Abstract
While it is well appreciated that receptors for secreted cytokines transmit ligand-induced signals, little is known about additional roles for cytokine receptor components in the control of ligand transport and secretion. Here, we show that interleukin-15 (IL-15) translocation into the endoplasmic reticulum occurs independently of the presence of IL-15 receptor α (IL-15Rα). Subsequently, however, IL-15 is transported through the Golgi apparatus only in association with IL-15Rα and then is secreted. This intracellular IL-15/IL-15Rα complex already is formed in the endoplasmic reticulum and, thus, enables the further trafficking of complexed IL-15 through the secretory pathway. Just transfecting IL-15Rα in cells, which transcribe but normally do not secrete IL-15, suffices to induce IL-15 secretion. Thus, we provide the first evidence of how a cytokine is chaperoned through the secretory pathway by complexing with its own high-affinity receptor and show that IL-15/IL-15Rα offers an excellent model system for the further exploration of this novel mechanism for the control of cytokine secretion.
Given the powerful biological properties of cytokines, the excessive secretion of which can have deleterious consequences, it is not surprising that not only the transcription and translation but also the secretion of cytokines are tightly controlled by a number of different mechanisms. In recent years, various secretory pathways that enable cells to control the release of cytokines into their tissue environment have been characterized (29, 41, 58). These pathways operate on the basis of recognized general principles for regulating protein secretion: secreted proteins commonly are translated as precursor proteins, which possess signal peptides at their N termini that target them to the secretory pathway (60, 67). Upon translocation across the endoplasmic reticulum (ER) membrane, this signal peptide is removed by signal peptidases and initial glycosylation can occur (16, 25). Subsequently, proteins are transported within vesicles to the Golgi compartments, in which additional glycosylation trimming occurs (50, 51, 55). Finally, secretory vesicles deliver mature proteins at the cell surface for secretion, either constitutively or upon specific stimuli (13, 47, 64).
Most cytokines exploit the same basic mechanisms for regulating their secretion. For example, interleukin-2 (IL-2), IL-3, and IL-7 carry a classical signal peptide and are glycosylated, suggesting secretion via the classical pathway (14, 24, 68). Consequently, such cytokines are constitutively secreted. However, increasing evidence suggests that cytokines utilize additional strategies for regulating their intracellular trafficking and/or secretion, which differ between cell types and depend on the specific tissue/signaling context in which cytokine secretion occurs. This is particularly evident for, e.g., IL-4, gamma interferon (IFN-γ), and tumor necrosis factor alpha, which are stored in granules or other secretory compartments at the proximity of the cell surface or within the cell membrane itself, allowing the rapid release/secretion of preformed, ready-to-act cytokines after specific stimulation (28, 27, 58). The compartmentalized storage of fully operative cytokines in the cell membrane even allows juxtacrine signaling, as shown for, e.g., tumor necrosis factor alpha and IL-15 (12, 56). Even though some progress has been made in defining how the subcellular localization and intracellular trafficking of cytokines are controlled, the precise nonclassical mechanisms and key regulatory proteins involved mainly remain obscure (29, 58). Here, we proposed that studying the intracellular interactions of IL-15 with its high-affinity receptor offers an intriguing and instructive model system for the further exploration of the as-yet insufficiently defined controls of cytokines.
IL-15 belongs to the four alpha-helix bundle family of cytokines and is a 15- to 17-kDa glycoprotein that displays multiple immunoregulatory functions and acts as a survival or growth factor for a large variety of cell types (10, 11, 19, 30, 34, 45, 53, 59, 65, 66). These activities are mediated by heterogeneous IL-15 receptor complexes (1, 5, 6, 8, 9, 22, 23, 46). IL-15 binding specificity is determined by the α subunit (IL-15Rα), which binds IL-15 with an extremely high affinity due to a unique, charge-dependent ligand-receptor interaction (35).
The interactions between IL-15Rα and its ligand are special in many respects. For example, monocytes express IL-15 on their cell surface in an IL-15Rα-independent manner (42). This cell surface IL-15 engages in reverse signaling, during which, intriguingly, the roles of cytokine and receptor are switched (4). Moreover, the priming of CD8+ T cells and NK cells by dendritic cells can occur via the trans presentation of IL-15 by IL-15Rα (18, 31, 54). This example for the receptor-dependent cell surface presentation of a cytokine already invites the hypothesis that IL-15Rα is somehow involved in controlling the presentation of its own ligand.
Although the IL-15 gene is transcribed constitutively by a large variety of cell types and tissues (e.g., keratinocytes, fibroblasts, and activated monocytes), it is extremely difficult to detect secreted IL-15 protein in the supernatants of cultured cells that express IL-15 mRNA. So far, only small amounts of secreted IL-15 could be detected in the supernatants of activated macrophages and epithelial cells (21, 49). In part, this may reflect the fact that secretion is isotype specific: in mice and humans, IL-15 exists in two isoforms that differ only in the length of their leader peptide (17, 20, 26, 37). The shorter isoform, which contains a 21-amino-acid leader peptide, remains in the cytoplasm or is translocated into the nucleus but is not secreted (43, 44, 61). In contrast, the longer IL-15 isoform, which displays a 48-amino-acid signal peptide, can be secreted (33, 42, 61).
In the current study, we have pursued the hypothesis that an additional control of IL-15 secretion exists that relies on interactions between IL-15 and its high-affinity receptor and that these intracellular ligand-receptor interactions are involved in the regulation of cytokine mobilization, trafficking, and/or secretion. If true, this would constitute a novel general mechanism for the control of cytokine secretion, with potential implications for general cytokine biology beyond that of IL-15.
By biochemical methods and in combination with mutational analyses, we therefore have examined the IL-15Rα-mediated regulation of IL-15 secretion in appropriate model cell systems. In addition, we have examined the secretory route, employing specific inhibitors and an analysis of N-linked glycosylation in membrane fractions and supernatants.
MATERIALS AND METHODS
Cytokines, recombinant proteins, and antibodies.
Recombinant IL-15 and TIMP-2 were purchased from PeproTech (London, United Kingdom) and Calbiochem (Merck KGaA, Darmstadt, Germany), respectively. The recombinant soluble IL-15Rα was produced as described earlier (9). Anti-GM130 (610822) and anti-green fluorescent protein (GFP) (JL-8) were purchased from BD Biotechnology. The anti-IL-15Rα antibodies (151308 and BAF847) were purchased from R&D Systems (Minneapolis, MN). Anti-CD81 (H-121) sc-9158, anti-c-Myc (C-19), and anti-IL-15 (L-20 and H-114) all were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin (A5441) was purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines, culture conditions, and transient transfection.
COS-7, HeLa, HEK293, and PC-3 cell lines were obtained from the ATCC and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin or Dulbecco's modified essential medium-high glucose supplemented with 10% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μg/ml penicillin, and 100 μg/ml streptomycin. For transient transfection, Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) was used according to the manufacturer's protocol. For this, cells were seeded at 6 × 105/well in 6-well plates, and after 24 h they were transfected with 2 μg of DNA. Sufficient transfection efficiency (>60%) was determined with fluorescent microscopy or fluorescence-activated cell sorter (FACS) analysis. An analysis of transfected cells and supernatants was performed 48 h after transfection. For inhibition experiments with GM6001, the medium was replaced with fresh medium containing 200 ng/ml phorbol myristate (PMA; Sigma-Aldrich) 48 h after transfection. After 2 h of prestimulation, 10 μg/ml GM6001 (final concentration) was added, and the cells were cultured for another 8 h.
Human monocytes and macrophages were obtained and cultured using the methods described earlier (48). A total of 10 × 106 cells were used for each measurement, and stimulation was performed with 10 U/ml IFN-γ. Subsequently, cells were incubated for an additional 6 (RNA isolation) or 10 h (IL-15 enzyme-linked immunosorbent assay [ELISA]).
