelEY08061. Ddc transcription was immune inducible in these each of these mutants (data not shown). Genetic data likewise fail to indicate a role for Toll signaling in Ddc immune induction. The Tl3 gain-of-function mutant, which constitutively induces AMP expression in the absence of infection (45), did not precociously induce Ddc transcription (data not shown). These data provide strong evidence that the NF-κB site, although perfectly conserved, is not required for Ddc induction following septic injury.
The p38c MAPK pathway is responsible for Ddc induction.
We have provided evidence that the major immune signaling pathways are dispensable for Ddc immune induction. Two other MAPK cascades have been implicated in Drosophila immunity and wound healing. The first, the ERK pathway, was shown by Mace et al. (49) to be necessary for robust induction of Ddc in the embryonic wound response. The Drosophila ERK is encoded by the gene rolled (rl) (6). We infected the hypomorphic rl1 mutant with E. coli and S. aureus and found that Ddc was induced normally (Fig. 8B).
The second, the p38 MAPK pathway, has been implicated in the attenuation of the immune response (27). There are two p38 MAPKs that have been described in Drosophila, p38a and p38b. In addition, there is a third p38 gene, termed p38c (CG33338), which has been annotated in FlyBase. The p38c gene is located immediately downstream of p38a and likely arose by gene duplication (G. Manning, personal communication). Although p38c shares 61% DNA sequence identity to p38a, there is some doubt that it, in fact, encodes a functional p38 MAPK. The p38c gene has a mutation that changes the TGY domain that is characteristic of p38 MAPKs (51) to a TDH (C. R. Craig, personal communication), thereby preventing this motif from serving as a substrate for dual phosphorylation. Secondly, it has 2 amino acid (aa) substitutions within the 8-aa catalytic domain that could prevent the active site from functioning. However, 4 expressed sequence tags (ESTs) have been identified in a cDNA library obtained from the larval fat body of infected organisms (J. Carlson, personal communication). Furthermore, the p38c MAPK protein coding sequence is conserved, suggesting that p38c is not a pseudogene, and may, in fact, be functional (G. Manning, personal communication).
The Mpk21 null allele of p38a (13), along with P-element insertions upstream of p38b (p38bKG01337 and p38bKG02737) and within p38c (p38cKG05834), was obtained, and flies were infected with E. coli or S. aureus (Fig. 9A). We find that Ddc transcription is induced normally in Mpk21, p38bKG01337 (Fig. 9A), and p38bKG02737 (data not shown) flies, but is not induced in p38cKG05834 flies (Fig. 9A). The p38cKG05834 allele contains an insertion of a P-element, in the center of the coding sequence, which most likely disrupts p38c function. However, this insertion is annotated in FlyBase as affecting p38a function. We performed RT-PCR for both p38a and p38c transcripts on RNA obtained from Mpk21 and p38cKG05834 mutants to determine the nature of the p38cKG05834 mutation. We find that p38a is transcribed in p38cKG05834 flies but not Mpk21 flies (Fig. 9B), and the p38c transcript is detectible in Mpk21 mutants, but not in p38cKG05834 flies (Fig. 9C). Thus, we conclude that the insertion in p38cKG05834 affects the expression of p38c and not p38a in these flies.
To confirm a role for p38c MAPK in the induction of Ddc transcription in the immune response, we undertook a P-element excision scheme to recover additional mutants of this gene. The P-element is inserted into the coding sequence of p38c, and we expected that most excisions would generate new mutants of p38c, since P-element excisions often leave remnants following transposition (24, 76, 77). Homozygous p38cKG05834 female flies (but not males) are sterile (M. Davis, unpublished observation). Since P{SUPor-P} contains a mini-white+ gene, we were able to detect P-element excisions by appearance of a white-eyed phenotype. We obtained 91 P-element excisions. In an attempt to create homozygous stocks, we found that 6 were homozygous lethal, 12 were homozygous viable and fertile, and 73 were homozygous viable but infertile. We assumed that the six lines with mutations that were homozygous lethal contained larger deletions that resulted in the loss of nearby essential genes and eliminated these lines from further analysis. Cloning and sequencing of the P-element excision region of p38c from several lines that yielded homozygous progeny revealed that those lines that were female fertile contained precise P-element excisions, which restored the correct reading frame of p38c. In contrast, those lines that displayed female infertility contained a mutation within p38c. Three revertant lines, p38c2A, p38c4C, and p38c8A, were kept for further analysis. Three mutant lines (p38c1A1, p38c7B1, and p38c19B1) were confirmed by sequencing to contain insertions that caused frameshift mutations that led to premature stop codons and truncated proteins. The full-length p38c MAPK is 356 aa long. The p38c1A1 allele contained a 36-bp insertion that resulted in a 234-aa protein, the p38c7B1 allele contained a 20-bp insertion that resulted in a 232-aa protein, and the p38c19B1 allele contained a 71-bp insertion that resulted in a 239-aa protein. All three p38c mutants were female sterile, but the homozygous males were fertile.
