Abstract
Chronic pain commonly accompanies long-term disabilities such as spinal cord injury (SCI). Research suggests that patient motivation to engage in adaptive pain coping strategies, such as exercise/stretching and task persistence, is an important factor in determining the impact that this pain will have on quality of life. One recently proposed model (the “Motivational Model of Pain Self-Management”) suggests that motivation to manage pain is influenced by two primary variables: beliefs about the importance of engaging in pain self-management (i.e., “perceived importance”) and beliefs about one's own ability to engage in these behaviors (i.e., “self-efficacy”). The purpose of this study was to provide a preliminary test of this model in a sample of 130 adults with SCI who completed a return by mail survey. Measures included a numerical rating scale of pain intensity and the revised version of the Multidimensional Pain Readiness to Change Questionnaire. Mediation analyses were performed using multiple regression. Results suggested that the effects of perceived importance and self-efficacy on exercise behavior were mediated by readiness to engage in exercise, consistent with the proposed model. However, the model could not be established for the outcome of task persistence. Perspective: This study tests a model describing motivation to engage in pain management behaviors (i.e., “readiness to change”) in adults with spinal cord injury. This model could potentially aid clinicians in their conceptualization of the factors that affect patient motivation to manage pain.
Introduction
Chronic pain commonly accompanies long-term disabilities such as spinal cord injury (SCI), amputation and neuromuscular disease.2,8 Although pharmacological interventions may be effective in managing certain sources of disability related pain (e.g., spasticity)38 they do not appear to provide meaningful pain relief for the majority of people with chronic pain secondary to disability.3,6,7,41,43
Consistent with the refractory nature of disability related pain, contemporary pain models and associated research suggest that adaptive pain management rests primarily with the patient - on the efficacy of his/her own attempts to change behaviors and cognitions associated with greater pain impact.5,26,35 In fact, several studies have demonstrated that a patient’s readiness to adopt a self-management approach predicts completion of pain self-management programs and program related goal accomplishment.4,16 Assessment of a patient’s readiness to engage in pain self-management may therefore have important implications for the tailoring of cognitive-behavioral and other interventions for pain.22, 23
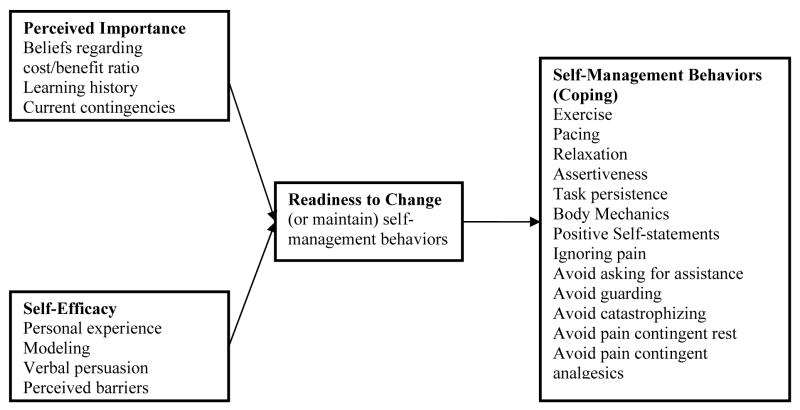
Recently a motivational model of pain self-management was presented that sought to describe the role of patient motivation on pain outcomes (see Fig 1).20 This model emphasizes motivation (or readiness to engage in pain management behaviors) as a final common pathway to adaptive coping. Motivation is in turn hypothesized to be influenced by two primary variables based on expectancy-value models of motivation: (1) beliefs about the importance of engaging versus not engaging in pain self-management (i.e., “perceived importance”) and (2) beliefs about one's own ability to engage in these behaviors (i.e., “self-efficacy”). The outcomes in this model are those coping behaviors or strategies that have been shown to be associated with positive outcomes in pain treatment (i.e., “self-management behaviors”).
Figure 1.