Cloning procedures and mutant construction.
For the cloning of IL-15 into the EcoRI site of the EGFP-N1 and pcDNA3.1(+) vectors (Invitrogen), the respective coding cDNA sequence was amplified by PCR using the primers IL-15F (5′-CCC GAA TTC CTT TTA GTA ATG AGA ATT TCG AAA CCA) and IL-15R (5′-CCC GAA TTC TAA AGA AGT GTT GAT GAA CAT). The vector pcDNA3.1 Myc/His, containing human IL-15Rα, was kindly provided by Y. Jacques. Vectors containing the human IL-2Rβ (IRATp970F0743D) and IL-2Rγ (IRAUp969A1273D) subunit genes were obtained from the German Resource Center for Genome Research (Berlin, Germany).
For the deletion of the sushi domain (ΔSD) and membrane anchor (ΔMD) of IL-15Rα, an inverse PCR strategy was adapted. Primers were designed in such a way that PCR amplification resulted in the amplification of the complete plasmid and the introduction of the desired mutation. For this, the following primers were used: IL-15RαΔMD-1 (5′-TAC CTC AAG TCA AGG CAA ACT C), IL-15RαΔMD-2 (5′-AGT GGT GTC GCT GTG GCC CTG), IL-15RαΔSD-1 (5′-AGA GAC CCT GCC CTG GTT CAC), and IL-15RαΔSD-2 (5′-CGT GAT GCC CCG CGT CGC). The sequence integrity of the cloned IL-15 and IL-15Rα deletion mutants used in this study was verified with the Perkin Elmer ABI PRISM 377 DNA sequencer (Waltham, MA) using the manufacturer's chemicals and protocols.
Protein isolation and Western blotting.
Membrane protein separations were performed with a membrane protein extraction kit from BioVision (Mountain View, CA). For this, 8 × 107 cells were transfected and collected by scraping. Membrane protein extraction was performed according to the manufacturer's protocol. After the extraction of the plasma membrane proteins, the remaining membrane proteins were considered the endomembrane proteins.
IL-15RαΔMD was isolated from the supernatants using the C-terminal polyhistidine tag fused to IL-15RαΔMD. For this, 5 × 107 cells were transfected, and after 48 h the supernatant was collected and concentrated. Subsequently, IL-15RαΔMD was isolated from the concentrated supernatant using the Probond purification system (Invitrogen) according the manufacturer's protocol for native conditions.
All obtained fractions were analyzed by Western blotting. For the analysis of cell lysates, cells were lysed for 20 min in 1% Nonidet P-40 extraction buffer consisting of 20 mM Tris-HCl, pH 8.0, 15 mM NaCl, 2 mM EDTA, 10 mM NaF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 10 mM phenylmethylsulfonyl fluoride (PMSF), and 100 μM vanadate. Detergent-insoluble material was removed by centrifugation at 13,000 rpm for 10 min. For Western blotting, nitrocellulose membranes (Invitrogen) were used and, depending on the antibody used, membranes were blocked with 5% bovine serum albumin (BSA) (c-myc) or with 0.5% skin milk-4.5% BSA for 2 h at room temperature. In all experiments, β-actin was used as the loading control.
Enzymatic analyses.
For N-glycosidase treatment, 1.2 × 106 cells were transfected, and cell lysates were prepared with 1% Nonidet P-40 extraction buffer. Lysates were divided in equal amounts and incubated for 3 h at 37°C with or without N-glycosidase F (1 U; Roche) and N-glycosidase H (0.5 mU; Roche) and were analyzed by Western blotting. For the proteinase K treatment of whole cells, 6 × 105 cells were transfected and cells were collected by scratching. The cells were incubated for 30 min on ice with proteinase K, after which the reaction was stopped by the addition of 10 mM PMSF (final concentration). Finally, the cells were lysed for 20 min in 1% Nonidet P-40 extraction buffer, and the obtained lysates were analyzed by Western blotting.
ELISA and FACS analysis.
For ELISA analysis, the supernatant of 6 × 105 cells was collected and concentrated 20 times with Vivaspin columns purchased from Vivascience AG (Hannover, Germany). The ELISA of IL-15 and IL-15Rα was performed using the DuoSet ELISA kit for human IL-15 or separate components purchased from R&D Systems. The absorbance at 450 nm was determined using chromogenic substrate obtained from R&D Systems with a Dynatech ELISA reader (Rückersdorf, Germany).
For FACS analysis, 6 × 105 cells were transfected and collected using Accutase, which was obtained from PAA Laboratories, GmbH (Pasching, Austria). Cells were washed twice in FACS buffer (2% newborn calf serum, 0.1% NaN3, 2 mM EDTA in phosphate-buffered saline) and stained with biotinylated antibodies against IL-15Rα (R&D Systems) after the incubation with phycoerythrin-labeled streptavidin (Dianova). To detect IL-15 on the cell surface, monoclonal anti-human IL-15 antibodies were labeled with an Alexa Fluor 647 monoclonal antibody labeling kit, purchased from Invitrogen, according to the manufacturer's instructions. The acidic stripping of transfected COS-7 cells was performed as described earlier (42). Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) according to standard protocols. For surface staining, gates on viable cells were set according to the exclusion of propidium iodide staining. IL-15 and/or IL15Rα expression on GFP-positive cells was analyzed.
RESULTS
The cell surface localization of IL-15 is IL-15Rα dependent.
To investigate whether IL-15Rα is required for cell surface localization, intracellular trafficking, and the secretion of IL-15, the following cell types were used as experimental models. COS-7 cells were selected because they express neither IL-15 nor IL-15Rα and can be transfected with high efficiency, PC-3 cells because they express membrane-bound IL-15 but no IL-15Rα, and HeLa cells because they express both the cytokine and the receptor. COS-7 cells were transfected with a plasmid coding for the long isoform of IL-15 fused to GFP, either in the absence or in the presence of the high-affinity IL-15Rα chain expression vector. IL-15 and/or IL-15Rα expression was analyzed by FACS.
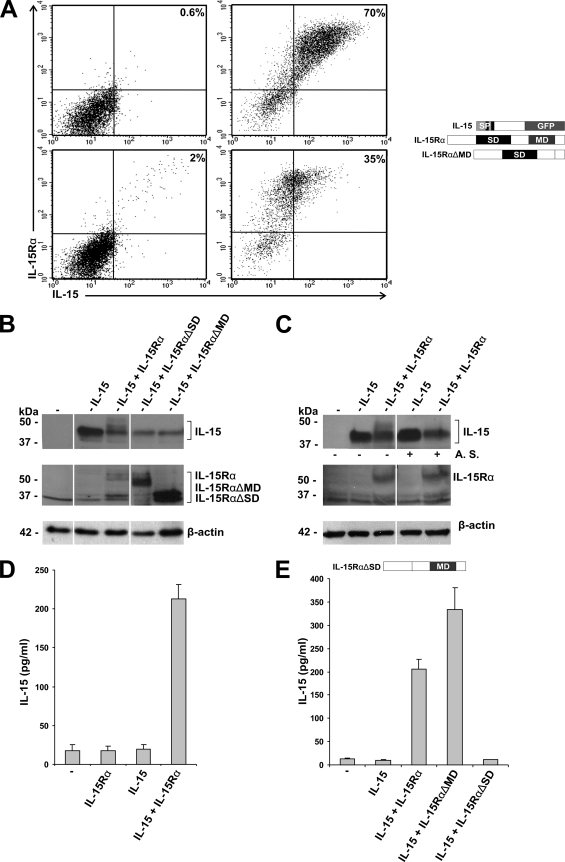
As shown in Fig. 1A (upper left), all GFP IL-15 mRNA-expressing (i.e., all GPF+) COS-7 cells failed to show IL-15 membrane immunoreactivity (i.e., membrane-bound IL-15 protein) if IL-15Rα was absent. However, upon IL-15Rα coexpression, COS-7 cells displayed membrane-associated IL-15 (Fig. 1A, upper right). This suggests that the formation of an IL-15/IL-15Rα complex is required for IL-15 membrane expression. To clarify whether the formation of an IL-15/IL-15Rα complex is essential for IL-15 membrane expression, we cotransfected IL-15 with a mutated IL-15Rα that lacks its membrane-spanning domain (IL-15RαΔMD). A FACS analysis of GFP-positive cells clearly demonstrated that, in the absence of membrane-bound IL-15Rα, no IL-15 could be detected on the cell surface (Fig. 1A, lower left). This confirms that binding to IL-15Rα is a prerequisite for the cell surface localization of IL-15 protein in COS-7 cells. This was underscored by the observation that cell surface IL-15 protein expression diminished after treatment with acidic buffer, which disrupts binding between IL-15 and IL-15Rα (Fig. 1A, lower right).
