Abstract
In nonprimate mammals, the dorsal cochlear nucleus (DCN) is thought to play a role in the orientation of the head towards sounds of interest by integrating acoustic and somatosensory information (May, 2000; Davis and Young, 2002; Ryugo et al., 2003). Humans and higher primates might not use this system because of reported phylogenetic changes in DCN cytoarchitecture (Moskowitz, 1969; Moore and Osen, 1979; Moore, 1980). In this study, we re-evaluated this question from a comparative perspective and examined the rhesus monkey (cercopithecoid primate) using more sensitive probes and higher resolution imaging methods. We used electron microscopy to identify parallel fibers and their synapses, and molecular markers to determine that primates exhibit the main components of excitatory neurotransmission as other mammals. We observed that characteristics of the monkey molecular layer resembled what has been reported for nonprimates: (1) immunohistochemistry revealed many unmyelinated, thin axons and en passant glutamatergic synapses on dendritic spines; (2) immunohistochemistry for phosphodiesterase (PDE10A) showed the nuclei of granule cells distributed in the external molecular layer and the deep layers in the DCN; (3) antibodies for the inositol trisphosphate receptor (IP3r) and calbindin immunostained cartwheel cells; (4) postembedding immunogold labeling revealed synaptic expression of AMPA and delta glutamate receptor subunits on spines in parallel fiber endings; and (5) parallel fibers use VGLUT1 to package glutamate into the synaptic vesicles and to mediate glutamate transport. These observations are consistent with the argument that the rhesus monkey DCN has similar neuronal features as other nonprimate mammals.
Keywords: spines, glutamate receptors, VGLUT1, calbindin, postembedding immunogold labeling, morphometry, ultrastructure, rhesus monkey
Introduction
The DCN receives direct input from the cochlea by way of the auditory nerve (Sando 1965; Osen 1970; Fekete et al. 1984). In nonprimate mammals, the cellular organization of the DCN is thought to play an important role in the orientation of the head towards sounds of interest by integrating acoustic and somatosensory information (May, 2000; Davis and Young, 2002). Whether this is also the case in man and higher primates is unclear because of reports that the DCN and associated granule cell regions undergo pronounced phylogenetic changes. Studies of the human cochlear nuclei described a complete and selective loss of the superficial layers of the nuclei, whereas deeper regions of the nuclei maintained the basic cytoarchitecture plan of mammals (Moskowitz, 1969; Moore and Osen, 1979; Heiman-Patterson and Strominger, 1985).
A transitional stage of the DCN granule cell system has been suggested for nonhuman primates because of its position between the full laminar development of the cochlear granule cell system in nonprimate mammals and its complete absence in humans (Moskowitz, 1969; Moore, 1980; Heiman-Patterson and Strominger, 1985). If granule cells are lost and not merely reorganized or relocated, then higher primates would have to replace integrating mechanisms for multimodal sensory inputs that occur in the superficial layers of the DCN. This loss would be most apparent by the absence of granule cells and their parallel fiber synapses (PF) on the apical dendrites of fusiform and cartwheel cells.
Studies in the DCN of nonprimate mammals have shown that the synapses of PF on fusiform cells and cartwheel cells express a unique composition of glutamate receptors and specific types of computational capabilities (Petralia et al., 1996; Rubio and Wenthold, 1997; Gardner et al., 1999, 2001; Fujino and Oertel, 2003; Tzounopolous et al., 2004, 2007). This feature would allow PF synapses to differ in their electrical properties and plasticity potential with their neuronal target. In the auditory system, plasticity at synapses is important for adaptation to normal fluctuations in the sensory environment (Molitor and Manis, 1997; Turecek and Trussell, 2000; Fujino and Oertel 2003), such as learning to recognize the calls of a new troop member and perhaps the processing of abnormal auditory input (Kaltenbach et al., 2005; Illing and Reisch, 2006). Therefore the comparative question regarding the superficial DCN with its synaptic circuitry was re-evaluated in order to better understand the substrates of normal hearing, deafness, tinnitus, and conditions where cochlear implants are needed.
In this study, we examined the rhesus monkey (cercopithecoid primate) to determine whether the superficial DCN of higher primates has similar synaptic components as nonprimate mammals. To do so, we made use of 1) ultrastructure to characterize parallel fibers and their synapses, and 2) molecular markers to determine whether primates exhibit the main components of excitatory neurotransmission. The data argue that the DCN of rhesus monkeys has similar neuronal features as nonprimate mammals.
Material and Methods
Tissue procedure
Tissue from rhesus monkeys was obtained after transcardial perfusion at the Johns Hopkins Medical School, Baltimore, MD or the Emory Primate Center, Atlanta, GA. At both institutions, animal protocols were approved by the institutional animal care and use committees and followed NIH guidelines.
Three rhesus monkeys (Macaca mulatta) of 3 years of age were perfused with 4% paraformaldehyde in 0.1M phosphate buffer (PB). Brains were removed and postfixed overnight with the same fixative. Brainstems were sectioned with a Vibratome and stored in 0.2M PB. Some of the brainstem slices were kept in a cryoagent solution until use, others were postfixed in 2% glutaraldehyde in 0.1M PB pH 7.2 for 4 hours, washed with 0.1M PB for 30 minutes, washed in 0.1M cacodylate buffer pH7.2 and postfixed in 1% osmium tetroxide in 0.1M cacodylate buffer for 45 minutes.
Three additional rhesus monkeys (Macaca mulatta), 6, 9, and 11 years of age were perfused with 4% paraformaldehyde and a low percentage of glutaraldehyde (e.g., 0.1 or 0.5% glutaraldehyde) in 0.1M PB. Brains were removed and immersed in buffer without postfixation. Brainstems were sectioned with a Vibratome at 70 or 300 µm in thickness. The thin sections were maintained in a cryoagent solution at −20°C until used. The 300 µm sections were cryoprotected in glycerol and followed the freeze-substitution procedure (see below).
Conventional transmission electron microscopy
Brainstem slices were dehydrated through a series of ethanol solutions (50%, 70%, 85%, 95% and 100%), infiltrated with epoxy resins and flat embedded. Blocks with cochlear nuclei were trimmed and mounted on blocks and cut with an ultramicrotome. Ultrathin sections (80 nm in thickness) were counterstained with uranyl acetate and lead citrate and analyzed with a TECNAI 12 Biotwin TEM. The images were captured with an AMT CCD camera. Image processing was performed with Adobe Photoshop using only the brightness and contrast commands.
Free floating and preembedding immunocytochemistry
Vibratome slices followed 2 cycles of freezing and thawing on dry ice, washed in 0.1M PB and were either stained for Nissl or incubated with the corresponding primary antibody [calbindin-D28k (1:3,000, Sigma, Saint Louis, MO), IP3r (inositol 1,4,5-triphosphate receptor; 0.1 µg/ml, Sharp et al., 1993; Ryugo et al., 1995); PDE10A (phosphodiesterase 10A; 1.2 µg/ml, Seeger et al., 2003); GluR1 (1.5 µg, Wenthold, 1992; Douyard et al., 2007), GluR2/3 (1.5 µg; Wenthold, 1992; Douyard et al., 2007), GluR4 (1.5 µg, Wenthold, 1992; Douyard et al., 2007), delta ½ (1.0 µg /ml; Mayat et al., 1995; Rubio and Wenthold, 1997), and VGLUT1 (1:2,000; Synaptic System, Göttingen, Germany)] in 0.1M PB for 48 hours at 4°C. All the antibodies used were polyclonal with the exception of calbindin and PDE10A. Slices were blocked for 1 hr with 10% normal goat or horse serum in buffer and followed the standard protocol for diaminobenzidine detection as previously described (Ryugo et al. 1995; Rubio and Wenthold, 1997). Nickel was added to intensify the immunoreaction of calbindin, VGLUT1 and some slices immunostained for delta ½. Some of the sections were mounted on glass slides, air dry and covered for further analysis with an Olympus BX51 research microscope. Other sections were postfixed for 1 hr in 1% osmium tetroxide, washed and embedded in EPON as described above. Ultrathin sections (80 nm in thickness) were counterstained with uranyl acetate and lead citrate and analyzed with a TECNAI 12 Biotwin TEM. The images were captured with an AMT CCD camera and processed with Adobe Photoshop using only the brightness and contrast commands.