Motivational Model of Pain Self-Management
The purpose of this study was to test the Motivational Model of Pain Self-Management in a sample of adults with spinal cord injury related pain. SCI is commonly associated with a number of chronic pain problems, including musculoskeletal pain,15 paresthesias (abnormal sensations such as burning or prickling), dysesthesias (unpleasant sensations produced by touch) and allodynia (painful sensations that result from stimuli that do not normally cause pain).8,10,11,18,36 Recent evidence suggests that the vast majority of patients with SCI report chronic painful sensations, and that as many as 26% of these report the pain as severe.18,43 For the purposes of model testing, we chose to select two self-management behaviors, exercise and task persistence. Physical activity and exercise are consistently associated with a range of positive outcomes following SCI, including greater emotional well-being24,29 improved physical fitness12,17 and better physical self-concept.27 Exercise has also been demonstrated to be an effective pain-management strategy for individuals with both tetra and paraplegias 17,30 and exercise induced decreases in pain have been shown to mediate later decreases in subjective stress.25 Task persistence, or and attempt to engage or follow through on a task, has also been shown to be an adaptive strategy for coping with chronic pain9 although the importance of this coping response in persons with SCI has not yet been established.
Materials and methods
Participants
Participants in this study were 130 adults with SCI who completed a return by mail survey asking about SCI related pain, coping efforts, comorbid health problems and overall quality of life, and who reported ongoing chronic pain problems. The procedure was approved by the University of Washington Institutional Review Board, and informed consent was obtained from each participant. Previous data from this survey concerning the frequency and impact of the pain in the sample have previously been reported.19 The participants were primarily Caucasian (89.5%) men (71.5%), and were an average of 10.1 years from their injury (SD = 10.0). They ranged in age from 18–82 (M = 45) and the majority reported completing at least a high school education (95.4%). Fifty-nine percent reported being unemployed due to disability, while only 29% reported either full or part time employment. Thirty-five percent reported complete SCI, and the most common levels of injury were at C5-C7 (approx 25%) and T10-L1 (approx 20%). Demographic and clinical variables for these participants are reported in Table 1.
Table 1.
Demographic and Clinical Data
| Variable | M | SD | n | % |
|---|---|---|---|---|
| Age | 45.0 | 14.4 | 130 | |
| Average pain (last week) | 5.3 | 2.6 | 130 | |
| Gender | ||||
| Male | 93 | 71.5 | ||
| Female | 37 | 28.5 | ||
| Marital status: | ||||
| Single | 36 | 27.7 | ||
| Married | 66 | 50.7 | ||
| Divorced/Widowed | 28 | 21.5 | ||
| Education | ||||
| < 12th grade | 6 | 4.6 | ||
| High school or GED | 34 | 26.1 | ||
| Voc/Tech school | 11 | 8.5 | ||
| Some college | 38 | 29.2 | ||
| College graduate | 41 | 31.6 | ||
| Employment status† | ||||
| Full time | 26 | 20.0 | ||
| Part time | 12 | 9.2 | ||
| Retired | 24 | 18.5 | ||
| Unemployed | ||||
| Due to pain | 20 | 15.4 | ||
| Due to disability | 57 | 43.8 | ||
| Level of injury† | ||||
| C1-C8 | 118 | 90.7 | ||
| T1-T12 | 105 | 80.8 | ||
| L1-L5 | 35 | 26.9 | ||
| S1-S5 | 5 | 4.8 | ||
| Injury status | ||||
| Complete | 46 | 35.4 | ||
| Incomplete | 68 | 52.3 | ||
| Don’t know | 16 | 12.3 | ||
| Cause of SCI | ||||
| Gunshot wound | 6 | 4.6 | ||
| Fall | 17 | 13.1 | ||
| MVA | 59 | 45.4 | ||
| Sport accident | 19 | 14.6 | ||
| Other | 29 | 22.3 | ||
Represents non-orthogonal groups.
Measures
Pain intensity
Average pain intensity during the past week was assessed using the 0–10 Numerical Rating Scale (NRS), with 0 indicating “no pain” and 10 indicating “pain as bad as it could be.”