FIG. 1.
Cell surface localization and secretion of IL-15 in COS-7 cells is IL-15Rα dependent. (A) FACS analysis of COS-7 cells transfected with IL-15 (upper left), IL-15 and IL-15Rα (upper right), IL-15 and IL-15RαΔMD (lower left), and IL-15 and IL-15Rα after being stripped with acidic buffer (lower right). Only GFP-positive cells were analyzed for the cell surface localization of IL-15 and IL-15Rα. (B) Western blot analysis of cell lysates from COS-7 cells transfected with empty vector (−), IL-15, IL-15 and IL-15Rα, IL-15 and IL-15RαΔSD, or IL-15 and IL-15RαΔMD. (C) Western blot analysis of cell lysates from COS-7 cells transfected with empty vector (−), IL-15, or IL-15 and IL-15Rα. After collection, half of the cells were left untreated (−) and half of the cells were stripped (+) with an acidic buffer (A. S.). (D) Specific ELISA showing IL-15 concentrations present in the supernatants of COS-7 cells transfected with empty vector (−), IL-15Rα, IL-15, or IL-15 and IL-15Rα. (E) Specific ELISA showing IL-15 concentrations present in the supernatants of COS-7 cells transfected with empty vector (−), IL-15, IL-15 and IL-15Rα, IL-15 and IL-15RαΔMD, or IL-15 and IL-15RαΔSD.
Western blot analysis revealed that, in the presence of IL-15Rα, a portion of the intracellular IL-15 appeared as higher-molecular-mass bands (Fig. 1B). Interestingly, cell lysates of COS-7 cells cotransfected with IL-15 and IL-15RαΔMD did not show such higher-molecular-mass bands for IL-15 in Western blot analysis (Fig. 1B). Moreover, acidic stripping (Fig. 1C) and incubation with proteinase K led to the specific disappearance (data not shown) of the higher-molecular-mass IL-15 bands that formed in the presence of IL-15Rα. This suggests that the higher-molecular-mass IL-15 bands correlate with the cell surface expression of IL-15. Taken together, these data show that, in COS-7 cells, membrane-associated IL-15 is directed to the cell surface and appears as a higher-molecular-mass IL-15 form in a strictly IL-15Rα-dependent manner.
IL-15 secretion specifically depends on the α-chain of the IL-15 receptor in a wide variety of cells.
Next, we asked whether the coexpression of IL-15Rα guarantees IL-15 secretion. To this end, the supernatant of transfected COS-7 cells was collected 48 h after transfection and subsequently analyzed by ELISA. As shown in Fig. 1D, only the cotransfection of IL-15 with IL-15Rα resulted in significant IL-15 secretion. In the absence of IL-15Rα, none or only very small amounts of cytokine were detected.
To further dissect the molecular requirements for an efficient IL-15 secretion by binding to IL-15Rα, IL-15 was transfected in COS-7 cells alone or together with an IL-15Rα expression vector that lacked the IL-15 binding domain, the so-called sushi domain (IL-15RαΔSD [34]), or with IL-15Rα lacking the membrane-spanning domain (IL-15RαΔMD). Supernatants were analyzed by specific ELISA (Fig. 1E). While IL-15 cotransfection with IL-15RαΔSD failed to induce any cytokine secretion, coexpression with IL-15RαΔMD resulted in an even higher level of secretion of IL-15 than coexpression with wild-type IL-15Rα. The observed differences did not reflect differential expression levels (Fig. 1B), and only the deletion of the IL-15 binding domain of IL-15Rα prevented IL-15 secretion. These results verify that IL-15 secretion requires IL-15-IL-15Rα binding, for which the sushi domain of IL-15Rα is critical.
Comparable results were obtained with an expression vector coding only for IL-15 and not for the GFP fusion protein (data not shown).
That only the presence of the IL-15Rα chain and not of other IL-15R components allowed efficient IL-15 secretion was shown in additional experiments: cotransfection with IL-2Rβ, IL-2Rγ, or both of these other IL-15 receptor chains together did not stimulate IL-15 secretion (data not shown).
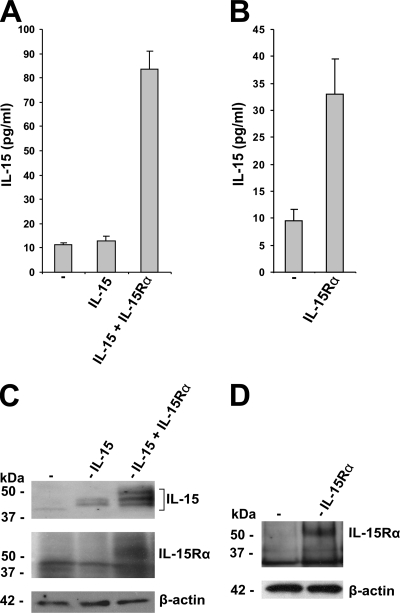
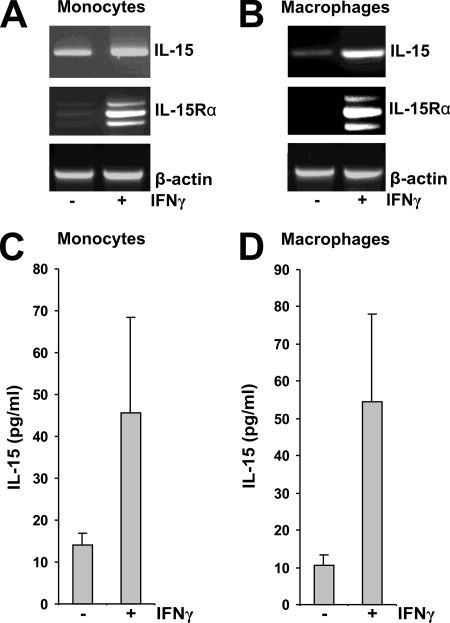
To assess whether the IL-15Rα dependence of IL-15 secretion is limited to COS-7 cells, additional cell types were examined. Comparable results were obtained with the human PC-3 cell line (Fig. 2A and C). Moreover, the transfection of PC-3 cells, which express only low levels of IL-15 (5), with IL-15Rα induced IL-15 secretion (Fig. 2B and D). In addition, PC-3 cells revealed an IL-15Rα-induced IL-15 pattern in Western blots upon transfection that was identical to that seen in COS-7 cells (Fig. 2C). In human HeLa and HEK293 cells, the secretion of IL-15 was found to be equally IL-15Rα dependent (data not shown). This suggests that the IL-15Rα dependence of IL-15 secretion represents a general mechanism of regulating IL-15 secretion in a wide variety of distinct cell types. Finally, we observed that in human monocytes and macrophages, IFN-γ stimulated the expression of both IL-15 and IL-15Rα (Fig. 3A and B), and this results in the augmented secretion of IL-15 (Fig. 3C and D). This not only further supports the concept that up-regulating the level of intracellular IL-15Rα availability also stimulates IL-15 secretion but also points to one concrete stimulus (IFN-γ in this instance) by which regulator-dependent cytokine secretion can be enhanced.