Freeze-substitution and postembedding immunogold labeling
For the detection of GluR1, GluR2/3 and GluR2, AMPA receptor subunits, delta ½ and VGLUT1 with immunogold labeling after freeze-substitution, a protocol similar to that described in detail elsewhere was used (Rubio and Wenthold, 1997; 1999; Rubio, 2006). The dorsal cochlear nucleus (DCN) was dissected and processed for freeze-substitution and low-temperature embedding. For postembedding immunocytochemistry, ultrathin sections (80 nm in thickness) on nickel grids were incubated in sodium borohydride and glycine in Tris-buffered saline solution with Triton X-100. After being pre-blocked with serum, the sections were incubated with affinity purified primary antibodies for GluR1 (1.5 µg), GluR2/3 (1.5 µg), the monoclonal antibody against GluR2 N- terminus (1.5 µg; Chemicon, Temecula, CA; Rubio 2006), delta ½ (1 µg), or VGLUT1 (1:300) for single postembedding immunogold labeling. Primary antibodies were detected with secondary antibodies conjugated to 5nm gold particles in diameter (1:20; Amersham GE Healthcare, Buckinghamshire, UK). No gold particles were observed at the postsynaptic density, synaptic endings of inhibitory synapses, or on mitochondria and myelin sheets. Controls sections were prepared either in the absence of the primary antibody during the incubation step or by preadsorption of GluR1, GluR2/3 and GluR2 antibodies with the corresponding peptides (Rubio and Wenthold, 1997; 1999; Matsui et al., 2005). No gold particles were observed on the ultrathin sections after any of the control procedures. The analysis of the localization of two proteins (GluR1 and GluR2 or GluR2 and VGLUT1) on the section was done using double post-embedding immunogold labeling and using antibodies conjugated to gold particles of two different sizes. Ultrathin sections were analyzed with a TECNAI 12 Biotwin TEM. The images were captured with an AMT CCD camera at 49,000x or 68,000x magnification. Image processing was performed with Adobe Photoshop using only the brightness and contrast commands to enhance gold particles.
Morphometric analysis of synaptic endings and vesicles
Morphometric analysis of synaptic endings and synaptic vesicles in the 3 and 9 year-old rhesus monkey was performed on electron micrographs using the ImageJ software. ImageJ is a public domain, Java-based image processing program developed at the National Institutes of Health (available at: http://rsb.info.nih.gov/ij/download.html). Parameters of the synaptic vesicles included: area, major and minor diameter, and circularity (where a ratio of 1 indicates round and smaller ratios indicate a progressively flatter structure). The size of the vesicle was represented by its approximate mean diameter, calculated by (major axis + minor axis)/2. In order to describe synaptic endings, the parameters included: area, major and minor axes and length of the postsynaptic density (PSD) at the postsynaptic membrane.
We analyzed a total of 90 synaptic endings in both ages (51 endings with round synaptic vesicles and 39 with flattened or pleomorphic vesicles) and, a minimum of 20 synaptic vesicles per ending (Total number = 1,913). The selection of the synaptic vesicles was random but limited to only those synaptic vesicles which plasma membrane was clearly seen. Means and standard errors of the mean are provided where appropriate. See Table 1 for more detail of the number of synaptic endings and vesicles analyzed per age and type. Student t-test was used to calculate the significance of the sample (P < 0.05). The morphometric analysis of the neuronal bodies and length of the DCN layers was performed with Olympus MicroSuite Five software. We also used this software to measure the VGLUT1 punctate immunostaining in the rat and primate DCN.
Table I.
Morphometric analysis of synaptic vesicle types in the 3 and 9 years old rhesus monkey DCN.
| Area ± SEM | Major Axis ± SEM | Minor Axis ± SEM | Size ± SEM | Circularity | |
|---|---|---|---|---|---|
| 3 years old | |||||
| Round synaptic vesicles | |||||
| LR | 2013.3 ± 9.9** | 54.9 ± 1.2 | 46.2 ± 1.2 | 50.5 ± 1.1** | 0.9 |
| N= 5; Nsv= 100 | |||||
| MR | 1027.6 ± 25.8** | 38.5 ± 0.5 | 33.5 ± 0.5 | 36.0 ± 0.4** | 0.9 |
| N= 5; Nsv= 109 | |||||
| SR | 743.5 ± 8.2** | 34.0 ± 0.2 | 28.0 ± 0.2 | 30.8 ± 0.1** | 0.9 |
| N= 17; Nsv= 413 | |||||
| Non-round synaptic vesicles | |||||
| LF | 1587.5 ± 80.2** | 61.5 ± 2.0 | 32.2 ± 1.1 | 47.0 ± 1.2** | 0.6 |
| N= 8; Nsv= 147 | |||||
| SF | 1123.1 ± 32.2** | 50.6 ± 0.9 | 28.1 ± 0.5 | 39.1 ± 0.5** | 0.7 |
| N= 9; Nsv= 167 | |||||
| SP | 804.0 ± 30.1** | 42.1 ± 0.8 | 24.4 ± 0.7 | 32.5 ± 0.5** | 0.7 |
| N= 7; Nsv= 123 | |||||
| 9 years old | |||||
| Round synaptic vesicles | |||||
| LR | 1966.3 ± 59.6** | 52.5 ± 0.8 | 47.3 ± 0.7 | 49.9 ± 0.7** | 0.9 |
| N= 4; Nsv= 80 | |||||
| MR | 1329.1 ± 56.2** | 43.1 ± 0.8 | 39.0 ± 0.1 | 41.1 ± 0.9** | 0.9 |
| N= 6; Nsv= 125 | |||||
| SR | 882.6 ± 30.3** | 35.3 ± 0.6 | 31.7 ± 0.6 | 32.1 ± 1.2** | 0.9 |
| N= 14; Nsv= 280 | |||||
| Non-round synaptic vesicles | |||||
| LF | 1003.2 ± 18.5** | 52.0 + 0.5 | 24.5 + 0.3 | 38.3 + 0.3** | 0.7 |
| N= 5; Nsv= 147 | |||||
| SF | 899.0 ± 43.7** | 45.7 ± 0.7 | 24.5 ± 0.7 | 35.1 ± 0.7** | 0.7 |
| N= 5; Nsv= 112 | |||||
| SP | 690.0 ± 23.2** | 37.1 ± 0.7* | 23.5 ± 0.4 | 30.3 ± 0.4** | 0.7 |
| N= 5; Nsv= 110 | |||||
N= number of synaptic endings analyzed; Nsv= number of synaptic vesicles analyzed
P < 0.05
Results
Granule cells and parallel fibers in the primate DCN
Nissl staining was used to determine whether the primate DCN had a laminar organization. Coronal sections revealed a layered organization in the rhesus DCN (Fig 1A). There was a superficial or molecular layer (layer I) with scattered small nuclei and a deeper region (layers II and III) with abundant nuclei of different shapes and sizes (8 – 20 µm). Large elongated cell bodies of fusiform cells (20 µm in size) were clearly observed distributed in layer II and formed a discrete but distinguishable layer. One of the main components of the molecular layer in nonprimate mammals is the existence of unmyelinated axons of granule cells running parallel to the long axis of the nucleus (Wouterlood and Mugnaini, 1984). To determine the presence and distribution of granule cells and their axons in the DCN we performed immunocytochemistry for phosphodiesterase (PDE10A), and an ultrastructural examination of the molecular layer (Fig 1B–E). Brainstem sections stained with PDE10A showed numerous small round nuclei labeled that had an average size of 8 µm, and distributed within the nucleus and also at the rim of molecular layer forming like an external strip (Fig. 1B).
Figure 1.