p38c1A1, p38c7B1, and p38c19B1 all failed to induce Ddc following infection with E. coli or S. aureus (data not shown). However, Ddc transcription was induced in p38c2A, p38c4C (data not shown), and p38c8A (Fig. 9D) flies following septic injury with E. coli and S. aureus. To eliminate the possibility of second-site effects, we also examined five heteroallelic combinations of p38c, each of which failed to induce Ddc transcription following infection with E. coli or S. aureus (Fig. 9D and data not shown). The p38c1A1/p38c19B flies shown in Fig. 9D demonstrate a typical heteroallelic result.
To analyze p38c expression, we used semiquantitative RT-PCR to detect p38c transcripts in uninjured and septically injured adult flies (Fig. 9E). We find that p38c transcription is not immune inducible; rather, levels of p38c transcripts are similar in infected and uninfected organisms.
Finally, to determine whether p38c is required for survival of bacterial infection, we assessed the survival of p38cKG05834 flies following infection with E. coli or S. aureus. We find that there is no difference in the survival of p38cKG05834 flies compared with p38cKG05834/TM3, Sb control flies following E. coli or S. aureus infection (Fig. 9F).
DISCUSSION
Microarray analysis has shown that Ddc transcription is induced by infection with gram-negative and gram-positive bacteria (9, 17, 34). We have confirmed that Ddc (Fig. 2), but not ple (data not shown), transcription is induced following gram-negative (E. coli) or gram-positive (S. aureus) septic injury with a small needle. The lack of ple transcriptional induction is not surprising, since PO activity can provide Dopa for DDC, thereby making TH redundant. In addition, transcripts for the genes encoding dihydropteridine reductase and GTP cyclohydrolase I, which are responsible for production of the essential TH cofactor, tetrahydrobiopterin, are significantly upregulated following septic injury (9, 17, 34). This induction is likely sufficient to increase basal TH activity levels.
Aseptic wounding of larvae or adults does not lead to Ddc transcriptional induction (Fig. 2), unlike in embryos where DDC activity at the edge of the wound contributes to the formation of a melanin clot (49). Our data show that robust induction of Ddc transcription is not apparent until 2 h after infection (Fig. 5), long after the melanin clot has already formed at the wound site. The profile of Ddc transcription differs in response to E. coli and S. aureus infection (Fig. 5). This discrepancy can be attributed to the virulence of S. aureus, since infection with heat-killed S. aureus recapitulates the profile of Ddc transcriptional induction with live E. coli (Fig. 5B).
Induction of Ddc transcription following septic injury apparently occurs within the epidermis (Fig. 3). Although we do not see a change in infection-induced Ddc transcription in domK08108 larvae (Fig. 3B), we cannot eliminate the possibility that the hemocytes also express Ddc. There is a possibility that our dissected epidermal tissues are contaminated with small amounts of these cells; however, we view the likelihood of their contributing to the Ddc response as slight. Induction of Ddc transcription in the epidermal tissues following infection (Fig. 3C), but not wounding (Fig. 2 and 3A), suggests a role for Ddc in the destruction of invading microorganisms. We find that mutations in Ddc or knockdown of Ddc expression by RNAi does not affect the ability of Drosophila to survive infection (Fig. 4). This suggests that the low level of DDC activity in these flies is sufficient for survival. However, the actions of enzymes such as PO in the epidermis may be able to compensate for the loss of DDC activity in the immune response. Since all null alleles of Ddc are embryonic lethal (84), it is impossible to determine whether DDC activity is essential for survival following infection.