FIG. 2.
IL-15 secretion is IL-15Rα dependent in PC-3 cells. (A) Specific ELISA showing IL-15 concentrations present in the supernatants of PC-3 cells transfected with empty vector (−), IL-15, or IL-15 and IL-15Rα. (B) Specific ELISA showing IL-15 concentrations present in the supernatants of PC-3 cells transfected with empty vector (−) or IL-15Rα. (C and D) Western blot analysis of the respective cell lysates.
FIG. 3.
IFN-γ-stimulated IL-15 and IL-15Rα expression results in the secretion of IL-15 in human monocytes and macrophages. (A and B) Reverse transcription-PCR showing IL-15 and IL-15Rα expression in unstimulated and IFN-γ (10 U/ml)-stimulated monocytes and macrophages. (C and D) Specific ELISA showing IL-15 concentrations present in the supernatants of unstimulated (−) and IFN-γ (10 U/ml)-stimulated (+) monocytes and macrophages.
IL-15 translocation into the ER is IL-15Rα independent.
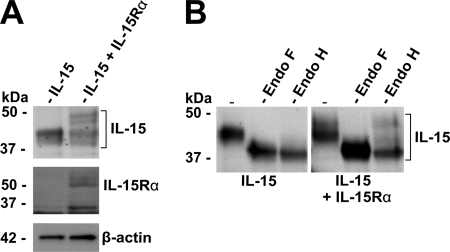
We next investigated the route and the mechanisms that IL-15Rα employs to efficiently drive the secretion of its own ligand. To determine, first, whether the IL-15 translocation into the ER is under the control of IL-15Rα, we began by analyzing the N glycosylation of IL-15. In the presence of IL-15Rα, at least two additional molecular mass bands could be detected that ran higher than those for IL-15 (Fig. 4A). Since IL-15 contains two functional N-glycosylation sites (32) and since the initial N glycosylation of secreted proteins takes place exclusively during the translocation into the ER (15), we determined if the different bands corresponded to N-glycosylated IL-15 products by incubation with N-glycosidases and Western blot analysis.
FIG. 4.
Translocation of IL-15 into the ER is IL-15Rα independent. (A) Western blot analysis of cell lysates from COS-7 cells transfected with IL-15 or IL-15 and IL-15Rα. (B) Western blot analysis of cell lysates from COS-7 cells transfected with IL-15 or IL-15 and IL-15Rα. Before analysis, the cell lysates were incubated alone (−), with Endo F, or with Endo H.
Interestingly, all cellular IL-15 turned out to be N glycosylated independently of the presence of IL-15Rα (Fig. 4B). However, only the IL-15 protein that had been modified in an IL-15Rα-dependent manner turned out to be endoglycosidase H (Endo H) resistant (Fig. 4B). Since such Endo H resistance is known to be acquired by additional modifications during transport through the Golgi apparatus (49), our data suggest that IL-15Rα is dispensable for IL-15 translocation into the ER but is required for the successful Golgi apparatus trafficking of IL-15. Thus, IL-15 does not need to interact with IL-15Rα to efficiently translocate into the ER.
Uncomplexed IL-15 is retained intracellularly.
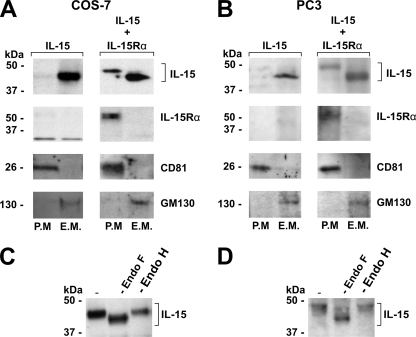
That uncomplexed IL-15 enters the ER but does not reach the cell surface suggests that, in the absence of IL-15Rα, IL-15 is retained within an intracellular compartment. To probe this concept, we isolated membrane proteins of transfected COS-7 and PC-3 cells, separated plasma membrane and endomembrane proteins, and determined the presence of IL-15 in both fractions. Indeed, in the absence of IL-15Rα, no IL-15 could be detected in the plasma membrane fraction, whereas the coexpression of IL-15Rα strongly enhanced the presence of IL-15 in the plasma membrane (Fig. 5A and B). Moreover, only the Endo H-resistant fraction was present in the plasma membrane (Fig. 5C and D). This confirms that the plasma membrane localization of IL-15 depends on IL-15Rα and involves Golgi apparatus transport. Nevertheless, even in the presence of IL-15Rα, a large portion of intracellular IL-15 still was present in the endomembrane fractions of both cell types. This implies that a portion of the cytokine remains intracellularly and membrane associated.
FIG. 5.
IL-15 is retained within an intracellular compartment. (A and B) Western blot analysis of isolated endomembrane proteins (E.M.) and plasma membrane proteins (P.M.) from COS-7 (A) and PC-3 (B) cells transfected with IL-15 (left columns) or IL-15 and IL-15Rα (right columns). The presence of GM130 (Golgi apparatus) and CD81 (plasma membrane) was determined to verify the purity of both fractions. (C and D) Western blot analysis of IL-15 present in the plasma membrane fraction of COS-7 (C) and PC-3 cells (D), cotransfected with IL-15 and IL-15Rα, prior to and after treatment with Endo F or Endo H.
Since this would have been well beyond the focus of the current study, we did not assess the effect of proteasome inhibitors extensively on the stability of IL-15 in the absence of the receptor chain. However, in COS-7 cells, the intracellularly retained IL-15 likely is targeted for degradation by the proteasome. Sometimes we saw a pattern of higher-molecular-mass bands in the Western blot analysis of cell lysates that had been obtained from COS-7 cells and that had been transfected with IL-15. This is typical for polyubiquitination (32). Furthermore, intracellular IL-15 also can be partially stabilized with the proteasome inhibitor lactacystin; however, this does not result in increased IL-15 secretion levels (data not shown). Therefore, one can presume that, in COS-7 cells, the intracellularly retained IL-15 is targeted for degradation by the proteasome.
IL-15 secretion depends on proteinase-mediated shedding of membrane IL-15/IL-15Rα complexes.
The results described above are in line with the concept that membrane-bound IL-15/IL-15Rα complexes represent an intermediate state during the IL-15Rα-dependent secretion of IL-15, and that the final release of IL-15 into the medium occurs by the dissociation of the complex and/or by the proteolytic release of the IL-15/IL-15Rα complexes. The latter had already been shown for uncomplexed IL-15Rα, which is released due to metalloproteinase activity (3). In COS-7 cells, IL-15Rα is constitutively released by an unknown proteolytic activity for which no inhibitor is known. Upon PMA stimulation, an additional proteolytic activity is induced, resulting in increased IL-15Rα release into the environment that can be inhibited by the general matrix metalloproteinase inhibitor GM6001 (7, 39). PMA stimulation did indeed increase the IL-15 secretion of COS-7 cells cotransfected with IL-15 and IL-15Rα and of cells cotransfected with IL-15RαΔMD (data not shown).