The rhesus DCN has a laminar structure, granule cells and unmyelinated fibers. The rhesus DCN has a laminar structure, defined by the distribution of granule cells and unmyelinated fibers. A, Nissl staining of a coronal section of the 3 years old brainstem at the level of the DCN. Arrows point to granule cells located in the most external rim of the nucleus. B, Light microscopic image shows a coronal section of a 3 years old brainstem after immunohistochemistry for PDE10A. Small cell bodies of labelled granule cells are observed distributed in the DCN. These stained cell bodies form an external strip to the molecular layer that would represent the superficial lamina in cats and rodents (white arrows). Abbreviations: ANr: auditory nerve root; AVCN: anteroventral cochlear nucleus; Cb: cerebellum; DCN: dorsal cochlear nucleus: ICP: inferior cerebellar peduncle; SpV, descending tract of the trigeminal. C, Electronmicrograph shows a low magnification of the molecular layer of the 3 years old rhesus monkey. Unmyelinated fibers are observed running parallel to the long axis of the nucleus. Intermingled are dendrites (dend) and spines (s) receiving synaptic endings. D–E, Electronmicrographs show high magnifications of the molecular layer, in which it is observed dendritic spines receiving en passant (*) asymmetric synaptic contacts of parallel fibers. Image in D corresponds to the box in C. Scale bars, A–B: 0.5 mm; C: 1 µm; D–E: 0.5 µm.
This external strip of cells could represent the superficial lamina in cats and rodents. The distribution and size of these labeled cells fit the criteria of granule cells. In addition, we also observed a dense staining of nuclei in the granular cell layer of cerebellum as previously shown (Seeger et al., 2003; Coskran et al., 2006). Ultrastructurally the molecular layer of the rhesus monkey DCN is characterized by the presence of numerous thin unmyelinated axons traveling parallel to the long axis of the nucleus (Fig. 1C–E). Dendritic profiles and abundant dendritic spines are distributed among these fibers, receiving small en passant varicosities containing clear, round synaptic vesicles. There are also small cell bodies scattered in the layer. Deeper in the nucleus was a robust net of myelinated fibers and many medium-to-large cell bodies. In summary, the DCN exhibits a distinct layering of cell bodies, a characteristic neuropil, and the presence of unmyelinated axons in a molecular layer.
Synaptic endings in the rhesus monkey DCN
Three distinct types of endings containing round synaptic vesicles
Three types of synaptic endings are identifiable on the basis of synaptic vesicle size, shape, and clarity in the rhesus monkey DCN (Fig. 2, Table 1). The three types were seen in both the young and adult primate. Two of the endings (Fig. 2 A–B and G–H) were medium (2.7 ± 0.2 µm) to large (5.5 ± 1.2 µm average size), distributed in the internal region of the molecular layer (layer I) and deeper in the nucleus (layers II and III) and made either single or multiple asymmetric synaptic contacts on cell bodies, dendrites of different sizes, and/or large spines (1.3 ± 0.1 µm average size). The third type (Fig. 2 D–F and J–L) was small (1.5 ± 0.1 µm average size), distributed in the most superficial or molecular layer of the DCN and made single and/or double asymmetric synaptic contacts preferentially on spines but also on dendritic shafts. Spines receiving these synaptic endings were seen varied in shape, had an average size of 0.7 ± 0.1 µm, were enriched with smooth endoplasmic reticulum, and arose from dendrites that also contained smooth endoplasmic reticulum and many mitochondria. The ultrastructure of the dendrites and spines receiving the small endings with round synaptic vesicles is similar to previous descriptions of dendrites and spines of cartwheel cells in the molecular layer of nonprimate mammals (Wouterlood and Mugnaini, 1984; Ryugo et al., 1995; Rubio and Juiz, 2004) and primates (owl monkey and rhesus monkey, Ryugo et al., 1995).
Figure 2.

Synaptic endings found in the rhesus DCN. A–F. Electronmicrographs show synaptic endings in the 3 years old monkey. A–B and D–F micrographs are examples of synaptic endings with round synaptic vesicles distributed in the deep DCN (A: LR, and B: MR), and superficial DCN (D–F: SR). C shows a typical ending with flattened synaptic vesicles (NR-Type 1) distributed in the central region of the DCN. S: spines; Dend: dendrites. Scale bar: A–B: 1 µm; C–F: 0.5 µm. G–L. Electronmicrographs show synaptic endings in the 9 years old monkey. G–H and J –L micrographs are examples of synaptic endings with round synaptic vesicles distributed in the deep DCN (G: LR, and H: MR), and superficial DCN (J–L: SR). I: shows a typical ending with flattened synaptic vesicles (NR-Type 1) distributed in the central region of the DCN. S: spines; Dend: dendrites. Scale bar: A–B: 1 µm; C–L: 0.5 µm.
Synaptic vesicle area and size are useful parameters to distinguish synaptic endings that presumably have different functional significance (Gray 1959; Uchizono 1965; Rubio and Juiz, 2004). Therefore, we performed a morphometric analysis of the round synaptic vesicles in the 3 and 9 years old primate DCN. In both ages analysis showed a concise division into three groups (Table 1). Because of this clear distinction in size of the round synaptic vesicles we named these endings as LR, MR and SR for large, medium and small, respectively (Table 1). The SR ending is the smallest, contained the smallest round synaptic vesicles, was distributed in the superficial zone of the DCN, and made synaptic contacts on dendritic spines (Fig. 1 and 2 D–F and J–L). RL and MR endings corresponded to the synaptic endings containing the largest and medium round synaptic vesicles, respectively. The ultrastructural characteristics, location in the nucleus, and synaptic targets of these three endings were similar to the auditory nerve (LR), mossy terminals (MR), and parallel fibers (SR), in nonprimate mammals.
Endings containing flattened or pleomorphic synaptic vesicles
In addition to the synaptic endings containing round synaptic vesicles, we also observed a large number of endings that contained pleomorphic or flattened synaptic vesicles (Fig. 2C, I). These endings made symmetric synaptic contacts on cell bodies and dendrites throughout the DCN but were distributed preferentially in the deep layers of the DCN. Morphometric analysis of the synaptic vesicles revealed three distinct types of endings (Table 1). Attending to the size of the vesicles, we categorized these endings as LF, SF and SP. LF endings contained large, flattened synaptic vesicles. SF also contained flattened synaptic vesicles but these were on average smaller than LF (P< 0.05). The SP endings contained pleomorphic synaptic vesicles that were the smallest (P< 0.05) of the three types of endings. Inhibition in the DCN plays a key role shaping auditory responses (Young and Spirou, 1991; Young et al., 1995; Spirou et al. 1999). Among the main inhibitory inputs into the DCN exist intrinsic inputs from cartwheel and vertical cells in the superficial and deeper DCN respectively, and extrinsic inputs from D-stellate cells in the ventral cochlear nucleus and as well as other auditory nuclei.
IP3r and calbindin-D28k immunoreaction in the molecular layer of the rhesus monkey
We have shown with Nissl and PDE10A staining a layered organization of the granule cells in the primate DCN (Fig. 1A). Furthermore, ultrastructural analysis revealed that the molecular layer was enriched with many unmyelinated “parallel” fibers and en passant synapses on spines of putative cartwheel cells in the molecular layer. Cartwheel cells are one of the two main neuronal targets of the parallel fibers in the molecular layer of the DCN, mediate multimodal information, and have specific/unique forms of synaptic plasticity (Fujino and Oertel, 2003; Tzounopolous et al., 2004, 2007). Therefore we addressed the presence, distribution and synaptic inputs on cartwheel cells spines in the rhesus DCN using molecular markers at the light and electron microscopic level.
We used an antibody for inositol 1,4,5-triphosphate receptor (Ip3r) that was previously shown to specifically and homogenously label cartwheel cells in the DCN of nonprimates and primates (Ryugo et al., 1995). We observed that IP3r labelled numerous medium size neurons whose somata were preferentially located on the border between layer II and III of the nucleus Rubio et al. (Fig. 3 B–C). The immunostained cells had a cell body that ranged in shape from round to polygonal and presented multiple dendrites emerging from the cell body in all directions as previously described cartwheel cells in primates, cats, bats, and rodents (Ryugo et al., 1995). These labelled dendrites for IP3r were smooth when viewed by light microscopy but ultrastructural analysis revealed unlabeled dendritic spines emerging from positively stained cartwheel cell dendrites (Ryugo et al., 1995).