In an effort to determine which Ddc promoter element is responsible for transcriptional induction during the innate immune response, we infected larvae and adults of fly stocks bearing various Ddc-GFP reporter constructs (Fig. 1 and 6). We confirmed that the immune response was mounted in these flies by showing that transcription from the endogenous Ddc gene was induced (Fig. 6A, bottom gels). Infection of flies bearing constructs revealed that a 302-bp sequence that was deleted in P[Ddc-GFP]PHΔBE-3 was necessary for Ddc induction (Fig. 6B). Alignment of the sequences from 10 Drosophila species for this 302-bp region (Fig. 7) revealed conserved binding sites for NF-κB and AP-1 transcription factors. Although the NF-κB site is highly conserved in the 10 Drosophila species (Fig. 7), this site is dispensable for immune induction of Ddc transcription (Fig. 6B).
The Toll and IMD pathways both activate the immune response through NF-κB transcription factors. Mutations in Toll (PGRP-SAseml, PGRP-SDΔ3, and Tl3) and IMD (PGRP-LCΔE, PGRP-LE112, ird5KG08072, ird5EY02434, and RelEY08061) pathway components had no effect on Ddc immune induction following E. coli or S. aureus infection (Fig. 8C and data not shown). This confirms that the NF-κB site is not necessary for Ddc immune induction in larvae and adults following bacterial infection. However, Ddc could be developmentally regulated by Toll signaling, particularly during embryogenesis when Toll signaling is very important for embryonic patterning (75) and the role of Ddc is not well understood.
Mutation of the consensus AP-1 site eliminated the immune induction of the Ddc-GFP reporter construct (Fig. 6B), suggesting that a transcription factor must bind this site to induce Ddc transcription. Drosophila AP-1 is a heterodimer composed of one JUN subunit and one FOS subunit (60). We demonstrated that AP-1 could bind its putative site within the Ddc promoter, but not a mutated site, and that binding could be eliminated by the addition of a specific competitor (see Fig. S2 in the supplemental material). AP-1 is activated by phosphorylation carried out by JNK (16). Using a hs-Gal4 driver, we induced ectopic expression of two kinases in the JNK pathway, HEP and BSK. Neither these nor a constitutively active HEP (UAS-hepAct) caused precocious induction of Ddc transcription in the absence of infection (see Fig. S3A and B in the supplemental material). In addition, induction of dominant-negative forms of HEP and BSK did not prevent induction of Ddc transcription following infection (see Fig. S3A and B in the supplemental material). Furthermore, heat shock induction of JUN and FOS expression did not activate Ddc transcription, and expression of a dominant-negative form of JUN or FOS did not eliminate Ddc immune induction (see Fig. S3C and D in the supplemental material). Most importantly, a mutant in Tak1, which lies at the branch point common to IMD and JNK signaling, fails to eliminate Ddc immune induction (Fig. 8A). Taken together, these results suggest that the JNK pathway is not responsible for Ddc transcriptional induction. Although AP-1 can bind the consensus binding site upstream of Ddc in vitro, our genetic data suggest that AP-1 does not induce Ddc transcription in vivo. Therefore, we conclude that a transcription factor other than AP-1 binds to this site to activate Ddc transcription.
We have provided extensive evidence that the JNK pathway and its transcription factor, AP-1, are not involved in the immune induction of Ddc transcription. The ERK pathway has been implicated in the induction of Ddc in the embryonic wound response, and treatment with a universal MAPK inhibitor reduces Ddc induction (49), suggesting that a MAPK is important for Ddc transcription. Ddc was immune inducible in the hypomorphic mutant of Drosophila ERK, rl1 (Fig. 8B), suggesting that the ERK pathway is not responsible for Ddc immune induction. However, analysis of a null rl mutant would be necessary to confirm this observation.
The p38 MAPK pathway has been implicated in attenuation of the Drosophila immune response (27). A null allele of p38a, Mpk21, did not affect immune induction of Ddc transcription. No mutant for p38b has been identified, but Ddc transcription is induced normally in two lines bearing P-element insertions immediately upstream of the p38b gene. However, the insertions likely do not eliminate p38b MAPK function, and the creation of a null allele of p38b would be useful. Interestingly, the only mutant we identified that affected Ddc transcriptional induction was p38cKG05834, which carries a P-element insertion in the coding sequence of an uncharacterized member of the p38 MAPK family, p38c (Fig. 9A). Furthermore, excision of this P-element restores immune inducibility of Ddc (Fig. 9D). We recovered three additional mutants in p38c, and Ddc immune induction is absent in these mutants, both as homozygotes (data not shown) or in heteroallelic combinations (Fig. 9D). The p38c gene is located immediately downstream of p38a and likely arose by gene duplication (G. Manning, personal communication). Four ESTs for p38c were detected in infected larval fat body cells (J. Carlson, personal communication). We have shown that this gene is expressed within epidermal tissues (data not shown), although its transcript is not induced in the immune response (Fig. 9E). Transcription of p38c is lost in p38cKG05834 (Fig. 9B), and the lack of p38c expression correlates with the loss of Ddc immune induction (Fig. 9A and D). The recovery of additional p38c mutants that fail to induce Ddc following infection (Fig. 9D) and the restoration of Ddc immune induction in flies bearing precise P-element excisions further confirm that p38c MAPK is responsible for Ddc transcriptional induction.