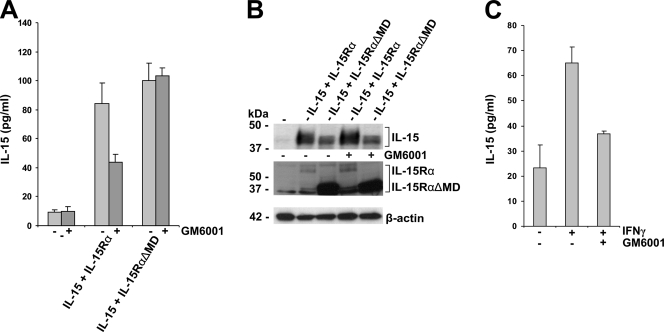
Therefore, to examine the contribution of the proteolytic cleavage of IL-15Rα to the actual secretion of IL-15, we investigated if the secretion of IL-15 upon PMA stimulation can be inhibited by the general matrix metalloproteinase inhibitor GM6001. For this, 48 h after transfection the COS-7 cells were stimulated for 2 h with PMA, after which the medium was exchanged with fresh medium with or without GM6001. The amount of secreted IL-15 was determined by specific ELISA after an additional 8 h. As shown in Fig. 6A and B, GM6001 inhibited the release of IL-15 in cells that coexpress IL-15 with wild-type IL-15Rα. That this was not the case when cells had been transfected with IL-15 and IL-15RαΔMD strongly suggested that the IL-15Rα-dependent secretion of IL-15 depends on the release of cytokine/cytokine receptor complexes by the proteolytic cleavage of IL-15Rα.
FIG. 6.
GM6001 inhibits IL-15Rα-dependent IL-15 secretion. (A) Specific ELISA showing IL-15 concentrations present in the medium of transfected and PMA (100 ng/ml)-stimulated COS-7 cells. After stimulation, the COS-7 cells were cultured for an additional 8 h in the presence (dark gray) or absence (light gray) of the general matrix metalloproteinase inhibitor GM6001 (10 μg/ml). The COS-7 cells were transfected with empty vector (−), IL-15 and IL-15Rα, or IL-15 and IL-15RαΔMD. (B) Western blot analysis of the respective cell lysates revealing the expression of IL-15 and IL-15Rα. (C) Specific ELISA showing IL-15 concentrations present in the medium of unstimulated and IFN-γ (10 U/ml)-stimulated monocytes in the absence and presence of the general matrix metalloproteinase inhibitor GM6001 (10 μg/ml).
Further, we have tested whether or not TIMP-2 inhibits the proteolytic release of IL-15 into the environment (TIMP-2 has been reported to be active against various matrix metalloproteinases and is expressed by a variety of cell types [16]). For this, 48 h after transfection, the COS-7 cells were stimulated for 2 h with PMA, after which the medium was exchanged by fresh medium with or without TIMP-2. The amount of secreted IL-15 was determined by specific ELISA after an additional 8 h. This experiment revealed that TIMP-2 addition did not reduce the amount of IL-15 in the supernatant (data not shown). Therefore, TIMP-2 is at least unlikely to be critically involved in the regulation of IL-15 proteolytic release into the environment.
To investigate whether or not the secretion of IL-15 in stimulated monocytes also is dependent on proteolytic release by matrix metalloproteinases, monocytes were stimulated with IFN-γ for 8 h in the absence or presence of GM6001, and IL-15 concentrations were determined in the supernatants by specific ELISA. As shown in Fig. 6C, the secretion of IL-15 by IFN-γ-stimulated monocytes is indeed strongly inhibited by GM6001, and the amount of IL-15 present in the supernatant is decreased almost to the level of unstimulated monocytes. This shows that, in monocytes, the final release of IL-15 into the environment depends on proteolytic cleavage by a matrix metalloproteinase.
All secreted IL-15 that is complexed with IL-15Rα is Endo H resistant.
We have shown that IL-15 passes the Golgi apparatus only in the presence of IL-15Rα and reaches the cell surface as an Endo H-resistant species (Fig. 5). This implies that the secreted IL-15 present in the supernatant is complexed with IL-15Rα and exhibits Endo H-resistant N glycosylation.
To confirm this, COS-7 and PC-3 cells were cotransfected with IL-15 and IL-15RαΔMD, and 48 h after transfection IL-15RαΔMD was isolated from the supernatant by making use of the C-terminal polyhistidine tag. Western blot analysis of the obtained fractions revealed that IL-15 was indeed coisolated with IL-15RαΔMD (Fig. 7A and B). Moreover, all of this IL-15 had a higher molecular mass than intracellular IL-15 (Fig. 7C and E). Incubation with Endo F and Endo H showed that the N glycosylation of the IL-15 present in the supernatant was Endo H resistant (Fig. 7D and F). Therefore, at least a large portion of the IL-15 present in the supernatant is complexed with IL-15Rα and then is secreted via the classical secretory pathway.
FIG. 7.
IL-15 secreted by COS-7 and PC-3 cells is complexed with IL-15Rα and is Endo H resistant. (A and B) Western blot analysis of the first seven fractions obtained after the isolation of IL-15RαΔMD from the supernatant of COS-7 (A) and PC-3 (B) cells cotransfected with IL-15 and IL-15RαΔMD. (C and E) Western blot analysis of intracellular and secreted IL-15 of COS-7 (C) and PC-3 (E) cells cotransfected with IL-15 and IL-15RαΔMD. (D and F) Western blot analysis of complexed IL-15, present in the supernatant of COS-7 (D) and PC-3 (F) cells, cotransfected with IL-15 and IL-15RαΔMD prior to and after Endo F or Endo H treatment.
DISCUSSION
Studying the IL-15/IL-15Rα system as a model, we demonstrate here for the first time that a cytokine receptor component, besides engaging in signal transduction, plays an important additional role in the control of ligand transport, cell surface expression, and secretion. By showing that IL-15Rα determines IL-15 trafficking and secretion, we provide the first evidence for cytokine chaperoning early in the secretory pathway via a cytokine complexing with a cognate receptor (Fig. 8). Specifically, we demonstrate in different cell types that IL-15Rα is essential for the secretion of IL-15 via the classical secretion pathway, involving the ER and Golgi passage, and provide evidence that besides transcriptional and translational regulation, an additional, unique mechanism exists by which IL-15 secretion ultimately can be regulated.
FIG. 8.
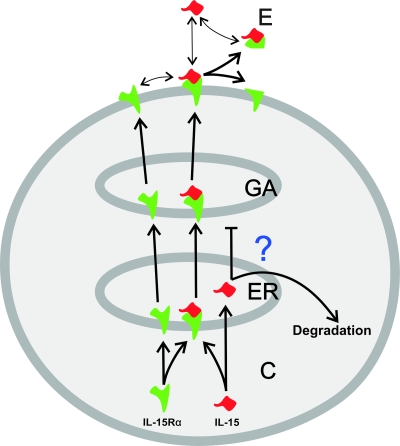
Receptor chaperoning model of the cytokine. Shown is a proposed model for the intracellular trafficking and IL-15Rα-dependent secretion of IL-15 in the investigated cell lines. The abbreviations represent cytoplasm (C), Golgi apparatus (GA), and environment (E).
Taken together, our data suggest the following mechanistic scenario by which IL-15Rα induces IL-15 secretion (Fig. 8). IL-15 is translocated into the ER independently of IL-15Rα. However, IL-15Rα then attains a crucial role, since IL-15 fails to continue its way through the secretory pathway in the absence of IL-15Rα (by an as-yet unknown mechanism). In the presence of IL-15Rα, IL-15 forms a complex with its own receptor, which then is further transported through the Golgi apparatus and translocated into the plasma membrane by using the conventional mechanisms employed for membrane proteins. Finally, the cell surface-localized complex is released due to the proteolytic activity of matrix metalloproteinases, resulting in soluble IL-15/IL-15Rα complexes in the environment. We do not know whether or not uncomplexed IL-15 is released due to the dissociation of the IL-15/IL-15Rα complex in unstimulated COS-7 and other cells. However, based on the extremely high binding affinity between IL-15 and IL-15Rα, this phenomenon can only play a minor role in the secretion of IL-15.
Recently, the stimulatory effect of IL-15Rα on IL-15 secretion was reported for human 293 cells. In that study, the authors observed a mutually stabilizing effect upon IL-15Rα/IL-15 complex formation that resulted in higher production levels in human 293 cells as well as in mice in vivo (2). Although we did not specifically study the stability of IL-15Rα and IL-15, in view of this study, we acknowledge the possibility that the stabilization of IL-15 by complexing with IL-15Rα had a stimulatory effect on the secretion of its ligand. Taking these possibilities together, we propose that two additive mechanisms, stabilization and mobilization by IL-15Rα, regulate the secretion of its ligand.