Figure 3.

IP3r and calbindin label cartwheel cells in the rhesus monkey DCN. A, Micrograph shows a coronal section of the brainstem at the level of the DCN stained for Nissl. Arrows point to medium to large cell bodies of cartwheel and fusiform cells distributed in the superficial layers of the nucleus. Note a clear layering organization of fusiform cell bodies. Cb: cerebellum.
B–C, Micrographs show labelled cartwheel cells after IP3r immunostaining in the 3 years old rhesus DCN. Micrograph in B corresponds to an image at low magnification of a coronal section of the DCN. Arrows point to numerous medium-size labelled somata of cartwheel cells distributed in the most superficial layers of the nucleus. C, micrograph shows a higher magnification of two cartwheel cells in the molecular layer. Labeling is observed filling the cell bodies and extends to distal dendrites of the cells. Scale bar: A–B: 0.5 mm; C: 25 µm. D–J, Micrographs show immunostaining for calbindin in the 9 years old rhesus monkey. D, micrographs shows at low magnification a saggital section of the brainstem with the cochlear nucleus complex (AVCN: anteroventral cochlear nucleus; PVCN: posteroventral cochlear nucleus; DCN: dorsal cochlear nucleus). Rectangles in D indicate the areas magnified in E–F and H–I. E–F and H–I images show calbindin positive cells in the molecular layer. Immunostaining is present in cell bodies (arrowheads), dendrites (arrows) as well as in the neuropil of the nucleus. G and J, Electron micrographs show preembedding immunolabeling for calbindin in the molecular layer of the DCN. The smooth membranes of endoplasmic reticulum in the cytoplasm of the spines are observed containing the electrondense reaction product for calbindin. Membranes associated to the postsynaptic density also contain immunolabeling. Labelled spines receive asymmetric synaptic contacts of endings containing round synaptic vesicles that are typical of parallel fiber synapses. Scale bar: A: 1mm; B–C and E–F: 50 µm; D–G: 0.5 µm.
In addition we used an antibody for calbindin-D28k. Calbindin belongs to the family of calcium binding proteins and has been found in different subsets of neurons in many brain regions. It is not yet known whether calbindin-D28k plays a ‘triggering’ role resembling calmodulin or merely serves as a buffer to modulate cytosolic calcium transients. Nevertheless, calbindin-D28k is a valuable marker of neuronal subpopulations for anatomical and developmental studies because of its selective staining properties (Baimbridge et al., 1992). Calbindin-D28k labels cartwheel cells in the rodent (Friauf, 1994) so we used calbindin-D28k to determine whether it was expressed in cartwheel cells of the rhesus (Fig. 3). Primate brainstem sections immunostained for calbindin-D28k showed that the molecular layer contained numerous immunopositive, medium size neurons (approximately 15 µm in size; Fig. 3 E–I). The immunoreaction product filled the cell bodies and extended to primary dendrites. Immunostained elements were also found in the neuropil. These positive cells shared the same location and size as cartwheel cells after IP3r staining (present study; Ryugo et al., 1995). As in rodents, neurons located at the superficial rim of the DCN that resembled ectopic Purkinje cells (Hurd and Feldman, 1994), and nerve fibers were also labelled (data not shown). In addition to cartwheel cells of the DCN, cerebellar Purkinje cells were clearly stained for calbindin (data not shown).
To determine whether calbindin was present in spines of stained cartwheel cells in the primate DCN, we examined the immunostaining at the electron microscopic level (Fig. 3 G and J). Electron-dense reaction product for calbindin was observed associated to membranes of smooth endoplasmic reticulum in cell bodies with an average size of 16 µm, and extended to dendrites and dendritic spines. These spines had an average diameter of 0.7 ± 0.1 µm, and received single asymmetric synaptic contacts from endings containing small and clear round synaptic vesicles, with similar area, size and circularity than the synaptic endings classified as SR (Table 1).
AMPA and delta glutamate receptors are expressed in the DCN of the rhesus monkey
AMPA glutamate receptors mediate fast synaptic transmission in the brain and in the auditory pathway. The expression and distribution of AMPA receptor subunits have been analyzed in detailed in the rodent DCN (Petralia et al., 1996; Rubio and Wenthold 1997, 1999; Rubio 2006). We asked whether AMPA receptors were expressed in the rhesus DCN and whether their cellular expression was similar to that in rodents. Using specific antibodies for GluR1, GluR2/3, and GluR4 receptor subunits we performed immunohistochemistry on the 11-year-old rhesus brainstem (Fig. 4). The antibodies used were the same as the ones previously used in rodents studies (Petralia et al., 1996; Rubio and Wenthold, 1997; Rubio, 2006). Our data showed that all the AMPA subunits were present in the primate DCN and had a similar cellular distribution as in rodents (Fig. 4 A–E).
Figure 4.

Ionotropic AMPA and delta ½ receptor subunits are present in the 9 years old rhesus monkey. A–E, Immunostaining for GluR1, GluR2/3, and GluR4 AMPA receptor subunits. A–B GluR1 labels medium oval in shape cells in the molecular layer. C–D, GluR2/3 labels medium cells (cartwheel; arrows: cell bodies) in the molecular layer (C) and larger cells (fusiform cells; arrows: cell bodies; arrowheads: dendrites) in deeper areas of the DCN (D). E, GluR4 immunostaining is light and preferentially in the neuropil. F–H, The antibody for delta ½ labelled fusiform and cartwheel cells. Labeling extended from the somata (arrows) to dendrites (arrowheads) and was observed in the neuropil. G and H show higher magnifications of cartwheel cells. Boxes in these figures are magnified in G’ and H’ Detail images show numerous and small labelled spines emerging from stained cartwheel cell dendrites. Scale bar: A–F: 50 µm; D and H: 25 µm G’ and H’: 5 µm.
GluR1
In rodents, GluR1 subunit is expressed in cell bodies and dendrites of cartwheel cells located in the ML (Petralia et al., 1996). In the rhesus DCN, immunostaining for GluR1 was observed preferentially in medium size neurons located superficially in the molecular layer of the nucleus (Fig. 4 A–B). These neurons resembled cartwheel cells in the rodent DCN.
GluR2/3
In rodents, GluR2/3 subunits are expressed in many cell types including those located superficially and deeper in the DCN. These cells have been identified as fusiform cells, cartwheel cells, stellate cells, and granule cells (Petralia et al., 1996). In the rhesus DCN, GluR2/3 labelled cells located in both the most superficial and deeper areas of the nucleus (Fig. 4 C–D). By the location and mean diameter of the cell body, these cells could be categorized as fusiform cells (23.8 ± 1.4 µm), cartwheel cells (15.6 ± 0.4 µm) and granule cells (8.4 ± 0.3). Staining was observed in cell bodies and also in primary dendrites. In fusiform cells, labeling extended from the cell body to apical and basal primary dendrites.
GluR4
In rodents, GluR4 labeling was distributed in the neuropil of the outer DCN and in large neurons in the deep layers (Petralia et al., 1996). In the primate, GluR4 immunostaining was very pale and was mainly found in the neuropil (Fig. 4 E). Cell bodies were not clearly visible due to the “fuzzy” outline of the cells. Large neurons in the deep DCN of the primate were also labelled for GluR4.
We also performed immunohistochemistry for the ionotropic glutamate receptor delta, with an antibody that recognizes the 1 and the 2 subunits (delta ½). In the rodent DCN, delta ½ is expressed in cartwheel and fusiform cells (Petralia et al., 1996; Rubio and Wenthold, 1997). Purkinje neurons in the cerebellum are also known to express delta ½ (Mayat et al., 1995; Zhao et al., 1998). In the rhesus DCN, delta ½ labelled medium and larger size neurons (cartwheel and fusiform cells, respectively) located in the more superficial layers (layers I and II) and also in large neurons distributed more deeply in the nucleus. Immunostaining was observed in cell bodies and dendrites of the cells and interestingly in dendritic spines of medium size neurons that were preferentially located superficially in the nucleus (Fig. 4 F–H). In the primate cerebellum, cell bodies, dendrites and spines of Purkinje cells were stained for delta ½ (data not shown).