Although we demonstrated that p38c MAPK is not required for survival of E. coli or S. aureus infection (Fig. 9F), this does not eliminate the possibility that it is required for survival of infection with E. coli and S. aureus under different conditions or infection with other bacterial species. A large repertoire of genes is induced following bacterial infection of Drosophila, primarily due to the activation of the Toll, IMD, and JNK pathways. This implies that a number of parallel defense responses are mounted simultaneously. Induction of the p38c MAPK pathway may only be critical to the survival of organisms under very specific circumstances, as was shown for the role of NF-κB-induced immunity in the gut epithelia (69). In this case, the NF-κB-induced immunity is only critical for survival of infections with reactive oxygen species-resistant bacteria that cannot be killed by the reactive oxygen species-dependent immunity that destroys the majority of gut-infecting organisms.
The identification of yet another signaling pathway that participates in the immune induction of gene transcription points to the evolutionary significance of innate immunity. The p38c MAPK pathway elicits its response through the activation of an unknown transcription factor, which acts through the conserved consensus AP-1 site upstream of the Ddc transcription start site. It has been suggested that p38c MAPK cannot be activated by phosphorylation by a MAPKK due to the mutation that converts the TGY dual-phosphorylation motif to TDH (C. R. Craig, personal communication). However, a clear role for p38c MAPK in Ddc immune induction has been demonstrated, although the mechanism of p38c activation is still to be elucidated. It is clear that p38c MAPK is not activated by signaling through PGRP-LC, -LE, -SA, or -SD, since Ddc is induced normally following infection of these mutants (Fig. 8C). However, the possibility exists that another PGRP is responsible for the detection of invading bacteria and signaling to p38c MAPK.
It has been suggested that the amino acid substitutions within the catalytic domain may render p38c MAPK nonfunctional as a kinase (C. R. Craig, personal communication). A clear role for another putative kinase, Tribbles, in regulating the Drosophila Cdc25 homolog, String, during morphogenesis has been shown, although researchers have failed to demonstrate any kinase activity of this protein or any of its homologs (26, 29, 52). Further work is required to ascertain the catalytic activity of p38c MAPK, although it is possible that this protein has a regulatory role in the absence of kinase activity.
Delineation of the other components of the p38c MAPK signaling pathway will aide in our understanding of this system. The MAPKKK, MEKK1, has been shown to be the major activator of peptidoglycan-induced p38 MAPK activation in Drosophila (88), although future research will determine if it is responsible for p38c MAPK activation in the immune response.
The importance of DDC to neuronal production of DA is well established. During development, DDC produces the DA that is required for melanin and sclerotin production (2, 83). Tanning of the pupal case at pupariation and the hardening and darkening of the adult cuticle following eclosion are the most obvious manifestations of DA metabolism. Ddc transcription is also induced in the Drosophila embryonic wound response, at the wound site, to produce a melanin plug (49). Melanotic encapsulation of the parasitic wasp egg also requires DDC (57). In addition to these well-established roles for DDC, here, we demonstrate a role for the activation of transcription of this gene in the Drosophila innate immune response to bacterial infection. The appearance of DDC in the epidermis will increase the production of DA. The DA is most likely metabolized to produce reactive quinones that are toxic to invading bacteria. Presumably, DA is metabolized by the enzymes responsible for the production of sclerotin since no melanin deposits are evident within the epidermis. Consistent with this prediction, the gene black, whose protein product is an enzyme involved in NBAD synthesis (30, 62, 82), is also upregulated in the Drosophila immune response (34). Future research will reveal the extent of the antimicrobial role of quinone production in the innate immune response.