Numerous studies have provided compelling evidence for the in vivo role of such an IL-15Rα-dependent trafficking of IL-15. IL-15 mainly functions as a membrane-associated cytokine, acting, e.g., at immunological synapses being presented in trans to the target cell during cell-cell contact (18, 36). It has been shown that, e.g., murine dendritic cells require the expression of IL-15Rα to prime NK cells, which suggests that membrane-bound IL-15/IL-15Rα complexes are essential for this cell-cell interaction (31). The mobilization mechanism described here results in cell surface-located IL-15 and represents an efficient way for IL-15 trans presentation.
As IL-15 mainly functions as a membrane-associated cytokine, differences in the glycosylation also might modulate cell differentiation and/or survival. For example, T-cell activation and differentiation are accompanied by changes in the expression of glycosyltransferases and glycosidases, Golgi enzymes that remodel N- and O-linked glycans. The conversion of activated T cells to memory cells or the differentiation of Th cells into Th1, Th2, or Th-17 subsets changes cell surface glycosylation profiles, cell functions, and their susceptibility to cell death (38, 62). Whether or not the glycosylation of IL-15 varies among cell types is not known. However, as recombinant IL-15 produced in Escherichia coli, which is not glycosylated, is active and capable of inducing, e.g., proliferation, one can presume that the N glycosylation is not essential for some of the reported IL-15 biological functions.
Our findings offer an explanation for the long-misunderstood low levels of IL-15 protein that are detectable upon stimulation in the supernatants of a limited number of cell types despite abundant IL-15 transcription (7, 40, 65). Strikingly, all IL-15-secreting cell types coexpress IL-15 and IL-15Rα (21, 40, 49), and in monocytes and macrophages IFN-γ-induced IL-15 secretion is associated with the up-regulation of the expression of both the cytokine and its receptor (Fig. 3). Moreover, the release of IL-15 by IFN-γ-stimulated monocytes is dependent on proteolytic cleavage and can be inhibited by the general matrix metalloproteinase inhibitor GM6001 (Fig. 6C).
Recently, we have shown that mouse fibroblasts, dendritic cells, and macrophages express an alternatively spliced, soluble isoform of IL-15Rα (7). Although such a soluble receptor isoform that lacks a membrane-spanning domain has not yet been identified for human IL-15Rα, in theory, the production of the IL-15Rα isoform is an efficient additional tool that enables cells to switch between cytokine trans presentation (e.g., for juxtacrine signaling) and paracrine signaling via soluble cytokine/receptor complexes. Both soluble IL-15Rα and soluble IL-15/IL-15Rα complexes have been reported to exhibit biological activities (7, 39, 52). Soluble IL-15Rα can function as a specific high-affinity IL-15 antagonist. In contrast, soluble IL-15/IL-15Rα complexes exhibit a strong agonistic activity. This agonistic activity is mediated through membrane-bound IL-15 receptor β and γ heterodimers and enables signaling to cells that only express the β- and γ-receptor chains, which also are part of the IL-2 receptor complex (22).
The mechanism described here results in the secretion of soluble IL-15/IL-15Rα complexes, which, based on this high binding affinity, will not dissociate and hence will not result in the secretion of uncomplexed IL-15. This raises the question of whether or not uncomplexed IL-15 is secreted at all. Earlier studies have reported the presence of low levels of IL-15 in the medium in the absence of IL-15Rα (32, 42), a phenomenon that we could observe only using higher DNA concentrations for transfection (≥4 μg) (data not shown). Therefore, it is possible that only with high IL-15 transcript concentration or in selected cell types does IL-15 manage to continue its way through the secretory pathway on its own. For example, monocytes, keratinocytes, and PC-3 cells possess another membrane-associated form of IL-15 (5, 40), which is insensitive to acidic stripping and not susceptible to proteolysis by trypsin and does not involve complex formation with IL-15Rα. This additional, still undiscovered, IL-15Rα-independent pathway by which uncomplexed IL-15 can be transported to the membrane could result in the secretion of uncomplexed IL-15.
The current mechanism by which the secretion of a cytokine is chaperoned through the secretory pathway by its receptor at a very early stage of secretion control is remarkable and unique. It has previously been shown that a cognate receptor chain (i.e., IL-4Rα) contributes to the mobilization and secretion of its ligand (IL-4) in eosinophils (58). In the case of IL-4, both receptor and ligand first are independently transported and stored in granules. In striking contrast, IL-15 requires complex formation with IL-15Rα for efficient secretion in the ER, and the secretion seems to be independent of secretory granules.
Nevertheless, later steps in the IL-15Rα-dependent secretion of IL-15 follow the well-recognized, conventional steps of secretion for heterodimeric cytokines. For example, IL-12 is a heterodimeric molecule composed of an α- and a β-chain, which are both glycosylated and encoded by genes located on separate chromosomes (57). In analogy to IL-15/IL-15Rα secretion, several studies report that the secretion of the α-chain is dependent on the intracellular formation of an α/β-chain heterodimer (63). In addition, the α-chain of both IL-12 and IL-15 possess a long signal peptide harboring two putative cleavage sites (40, 61).
In summary, we present a unique mechanism by which the cytokine IL-15 is chaperoned through the secretory pathway by its high-affinity receptor α-chain. Although at first glance this seems to be cumbersome, it provides a sophisticated mechanism by which to fine-tune the release of IL-15. In addition, it equips the cell with an efficient tool for regulating juxtacrine signaling via IL-15 trans presentation and enables signaling toward recipient cells that do not express IL-15Rα but do express the heterodimeric IL-2Rβ and γ-chain receptor complex.
Acknowledgments
We thank Yannick Jacques for kindly providing the plasmid pcDNA3.1 Myc/His, containing human IL-15Rα, and Enno Hartmann and Kai-Uwe Kalies for excellent discussions. Furthermore, we are grateful to Jürgen Bernhagen and Niko Föger for stimulating discussions and helpful editorial advice. Finally, the expert technical assistance of Martina and Manuel Hein is most gratefully acknowledged.
This work was supported by the Deutsche Forschungs Gemeinschaft grant SFB415, A10 to S.B.P.
Footnotes
Published ahead of print on 27 May 2008.