AMPA and delta receptor subunits enrich the postsynaptic membrane of cartwheel cells spines receiving parallel fiber-like synaptic endings
We asked whether GluR1, GluR2, GluR3 AMPA subunits and, delta ½ glutamate receptors enriched the postsynaptic densities of spines located in the more superficial layers of the DCN receiving the SR synaptic endings (parallel fiber-like ending) described in this study. To do so we performed single and double postsynaptic immunogold labeling for these subunits on the 11-year-old rhesus DCN (Fig. 5). The antibodies used were the same as the ones used in the previous rodent studies (Rubio and Wenthold, 1997; Rubio, 2006). Our data showed that approximately 85% of the PSDs of dendritic spines with an average diameter of 0.8 ± 0.1 µm and located in the molecular layer of the DCN were enriched with gold particles for all of these receptor subunits. Synapses formed by the LR and MR endings on cell bodies, dendrites and/or large spines were observed immunogold labelled for GluR2 and GluR2/3 subunits (data not shown). Symmetric synapses formed by FL, FS and PS of the inhibitory endings lacked of immunogold labeling for any of the AMPA subunits (supplemental Fig. 1).
Figure 5.
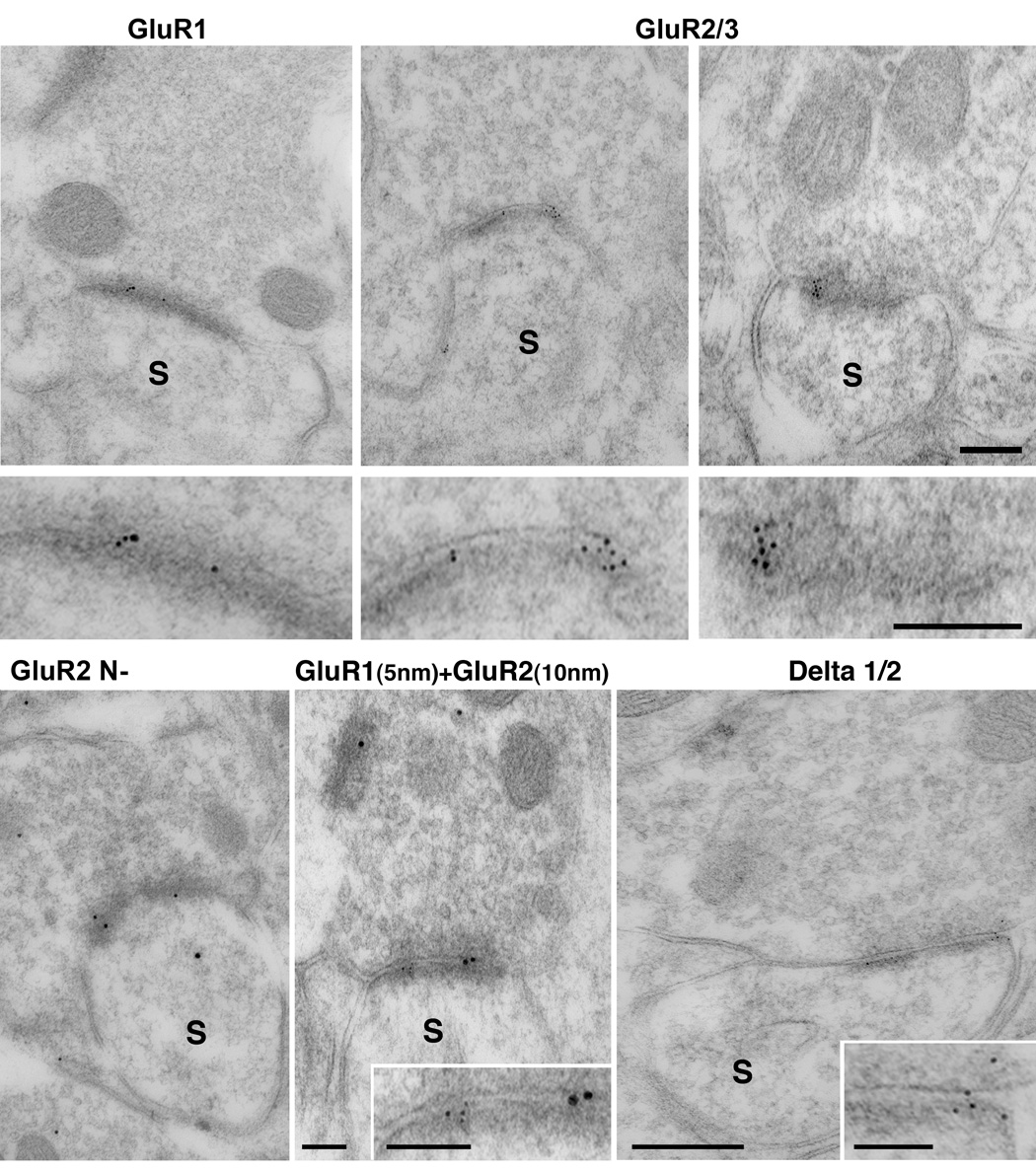
Electronmicrographs show single and double postembedding immunogold labeling for GluR1, GluR2/3, GluR2 and delta ½ glutamate receptor subunits in the 11 years old Rhesus monkey. Images correspond to synapses of parallel fibers on cartwheel cell spines distributed in the molecular layer. Postembedding immunogold labeling revealed 5 nm in diameter gold particles for GluR1, GluR2/3, GluR2 and delta ½ are present at the postsynaptic density. The same synapses also co-localize GluR1 (5 nm) and GluR2 (10 nm) at the postsynaptic density. Scale bars: 0.25 µm.
VGLUT1 immunolabeling is concentrated in the molecular layer of the DCN rhesus monkey
Vesicular glutamate transporters (VGLUTs) selectively package glutamate into synaptic vesicles and mediate glutamate transport. Three subtypes of vesicular glutamate transporters (VGLUT 1, 2 and 3) have been cloned so far, and only the types 1 and 2 (VGLUT1 and VGLUT2) are expressed in the cochlear nucleus (Kaneko et al., 2002; Herzog et al., 2004; Zhou et al., 2007). In the rodent cochlear nucleus, these two types present different patterns of immunolabeling (Kaneko et al., 2002; Herzog et al., 2004; Zhou et al., 2007). In the DCN, the molecular layer only shows strong immunolabeled for VGLUT1 (Zhou et al., 1997). This layer is mainly enriched by only one type of excitatory synaptic endings in the DCN, the parallel fiber synapses of the granule cells (Wouterlood and Mugnaini, 1984). Therefore VGLUT1 represents an excellent maker to determine the location of parallel fiber endings in the rhesus DCN. We first determined the expression of VGLUT1 in rats using immunohistochemistry (Fig 6 A). VGLUT1 immunostaining concentrated in the molecular layer of the DCN as described in the guinea pig (Zhou et al., 2997). In the primate, we found strong VGLUT1 immunostaining in the most superficial layer of the DCN (Fig. 6), as observed in rodents. The labeling for VGLUT1 was granular throughout the nucleus. We measured the stained granules and found that the average diameter in the superficial DCN was approximately 1.2 ± 0.1 µm. The stained granules in deeper regions of the nucleus were larger with an average size of 2.8 ± 0.3 µm, and were observed on unstained cell bodies and primary dendrites (Fig. 6 E).
Figure 6.

VGLUT1 is present in the rhesus DCN, concentrates in the molecular layer and labels synaptic vesicles of parallel fibers. A. VGLUT1 immunohistochemistry in the rat DCN. Immunoreaction is observed granular along the nucleus. In the molecular layer (ML) labeling forms an external dark band approximately 55 µm in thickness. Granular labeling distributes also in the fusiform cell layer (FCL). Scale bar: 55 µm. B–E. Distribution of VGLUT1 immunohistochemistry in the 9 years old rhesus DCN. B: sagittal section of the brainstem shows immunostaining for VGLUT1 in the cochlear nucleus complex. In the DCN labeling forms a dark band in the molecular layer. C: higher magnification of the boxed area in B. VGLUT1 immunostaining appears granular along the nucleus. In the brainstem (ML) staining forms a dark band with a thickness of approximately 67 µm. D and E micrographs show details of the ML and a deeper layer (DL) of the DCN. Granular labeling is observed around unlabeled neurons (*). The granules have a range of sizes that correspond to their distribution. The granules located in the ML are small (1.0 µm in size) and round in shape. In deeper layers, granules have a irregular shape and are larger with an average size range from 1.6 µm to 5.5 µm. Scale bar: 25 µm.