Readiness to change
Readiness to change was assessed using a revised version of the Multidimensional Pain Readiness to Change Questionnaire (MPRCQ).31 The revised version (MPRCQ.v2)32 is a 69-item measure that assesses willingness to adopt pain management skills in 9 areas, including exercise (7 items) and task persistence (5 items) utilized in the present study. The MPRCQ.v2 is divided into two sections, containing items describing adaptive and maladaptive coping. The MPRCQ has demonstrated adequate reliability and validity in samples of patients with fibromyalgia and SCI related pain.31 In the present sample, exercise and task persistence subscales of the MPRCQ.v2 demonstrated adequate internal consistency (Cronbach’s alpha = .83 and .75, respectively).
Perceived importance, self-efficacy and self-management behavior
In order to measure the key components of the Motivational Model of Pain Self-Management (perceived importance of and self-efficacy relating to self-management behaviors), a new 10-item scale was created. Perceived importance was assessed using the mean of three of these items, based on a 0–10 numeric rating scale. For exercise, the items were as follows: (1) “To what extent do you believe that regular exercise is important for managing your health and pain problem?” (2) “To what extent have you experienced direct and immediate benefits of exercise (such as encouragement from someone important to you, or feeling better right after you exercise) in the past?” and (3) “To what extent do you currently receive encouragement or other benefits when you exercise?” Internal consistency for these 3 items was excellent (Cronbach’s alpha = .84).
Perceived importance of task persistence was also assessed using the average of three items: (1) “How important is it to you, in managing your health and pain problem, to keep going despite the pain?” (2) “To what extent have you experienced direct and immediate benefits when you keep doing what you need to do despite pain in the past?” (3) “To what extent do you currently receive encouragement or other benefits when you keep going despite pain?” Internal consistency for the task persistence scale was marginal (Cronbach’s alpha = .72).
Self-efficacy for exercise and task persistence was assessed using one 0 – 10 item each. For task persistence, the item was “To what extent do you see yourself as having the ability to keep going with what you need to do despite any pain you might feel?” For exercise, it was “To what extent do you see yourself as having the resources (such as the time and energy) to exercise regularly if you choose to?”
Finally, self-management behaviors were assessed using a single item that asked the number of months in a row that a patient has been persisting with tasks despite the pain (task persistence) or exercising regularly (exercise).
Procedures
A survey that included the study measures was mailed to a total 426 individuals, identified through a combination of sources including study brochures and flyers, physician referrals, and the mailing list of the Northwest Regional Spinal Cord Injury System (NWRSCIS), a service delivery model system funded in part by the National Institute on Disability and Rehabilitation Research. A subset (223) had also responded to one of two previous surveys of chronic pain problems in persons with SCI 4–8 years prior to the current study.39,40 Of the 426 surveys, 163 were returned, yielding a response rate of 38.2%. All participants signed an informed consent document approved by the University of Washington IRB and were paid $25 for participation. Of the 163 individuals who returned the survey, thirty-three reported experiencing no problems with pain in the past 3 months, and were excluded in subsequent analyses, yielding a final n of 130.
Preliminary data analysis
Prior to further statistical analysis, we evaluated self-management behaviors (i.e., the outcomes) and potential mediators for normality. Exercise behavior, perceived importance of exercise and exercise self-efficacy all demonstrated normality statistics that were acceptable for use as continuous variables in multiple regression, although exercise behavior did tend towards a bimodal distribution (31.2% indicated no exercise, and 38.5% indicated 12 months of exercise or more). Task persistence behavior demonstrated significant negative skew and leptokurtosis (Skewness = −1.9; Kurtosis = 2.3). On closer examination, it was apparent that the vast majority of individuals (more than 75%) indicated that they “have used the approach ‘keep going despite the pain’ for 12 or more months.” Attempts were made to normalize this distribution using exponential and natural log transformations, but given the severity of this ceiling effect, we were unable to create a normal distribution. As a result of this skew, as well as marginal internal consistency for this scale, we were unable to perform mediation analyses on task persistence, and so continued with the planned analyses only for exercise. This issue is discussed further in the Discussion section, below.