Supplementary Material
Acknowledgments
We thank Edan Foley for the yw; Tak1 flies and critical review of the manuscript, Robert Tjian for the JUN- and FOS-containing plasmids, Julien Royet and Shoichiro Kurata for the PGRP mutant flies, and Mattea Bujold and Kirst King-Jones for assistance with real-time PCR.
This work was funded by an NSERC grant (R.B.H.) and studentship (M.M.D.). The Parkinson's Society of Alberta also supported M.M.D.
Footnotes
Published ahead of print on 2 June 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with Image J. Biophotonics Int. 1136-42. [Google Scholar]
- 2.Andersen, S. O., P. Hojrup, and P. Roepstorff. 1995. Insect cuticular proteins. Insect Biochem. Mol. Biol. 25153-176. [DOI] [PubMed] [Google Scholar]
- 3.Barolo, S., L. A. Carver, and J. W. Posakony. 2000. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques 29726-732. [DOI] [PubMed] [Google Scholar]
- 4.Bidla, G., M. S. Dushay, and U. Theopold. 2007. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci. 1201209-1215. [DOI] [PubMed] [Google Scholar]
- 5.Bidla, G., M. Lindgren, U. Theopold, and M. S. Dushay. 2005. Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev. Comp. Immunol. 29669-679. [DOI] [PubMed] [Google Scholar]
- 6.Biggs, W. H., III, K. H. Zavitz, B. Dickson, A. van der Straten, D. Brunner, E. Hafen, and S. L. Zipursky. 1994. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 131628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff, V., C. Vignal, I. G. Boneca, T. Michel, J. A. Hoffmann, and J. Royet. 2004. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat. Immunol. 51175-1180. [DOI] [PubMed] [Google Scholar]
- 8.Bosch, M., F. Serras, E. Martin-Blanco, and J. Baguna. 2005. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 28073-86. [DOI] [PubMed] [Google Scholar]
- 9.Boutros, M., H. Agaisse, and N. Perrimon. 2002. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 3711-722. [DOI] [PubMed] [Google Scholar]
- 10.Braun, A., J. A. Hoffmann, and M. Meister. 1998. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. USA 9514337-14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse, M. S., C. P. Arnold, P. Towb, J. Katrivesis, and S. A. Wasserman. 2007. A kappaB sequence code for pathway-specific innate immune responses. EMBO J. 263826-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbo, J. C., and M. Levine. 1996. Characterization of an immunodeficiency mutant in Drosophila. Mech. Dev. 55211-220. [DOI] [PubMed] [Google Scholar]
- 13.Craig, C. R., J. L. Fink, Y. Yagi, Y. T. Ip, and R. L. Cagan. 2004. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 51058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, M. M., S. L. O'Keefe, D. A. Primrose, and R. B. Hodgetts. 2007. A neuropeptide hormone cascade controls the precise onset of post-eclosion cuticular tanning in Drosophila melanogaster. Development 1344395-4404. [DOI] [PubMed] [Google Scholar]
- 15.Davis, M. M., P. Yang, L. Chen, S. L. O'Keefe, and R. B. Hodgetts. 2007. The orphan nuclear receptor DHR38 influences transcription of the DOPA decarboxylase gene in epidermal and neural tissues of Drosophila melanogaster. Genome 501049-1060. [DOI] [PubMed] [Google Scholar]
- 16.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103239-252. [DOI] [PubMed] [Google Scholar]
- 17.De Gregorio, E., P. T. Spellman, G. M. Rubin, and B. Lemaitre. 2001. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA 9812590-12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaney, J. R., S. Stoven, H. Uvell, K. V. Anderson, Y. Engstrom, and M. Mlodzik. 2006. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 253068-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dushay, M. S., B. Asling, and D. Hultmark. 1996. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl. Acad. Sci. USA 9310343-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrandon, D., A. C. Jung, M. Criqui, B. Lemaitre, S. Uttenweiler-Joseph, L. Michaut, J. Reichhart, and J. A. Hoffmann. 1998. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 171217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galko, M. J., and M. A. Krasnow. 2004. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giordano, E., R. Rendina, I. Peluso, and M. Furia. 2002. RNAi triggered by symmetrically transcribed transgenes in Drosophila melanogaster. Genetics 160637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glise, B., H. Bourbon, and S. Noselli. 1995. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83451-461. [DOI] [PubMed] [Google Scholar]