F–H. Electronmicrographs show double postembedding immunogold labeling for VGLUT1 (5 nm) and GluR2 (10 nm) in the 11 years old rhesus DCN. Gold particles for VGLUT1 are observed associated to membranes of round synaptic vesicles in presynaptic endings (arrows and inset). The synaptic endings containing gold particles for VGLUT1 are observed making asymmetric synaptic contacts on dendritic spines, which PSD present gold particles for GluR2. Scale bar: 0.1 µm; Inset: 0.05 µm.
To determine whether the SR synaptic endings on dendritic spines contained VGLUT1, and whether the synapse contained AMPA receptors at the postsynaptic membrane, we performed double postembedding immunogold labeling for VGLUT1 and the GluR2 subunit (Fig. 6 F–H). Our data showed that gold particles labeling VGLUT1 were associated with clear, round synaptic vesicles in endings located in the molecular layer of the rhesus DCN. These endings made single asymmetric contacts with dendritic spines whose postsynaptic membranes contained gold particles for GluR2. No gold particles were seen associated with flattened or pleomorphic synaptic vesicles of the inhibitory synaptic endings (supplemental Fig. 1).
Discussion
By using electron microscopy and immunohistochemistry at the light and electron microscopic level we observed that the organization of the primate DCN is similar to that of the nonprimate mammal. The finding of many unmyelinated thin axons and the characteristic en passant glutamatergic synapses on dendritic spines within the molecular layer strongly support these arguments. Furthermore, we showed that the primate DCN share the main molecular components of excitatory neurotransmission with rodents. We demonstrated that the synaptic expression of AMPA glutamate receptor subunits on spines in the molecular layer resembles that of nonprimate mammals, and that parallel fibers use VGLUT1 to package glutamate into the synaptic vesicles and to mediate glutamate transport. These observations indicate that the DCN of an Old World monkey has the necessary structural organization to integrate multimodal somatosensory information through granule cells.
Primates may have the capability of integrating multimodal sensory information in the superficial layers of the DCN
In this study we asked whether the superficial DCN of higher primates had similar synaptic components as nonprimate mammals. This question needed to be re-evaluated because it was suggested that humans and primates exhibited a loss of lamination due to failure of inward migration and increasing cell death during ontogeny of the cochlear external granular layer (Moore, 1980). By using a variety of staining techniques we presented evidence that the DCN of higher primates has a laminar structure similar to nonprimate mammals. We also revealed that granule cell bodies whose nuclei labelled for phosphodiesterase were abundant and distributed within the deep DCN as well as along a superficial band similar to the cap found over the anteroventral cochlear nucleus in cats. In addition, cartwheel cells labeled for IP3r, and fusiform cells in Nissl stained sections showed a distinct layered organization. Ultrastructurally we revealed that the primate DCN has a superficial molecular layer containing many thin unmyelinated axons traveling along the long axis of the nucleus. Along these fibers, numerous en passant varicosities typical of the parallel fibers from the granule cells synapsed on dendritic spines of cartwheel cells immunolabeled for calbindin.
The difference between our results and previous reports is that we had more sensitive probes with which to examine the tissue. Whereas previous studies relied on light microscopic Nissl stains, we were able to show lamination by using immunohistochemical methods, and to identify fiber and terminal types using electron microscopy. Collectively, our data demonstrate that the primate DCN is a layered structure and that molecular layer contains the basic cytoarchitectonic elements that are necessary to integrate and process somatosensory information.
Excitatory and inhibitory synaptic endings in the primate DCN
The balance of excitation and inhibition is important for acoustic and somatosensory processing in the DCN. Using ultrastructural analyses we found six distinct types of synaptic endings in the primate DCN. By the morphology of their synaptic vesicles and type of synaptic specializations three of them (LR, MR and SR) were classified as putative excitatory contained clear round vesicles and formed asymmetric synaptic contacts preferentially on dendrites and/or dendritic spines. The other three (LF, SF and SP) were classified as inhibitory because they contained flattened or pleomorphic synaptic vesicles and made symmetric synaptic contacts on cell bodies and dendrites. In rodents, by using a combination of postembedding immunogold labeling for excitatory and inhibitory neurotransmitters with conventional ultrastructural analysis, evidence reported nine subtypes of synaptic endings in the DCN (Rubio and Juiz, 2004). Therefore, it is possible that there are more subtypes of endings in the primate DCN than only those described in this study.
Our work represent the first ultrastructural analysis of the synaptic circuitry in the primate cochlear nucleus, and it is evident that more studies need to be done to characterize in detail and identify all the synaptic inputs. Nevertheless, in this study we present clear evidence that the six types of endings found in the rhesus DCN shared similar ultrastructural parameters and distribution as the main subtypes of endings previously described in nonprimates. It is important to note that quantitative morphometry is necessary to make these distinctions because the subjective “visual inspection” of vesicles is simply not reliable except in the most obvious instances. It is also important to note that comparing quantitative data across different animals whose tissue preparation might differ even slightly (e.g., differences in aldehydes concentrations, buffer molarity, pH, calcium concentration) can have a large effect on vesicle shape and/or tissue shrinkage, and thus, the results.
The location and synaptic targets of the six types of synaptic endings found in the primate DCN differed. Among the three types of excitatory endings only the smallest, the SR (which also contained the smallest synaptic vesicles), was found in the unmyelinated superficial layer of the DCN where it mainly synapsed on dendritic spines. This same type of ending was described for the parallel fiber synapses of the granule cells in nonprimate mammals (Wouterlood and Mugnaini, 1984; Ryugo et al., 1995; Rubio and Wenthold, 1997; Rubio and Juiz, 1998, 2004; Rubio, 2006). The other two endings (LR and MR) were found among many myelinated fibers in deeper regions of the nucleus. The medium-large LR ending mainly targeted large-to-medium size dendrites, whereas the large and irregularly shaped MR endings synapsed on small dendrites and/or on spines with a claw-like morphology. It is known that the auditory nerve of nonprimate mammals enters in the DCN and distributes endings in the deeper layers to synapse on dendrites of projection neurons and interneurons (May and Ryugo, 1993; Rubio and Wenthold, 1997). By morphology and distribution the LR endings of the rhesus probably arise from the auditory nerve. This interpretation is consistent with previous reports indicating that the DCN of humans and primates would process acoustic information because the ascending auditory pathway is maintained through the phylogenetic process (Moore and Osen, 1979; Heiman-Patterson and Strominger, 1985). Similarly, mossy terminals from a variety of somatosensory sources enter the nonprimate DCN to distribute along the fusiform cell layer where they synapse on small dendrites and spines of granule cells (Mugnaini et al., 1980; Wright and Ryugo, 1996; Rubio and Juiz, 1998). Therefore, the MR ending is likely to be mossy terminals. Identifying and locating the neuronal sources of these endings would be relevant to understand the synaptic circuitry of the primate DCN. Nevertheless, the presence of parallel fiber synapses of the granule cells in the superficial DCN and mossy terminals within the nucleus represent a strong argument favoring the hypothesis that the primate DCN can convey information from widespread areas of the brain that are associated with multisensory modalities.