Mediation analysis
To evaluate the model, we performed mediation analyses described by Baron & Kenny1 and following more recent guidelines presented by Preacher & Hayes.2354 In traditional mediation, multiple regression is used to determine the strength of the association between predictor and outcome variables both before and after the inclusion of a third (mediating) variable. Although Baron & Kenny describe mediation as resulting when the effect of the predictor on the outcome (i.e., the total effect) is reduced to zero after inclusion of the mediating variable, Preacher & Hayes emphasize the importance of measuring the size and statistical significance of the indirect effect (i.e., the effect of the predictor through the mediator) as opposed to simple reduction of the total effect. The Sobel statistic2376 is a widely used test of the strength of the indirect effect, and was included in our analyses.
Separate mediation analyses were performed on the outcome of exercise behavior (months of regular exercise) for each of two predictor variables: perceived importance of exercise and exercise self-efficacy. Readiness to change exercise behavior served as the mediator in each model. Given that this plan called for the estimation of coefficients in two separate mediational models, alpha was adjusted to p < .025 (i.e., .05 /2) accordingly.
Consistent with the Baron & Kenny approach, we first established the assumptions of mediation in each model in three steps: (1) first by regressing the outcome variable on the predictor variable, (2) second by regressing the mediator on the predictor, and (3) finally by regressing the outcome on the mediator after controlling for the predictor. After establishing the existence of relationships among these variables, we tested mediation via evaluation of the indirect path using the Sobel statistic.
It is important to note that the use of cross-sectional data poses significant limitations for mediation analyses, as one cannot make statements about causality in correlations determined at a single time point. Although mediation is perhaps the most appropriate test given the model to be tested, the results need to be interpreted with caution.
Results
Demographics
Demographic and SCI-related descriptive information are presented in Table 1. Consistent with previous data (e.g., those reported by Jensen, Hoffman & Cardenas),19 130 of 163 participants (79%) in this study reported chronic pain associated with SCI. Of the 130 reporting pain problems, 51% reported chronic “severe” pain (i.e., ≥7). The most common sites of pain were the shoulder (44%) the lower back (46%) and the legs (37%).
Regression analyses: Predicting exercise behavior
Perceived importance
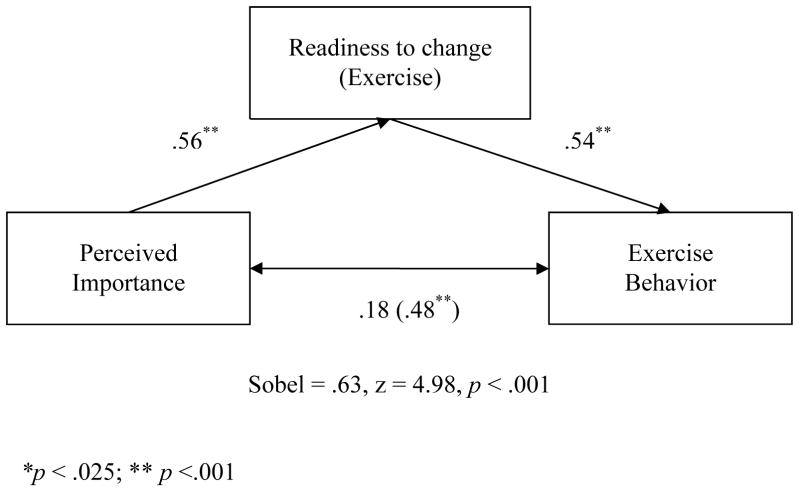
In simple regression, the effect of perceived importance was significant (β = .48, p < .001) and accounted for 23% of the variance in exercise behavior. The effect of perceived importance on the mediator (readiness to exercise) was also significant (β =.56, p < .001), accounting for 31% of the variance. With the inclusion of readiness to exercise, the effect of perceived importance on exercise behavior decreased considerably to β = .18 (p = .04; insignificant after alpha correction) while the effect of readiness to exercise on exercise behavior was significant (β = .54, p < .001). Finally, the Sobel statistic testing the strength of the indirect path was significant (Sobel = .63, z = 4.98, p < .001) supporting mediation. These results are presented graphically in Figure 2.
Figure 2.