- 24.Gloor, G. B., J. Moretti, J. Mouyal, and K. J. Keeler. 2000. Distinct P-element excision products in somatic and germline cells of Drosophila melanogaster. Genetics 1551821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottar, M., V. Gobert, T. Michel, M. Belvin, G. Duyk, J. A. Hoffmann, D. Ferrandon, and J. Royet. 2002. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416640-644. [DOI] [PubMed] [Google Scholar]
- 26.Grosshans, J., and E. Wieschaus. 2000. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101523-531. [DOI] [PubMed] [Google Scholar]
- 27.Han, Z. S., H. Enslen, X. Hu, X. Meng, I.-H. Wu, T. Barrett, R. J. Davis, and Y. T. Ip. 1998. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol. Cell. Biol. 183527-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedengren, M., B. Asling, M. S. Dushay, I. Ando, S. Ekengren, M. Wihlborg, and D. Hultmark. 1999. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 4827-837. [DOI] [PubMed] [Google Scholar]
- 29.Hegedus, Z., A. Czibula, and E. Kiss-Toth. 2007. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell. Signal. 19238-250. [DOI] [PubMed] [Google Scholar]
- 30.Hodgetts, R. B. 1972. Biochemical characterization of mutants affecting the metabolism of β-alanine in Drosophila. J. Insect Physiol. 18937-947. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 2841313-1318. [DOI] [PubMed] [Google Scholar]
- 32.Imler, J. L., and P. Bulet. 2005. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem. Immunol. Allergy 861-21. [DOI] [PubMed] [Google Scholar]
- 33.Ip, Y. T., M. Reach, Y. Engstrom, L. Kadalayil, H. Cai, S. Gonzalez-Crespo, K. Tatei, and M. Levine. 1993. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 75753-763. [DOI] [PubMed] [Google Scholar]
- 34.Irving, P., L. Troxler, T. S. Heuer, M. Belvin, C. Kopczynski, J. M. Reichhart, J. A. Hoffmann, and C. Hetru. 2001. A genome-wide analysis of immune responses in Drosophila. Proc. Natl. Acad. Sci. USA 9815119-15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallio, J., A. Leinonen, J. Ulvila, S. Valanne, R. A. Ezekowitz, and M. Ramet. 2005. Functional analysis of immune response genes in Drosophila identifies JNK pathway as a regulator of antimicrobial peptide gene expression in S2 cells. Microbes Infect. 7811-819. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson, C., A. M. Korayem, C. Scherfer, O. Loseva, M. S. Dushay, and U. Theopold. 2004. Proteomic analysis of the Drosophila larval hemolymph clot. J. Biol. Chem. 27952033-52041. [DOI] [PubMed] [Google Scholar]
- 37.Kent, W. J. 2002. BLAT—the BLAST-like alignment tool. Genome Res. 12656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim, L. K., U. Y. Choi, H. S. Cho, J. S. Lee, W. B. Lee, J. Kim, K. Jeong, J. Shim, J. Kim-Ha, and Y. J. Kim. 2007. Down-regulation of NF-kappaB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 5e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, M. H., C. H. Joo, M. Y. Cho, T. H. Kwon, K. M. Lee, S. Natori, T. H. Lee, and B. L. Lee. 2000. Bacterial-injection-induced syntheses of N-beta-alanyldopamine and Dopa decarboxylase in the hemolymph of coleopteran insect, Tenebrio molitor larvae. Eur. J. Biochem. 2672599-2608. [DOI] [PubMed] [Google Scholar]
- 40.Kockel, L., J. G. Homsy, and D. Bohmann. 2001. Drosophila AP-1: lessons from an invertebrate. Oncogene 202347-2364. [DOI] [PubMed] [Google Scholar]
- 41.Lazareva, A. A., G. Roman, W. Mattox, P. E. Hardin, and B. Dauwalder. 2007. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 3e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, Y. S., and R. W. Carthew. 2003. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30322-329. [DOI] [PubMed] [Google Scholar]
- 43.Lemaitre, B., and J. Hoffmann. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25697-743. [DOI] [PubMed] [Google Scholar]
- 44.Lemaitre, B., E. Kromer-Metzger, L. Michaut, E. Nicolas, M. Meister, P. Georgel, J. M. Reichhart, and J. A. Hoffmann. 1995. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. USA 929465-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86973-983. [DOI] [PubMed] [Google Scholar]
- 46.Levashina, E. A., S. Ohresser, B. Lemaitre, and J. L. Imler. 1998. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide metchnikowin. J. Mol. Biol. 278515-527. [DOI] [PubMed] [Google Scholar]
- 47.Lindsley, D. L., and G. G. Zimm. 1992. The genome of Drosophila melanogaster, vol. 8. Academic Press, San Diego, CA.