We identified three distinct categories of inhibitory endings in the rhesus DCN that distributed within the superficial and deep layers of the nucleus. These endings made symmetric synapses on dendrites and cell bodies and contained flattened (LF and SF) or pleomorphic (SP) synaptic vesicles. The existence of these endings indicates the presence of extrinsic and/or intrinsic inhibitory inputs onto neurons of the rhesus DCN. Reports on nonprimate mammals presented evidence that cartwheel and vertical cells are the most abundant inhibitory inputs in the superficial and deep DCN, respectively (Zhang and Oertel, 1993; Golding and Oertel, 1997; Davis and Young, 2000). On the other hand, the main extrinsic inhibitory inputs are represented by the projection of D-stellate cells of the ventral cochlear nucleus, as well as descending projections from upper auditory nuclei (Saint-Marie et al., 1991; Potashner et al., 1993; Ostapoff et al., 1997). Cartwheel cells colocalize GABA and glycine, meanwhile vertical cells label for glycine in rodents (Kolston et al., 1992). In the ventral cochlear nucleus D-stellate cells also stained for glycine, although it is unclear whether the descending projections uses either glycine or GABA, or both. Previous studies reported the existence of a correlation between the shape of synaptic vesicles and neurotransmitter content (Rubio and Juiz, 2004). Pure glycinergic endings have shown to contain flattened synaptic vesicles, whereas those colocalizing GABA and glycine have pleomorphic endings. Therefore, vertical and D-stellate cells may be considered as putative neuronal sources for the LF and SF endings found in our study. Additionally, SP endings that distributed in more superficial layers and contained pleomorphic vesicles might correspond to cartwheel cells axons (Berrebi and Mugnaini 1991). There is only one report at the light microscopic level that addressed the presence of inhibitory inputs in higher primates.
Moore et al. (1996) using postembedding immunostaining for GABA and glycine on semithin sections suggested that inhibitory inputs in the baboon cochlear nucleus complex existed but they were otherwise distinct to nonprimates. For example, they argue that the commissural and tuberculoventral projections from D-stellate cells and vertical cells respectively were identical in size and location to those observed in cat and guinea pig (Osen et al., 1990; Kolsten et al., 1992). Although they provide no evidence regarding descending inhibitory projections into the nucleus, they also speculate that any inhibition mediated by cartwheel cells in the molecular layer would be affected because they found a decrease in the number of this cell type. On the basis of these limited data, they concluded that the modulation of the ascending auditory pathway of the baboon differed from that occurring in rodents and cats. Our data both support and contradict the conclusions of Moore and colleagues because we did find numerous cartwheel cells in the DCN, and we identified numerous endings containing pleomorphic synaptic vesicles with the same distribution as previously described for cartwheel cell axons in nonprimates (Berrebi and Mugnaini, 1991; Rubio and Juiz, 2004). Because rhesus monkeys and baboons belong to the same superfamily Cercopithecoidea, and because (aside from apes) they represent the animals most closely related to humans, further studies are needed to address this particular issue.
Spines in the molecular layer of the primate DCN share key components of excitatory neurotransmission as nonprimate mammals
Spiny dendrites of fusiform and cartwheel cells in the molecular layer integrate multimodal somatosensory information carried out by glutamatergic synapses of the parallel fibers (Manis and Molitor, 1996; Kanold and Young, 2001). These synapses are subject to specific types of computational capabilities (Fujino and Oertel, 2003; Tzounopoulos et al., 2004; 2007). In the primate we found that spines of cartwheel and probably fusiform cells receive glutamatergic innervation from parallel fiber synapses that are enriched with VGLUT1. This relationship suggests that in the rhesus those synapses might be subject of plasticity as occurs in rodents. Cartwheel cells contain calbindin, and IP3r receptors associated to membranes of smooth endoplasmic reticulum in the cell body and dendrites. Interestingly, only membranes of smooth endoplasmic reticulum within the spine present immunolabeling for calbindin. This result supports the idea that Ca++ released from intracellular stores could act within individual spines (Wouterlood and Mugnaini, 1984; Ryugo et al., 1995). In nonprimate mammals Ca++- induced Ca++ release through ryanodine receptors is required for the induction of long-term potentiation and long-term depression in cartwheel as well as fusiform cells (Fujino and Oertel, 2002).
Glutamate mediates fast synaptic transmission along the auditory pathway through the activation of AMPA glutamate receptor subunit complexes. Activation of AMPA receptor channels is linked to synaptic activity because they can activate Ca++ channels in neurons (Soler-Llavina and Sabatini, 2006). In the same manner, it is known that parallel fibers are glutamatergic (Manis and Molitor, 1996; Rubio and Juiz, 2004) and activate postsynaptic GluR1, GluR2 and GluR3 AMPA glutamate receptor channels (Rubio and Wenthold, 1997, Gardner et al., 1999, 2001; Rubio, 2006). In this study of the primate DCN we have clearly shown that postsynaptic densities of dendritic spines within the molecular layer that are apposed to VGLUT1 positive parallel fibers contain GluR1, GluR2 and GluR3 subunits of the AMPA receptors. This expression pattern is remarkably similar to previous report in rodents (Rubio and Wenthold, 1997; Gardner et al., 1999, 2001; Rubio, 2006). Also as in nonprimate mammals, the presence of synaptic GluR2 at those spines indicates that the synapses formed by the parallel fibers on cartwheel cell spines are calcium impermeable (Gardner et al., 1999, 2001). Therefore we can speculate that the parallel fiber synapses of higher primates might modulate glutamatergic transmission in the same manner as nonprimates. These rhesus spines within the molecular layer also contained synaptic delta ½ subunits as shown in rodents (Petralia et al., 1996; Rubio and Wenthold, 1997). Although the function of delta glutamate receptors in the cochlear nucleus is not clear, recent genetic and electrophysiological studies in the cerebellum reported new evidence of the role of this receptor type in synaptogenesis and synaptic plasticity (Yuzaki, 2003; Hirai et al., 2003). In the cerebellum, the delta 2 subunit is predominantly expressed in the parallel fiber/Purkinje cell synapse and it may have a unique role in regulating endocytosis of postsynaptic AMPA receptors during long term depression. All together, our data advocate for the existence of synaptic plasticity at parallel fiber synapses on dendritic spines within a well define molecular layer in the rhesus DCN.
Supplementary Material
Supplemental Figure 1. Electronmicrographs show two inhibitory synaptic endings (SP) after single postembedding immunogold for GluR2/3 and double postembedding immunogold for GluR2 with VGLUT1. None of the synapses present gold particles for the AMPA subunits at the PSDs or contained VGLUT1 associated to the synaptic vesicles. Scale bar: 0.50 µm.