REFERENCES
- 1.Bamford, R. N., A. J. Grant, J. D. Burton, C. Peters, G. Kurys, C. K. Goldman, J. Brennan, E. Roessler, and T. A. Waldmann. 1994. The interleukin (IL) 2 receptor β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA 914940-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergamaschi, C., M. Rosati, R. Jalah, A. Valentin, V. Kulkarni, C. Alicea, G. M. Zhang, V. Patel, B. K. Felber, and G. N. Pavlakis. 2008. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J. Biol. Chem. 2834189-4199. [DOI] [PubMed] [Google Scholar]
- 3.Budagian, V., E. Bulanova, Z. Orinska, A. Ludwig, S. Rose-John, P. Saftig, E. C. Borden, and S. Bulfone-Paus. 2004. Natural soluble interleukin-15Rα is generated by cleavage that involves the tumor necrosis factor-alpha-converting enzyme (TACE/ADAM17). J. Biol. Chem. 27940368-40375. [DOI] [PubMed] [Google Scholar]
- 4.Budagian, V., E. Bulanova, Z. Orinska, T. Pohl, E. C. Borden, R. Silverman, and S. Bulfone-Paus. 2004. Reverse signaling through membrane-bound interleukin-15. J. Biol. Chem. 27942192-42201. [DOI] [PubMed] [Google Scholar]
- 5.Budagian, V., E. Bulanova, Z. Orinska, A. Tho, U. Mamat, P. Bellosta, C. Baslico, D. Adam, R. Paus, and S. Bulfone-Paus. 2005. A promiscuous liaison between IL-15 receptor and Axl receptor tyrosine kinase in cell death control. EMBO J. 244260-4270. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Budagian, V., E. Bulanova, R. Paus, and S. Bulfone-Paus. 2006. IL15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 4259-280. [DOI] [PubMed] [Google Scholar]
- 7.Bulanova, E., V. Budagian, E. H. Duitman, Z. Orinska, H. Krause, R. Rückert, N. Reiling, and S. Bulfone-Paus. 2007. Soluble interleukin (IL)-15Rα is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem. 28213167-13179. [DOI] [PubMed] [Google Scholar]
- 8.Bulanova, E., V. Budagian, T. Pohl, H. Krause, H. Dürkop, R. Paus, and S. Bulfone-Paus. 2001. The IL-15Rα chain signals through association with Syk in human B cells. J. Immunol. 1676292-6302. [DOI] [PubMed] [Google Scholar]
- 9.Bulanova, E., V. Budagian, Z. Orinska, H. Krause, R. Paus, and S. Bulfone-Paus. 2003. Mast cells express novel functional IL-15 receptor alpha isoforms. J. Immunol. 1705045-5055. [DOI] [PubMed] [Google Scholar]
- 10.Bulfone-Paus, S., D. Ungureanu, T. Pohl, G. Lindner, R. Paus, R. Rückert, H. Krause, and U. Kunzendorf. 1997. Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 31124-1128. [DOI] [PubMed] [Google Scholar]
- 11.Bulfone-Paus, S., E. Bulanova, T. Pohl, V. Budagian, H. Dürkop, R. Rückert, U. Kunzendorf, R. Paus, and H. Krause. 1999. Death deflected: IL-15 inhibits TNFα-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J. 131575-1585. [DOI] [PubMed] [Google Scholar]
- 12.Bulfone-Paus, S., E. Bulanova, V. Budagian, and R. Paus. 2006. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays 4362-377. [DOI] [PubMed] [Google Scholar]
- 13.Burgess, T. L., and R. B. Kelly. 1987. Constitutive and regulated secretion of proteins. Annu. Rev. Cell Biol. 3243-293. [DOI] [PubMed] [Google Scholar]
- 14.Conradt, H. S., M. Ausmeier, K. E. Dittmar, H. Hauser, and W. Lindenmaier. 1986. Secretion of glycosylated human interleukin-2 by recombinant mammalian cell lines. Carbohydr. Res. 149443-450. [DOI] [PubMed] [Google Scholar]
- 15.Dempski, R. E., and B. Imperriali. 2002. Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr. Opin. Chem. Biol. 6844-850. [DOI] [PubMed] [Google Scholar]
- 16.Docherty, A., J. Lyons, A. Smith, B. J. Wright, E. M. Stephens, P. E. Harris, T. J. G. Murphy, and J. J. Reynolds. 1985. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature 31866-69. [DOI] [PubMed] [Google Scholar]
- 17.Dubois, S., F. Magrangeas, P. Lehours, S. Raher, J. Bernard, O. Boisteau, S. Leroy, S. Minvielle, A. Godard, and Y. Jacques. 1999. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J. Biol. Chem. 27426978-26984. [DOI] [PubMed] [Google Scholar]
- 18.Dubois, S., J. Mariner, T. A. Waldmann, and T. Tagaya. 2002. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity 17537-547. [DOI] [PubMed] [Google Scholar]
- 19.Farag, S. S., and M. A. Caligiuri. 2006. Human natural killer cell development and biology. Blood Rev. 20123-137. [DOI] [PubMed] [Google Scholar]
- 20.Gaggero, A., B. Azzarone, C. Andrei, Z. Mishal, R. Meazza, E. Zappia, A. Rubartelli, and F. Ferrini. 1999. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur. J. Immunol. 291265-1274. [DOI] [PubMed] [Google Scholar]
- 21.Ge, N., Y. Nishioka, Y. Nakamura, Y. Okano, K. Yoneda, H. Ogawa, A. Sugita, H. Yanagawa, and S. Sone. 2004. Synthesis and secretion of interleukin-15 by freshly isolated human bronchial epithelial cells. Int. Arch. Allergy. Immunol. 135235-242. [DOI] [PubMed] [Google Scholar]
- 22.Giri, J. G., M. Ahdieh, J. Eisman, K. Shanebeck, K. Grabstein, S. Kumaki, A. Namen, L. S. Park, D. Cosman, and D. M. Anderson. 1994. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 132822-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giri, J. G., S. Kumaki, M. Ahdieh, D. J. Friend, A. Loomis, K. Shanebeck, R. DuBose, D. Cosman, L. S. Park, and D. M. Anderson. 1995. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 143654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin, R. G., S. Lupton, A. Schmierer, K. J. Hjerrild, R. Jerzy, W. Clevenger, S. Gillis, D. Cosman, and A. E. Namen. 1989. Human interleukin 7: molecular cloning and growth factor activity on human and murine B lineage cells. Proc. Natl. Acad. Sci. USA 86302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Görlich, D., S. Prehn, E. Hartmann, J. Herz, A. Otto, R. Kraft, M. Wiedmann, S. Knespel, B. Dobberstein, and T. A. Rapoport. 1990. The signal sequence receptor has a second subunit and is part of a translocation complex in the endoplasmic reticulum as probed by bifunctional reagents. J. Cell Biol. 1112283-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabstein, K. H., J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M. A. Schoenborn, M. Ahdieh, L. Johnson, M. R. Alderson, J. D. Watson, D. M. Anderson, and J. G. Giri. 1994. Cloning of a T-cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264965-968. [DOI] [PubMed] [Google Scholar]
- 27.Hansson, M., Y. Gao, H. Rosen, H. Tapper, and I. Olsson. 2004. Hematopoietic secretory granules as vehicles for the local delivery of cytokines and soluble cytokine receptors at sites of inflammation. Eur. Cytokine Netw. 15167-176. [PubMed] [Google Scholar]
- 28.Hofsli, E., O. Bakke, U. Nonstad, and T. Espevik. 1989. A flow cytometric and immunofluorescence microscopic study of tumor necrosis factor production and localization in human monocytes. Cell Immunol. 122405-415. [DOI] [PubMed] [Google Scholar]
- 29.Huse, M., B. F. Lillemeier, M. S. Kuhns, D. S. Chen, and M. M. Davis. 2006. T cells us e two directionally distinct pathways for cytokine secretion. Nat. Immunol. 7247-255. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuk, K. Carrier, L. Sedgers, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191753-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koka, R., P. Burkett, M. Chien, S. Chai, D. L. Boone, and A. Ma. 2004. Cutting edge: murine dendritic cells require IL-15Rα to prime NK cells. J. Immunol. 1733594-3598. [DOI] [PubMed] [Google Scholar]
- 32.Kostova, Z., Y. C. Tsai, and W. A. Weissman. 2007. Ubiquitin ligases, critical mediators of endoplasmic reticulum-associated degradation. Semin. Cell Dev. Biol. 18770-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurys, G., Y. Tagaya, R. Bamford, J. A. Hanover, and T. A. Waldmann. 2000. The long signal peptide isoform and its alternative processing directs the intracellular trafficking of interleukin 15. J. Biol. Chem. 27530653-30659. [DOI] [PubMed] [Google Scholar]