Mediation of Exercise Behavior/Perceived Importance Relationship by Readiness
Self-efficacy
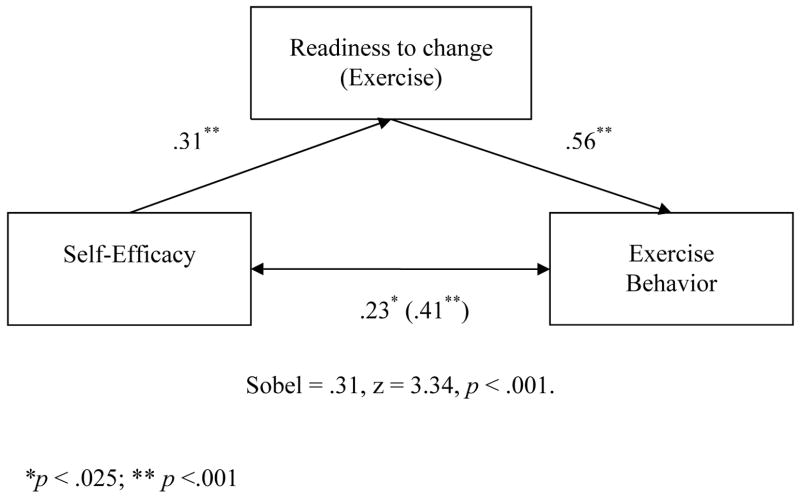
As was true for perceived importance, the effect of self-efficacy on exercise behavior was significant (β = .41, p < .001) and accounted for 16% of the variance in the model. The effect of self-efficacy on readiness to exercise was also significant (β = .31, p < .001), accounting for 9% of the variance. In the mediational model, the effect of readiness to exercise on exercise behavior was significant (β = .56, p < .001) while the effect of self-efficacy on exercise behavior decreased, but remained significant (β = .23, p = .01). The Sobel statistic testing the strength of the indirect path was significant (Sobel = .31, z = 3.34, p < .001) supporting mediation. These results are presented graphically in Figure 3.
Figure 3.
Mediation of Exercise Behavior/Self-Efficacy Relationship by Readiness
Discussion
Comprehensive management of chronic pain is perhaps one of the most demanding and complicated challenges in modern rehabilitation medicine. Chronic pain is ubiquitous in populations with disabilities such as spinal cord injury, and in many cases does not readily respond to pharmacological intervention. Rather, pain physicians and psychologists are faced with the difficult balancing act of providing external support (e.g., medication, education, and physical therapy, as indicated) while stimulating and nurturing a patient’s internal resources to change behaviors associated with pain. Given that readiness and motivation play an important role in whether or not people make and follow-through with behavioral changes, there has been considerable recent interest on methods for increasing patient motivation and readiness to change regarding pain management20,21 and for models accounting for patient motivational factors.20
Results of this study provide additional preliminary support for the Motivational Model of Pain Self-Management.20 Specifically, we found that the vast majority of individuals in this study reported feeling that exercise as pain management was important to them, and that the felt they had the time and resources to exercise regularly. Further, patient self-efficacy, perceived importance and patient readiness to change were positively associated with self-reports of exercise behavior to reduce pain. As predicted, we also found that the effects of self-efficacy and perceived importance were mediated by readiness to change, as reflected in a measure of the patient’s expressed interest in exercise. These data can be interpreted to suggest that patient readiness to make changes in exercise behavior is influenced by their perception of the importance of exercise and their perception of their own ability to exercise. In turn, patient readiness to exercise is a strong predictor of reported exercise behavior.
Contrary to our expectations, the motivational model could not be established for the coping behavior of task persistence. It appears that the measures of task persistence used here, while reasonably well validated in other groups, did a poorer job of measuring task persistence in the present sample. Internal consistency for our measure of motivation to change task persistence behavior was marginal, and the outcome of actual task persistence demonstrated a dramatic negative skew; a large majority of the participants in this study reported that they were using this adaptive coping strategy. It may be that response bias played a role for these participants, who felt that the answer “I have been persisting with tasks despite pain for at least 12 months” was simply the most appropriate response. Alternately, it could be that the experience of SCI forces an individual to adopt a stance of task persistence, and these data reflect actual rates of this coping behavior. In any case, poor measurement and statistical considerations interfered with our ability to test our hypotheses related to task persistence in this sample.