Acknowledgments
We acknowledge NIH/NIDCD RO1 DC006881 to M.E.R., RO1 DC004395 to D.K.R. and NSF DBI-0420580 for funds to purchase the Tecnai 12 Biotwin electron microscope. Antibodies directed against PDF10A were generously provided by Pfizer, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends in Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Mugnaini E. Distribution and targets of the cartwheel cell axon in the dorsal cochlear nucleus of the guinea pig. Anat Embryol (Berl) 1991;183:427–454. doi: 10.1007/BF00186433. [DOI] [PubMed] [Google Scholar]
- Coskran TM, Morton D, Menniti FS, Adamowicz WO, Kleiman RJ, Ryan AM, Strick CA, Schmidt CJ, Stephenson DT. Immunohistochemical Localization of Phosphodiesterase 10A in Multiple Mammalian Species. J Histochem and Cytochem. 2006;54:1205–1213. doi: 10.1369/jhc.6A6930.2006. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83:926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- Douyard J, Shen L, Huganir RL, Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit, and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J Comp Neurol. 2007;502:141–156. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Roullier EM, Liberman MC, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol. 1984;229:432–450. doi: 10.1002/cne.902290311. [DOI] [PubMed] [Google Scholar]
- Friauf E. Distribution of calcium-binding pretein calbindin-D28k in the auditory system of adult and developing rats. J Comp Neurol. 1994;349:193–221. doi: 10.1002/cne.903490204. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Nat Aca Sci. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. J Neurosci. 1999;19:8721–8729. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci. 2001;21:7428–7437. doi: 10.1523/JNEUROSCI.21-18-07428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78:248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebralcortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson TD, Strominger NL. Morphological changes in the cochlear complex in primate phylogeny and development. J Morphol. 1985;186:289–306. doi: 10.1002/jmor.1051860306. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, EL Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Hirai H, Launey T, Mikawa S, Torashima T, Yanagiraha D, Kasura T, Miyamoto A, Yuzaki M. New role of δ2-glutamate receptors in AMPA trafficking and cerebellar function. Nat Neurosci. 2003;6:869–876. doi: 10.1038/nn1086. [DOI] [PubMed] [Google Scholar]
- Hurd LB, 2nd, Feldman ML. Purkinje-like cells in rat cochlear nucleus. Hear Res. 1994;72:143–158. doi: 10.1016/0378-5955(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Illing RB, Reisch A. Specific plasticity responses to unilaterally decreased or increased hearing intensity in the adult cochlear nucleus and beyond. Hear Res. 2006;216–217:189–197. doi: 10.1016/j.heares.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206:200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci. 2001;21:7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. An atlas of glycine-like and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat embryol. 1992;186:443–465. doi: 10.1007/BF00185459. [DOI] [PubMed] [Google Scholar]
- Manis PB, Molitor SC. N-methyl-D-aspartate receptors at parallel fiber synapses in the dorsal cochelar nucleus. J Neurophysiol. 1996;76:1639–1659. doi: 10.1152/jn.1996.76.3.1639. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE, Rubio ME. High concentration rapid transient of glutamate mediates neuron-glia communication via ectopic release. Journal of Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayat E, Petralia RS, Wang XY, Wenthold RJ. Immunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor delta subunits form novel postsynaptic receptor complexes. J Neurosci. 1995;15:2533–2546. doi: 10.1523/JNEUROSCI.15-03-02533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behaviour of cats. Hear Res. 2000;148:74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Molitor SC, Manis PB. Evidence for functional metabotropic glutamate receptors in the dorsal cochlear nucleus. J Neurophysiol. 1997;77:1889–1905. doi: 10.1152/jn.1997.77.4.1889. [DOI] [PubMed] [Google Scholar]
- Moore JK. The primate cochlear nuclei: loss of lamination as a phylogenetic process. J Comp Neurol. 1980;193:609–629. doi: 10.1002/cne.901930303. [DOI] [PubMed] [Google Scholar]
- Moore JK, Osen KK. The cochlear nuclei in man. Am. J. Anat. 1979;154:393–418. doi: 10.1002/aja.1001540306. [DOI] [PubMed] [Google Scholar]
- Moskowitz N. Comparative aspects of some features of the central auditory system of primates. Ann New York Acad Sci. 1969;167:357–369. [Google Scholar]
- Mugnaini E, Osen KK, Dahl AL, Friedrich VL, JR, Korte G. Fine structure of granule cells and related interneurons (termed Golgi cells) in the cochlear nuclear comples of the cat, rat and mouse. J Neurocytol. 1980;9:225–238. doi: 10.1007/BF01204841. [DOI] [PubMed] [Google Scholar]
- Osen KK. Course and termination of the primary afferents in the cochlear nuclei of the cat. An experimental anatomical study. Arch Ital Biol. 1970;108:21–51. [PubMed] [Google Scholar]
- Osen KK, Ottersen OP, Storm-Mathissen J. Colocalization of glycine-like and GABA immunoreactivities: semiquantitative study of individual neurons in the dorsal cochelar nucleus. In: ottersen OP, storm-Mathisen J, editors. Glycine neurotransmission. Chichester, UK: John Wiley & Sons; 1990. pp. 417–451. [Google Scholar]
- Ostapoff EM, Benson CG, Saint Marie RL. GABA- and glycine- immunoreactivity projections from the superior olivary complex to the cochlear nucleus in guinea pig. J Comp Neurol. 1997;381:500–512. doi: 10.1002/(sici)1096-9861(19970519)381:4<500::aid-cne9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Zhao H-M, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic and glial localization in the dorsal cochlear nucleus. J Comp Neurol. 1996;372:356–383. doi: 10.1002/(SICI)1096-9861(19960826)372:3<356::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Benson CG, Ostapoff E-M, Lindberg N, Morest DK. Glycine and GABA: transmitter candidates of projections descending to the cochlear nucleus. In: Merchan M, Juiz J, Godfrey D, Mugnaini E, editors. The mmamalian cochlear nuclei: organization and function. New York: Plenum Press; pp. 195–210. [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in the rat. Hearing Research. 2006;216–217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Chemical anatomy of excitatory endings in the dorsal cochlear nucleus of the rat: differential synaptic distribution of aspartate aminotransferase, glutamate, and vesicular zinc. J Comp Neurol. 1998;399:341–358. doi: 10.1002/(sici)1096-9861(19980928)399:3<341::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Juiz JM. Differential distribution of synaptic endings containing glutamate, glycine, and GABA in the rat dorsal cochlear nucleus. J Comp Neurol. 2004;477:253–272. doi: 10.1002/cne.20248. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Haenggeli C-A, Doucet JR. Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res. 2003;153:477–485. doi: 10.1007/s00221-003-1605-3. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, May SK. The projections of intracellularly labelled auditory fibers to the dorsal cochlear nucleus in cats. J Comp Neurol. 1993;329:20–35. doi: 10.1002/cne.903290103. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Wright DD, Sharp AH. Inositol 1,4,5-triphosphate receptors: immunocytochemical localization in the dorsal cochlear nucleus. J Comp Neurol. 1995;358:102–118. doi: 10.1002/cne.903580107. [DOI] [PubMed] [Google Scholar]
- Sando I. The anatomical interrelationships of the cochlear nerve fibers. Acta otolaryngol. 1965;59:417–436. [Google Scholar]
- Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–126. doi: 10.1016/s0006-8993(03)02754-9. [DOI] [PubMed] [Google Scholar]
- Saint-Marie R, Benson CG, Ostapoff E-M, Morest K. Glycine immunoreactive projections from the dorsal to the anteroventral cochlear nucleus. Her Res. 1991;51:11–28. doi: 10.1016/0378-5955(91)90003-r. [DOI] [PubMed] [Google Scholar]
- Sharp AH, Dawson TM, Ross CA, Fotuhi M, Mourney RJ, Snyder SH. Inositol 1,4,5-triphosphate receptors: immunohistochemical localization to discrete areas of rat central nerous system. Neuroscience. 1993;53:927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Davis KA, Nelken I, Young ED. Spectral integration by type II interneurons in dorsal cochlear nucleus. J Neurophysiol. 1999;82:648–663. doi: 10.1152/jn.1999.82.2.648. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Young ED. Organization of dorsal cochlear nucleus type IV unit response maps and their relationship to activation by bandlimited noise. J Neurophysiol. 1991;66:1750–1768. doi: 10.1152/jn.1991.66.5.1750. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Control of synaptic depression by glutamate transporters. J Neurosci. 2000;20:2054–2063. doi: 10.1523/JNEUROSCI.20-05-02054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timingdependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchizono K. Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature. 1965;207:642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Mugniani E. Cartwheel neurons of the dorsal cochlear nucleus: A Golgi-electron microscopic study in rat. J Comp Neurol. 1984;227:136–157. doi: 10.1002/cne.902270114. [DOI] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365:159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol. 1995;73:743–765. doi: 10.1152/jn.1995.73.2.743. [DOI] [PubMed] [Google Scholar]
- Young ED, Davis KA. Circuitry and function of the dorsal cochlear nucleus. In: Oertel D, et al., editors. Integrative functions in the mammal auditory pathway. New York: Springer; 2002. pp. 160–206. [Google Scholar]
- Yuzaki M. The δ2 glutamate receptor: 10 years later. Neurosci Research. 2003;46:111–122. doi: 10.1016/s0168-0102(03)00036-1. [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Tuberculoventral cells of the dorsal cochlear nucleus of mice: intracellular recordings in slices. J Neurophysiol. 1993;69:1409–1421. doi: 10.1152/jn.1993.69.5.1409. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Petralia RS. Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. J Neurosci. 1998;18:5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Electronmicrographs show two inhibitory synaptic endings (SP) after single postembedding immunogold for GluR2/3 and double postembedding immunogold for GluR2 with VGLUT1. None of the synapses present gold particles for the AMPA subunits at the PSDs or contained VGLUT1 associated to the synaptic vesicles. Scale bar: 0.50 µm.