- 34.Lodolce, J. P., D. L. Boone, S. Chai, R. E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9669-976. [DOI] [PubMed] [Google Scholar]
- 35.Lorenzen, I., A. J. Dingley, Y. Jacques, and J. Grötzinger. 2006. The structure of the interleukin-15 alpha receptor and its implications for ligand binding. J. Biol. Chem. 2816642-6647. [DOI] [PubMed] [Google Scholar]
- 36.Lucas, M., W. Schachterle, K. Oberle, P. Aichele, and A. Diefenbach. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26503-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meazza, R., S. Verdiani, R. Biassoni, M. Coppolecchia, A. Gaggero, A. M. Orengo, M. P. Colombo, B. Azzarone, and S. Ferrini. 1996. Identification of a novel interleukin-15 (IL-15) transcript isoform generated by alternative splicing in human small cell lung cancer cell lines. Oncogene 122187-2192. [PubMed] [Google Scholar]
- 38.Morgan, R., G. Gao, J. Pawling, J. W. Dennis, M. Demetrious, and B. Li. 2004. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J. Immunol. 1737200-7208. [DOI] [PubMed] [Google Scholar]
- 39.Mortier, E., J. Bernard, A. Plet, and Y. Jacques. 2004. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J. Immunol. 1731681-1688. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, F. J., M. P. Hayes, and P. R. Burd. 2000. Dispartate intracellular processing of human IL-12 preprotein subunits: atypical processing of the P35 signal peptide. J. Immunol. 164839-847. [DOI] [PubMed] [Google Scholar]
- 41.Murray, R. Z., J. G. Kay, D. G. Sangermani, and J. L. Stow. 2005. A role for the phagosome in cytokine secretion. Science 3101492-1495. [DOI] [PubMed] [Google Scholar]
- 42.Musso, T., L. Calosso, M. Zucca, M. Millesimo, D. Ravarino, M. Giovarelli, F. Malavasi, A. Ponzi, R. Paus, and S. Bulfone-Paus. 1999. Human monocytes constitutively express membrane-bound, biologically active, and interferon-γ-upregulated interleukin-15. Blood 933531-3539. [PubMed] [Google Scholar]
- 43.Nishimura, H., A. Fujimoto, N. Tamura, T. Yajima, W. Wajjwalku, and Y. Yoshikai. 2005. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J. 1919-28. [DOI] [PubMed] [Google Scholar]
- 44.Onu, A., T. Pohl, H. Krause, and S. Bulfone-Paus. 1997. Regulation of Il-15 secretion via the leader peptide of two IL-15 isoforms. J. Immunol. 158255-262. [PubMed] [Google Scholar]
- 45.Orinska, Z., M. Maurer, F. Mirghomizadeh, E. Bulanova, M. Metz, N. Nashkevich, F. Schiemann, J. Schulmistrat, V. Budagian, J. Giron-Michel, E. Brandt, R. Paus, and S. Bulfone-Paus. 2007. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 13927-934. [DOI] [PubMed] [Google Scholar]
- 46.Pereno, R., J. A. Giron-Michel, A. Gaggero, E. Cazes, R. Meazza, M. Monetti, E. Monaco, Z. Mishal, C. Jasmin, F. Indiveri, S. Ferrini, and B. Azzarone. 2000. IL-15/IL-15Rα intracellular trafficking in human melanoma cells and signal transduction through the IL-15Rα. Oncogene 195153-5162. [DOI] [PubMed] [Google Scholar]
- 47.Ponnambalam, S., and S. A. Baldwin. 2003. Constitutive protein secretion from the trans-Golgi apparatus to the plasma membrane. Mol. Membr. Biol. 20129-139. [DOI] [PubMed] [Google Scholar]
- 48.Reiling, N., A. Blumenthal, H. D. Flad, M. Ernst, and S. Ehlers. 2001. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J. Immunol. 1673339-3345. [DOI] [PubMed] [Google Scholar]
- 49.Revell, P. A., and S. E. Jellie. 1998. Interleukin 15 production by macrophages in the implant interface membrane of aseptically loosened joint replacements. J. Mater. Sci. Mater. Med. 9727-930. [DOI] [PubMed] [Google Scholar]
- 50.Roth, J. 2002. Protein N-glycosylation along the secretory pathway: relationship to organelle topography and function, protein quality control, and cell interactions. Chem. Rev. 102285-303. [DOI] [PubMed] [Google Scholar]
- 51.Rothman, J. E., and R. E. Fine. 1980. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc. Natl. Acad. Sci. USA 77780-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinstein, M. P., M. Kovar, J. F. Purton, J. H. Cho, O. Boymqan, C. D. Surh, and J. Sprent. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl. Acad. Sci. USA 1039166-9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rückert, R., K. Asadullah, M. Seifert, V. Budagian, R. Arnold, C. Trombotto, R. Paus, and S. Bulfone-Paus. 1998. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J. Immunol. 1652240-2250. [DOI] [PubMed] [Google Scholar]
- 54.Sato, N., H. J. Patel, T. A. Waldmann, and Y. Tagaya. 2007. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl. Acad. Sci. USA 104588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schachter, H. 1984. Coordination between enzyme specificity and intracellular compartmentation in the control of protein-bound oligosaccharide biosynthesis. Biol. Cell 51133-145. [DOI] [PubMed] [Google Scholar]
- 56.Schmid, E. F., K. Binder, M. Grell, P. Scheurich, and K. Pfizenmaier. 1995. Both tumor necrosis factor receptors, TNFR60 and TNFR80, are involved in signaling endothelial tissue factor expression by juxtacrine tumor necrosis factor alpha. Blood 861836-1841. [PubMed] [Google Scholar]
- 57.Sieburth, D. E. W., J. A. Jabs, X. Warrington, J. Li, S. Lasota, K. La Forgia, K. Kelleher, K. Huebner, J. J. Wasmuth, and S. F. Wolf. 1992. Assignment of genes encoding a unique cytokine (IL12) composed of two unrelated subunits to chromosomes 3 and 5. Genomics 1459-62. [DOI] [PubMed] [Google Scholar]
- 58.Spencer, L. A., R. C. N. Melo, S. A. C. Perez, S. P. Bafford, A. M. Dvorak, and P. F. Weller. 2006. Cytokine receptor-mediated trafficking of preformed IL-4 eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl. Acad. Sci. USA 1033333-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sprang, S. R., and J. F. Bazan. 1993. Cytokine structural taxonomy and mechanisms of receptor engagement. Curr. Opin. Struct. Biol. 3815-827. [Google Scholar]
- 60.Swanton, E., and S. High. 2006. ER targeting signals: more than meets the eye? Cell 127877-879. [DOI] [PubMed] [Google Scholar]
- 61.Tagaya, Y., G. Kurys, T. A. Thies, J. M. Losi, N. Azimi, J. A. Hanover, R. N. Bamford, and T. A. Waldmann. 1997. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc. Natl. Acad. Sci. USA 9414444-14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toscano, M. A., G. A. Bianco, J. M. Ilarregui, D. O. Croci, J. Correale, J. D. Hernandez, N. W. Zwirner, F. Poirier, E. M. Riley, L. G. Baum, and G. A. Rabinovich. 2007. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8825-834. [DOI] [PubMed] [Google Scholar]
- 63.Vaidyanathan, H., Y. Zhou, T. M. Petro, and S. D. Schwartzbach. 2003. Intracellular localization of the p35 subunit of murine IL-12. Cytokine 7120-128. [DOI] [PubMed] [Google Scholar]
- 64.Verhage, M., and R. F. Toonen. 2007. Regulated exocytosis: merging ideas on fusing membranes. Curr. Opin. Cell Biol. 19402-408. [DOI] [PubMed] [Google Scholar]
- 65.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular patogenes. Annu. Rev. Immunol. 1719-49. [DOI] [PubMed] [Google Scholar]
- 66.Waldmann, T. A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6595-601. [DOI] [PubMed] [Google Scholar]
- 67.Walter, P., and G. Blöbel. 1981. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J. Cell Biol. 91557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang, Y. C., B. A. B. Ciarletta, P. A. Temple, M. P. Chung, S. Kovacic, J. S. Witek-Giannotti, A. C. Leary, R. Kriz, R. E. Donahue, G. G. Wong, and S. C. Clark. 1986. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell 473-10. [DOI] [PubMed] [Google Scholar]