We emphasize that this study is preliminary, and was designed to provide some pilot information for further testing and development of the motivational model. As such, this study has some significant limitations that should be considered. Most importantly, our sample was cross-sectional, which limits our ability to make causal or directional statements regarding our mediational analyses. Relatedly, the results of our mediational analyses would be considered only “partial mediation” by the traditional approach described in Baron and Kenny.1 However, more recent work in this area emphasizing the size of the indirect effect (over simple drop in the beta coefficient) supports our position of full mediation.35 In any case, the remaining effects of self-efficacy and perceived importance on exercise behavior were quite small after inclusion of patient readiness to change (i.e., β’s < .20), suggesting that much of the effects of self-efficacy on actual exercise behavior is indeed mediated via its effects on patient motivation.
Other limitations are noted in the selection of survey instruments. While survey scales were selected to minimize participant burden, use of single-item measures of key constructs such as exercise falls well short of what could be hoped for. We also relied on a new scale (the MPRCQv2)32 which will require additional psychometric evaluation in future studies. The homogeneity of our sample (i.e., predominantly Caucasian men) also limits the generalizability of the findings to other SCI populations, including samples of women and minority groups with SCI. Future work should include more demographically and clinically diverse populations and utilize well-validated measures in a longitudinal design. Finally, the response rate of 38.2%, while consistent with other survey studies in this population, leads to the possibility of sampling bias. Unfortunately, no information was available regarding individuals who declined to participate as these individuals did not provide informed consent or complete any study measures.
Limitations notwithstanding, these data add to a growing literature emphasizing the importance of patient readiness to alter behavior in the management of chronic pain. Results also suggest that the many patients with SCI understand the importance of exercise and have interest in using physical activity to manage pain. This understanding may be adaptive for many persons with SCI and pain, given the evidence that exercise can reduce pain and improved quality of life in this population.24,30,33
The findings from this study also have important clinical implications, suggesting that motivation or readiness to change behavior may be a useful target for interventions designed to increase adaptive coping responses. The findings are also consistent with the idea that readiness to change may be influenced using approaches that target perceived importance and/or coping self-efficacy directly. One such approach is Motivational Interviewing,28 which may be helpful for this population.13 However, few clinical trials of this approach have been published in persons with SCI, and to date no interventions based directly on the Motivational Model of Pain Self-Management have yet been established. Our findings suggest that such research is warranted.
Acknowledgments
This research was supported by grant number P01 HD33988 from the National Institutes of Health, National Institute of Child Health and Human Development (National Center for Medical Rehabilitation Research). The authors gratefully acknowledge the contributions of Kevin Gertz, Katherine Raichle, Travis Osborne, Amy Hoffman, Beth Gerrard, Bridget Bjork, Catherine McClellan, Erica Tyler, Cindy Davis, and Kim McKearnan, University of Washington Department of Rehabilitation Medicine, in data collection and database management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 2.Benrud-Larson L, Wegener S. Chronic pain in neurorehabiolitation populations: prevalence, severity and impact. Neurorehabilitation. 2000;14:127–137. [PubMed] [Google Scholar]
- 3.Beric A. Post-spinal cord injury pain states. Pain. 1997;72:295–298. [PubMed] [Google Scholar]