- 48.Lu, Y., L. P. Wu, and K. V. Anderson. 2001. The antibacterial arm of the Drosophila innate immune response requires an IkappaB kinase. Genes Dev. 15104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mace, K. A., J. C. Pearson, and W. McGinnis. 2005. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 308381-385. [DOI] [PubMed] [Google Scholar]
- 50.Manfruelli, P., J. M. Reichhart, R. Steward, J. A. Hoffmann, and B. Lemaitre. 1999. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J. 183380-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin-Blanco, E. 2000. p38 MAPK signalling cascades: ancient roles and new functions. Bioessays 22637-645. [DOI] [PubMed] [Google Scholar]
- 52.Mata, J., S. Curado, A. Ephrussi, and P. Rorth. 2000. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101511-522. [DOI] [PubMed] [Google Scholar]
- 53.Meister, M. 2004. Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 1610-15. [DOI] [PubMed] [Google Scholar]
- 54.Meister, M., and M. Lagueux. 2003. Drosophila blood cells. Cell. Microbiol. 5573-580. [DOI] [PubMed] [Google Scholar]
- 55.Meng, X., B. S. Khanuja, and Y. T. Ip. 1999. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 13792-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michel, T., J. M. Reichhart, J. A. Hoffmann, and J. Royet. 2001. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414756-759. [DOI] [PubMed] [Google Scholar]
- 57.Nappi, A. J., Y. Carton, and E. Vass. 1992. Reduced cellular immune competence of a temperature-sensitive Dopa decarboxylase mutant strain of Drosophila melanogaster against the parasite Leptopilina boulardi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 101453-460. [DOI] [PubMed] [Google Scholar]
- 58.Nappi, A. J., and E. Ottaviani. 2000. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays 22469-480. [DOI] [PubMed] [Google Scholar]
- 59.Perkins, K. K., A. Admon, N. Patel, and R. Tjian. 1990. The Drosophila Fos-related AP-1 protein is a developmentally regulated transcription factor. Genes Dev. 4822-834. [DOI] [PubMed] [Google Scholar]
- 60.Perkins, K. K., G. M. Dailey, and R. Tjian. 1988. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 74265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen, U. M., G. Bjorklund, Y. T. Ip, and Y. Engstrom. 1995. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. EMBO J. 143146-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips, A. M., R. Smart, R. Strauss, B. Brembs, and L. E. Kelly. 2005. The Drosophila black enigma: the molecular and behavioural characterization of the black1 mutant allele. Gene 351131-142. [DOI] [PubMed] [Google Scholar]
- 63.Ramet, M., R. Lanot, D. Zachary, and P. Manfruelli. 2002. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 241145-156. [DOI] [PubMed] [Google Scholar]
- 64.Riesgo-Escovar, J. R., M. Jenni, A. Fritz, and E. Hafen. 1996. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 102759-2768. [DOI] [PubMed] [Google Scholar]
- 65.Rizki, T. M., R. M. Rizki, and R. A. Bellotti. 1985. Genetics of a Drosophila phenoloxidase. Mol. Gen. Genet. 2017-13. [DOI] [PubMed] [Google Scholar]
- 66.Rutschmann, S., A. C. Jung, C. Hetru, J. M. Reichhart, J. A. Hoffmann, and D. Ferrandon. 2000. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12569-580. [DOI] [PubMed] [Google Scholar]
- 67.Rutschmann, S., A. C. Jung, R. Zhou, N. Silverman, J. A. Hoffmann, and D. Ferrandon. 2000. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat. Immunol. 1342-347. [DOI] [PubMed] [Google Scholar]
- 68.Rutschmann, S., A. Kilinc, and D. Ferrandon. 2002. Cutting edge: the toll pathway is required for resistance to gram-positive bacterial infections in Drosophila. J. Immunol. 1681542-1546. [DOI] [PubMed] [Google Scholar]
- 69.Ryu, J. H., E. M. Ha, C. T. Oh, J. H. Seol, P. T. Brey, I. Jin, D. G. Lee, J. Kim, D. Lee, and W. J. Lee. 2006. An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 253693-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schachter, J., M. M. Perez, and L. A. Quesada-Allue. 2007. The role of N-beta-alanyldopamine synthase in the innate immune response of two insects. J. Insect Physiol. 531188-1197. [DOI] [PubMed] [Google Scholar]