- 4.Biller N, Arnstein P, Caudill M, Federman C, Guberman C. Predicting completion of a cognitive-behavioral pain management program by initial measures of a chronic pain patient’s readiness for change. Clinical Journal of Pain. 16:352–359. doi: 10.1097/00002508-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Bradley L. Cognitive-behavioral therapy for chronic pain. In: Gatchel R, Turk D, editors. Psychological Approaches to Pain Management: A Practitioner’s Handbook. New York, NY: 1996. [Google Scholar]
- 6.Cardenas D, Warms C, Turner J, Marshall H, Brooke M, Loeser J. Efficacy of amitryptiline for relief of pain in spinal cord injury: Results of a randomized controlled trial. Pain. 2002;96:127–134. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 7.Davidoff G, Guarrancini M, Roth E, Sliewa J, Yarkony G. Trazodone hydrochloride in the treatment of dysesthetic pain in traumatic myelopathy: A randomized, double-blind, placebo-controlled study. Pain. 1987;29:151–161. doi: 10.1016/0304-3959(87)91032-3. [DOI] [PubMed] [Google Scholar]
- 8.Ehde D, Jensen M, Engel J, Turner J, Hoffman A, Cardenas D. Chronic pain secondary to disability: A review. Clinical Journal of Pain. 2003;19:197–210. doi: 10.1097/00002508-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ersek M, Turner J, Kemp C. Use of the chronic pain coping inventory to assess older adults’ pain coping strategies. Journal of Pain. 2006;7:833–842. doi: 10.1016/j.jpain.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Finnerup N, Johannesen I, Sindrup S, Bach F, Jensen T. Pain and dysethesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 11.Frisbie J, Aguilera E. Chronic pain after spinal cord injury: An expedient diagnostic approach. Paraplegia. 1990;28:460–465. doi: 10.1038/sc.1990.62. [DOI] [PubMed] [Google Scholar]
- 12.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- 13.Habib S, Morrissey S, Helmes E. Preparing for pain management: a pilot study to enhance engagement. Journal of Pain. 2005;6:48–54. doi: 10.1016/j.jpain.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hadjistavropoulos H, Shymkiw J. Predicting readiness to self-manage pain. Clinical Journal of Pain. 2007;23:259–266. doi: 10.1097/AJP.0b013e31802f67f3. [DOI] [PubMed] [Google Scholar]
- 15.Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Post MW, Bussman JB. Complications following spinal cord injury: Occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. Journal of Rehabilitation Medicine. 2007;39:393–398. doi: 10.2340/16501977-0067. [DOI] [PubMed] [Google Scholar]
- 16.Heapy A, Otis J, Stein Marcus K, Frantsve LM, Janke EA, Shulman M, Bellmore W, Kerns RD. Intersession coping skill practice mediates the relationship between readiness for self-management treatment and goal accomplishment. Pain. 118:360–368. doi: 10.1016/j.pain.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, McCatney N. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- 18.Jensen M, Hanley M, Turner J, Cardenas D. Pain and other sensation in persons with spinal cord injury: Frequency and association with depression and pain interference. Psychologica. 2004;37:129–143. [Google Scholar]
- 19.Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43:704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- 20.Jensen M, Nielson W, Kerns R. Toward the development of a motivational model of pain self-management. The Journal of Pain. 2003;4:477–492. doi: 10.1016/s1526-5900(03)00779-x. [DOI] [PubMed] [Google Scholar]
- 21.Kerns R, Bayer L, Findley J. Motivation and adherence in the management of chronic pain. In: Block A, Kremer E, Fernandex E, editors. Handbook of pain syndromes: Biopsychosocial perspectives. Mahwah, NY: 1999. [Google Scholar]
- 22.Kerns RD, Habib S. A critical review of the pain readiness to change model. The Journal of Pain. 2004;5:357–367. doi: 10.1016/j.jpain.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Kerns RD, Rosenberg R. Predicting responses to self-management treatments for chronic pain: Application of the pain stages of change model. Pain. 2000;84:49–55. doi: 10.1016/S0304-3959(99)00184-0. [DOI] [PubMed] [Google Scholar]
- 24.Latimer AE, Ginis KA, Hicks AL. Buffering the effects of stress on well-being among individuals with spinal cord injury: A potential role for exercise. Therapeutic Recreation Journal. 2005;39:131–138. [Google Scholar]
- 25.Latimer AE, Ginis KA, Hicks AL, McCartney N. An examination of the mechanisms of exercise-induced change in psychological well-being among people with spinal cord injury. Journal of Rehabilitation Research and Development. 2004;41:643–652. doi: 10.1682/jrrd.2003.04.0043. [DOI] [PubMed] [Google Scholar]
- 26.Loeser J, Turk D. Multidisciplinary pain management. In: Loeser J, Batler S, Chapman C, Turk D, editors. Bonica’s Management of Pain. Philadelphia, PA: 2003. [Google Scholar]
- 27.Martin Ginis KA, Latimer AE, McKecknie K, Ditor DS, McCartney N, Hicks AL, Bugaresti J, Craven BC. Using exercise to enhance subjective well-being among people with spinal cord injury: The mediating influences of stress and pain. Rehabilitation Psychology. 2003;48:157–164. [Google Scholar]
- 28.Miller W, Rollnick S. Motivational Interviewing: Preparing people for change. 2. New York: NY: Guilford Press; 2002. [Google Scholar]
- 29.Muraki S, Tsunawake N, Hiramatsu S, Yamasaki M. The effects of frequency and mode of sports activity on the psychological status in tetraplegics and paraplegics. Spinal Cord. 2000;38:309–341. doi: 10.1038/sj.sc.3101002. [DOI] [PubMed] [Google Scholar]
- 30.Nawoczenski D, Ritter-Soronen J, Wilson C, Howe B, Ludewig P. Clinical trial of exercise for shoulder pain in chronic spinal injury. Physical Therapy. 2006;86:1604–1618. doi: 10.2522/ptj.20060001. [DOI] [PubMed] [Google Scholar]
- 31.Nielson W, Jensen M, Kerns R. Initial development and validation of a multidimensional pain readiness to change questionnaire. The Journal of Pain. 2003;4:148–158. doi: 10.1054/jpai.2003.436. [DOI] [PubMed] [Google Scholar]
- 32.Nielson WR, Jensen MP, Ehde DM, Kerns RD, Molton IR. Further development of the Multidimensional Pain Readiness to Change Questionnaire: The MPRCQ2. The Journal of Pain. 2008 doi: 10.1016/j.jpain.2008.01.327. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrbrink B, Kowalski J, Lundeberg T. A comprehensive pain management programme comprising educational, cognitive and behavioral interventions for neuropathic pain following spinal cord injury. Journal of Rehabilitation Medicine. 2006;38:172–180. doi: 10.1080/16501970500476258. [DOI] [PubMed] [Google Scholar]
- 34.Novy D, Nelson D, Francis D, Turk D. Perspectives of chronic pain: An evaluative comparison of restrictive and comprehensive models. Psychological Bulletin. 1995;118:238–247. doi: 10.1037/0033-2909.118.2.238. [DOI] [PubMed] [Google Scholar]
- 35.Preacher K, Hayes A. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments & Computers. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 36.Siddal P, Loeser J. Pain following spinal cord injury. Spinal Cord. 2001;39:63–77. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- 37.Sobel M. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhart S, editor. Sociological Methodology 1982. San Francisco, CA: 1982. pp. 290–312. [Google Scholar]
- 38.Taricco M, Pagliacci M, Telaro E, Adone R. Pharmacological interventions for spasticity following spinal cord injury: Results of a Cochrane systematic review. Europa Medicophysica. 2006;42:5–15. [PubMed] [Google Scholar]
- 39.Turner J, Cardenas D. Chronic pain problems in individuals with spinal cord injuries. Seminars in Clinical Neuropsychiatry. 1999;4:16–194. doi: 10.153/SCNP00400186. [DOI] [PubMed] [Google Scholar]
- 40.Turner J, Cardenas D, Warms C, McClellan C. Chronic pain associated with spinal cord injuries: A community survey. Archives of Physical Medicine and Rehabilitation. 2001;82:501–508. doi: 10.1053/apmr.2001.21855. [DOI] [PubMed] [Google Scholar]
- 41.Warms C, Turner J, Marshall H, Cardenas D. Treatments for chronic pain associated with spinal cord injuries: Many are tried, few are helpful. Clinical Journal of Pain. 2002;18:154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Widerstrom-Noga E, Felipe-Cuervo E, Broton J, Duncan R, Yezierski R. Perceived difficulty in dealing with consequences of spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1999;80:580–586. doi: 10.1016/s0003-9993(99)90203-4. [DOI] [PubMed] [Google Scholar]
- 43.Widerstrom-Noga E, Turk D. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: Influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–609. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]