- 71.Scherfer, C., C. Karlsson, O. Loseva, G. Bidla, A. Goto, J. Havemann, M. S. Dushay, and U. Theopold. 2004. Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Curr. Biol. 14625-629. [DOI] [PubMed] [Google Scholar]
- 72.Schug, J. 2003. Using TESS to predict transcription factor binding sites in DNA sequence, chapter 2.6. In A. D. Baxevanis (ed.), Current protocols in bioinformatics. J. Wiley and Sons, Hoboken, NJ. [DOI] [PubMed]
- 73.Silverman, N., R. Zhou, R. L. Erlich, M. Hunter, E. Bernstein, D. Schneider, and T. Maniatis. 2003. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J. Biol. Chem. 27848928-48934. [DOI] [PubMed] [Google Scholar]
- 74.Silverman, N., R. Zhou, S. Stoven, N. Pandey, D. Hultmark, and T. Maniatis. 2000. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 142461-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stathopoulos, A., and M. Levine. 2002. Dorsal gradient networks in the Drosophila embryo. Dev. Biol. 24657-67. [DOI] [PubMed] [Google Scholar]
- 76.Staveley, B. E., T. R. Heslip, R. B. Hodgetts, and J. B. Bell. 1995. Protected P-element termini suggest a role for inverted-repeat-binding protein in transposase-induced gap repair in Drosophila melanogaster. Genetics 1391321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takasu-Ishikawa, E., M. Yoshihara, and Y. Hotta. 1992. Extra sequences found at P element excision sites in Drosophila melanogaster. Mol. Gen. Genet. 23217-23. [DOI] [PubMed] [Google Scholar]
- 78.Takehana, A., T. Yano, S. Mita, A. Kotani, Y. Oshima, and S. Kurata. 2004. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 234690-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tzou, P., S. Ohresser, D. Ferrandon, M. Capovilla, J. M. Reichhart, B. Lemaitre, J. A. Hoffmann, and J. L. Imler. 2000. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13737-748. [DOI] [PubMed] [Google Scholar]
- 80.Vidal, S., R. S. Khush, F. Leulier, P. Tzou, M. Nakamura, and B. Lemaitre. 2001. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 151900-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walter, M. F., L. L. Zeineh, B. C. Black, W. E. McIvor, T. R. Wright, and H. Biessmann. 1996. Catecholamine metabolism and in vitro induction of premature cuticle melanization in wild type and pigmentation mutants of Drosophila melanogaster. Arch. Insect Biochem. Physiol. 31219-233. [DOI] [PubMed] [Google Scholar]
- 82.Weber, J. P., R. J. Bolin, M. S. Hixon, and A. F. Sherald. 1992. Beta-alanine transaminase activity in black and suppressor of black mutations of Drosophila melanogaster. Biochim. Biophys. Acta 1115181-186. [DOI] [PubMed] [Google Scholar]
- 83.Wright, T. R. 1987. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Adv. Genet. 24127-222. [PubMed] [Google Scholar]
- 84.Wright, T. R., G. C. Bewley, and A. F. Sherald. 1976. The genetics of dopa decarboxylase in Drosophila melanogaster. II. Isolation and characterization of dopa-decarboxylase-deficient mutants and their relationship to the α-methyl-dopa-hypersensitive mutants. Genetics 84287-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wright, T. R., R. Steward, K. W. Bentley, and P. N. Adler. 1981. The genetics of Dopa decarboxylase in Drosophila melanogaster. III. Effect of a temperature sensitive Dopa decarboxylase deficient mutation of female fertility. Dev. Genet. 2223-235. [Google Scholar]
- 86.Zettervall, C. J., I. Anderl, M. J. Williams, R. Palmer, E. Kurucz, I. Ando, and D. Hultmark. 2004. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 10114192-14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang, K., J. R. Chaillet, L. A. Perkins, T. D. Halazonetis, and N. Perrimon. 1990. Drosophila homolog of the mammalian jun oncogene is expressed during embryonic development and activates transcription in mammalian cells. Proc. Natl. Acad. Sci. USA 876281-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhuang, Z. H., Y. Zhou, M. C. Yu, N. Silverman, and B. X. Ge. 2006. Regulation of Drosophila p38 activation by specific MAP2 kinase and MAP3 kinase in response to different stimuli. Cell. Signal. 18441-448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.